Molecular engineering of face-on oriented dopant-free hole transporting material for perovskite solar cells with 19% PCE†
Abstract
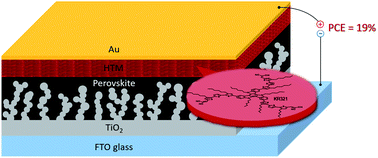
Through judicious molecular engineering, novel dopant-free star-shaped D–π–A type hole transporting materials coded KR355, KR321, and KR353 were systematically designed, synthesized and characterized. KR321 has been revealed to form a particular face-on organization on perovskite films favoring vertical charge carrier transport and for the first time, we show that this particular molecular stacking feature resulted in a power conversion efficiency over 19% in combination with mixed-perovskite (FAPbI3)0.85(MAPbBr3)0.15. The obtained 19% efficiency using a pristine hole transporting layer without any chemical additives or doping is the highest, establishing that the molecular engineering of a planar donor core, π-spacer and periphery acceptor leads to high mobility, and the design provides useful insight into the synthesis of next-generation HTMs for perovskite solar cells and optoelectronic applications.
- This article is part of the themed collections: 2020 Journal of Materials Chemistry Lectureship Winner: Giulia Grancini and International Year of the Periodic Table : From Pb and Sn Perovskites to the Next Generation


 Please wait while we load your content...
Please wait while we load your content...