Can γ-MgH2 improve the hydrogen storage properties of magnesium?†
Abstract
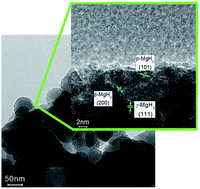
Upon hydrogen absorption, magnesium (Mg) spontaneously converts to the thermodynamically favoured β-MgH2 phase, but this leads to hydrogen release/uptake far from the desired low temperatures and fast kinetics. Conversion to the metastable γ-MgH2 phase should lead to improved hydrogen sorption properties. However, experimental verification of such a hypothesis remained distant. In this work, we report the electrochemical synthesis of nanosized Mg leading to the formation of a mixed γ/β hydride phase upon hydrogen absorption with a high γ-MgH2 content (29.6%). More remarkably, full release of hydrogen from these phases occurred at 200 °C only and upon hydrogen reabsorption at 100 °C, the γ/β-MgH2 mixture was restored. It was thus possible to determine for the first time the effect of γ-MgH2 on the kinetic and thermodynamic properties of the Mg/H2 reaction. The presence of γ-MgH2 was found to lead to a significant reduction of the apparent activation energy from 106.2 ± 4.0 to 69.1 ± 2.9 kJ mol−1 and a reduction of the Mg/H2 reaction enthalpy from 74.8 ± 1.0 to 57.7 ± 5.3 kJ mol−1 H2. However, this was accompanied by a concomitant decrease in entropy limiting the reduction of hydrogen desorption temperatures. γ-MgH2 can thus lead to improve hydrogen kinetics but to reach ambient hydrogen uptake and release ways to tune the enthalpy/entropy compensation effects have to be devised.


 Please wait while we load your content...
Please wait while we load your content...