Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reversible hydrogen storage in yttrium aluminum hydride†
Zhijie
Cao
abc,
Liuzhang
Ouyang
*abd,
Hui
Wang
ab,
Jiangwen
Liu
ab,
Michael
Felderhoff
 *c and
Min
Zhu
ab
*c and
Min
Zhu
ab
aSchool of Materials Science and Engineering, Key Laboratory of Advanced Energy Storage Materials of Guangdong Province, South China University of Technology, Guangzhou, 510641, PR China. E-mail: meouyang@scut.edu.cn; Fax: +86-20-87112762
bChina-Australia Joint Laboratory for Energy & Environmental Materials, South China University of Technology, Guangzhou, 510641, PR China
cMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany. E-mail: felderhoff@mpi-muelheim.mpg.de; Tel: +49-2083062368
dKey Laboratory for Fuel Cell Technology in Guangdong Province, Guangzhou, 510641, PR China
First published on 17th March 2017
Abstract
Reversible hydrogen storage has been found in transition metal alanates, Y(AlH4)3, for the first time. An amount of 3.4 wt% H2 can be released at 140 °C from the first dehydrogenation step of Y(AlH4)3, and 75% of it is reversible at 145 °C and 100 bar H2, which holds promise for low-temperature applications.
Pure hydrogen is an ideal energy source for proton exchange membrane (PEM) fuel cell vehicles. However, large-scale hydrogen application in the field of PEM fuel cells, especially for those with operating temperatures below 100 °C, is limited due to the absence of a safe and effective hydrogen storage approach.1,2 A possible solution could be a novel hybrid tank system, which combines a high-pressure tank with unstable metal hydrides. This system shows obvious advantages in terms of gravimetric and/or volumetric hydrogen density compared to high pressure, solid state or liquid hydrogen storage techniques.3
Unstable metal hydrides for hybrid tank systems have the characteristic of high desorption plateau pressures, meaning that they don't exist under ambient conditions (room temperature, 1 bar pressure). This high desorption plateau pressure is the result of a decomposition enthalpy (ΔH) of the metal hydrides of less than 20 kJ mol−1 H2. Consequently, these unstable hydrides can be synthesized only at low temperatures and/or under very high hydrogen pressure conditions.4 To achieve needs for future metal hydride/fuel cell applications, it is important to consider the overall system performance and not just the properties of materials. Nevertheless, large hydrogen capacity, sufficient kinetics at low temperatures and excellent long-term stability are also very important material properties. To date, several unstable metal hydrides including AB2-type5–7 and V-based BCC alloys8,9 have been investigated for a hybrid tank system. Until now, their reversible hydrogen capacity (<2 wt%) is too low to meet practical requirements. It is believed that an unstable metal hydride with a reversible capacity of 4 wt% can bring this hybrid system close to an attractive level.10 However, novel unstable hydrides like light weight alloys or complex hydride systems with higher hydrogen capacity are more favorable.
During the past two decades, light complex aluminium hydrides like NaAlH4, LiAlH4, KAlH4, etc., have been intensively investigated as potential candidates for solid-state hydrogen storage due to their relatively high capacity, moderate absorption/desorption conditions and good reversibility in the presence of catalysts.11–15 For example, TiCl3-doped NaAlH4 showed a high reversible capacity of ∼4 wt% H2 during 100 de/rehydrogenation cycles at relatively low temperatures of 70 and 270 °C.11 On the other hand, LiAlH4 can't be synthesized directly in the solid state from commercially available LiH and Al powders. The synthesis is only possible in an ether solution. LiAlH4 can release more than 7 wt% H2 with an onset dehydrogenation temperature of 80 °C, and the dehydrogenated sample can be recycled in an ether solution with a retention capacity of 6.4 wt% even after 3 cycles.12 For these light aluminium hydrides, the hydrogen capacities of the first decomposition steps are not sufficient, while the decomposition temperatures for the second decomposition steps are too high, which makes them inappropriate for low temperature fuel cells.16 Besides these light aluminium hydrides, several thermodynamically unstable transition metal aluminium hydrides with a high hydrogen content like Ti(AlH4)4 (9.3 wt% H2), Fe(AlH4)2 (5.8 wt% H2), or Y(AlH4)3 (6.6 wt% H2) are described, but have attracted less attention.17 The general synthesis of these transition metal complex aluminium hydrides M(AlH4)n (M = Ti, V, Co, Mn, Fe, Cu, Zr, Nb, Ag, Ce, Ta, etc.) was carried out at very low temperatures between −110 and −80 °C because the decomposition and hydrogen release start in most cases at −50 °C or below.18 For instance, Ti(AlH4)4 and Fe(AlH4)2 start to decompose slowly above −80 °C and two hydrogen atoms are liberated while heating to room temperature.19 Compounds [RE(AlH4)3] (RE = La, Ce, Pr) already start to evolve hydrogen and form RE aluminium hydride REAlH6 and Al metal during the ball milling process of rare earth chlorides and sodium aluminium hydride.16 Among all these transition metal complex aluminium hydrides, the relatively high stability of TaH2(AlH4)2 and Y(AlH4)3 is remarkable.20–22 The thermal decomposition of TaH2(AlH4)2 occurs in the interval of 135–195 °C, and the metal–hydrogen bonds are even retained after hydrolysis.20,21 Y(AlH4)3 was reported to be quite stable and starts to decompose at 50 °C.22 Y(AlH4)3 has a high theoretical hydrogen content of 6.6 wt%, while until now no further information is known about its hydrogen storage properties and reversibility. In this work, in order to continue to explore new unstable high capacity hydrides (>4 wt%), Y(AlH4)3 was prepared via a mechanochemical reaction. The dehydrogenation mechanism, hydrogen storage properties, reversibility and prospect for hydrogen storage were systematically evaluated. The preliminary results of the kinetics and reversibility of this alanate are quite interesting, which encourages us to place more effort into exploring new types of unstable hydrides with favorable hydrogen storage properties.
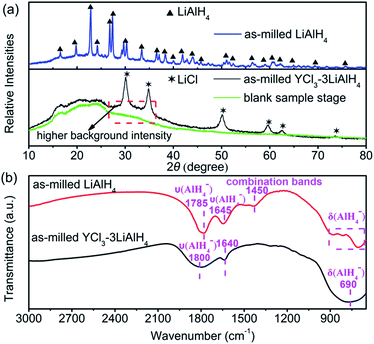
The mechanochemical metathesis reaction is a convenient and efficient procedure for the synthesis of complex aluminium hydrides.23Fig. 1(a) presents the XRD patterns of the as-milled YCl3–3LiAlH4 mixture and LiAlH4 milled under the same conditions. For comparison the XRD pattern of the blank sample stage was also presented. Pure LiAlH4 remains in a highly crystalline state after ball milling for 6 h. For the as-milled YCl3–3LiAlH4 mixture, an exchange reaction between 3LiAlH4 and YCl3 initiated by ball milling would theoretically lead to the formation of Y(AlH4)3 and 3LiCl, while here only the diffraction peaks of LiCl can be observed with the absence of any other phases. Moreover, it is noteworthy that the background intensity of the as-milled YCl3–3LiAlH4 sample between 26° and 36° is obviously higher than that of the blank sample stage, suggesting a possible amorphous nature of Y(AlH4)3. Actually the amorphous structure was verified after the removal of LiCl via an extraction with diethylether and drying procedure (Fig. S1†). This result is consistent with the observation by Kost et al.,22 who also demonstrated the amorphous nature of Y(AlH4)3. As shown in Fig. S1,† the presence of traceable diffraction peaks of Al indicated a minimal decomposition of Y(AlH4)3 during the purification process. Most of the transition tetrahydroaluminates M(AlH4)n decompose at −50 °C or even below while yttrium tetrahydroaluminate is an exception.18 The appearance of the diffraction peaks of Al metal and the increase of pressure inside the jar during the milling process implied the decomposition of unstable RE(AlH4)3 hydrides.16 In this study, Y(AlH4)3 still stays in an amorphous state after ball milling without the observation of any decomposition products, further confirming its relatively high stability under normal conditions. Fig. 1(b) shows the FT-IR patterns of the as-milled LiAlH4 and YCl3–3LiAlH4 sample. The as-milled LiAlH4 shows two Al–H stretching vibration frequencies at 1785 cm−1 and 1645 cm−1, consistent with the reported values (1757 cm−1 and 1615 cm−1).24 In the fingerprint region, the bands at 885, 790, and 704 cm−1 correspond to the deformational modes; meanwhile the combination band is at 1450 cm−1.25 The shift of the Al–H stretching band towards a higher frequency (about 30 cm−1) in the spectrum of the as-milled LiAlH4 was thought to be related to the strain effect induced by milling treatment,26 which was also observed in the as-milled KAlH4 (ref. 27) and LiAlH4 under a high static pressure (GPa).28 Distinct differences exist in the FT-IR spectra of the as-milled YCl3–3LiAlH4 and LiAlH4 due to the different chemical environments of the Al–H bonds. The as-milled YCl3–3LiAlH4 sample exhibits only one stretching vibration (1800 cm−1) and one broad deformational band at 690 cm−1 for the Al–H bond. The peak at 1640 cm−1 corresponds to the water bending vibration.29 No bands for the LiAlH4 can be detected in the FT-IR spectrum of the YCl3–3LiAlH4 sample, implying the complete transformation from the starting materials into the product (a mixture of Y(AlH4)3 and 3LiCl, donates as Y(AlH4)3–3LiCl) after 6 h of ball milling. Such a result is in good agreement with the results of XRD analysis.
The thermal dehydrogenation properties of Y(AlH4)3 were measured by TPD, MS and DSC, and the results are shown in Fig. 2. Three endothermic dehydrogenation peaks are observed during the heating process. All these dehydrogenation peaks combined with the release of hydrogen gas. These three steps with different slopes can be distinguished in the TPD volumetric release curve (labeled with a serial number). One additional exothermic peak at ∼395 °C can be observed in the DSC curve, besides the aforementioned three endothermic dehydrogenation stages (as shown by arrows). These results indicate that altogether four different steps are involved in the thermal decomposition process of Y(AlH4)3. As shown in the TPD curve, hydrogen release from Y(AlH4)3 starts at around 80 °C, accelerates at ∼120 °C and first peaks at ∼140 °C. The first dehydrogenation stage is finished at ∼170 °C with a desorption capacity of ∼3.9 wt% H2. In the temperature range from 170 to 350 °C, two dehydrogenation reactions with maxima at 245 °C and 290 °C can be observed. The quantitative measurement of these two processes delivers a hydrogen amount of ∼1.0 wt% and ∼0.7 wt%, respectively. A total hydrogen amount of ∼5.6 wt% H2 can be released from the Y(AlH4)3 within the temperature range of 80–400 °C. These desorbed hydrogen values were normalized to reflect the weight of Y(AlH4)3 (overall hydrogen amount is 6.6 wt%) considering the theoretical ratio of Y(AlH4)3 and LiCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in the ball milled product.
3) in the ball milled product.
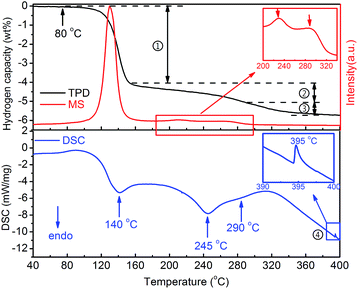 | ||
| Fig. 2 TPD, MS and DSC curves of the Y(AlH4)3–3LiCl sample. The wt% H2 values are normalized to the Y(AlH4)3 percentage. | ||
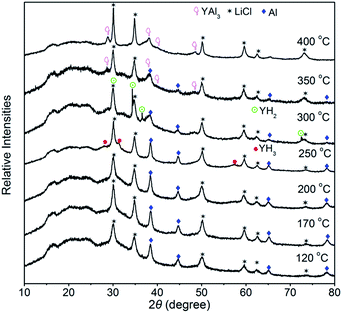
For further understanding thermal decomposition of Y(AlH4)3, XRD analyses of the dehydrogenated samples at different temperatures were performed (see Fig. 3). Kost et al. proposed that, thermal decomposition of Y(AlH4)3 proceeds directly through YH3 and AlH3 derived on the basis of evolved hydrogen capacity and thermographic data.22 However, here only the characteristic diffraction pattern of Al metal arises after dehydrogenation at 120 °C, and no information concerning these reported intermediates can be detected. This result suggests that the dehydrogenation Y(AlH4)3 maybe occur in a way different from this description. The generally accepted decomposition pathway of aluminium tetrahydrides is a two-stage reaction. Starting from AlH4− units an aluminium hexahydride compound in combination with aluminium metal is produced in the first step. Afterwards the hexahydride compound decomposes into a metal hydride and aluminium metal. For metal elements in their stable +1 and +3 oxidation states, the intermediates would be M3AlH6 (M = Li, Na, K)15 or MAlH6 (M = RE),16 while for the stable +2 oxidation state, these hydrides usually formed compounds with the formula MAlH5.23,30 For instance, a new intermediate MeAlH5 (Me = Eu, Sr) with a zigzag chain formed by corner-sharing octahedra was identified during the decomposition process of the complex aluminium hydride, Me(AlH4)2.31 For unstable RE(AlH4)3 hydrides, a REAlH6 intermediate compound with isolated octahedra altering with the RE cations is formed before transforming to REHx hydrides.16 Here in Y(AlH4)3, however, no other Y–Al–H containing decomposition products can be identified even up to the temperature range between 170 °C and 200 °C by means of XRD, suggesting that this new YAlHx hydride may also be amorphous. We propose this YAlHx hydride to be YAlH6 based on two reasons: (1) the yttrium element has a stable +3 oxidation state, which is more likely to result in the formation of [AlH6]3−; (2) for the formation of the hexahydride, 50% of the hydrogen was expected to be released from the first dehydrogenation step.16 According to the TPD curve of Y(AlH4)3 in Fig. 2, a capacity of ∼3.9 wt% H2 (59% of the total hydrogen) is liberated from the first desorption reaction, which is in reasonable agreement with the results observed for the first dehydrogenation step of RE(AlH4)3 (La 51%, Ce 60%, Pr 56%, and Nd 57%) with REAlH6 as the intermediate phase.16 With further increase of the temperature to 250 °C, diffraction peaks of YH3 show up; meanwhile the intensities of visible free Al metal obviously increase. This result demonstrates that the newly formed YAlH6 decomposes into YH3, Al and H2 during the second dehydrogenation stage. Above 250 °C, YH3 starts to transform into YH2 and H2. When the temperature increases to 300 °C, the diffraction peaks of YH3 disappear and only those of YH2 can be observed. As the temperature reaches 350 °C, it is noted that the diffraction peaks of YH2 completely disappear and the intensity of free Al metal also significantly decreases. Meanwhile new intermetallic YAl3 is formed, which results from the reaction between YH2 and Al. Further heating the sample up to 400 °C results in the complete dehydrogenation of YH2; hence YAl3 is dominant in the XRD pattern.
According to the above analysis, Y(AlH4)3 is most probably made up of isolated tetrahedral [AlH4]−, while the intermediate decomposition product consists of octahedral [AlH6]3−, which is very similar to the crystal structure of REAlH6.16 Therefore, the experimental results obtained in the present case suggest a reaction mechanism as follows:
| First step (80–170 °C): Y(AlH4)3 → YAlH6 + 2Al + 3H2 |
| Second step (170–250 °C): YAlH6 → YH3 + Al + 1.5H2 |
| Third step (250–350 °C): YH3 → YH2 + 0.5H2 |
| Fourth step (>350 °C): YH2 +3Al → YAl3 + H2 |
It must be mentioned that other intermediates of the general formula YAlxHy can't be excluded, which leads to the fact that the overall hydrogen capacity is reduced and that these intermediates may contribute to additional dehydrogenation steps.
To realize the low temperature (25–150 °C) application of Y(AlH4)3 in hybrid tanks, only the first dehydrogenation step can be possible. Thus isothermal desorption kinetic curves of Y(AlH4)3 in a temperature range from 80 to 140 °C were studied, and the results are displayed in Fig. S3.† The wt% H2 values are normalized to the Y(AlH4)3 percentage. Y(AlH4)3 can release ∼3.4 wt% H2 within 60 min at 140 °C, and ninety percent of the hydrogen can be liberated in 30 min at this temperature. The activation energy for the dehydrogenation process of Y(AlH4)3 is estimated to be 91.7 kJ mol−1 (Fig. S3d†), which is much smaller compared to that of other nanocrystalline complex metal alanates, e.g. rod Mg(AlH4)2 with 123.0 kJ mol−1.32 A possible explanation for the low on-set dehydrogenation temperature of 80 °C could be this low kinetic barrier.
After dehydrogenation at 145 °C, a series of recharge/discharge experiments were performed to demonstrate the reversibility of the first dehydrogenation step of Y(AlH4)3. After each dehydrogenation, the samples were rehydrogenated at a pressure of 100 bar H2, and afterwards dehydrogenated in a vacuum at the same temperature. Fig. 4 shows the isothermal (a) absorption and (b) desorption kinetic curves of Y(AlH4)3 for three consecutive cycles at 145 °C. As shown in Fig. 4(a), during rehydrogenation cycles an amount of ∼2.6 wt% H2 can be absorbed at 145 °C and the whole amount of absorbed hydrogen can be liberated during the corresponding dehydrogenation processes (Fig. 4(b)). In general, the reversible hydrogen storage capacity at 145 °C can reach 2.6 wt%, indicating the reversibility of the first dehydrogenation step of Y(AlH4)3. This corresponds to ∼75% of the theoretical hydrogen capacity of Y(AlH4)3 for the first step. To our knowledge, this is the first time that reversible hydrogen storage is found in any transition metal alanates. To check whether Y(AlH4)3 has a higher reversibility or not, its final thermal decomposition product (YAl3) was prepared, and was subjected to a high pressure of 300 bar. As shown in Fig. S5,† not any exothermic peaks for hydrogen absorption can be observed, indicating that the YAl3 alloy can't absorb hydrogen in a temperature range from −40 °C to 100 °C and at a high pressure of 300 bar. This is also the case for the YH3 + 3Al mixture, which cannot be hydrogenated at a pressure of 100 bar H2 at 145 °C (Fig. S6†). These results demonstrate that only the first dehydrogenation step of Y(AlH4)3 can be rehydrogenated. Further attempts to improve the reversibility of unstable transition complex alanates are underway in our laboratories.
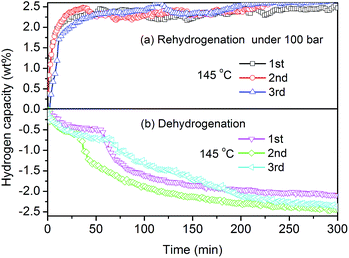 | ||
| Fig. 4 (a) Isothermal absorption and (b) desorption kinetic curves of Y(AlH4)3 for three consecutive cycles at 145 °C. The wt% H2 values are normalized to the Y(AlH4)3 percentage. | ||
Conclusions
A new complex metal aluminium hydride with the composition of Y(AlH4)3 was prepared via the mechanochemical reaction of YCl3 + 3LiAlH4. Upon heating, the Y(AlH4)3 sample decomposes via a four-stage dehydrogenation process over the temperature range of 80–400 °C. At 80–170 °C, Y(AlH4)3 is first decomposed into an intermediate hydride, YAlH6, 2Al and 3H2. With increasing temperature up to 250 °C, YAlH6 continues to release hydrogen to form YH3 and additional Al metal. Upon further increasing the temperature to 300 °C, YH3 starts to decompose into YH2 and H2. As the temperature reaches 350 °C, the newly formed YH2 starts to react with Al to generate YAl3, and this reaction proceeds completely upon further heating the sample up to 400 °C. An amount of 3.4 wt% H2 can be released from the sample within ∼60 min at 140 °C during the first dehydrogenation step. The apparent activation energy of the first dehydrogenation step of Y(AlH4)3 is 92.1 kJ mol−1. Rehydrogenation experiments indicate that the first dehydrogenation step shows a reversibility of 75% even at a low temperature of 145 °C. This is the first example that a transition metal alanate can reversibly absorb hydrogen. Further improvements on the hydrogen storage properties of Y(AlH4)3 would make it a possible and promising candidate for hybrid tank system applications.Acknowledgements
This work was supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. NSFC51621001), National Natural Science Foundation of China Projects (No. 51431001), by the International Science & Technology Cooperation Program of China (2015DFA51750) and by the Project Supported by the Natural Science Foundation of Guangdong Province of China (2014GKXM011 and 2014A030311004). The Project Supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014) is also acknowledged. The support from the China Scholarship Council is specially acknowledged. Open Access funding provided by the Max Planck Society.Notes and references
- P. M. Grant, Nature, 2003, 424, 129–130 CrossRef CAS PubMed.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- N. Takeichi, H. Senoh, T. Yokota, H. Tsuruta, K. Hamada, H. T. Takeshita, H. Tanaka, T. Kiyobayashi, T. Takano and N. Kuriyama, Int. J. Hydrogen Energy, 2003, 28, 1121–1129 CAS.
- C. Weidenthaler and M. Felderhoff, Energy Environ. Sci., 2011, 4, 2495–2502 CAS.
- Z. Chen, X. Xiao, L. Chen, X. Fan, L. Liu, S. Li, H. Ge and Q. Wang, J. Alloys Compd., 2014, 585, 307–311 CrossRef CAS.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, D. Sun, Q. Zhang and M. Zhu, Int. J. Hydrogen Energy, 2015, 40, 2717–2728 CrossRef CAS.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, L. Sun and M. Zhu, J. Alloys Compd., 2015, 639, 452–457 CrossRef CAS.
- T. Matsunaga, M. Kon, K. Washio, T. Shinozawa and M. Ishikiriyama, Int. J. Hydrogen Energy, 2009, 34, 1458–1462 CrossRef CAS.
- T. Kuriiwa, T. Maruyama, A. Kamegawa and M. Okada, Int. J. Hydrogen Energy, 2010, 35, 9082–9087 CrossRef CAS.
- D. Mori and K. Hirose, Int. J. Hydrogen Energy, 2009, 34, 4569–4574 CrossRef CAS.
- B. Bogdanović and M. Schwickardi, J. Alloys Compd., 1997, 253–254, 1–9 CrossRef.
- X. Liu, H. W. Langmi, S. D. Beattie, F. F. Azenwi, G. S. McGrady and C. M. Jensen, J. Am. Chem. Soc., 2011, 133, 15593–15597 CrossRef CAS PubMed.
- R. Gremaud, A. Borgschulte, W. Lohstroh, H. Schreuders, A. Züttel, B. Dam and R. Griessen, J. Alloys Compd., 2005, 404–406, 775–778 CrossRef CAS.
- B. Bogdanović, M. Felderhoff, A. Pommerin, F. Schüth and N. Spielkamp, Adv. Mater., 2006, 18, 1198 CrossRef.
- S. I. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111–4132 CrossRef CAS PubMed.
- C. Weidenthaler, A. Pommerin, M. Felderhoff, W. Sun, C. Wolverton, B. Bogdanović and F. Schüth, J. Am. Chem. Soc., 2009, 131, 16735–16743 CrossRef CAS PubMed.
- H. Neumaier, D. Büchel and G. Ziegelmaier, Z. Anorg. Allg. Chem., 1966, 345, 46–52 CrossRef CAS.
- G. L. Soloveichik and B. M. Bulychev, Russ. Chem. Rev., 1983, 52, 43–60 CrossRef.
- M. E. Kost and A. L. Golovanova, Russ. Chem. Bull., 1975, 24, 905–907 CrossRef.
- A. I. Golovanova, M. E. Kost and V. I. Mikheeva, Russ. Chem. Bull., 1973, 22, 1410–1413 CrossRef.
- A. I. Golovanova, M. E. Kost and V. I. Mikheeva, Izv. Akad. Nauk SSSR, 1973, 5, 1448–1452 Search PubMed.
- M. E. Kost and A. I. Golvanova, Inorg. Mater., 1978, 14, 1348–1350 Search PubMed.
- M. Mamatha, B. Bogdanović, M. Felderhoff, A. Pommerin, W. Schmidt, F. Schüth and C. Weidenthaler, J. Alloys Compd., 2006, 407, 78–86 CrossRef CAS.
- C. Li, X. Xiao, L. Chen, K. Jiang, S. Li and Q. Wang, J. Alloys Compd., 2011, 509, 590–595 CrossRef CAS.
- J. R. Ares, K. F. Aguey-Zinsou, F. Leardini, I. J. Ferrer, J. F. Fernandez, Z. X. Guo and C. Sánchez, J. Phys. Chem. C, 2009, 113, 6845–6851 CAS.
- J. R. Ares, K. F. Aguey-Zinsou, M. Porcu, J. M. Sykes, M. Dornheim, T. Klassen and R. Bormann, Mater. Res. Bull., 2008, 43, 1263–1275 CrossRef CAS.
- J. R. Ares Fernandez, F. Aguey-Zinsou, M. Elsaesser, X. Z. Ma, M. Dornheim, T. Klassen and R. Bormann, Int. J. Hydrogen Energy, 2007, 32, 1033–1040 CrossRef CAS.
- A. V. Talyzin and B. Sundqvist, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 180101 CrossRef.
- X. Z. Xiao, C. X. Li, L. X. Chen, X. L. Fan, H. Q. Kou and Q. D. Wang, J. Alloys Compd., 2011, 509, S743–S746 CrossRef CAS.
- A. Marashdeh and T. J. Frankcombe, J. Chem. Phys., 2008, 128, 234505 CrossRef PubMed.
- A. Pommerin, A. Wosylus, M. Felderhoff, F. Schüth and C. Weidenthaler, Inorg. Chem., 2012, 51, 4143–4150 CrossRef CAS PubMed.
- Y. Liu, Y. Pang, X. Zhang, Y. Zhou, M. Gao and H. Pan, Int. J. Hydrogen Energy, 2012, 37, 18148–18154 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta10928d |
| This journal is © The Royal Society of Chemistry 2017 |