Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymorphism in the family of Ln6−xMoO12−δ (Ln = La, Gd–Lu; x = 0, 0.5) oxygen ion- and proton-conducting materials
A. V.
Shlyakhtina
 *a,
S. N.
Savvin
b,
N. V.
Lyskov
c,
I. V.
Kolbanev
a,
O. K.
Karyagina
d,
S. A.
Chernyak
e,
L. G.
Shcherbakova
a and
P.
Núñez
b
*a,
S. N.
Savvin
b,
N. V.
Lyskov
c,
I. V.
Kolbanev
a,
O. K.
Karyagina
d,
S. A.
Chernyak
e,
L. G.
Shcherbakova
a and
P.
Núñez
b
aSemenov Institute of Chemical Physics, Russian Academy of Sciences, ul. Kosygina 4, Moscow, 119991, Russia. E-mail: annashl@inbox.ru; annash@chph.ras.ru
bDepartment of Inorganic Chemistry, Institute of Materials and Nanotechnology, University of La Laguna, Tenerife, La Laguna, 38200, Spain
cInstitute of Problems of Chemical Physics, Russian Academy of Sciences, Academician Semenov ave. 1, Chernogolovka 142432, Moscow region, Russia
dEmanuel Institute of Biochemical Physics, Russian Academy of Sciences, ul. Kosygina 4, Moscow 119991, Russia
eDepartment of Chemistry, Lomonosov Moscow State University, 1–3 Leninskie Gory, Moscow, 119991, Russia
First published on 27th March 2017
Abstract
The formation of Ln6−xMoO12−δ (Ln = La, Gd, Dy, Ho, Er, Tm, Yb, Lu; x = 0, 0.5) rare-earth molybdates from mechanically activated oxide mixtures has been studied in the range 900–1600 °C. The morphotropy and polymorphism (thermodynamic phase and kinetic (growth-related) transitions) of the Ln6−xMoO12−δ (Ln = La, Gd–Lu; x = 0, 0.5) molybdates have been analyzed in detail. As a result we have observed two new types of oxygen ion- and proton-conducting materials with bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , no. 206) and rhombohedral (R
, no. 206) and rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , no. 148) structures in the family of Ln6−xMoO12−δ (Ln = La, Gd–Lu; x = 0, 0.5) molybdates. The heavy rare-earth molybdates Ln6−xMoO12−δ (Ln = Er, Tm, Yb; x = 0, 0.5) have been shown for the first time to undergo an order–disorder (rhombohedral–bixbyite) phase transition at 1500–1600 °C, and we have obtained compounds and solid solutions with the bixbyite structure (Ia
, no. 148) structures in the family of Ln6−xMoO12−δ (Ln = La, Gd–Lu; x = 0, 0.5) molybdates. The heavy rare-earth molybdates Ln6−xMoO12−δ (Ln = Er, Tm, Yb; x = 0, 0.5) have been shown for the first time to undergo an order–disorder (rhombohedral–bixbyite) phase transition at 1500–1600 °C, and we have obtained compounds and solid solutions with the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ). The stability range of the rhombohedral phase (R
). The stability range of the rhombohedral phase (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) increases with decreasing Ln ionic radius across the Ln6−xMoO12−δ (Ln = Er, Tm, Yb, Lu) series. We have detected a proton contribution to the conductivity of the rhombohedral La5.5MoO11.25 (2 × 10−4 S cm−1 at 600 °C in wet air) and high-temperature polymorph Yb6MoO12−δ bixbyite structure, (Ia
) increases with decreasing Ln ionic radius across the Ln6−xMoO12−δ (Ln = Er, Tm, Yb, Lu) series. We have detected a proton contribution to the conductivity of the rhombohedral La5.5MoO11.25 (2 × 10−4 S cm−1 at 600 °C in wet air) and high-temperature polymorph Yb6MoO12−δ bixbyite structure, (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) below 600 °C. At these temperatures, rhombohedral (R
) below 600 °C. At these temperatures, rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 seems to be an oxygen ion conductor (Ea = 0.53–0.58 eV). The total conductivity of rhombohedral (R
) Yb6MoO12 seems to be an oxygen ion conductor (Ea = 0.53–0.58 eV). The total conductivity of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 exceeds that of bixbyite Yb6MoO12−δ by more than one order of magnitude and is 3 × 10−5 S cm−1 at 500 °C. According to their high-temperature (T > 600 °C) activation energies, the lanthanum and ytterbium molybdates studied here are mixed electron–ion conductors.
) Yb6MoO12 exceeds that of bixbyite Yb6MoO12−δ by more than one order of magnitude and is 3 × 10−5 S cm−1 at 500 °C. According to their high-temperature (T > 600 °C) activation energies, the lanthanum and ytterbium molybdates studied here are mixed electron–ion conductors.
Introduction
Ln6MoO12, Ln6WO12, and solid solutions based on these compounds have recently been the subject of intense research as promising environmentally friendly dyes, luminescent materials, effective catalysts, and oxygen ion and proton conductors.1–11 Tungstates and molybdates in these series are known to undergo various structural transformations with decreasing Ln ionic radius. The compounds in the Ln6WO12 series have the fluorite structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, no. 225) at Ln = La–Pr, a pseudotetragonal structure at Ln = Nd–Gd, and a rhombohedral structure (R
m, no. 225) at Ln = La–Pr, a pseudotetragonal structure at Ln = Nd–Gd, and a rhombohedral structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , no. 148) at Ln = Tb–Lu. The Ln6MoO12 series has a morphotropic phase transition from the fluorite structure at Ln = La–Ho to a rhombohedral structure (R
, no. 148) at Ln = Tb–Lu. The Ln6MoO12 series has a morphotropic phase transition from the fluorite structure at Ln = La–Ho to a rhombohedral structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) at Ln = Ho–Lu. It is also known that the molybdates exhibit a rich polymorphism, with polymorphic transformations between phases belonging to the fluorite series. In particular, both the Fm
) at Ln = Ho–Lu. It is also known that the molybdates exhibit a rich polymorphism, with polymorphic transformations between phases belonging to the fluorite series. In particular, both the Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and R
m and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) polymorphs were obtained at high temperatures for the lanthanide molybdates with Ln = La–Sm and Ho, whereas among the tungstates only for Y6WO12 the formation of an R
polymorphs were obtained at high temperatures for the lanthanide molybdates with Ln = La–Sm and Ho, whereas among the tungstates only for Y6WO12 the formation of an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) phase (above 1200 °C) and an Fm
phase (above 1200 °C) and an Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m fluorite phase (at 1765 °C) was reported.12 It may be that the relatively poor polymorphism of the tungstates is due to the extremely high temperatures of thermodynamic order–disorder transitions in Ln2O3–WO3 systems. However especially for Ln6WO12 (Ln = Y, La–Yb except Ce, Pm, Eu, Tb, Tm) the cubic high-temperature (Tsyn. > 1960 °C) bixbyite polymorph (Ia
m fluorite phase (at 1765 °C) was reported.12 It may be that the relatively poor polymorphism of the tungstates is due to the extremely high temperatures of thermodynamic order–disorder transitions in Ln2O3–WO3 systems. However especially for Ln6WO12 (Ln = Y, La–Yb except Ce, Pm, Eu, Tb, Tm) the cubic high-temperature (Tsyn. > 1960 °C) bixbyite polymorph (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , no. 206) was reported by Foex.13
, no. 206) was reported by Foex.13
At the same time, a number of reports mention low-temperature cubic Ln6MoO12 and Ln6WO12 phases, which exist, as a rule, in the range 700–1000 °C and have rather broad diffraction lines. The formation of such low-temperature, poorly crystallized cubic phases was observed in the synthesis of compounds by various wet-chemical methods followed by annealing between 700 and 1000 °C. For example, a low temperature fluorite phase was observed in the synthesis of Y6WO12 at 600 °C using a polymerized complex method.14 A fluorite phase was detected in the range 800–1100 °C for Lu6WO12 and 800–1000 °C for Lu6MoO12 with the use of the citrate complexation method, followed by calcinations at these temperatures.6 Recently, a low-temperature fluorite-like bixbyite polymorph (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) has been obtained using the solution combustion reaction between 700 and 1000 °C in Ln6MoO12 (Ln = Tm, Yb, Lu).15 In a study of the formation of Ln6WO12 (Ln = Nd, Eu, Er) tungstates between 700 and 1480 °C using a sol–gel complexation synthesis method, Escolastico et al.16 obtained low-temperature fluorites at temperatures from 700 to 1000 °C.
) has been obtained using the solution combustion reaction between 700 and 1000 °C in Ln6MoO12 (Ln = Tm, Yb, Lu).15 In a study of the formation of Ln6WO12 (Ln = Nd, Eu, Er) tungstates between 700 and 1480 °C using a sol–gel complexation synthesis method, Escolastico et al.16 obtained low-temperature fluorites at temperatures from 700 to 1000 °C.
A distinction is made between thermodynamic phase transitions and kinetic (growth-related) order–disorder transitions. In the thermodynamic phase transitions, structural changes result from changes in thermodynamic conditions (pressure p and temperature T). The basic trends of structural changes caused by an increase in temperature or pressure were formulated by Goldschmidt:17 the average coordination numbers (CNs) of atoms increase with increasing pressure and density and decrease with increasing temperature and decreasing density. High-temperature phases typically have higher symmetries than do low-temperature phases. In particular, many high-temperature phases have cubic structures, whereas low-temperature phases have lower symmetry. There are, however, exceptions. For example, the CN of one of the lanthanum atoms in La2S3 increases from 7 to 8 as a result of the α-La2S3 → γ-La2S3 phase transition at 1570 °C.18
There are also kinetic (growth-related) order–disorder transitions. These include the formation of a phase of a particular composition in the thermodynamic stability region of another phase with the same composition under the effect of kinetic factors such as crystal growth rate, particle attachment selectivity, etc.19 This term was introduced by Chernov for describing the growth of a disordered crystal instead of an ordered, equilibrium crystal when slight differences in energy between sites become insignificant because of high growth rates.20,21
Solid solutions with a particular structure can result from kinetic ordering and disordering transitions.19 Kinetic disordering transitions may be due to poor statistical selection of different atoms at high crystal growth rates (atoms in the surface layer do not have enough time to occupy sites corresponding to an ordered state before the formation of the next layer). The result is a disordered solid solution (typically with the fluorite structure according to X-ray diffraction (XRD) data). The formation of low-symmetry metastable phases (desymmetrization of crystals) in the case of ordered solid solutions is possible because the surface structure of a growing face differs significantly from the bulk structure of the crystal.
The kinetic (growth-related) order–disorder transitions considered above are characteristic of inorganic crystalline solid solutions.
Recently, kinetic (growth-related) metastable fluorite–pyrochlore (F*–P) transitions have also been classified with application to the pyrochlore family Ln2M2O7 (Ln = La–Lu; M = Ti, Zr, Hf) oxygen-ion conductors.22 The morphotropy and polymorphism (thermodynamic phase and kinetic (growth-related) transitions) of the Ln2M2O7 (Ln = La–Lu; M = Ti, Zr, Hf) rare-earth pyrochlores have been analyzed in detail.22,23
The purpose of this work was to study the formation of rare-earth molybdate Ln6−xMoO12−δ (Ln = La, Gd, Dy, Ho, Er, Tm, Yb, Lu; x = 0, 0.5) solid solutions in a wide temperature range, from 900 to 1650 °C. To this end, precursors were synthesized using mechanical activation, a method that allows one to obtain homogeneous, highly dispersed oxide mixtures and occasionally leads to the formation of compounds at room temperature, i.e. ensures mechanochemical synthesis of mixed oxides.24,25 Since a number of zirconium-doped rare-earth molybdates, Ln5.4Zr0.6MoO12.3 (Ln = Nd, Sm, Dy) and La5.8Zr0.2MoO12.1, were shown to possess oxygen ion and proton conductivity,10,11 the total conductivity of some polymorphs of undoped Ln6−xMoO12−δ (Ln = La, Yb; x = 0, 0.5) was also measured in dry and wet air.
Experimental section
The Ln6−xMoO12−δ (Ln = La, Gd, Dy, Ho, Er, Tm, Yb, Lu; x = 0, 0.5) materials were prepared using the mechanical activation of starting oxides followed by high-temperature heat treatment of green compacts. All of the rare-earth oxides and molybdenum oxide used in our preparations were 99.9% pure.After preheating the starting Ln2O3 (Ln = La, Gd, Dy, Ho, Er, Tm, Yb, Lu) oxides at 1000 °C for 2 h, they were mixed with MoO3 and co-milled in a SPEX 8000 ball mill for 1 h. MoO3 was previously activated in a high energy Aronov ball mill for 4 min. The mechanically activated mixtures of the oxides were uniaxially pressed at 680–914 MPa and sintered at 900, 1100, and 1200 °C for 4 h, and 1400, 1500 °C and 1600 °C for 3 h.
The geometric density of the as-prepared ceramics ranged from 64 to 96.38% of the theoretical one (Table 1). All samples were characterized structurally. X-ray diffraction (XRD) patterns of the polycrystalline samples were collected at room temperature on a DRON-3M automatic diffractometer (Cu Kα radiation, λ = 1.5418 Å, Bragg-reflection geometry, 35 kV, 28 mA) in the 2θ range 13° to 65° (scan step 0.1°). Table 1 summarizes the color, density, and crystallographic characteristics of the samples.
| Sample no. | Composition | Synthesis temperature | Color | Relative density, % | Structure | Unit-cell parameters, Å |
|---|---|---|---|---|---|---|
| 1 | La5.5MoO11.25 | 900 °C-3 h | Grey | — |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (metastable) m (metastable) |
a = 5.478(1) |
| 2 | La5.5MoO11.25 | 1600 °C-3 h | Dirty-yellow | 91.1 | Complex rhombohedral phase | |
| 3 | Gd6MoO12−δ | 1600 °C-3 h | Speckled yellow | 90.05 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m + traces of admixtures m + traces of admixtures |
a = 5.382(3) |
| 4 | Gd5.5MoO11.25−δ | 1500 °C-3 h | Speckled yellow | 95.68 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.378(2) |
| 5 | Gd5.5MoO11.25−δ | 1600 °C-3 h | Speckled yellow | 96.25 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.379(1) |
| 6 | Dy6MoO12−δ | 1500 °C-3 h | Speckled orange | 84.32 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m + traces of admixtures m + traces of admixtures |
a = 5.312(2) |
| 7 | Dy5.5MoO11.25−δ | 1500 °C-3 h | Speckled orange | 92.86 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.313(1) |
| 8 | Dy5.5MoO11.25−δ | 1600 °C-3 h | Speckled brown | 96.38 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.313(1) |
| 9 | Ho6MoO12−δ | 1400 °C-3 h | Yellow with separate spots | 74.26 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.579(2) |
| 10 | Ho6MoO12−δ | 1600 °C-3 h | Yellow with separate spots | 85.2 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.583(4) |
| 11 | Ho5.5MoO11.25−δ | 1400 °C-3 h | Speckled yellow | 82.65 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.292(3) |
| 12 | Ho5.5MoO11.25−δ | 1500 °C-3 h | Speckled yellow | 89.27 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.291(2) |
| 13 | Ho5.5MoO11.25−δ | 1600 °C-3 h | Speckled yellow | 94.44 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.291(2) |
| 14 | Er6MoO12 | 1200 °C-40 h | Pink | — |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.695(4) |
| c = 9.242(4) | ||||||
| 15 | Er6MoO12 | 1400 °C-3 h | Pink | 78.76 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.697(4) |
| c = 9.249(6) | ||||||
| 16 | Er6MoO12−δ | 1600 °C-3 h | Orange with separate spots | 77.64 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.526(4) |
| 17 | Er5.5MoO11.25−δ | 1600 °C-3 h | Speckled orange | 96.25 |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
a = 5.267(2) |
| 18 | Tm6MoO12 | 1400 °C-3 h | Lemon | 71.32 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.69(3) |
| c = 9.234(15) | ||||||
| 19 | Tm6MoO12−δ | 1600 °C-3 h | Yellow with separate spots | 76.58 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.474(14) |
| 20 | Yb6MoO12 | 1200 °C-3 h | Lemon | 65.86 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.614(2) |
| c = 9.170(2) | ||||||
| 21 | Yb6MoO12 | 1400 °C-3 h | Lemon | 75.06 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.610(2) |
| c = 9.169(2) | ||||||
| 22 | Yb6MoO12 | 1500 °C-3 h | Lemon | 78.12 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.612(2) |
| c = 9.166(2) | ||||||
| 23 | Yb6MoO12−δ | 1600 °C-3 h | Yellow with separate spots | 81.19 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.421(5) |
| 24 | Yb5.5MoO11.25 | 1200 °C-3 h | Lemon | 65.86 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.618(2) |
| c = 9.172(2) | ||||||
| 25 | Yb5.5MoO11.25 | 1400 °C-3 h | Lemon | 75.06 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.615(2) |
| c = 9.168(2) | ||||||
| 26 | Yb5.5MoO11.25 | 1500 °C-3 h | Lemon | 85.21 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.616(2) |
| c = 9.168(2) | ||||||
| 27 | Yb5.5MoO11.25−δ | 1600 °C-3 h | Yellow with separate spots | 87.2 |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 10.439(6) |
| 28 | Lu6MoO12 | 1400 °C-3 h | Lemon | 64 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.594(6) |
| c = 9.127(4) | ||||||
| 29 | Lu6MoO12 | 1600 °C-3 h | Light yellow | 66.42 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
a = 9.594(13) |
| c = 9.123(8) |
Energy dispersive X-ray spectroscopy (EDX) was realized by using a JEOL JSM-6390LA scanning electron microscope (SEM). An excitation energy of 20 keV was chosen as the accelerating voltage. The L-shells of Mo, La, Gd, Dy, Ho, Er, and Tm, and the M-shells of Yb and Lu were used for EDX analysis. 5–14 spectra were collected for each sample for Ln/Mo ratio determination. EDX measurements, as well as recording of the SEM images, were carried out in high vacuum mode (pressure p ≈ 4 × 10−6 mbar). To prevent shifts in the images and heating of the sample caused by charging effects, the sample was coated with a 4 nm gold layer (Quorum Q150R ES) before the analysis. Microanalysis data of the Ln and Mo-containing samples are presented in Table 2.
| Sample no. | Composition | Synthesis temperature | Average nominal Ln/Mo atomic ratio | Average measured Ln/Mo atomic ratio |
|---|---|---|---|---|
| 2 | La5.5MoO11.25 | 1600 °C-3 h | 5.5 | 5.7 ± 0.6 |
| 3 | Gd6MoO12−δ | 1600 °C-3 h | 6 | 6.5 ± 0.2 |
| 6 | Dy6MoO12−δ | 1500 °C-3 h | 6 | 6.4 ± 0.5 |
| 9 | Ho6MoO12−δ | 1400 °C-3 h | 6 | 6.5 ± 0.6 |
| 10 | Ho6MoO12−δ | 1600 °C-3 h | 6 | 6.3 ± 0.9 |
| 11 | Ho5.5MoO11.25−δ | 1400 °C-3 h | 5.5 | 6.1 ± 0.5 |
| 12 | Ho5.5MoO11.25−δ | 1500 °C-3 h | 5.5 | 5.5 ± 0.2 |
| 15 | Er6MoO12 | 1400 °C-3 h | 6 | 6.5 ± 0.5 |
| 16 | Er6MoO12−δ | 1600 °C-3 h | 6 | 6.3 ± 0.2 |
| 17 | Er5.5MoO11.25−δ | 1600 °C-3 h | 5.5 | 6.1 ± 0.3 |
| 19 | Tm6MoO12−δ | 1600 °C-3 h | 6 | 6.3 ± 0.4 |
| 21 | Yb6MoO12 | 1400 °C-3 h | 6 | 5.8 ± 0.2 |
| 23 | Yb6MoO12−δ | 1600 °C-3 h | 6 | 5.3 ± 0.4 |
| A | Er5.5MoO11.25 | 1200 °C-40 h | 5.5 | 6.6 ± 0.7 |
| B | Tm6MoO12 | 1200 °C-40 h | 6 | 6.6 ± 0.3 |
The electrical conductivity of Ln6−xMoO12−δ (Ln = La, Yb; x = 0, 0.5) was characterized by two-probe AC impedance spectroscopy. Both faces of the disk-shaped polycrystalline samples sintered as described above (2–3 mm thick with a diameter of 9–10 mm) were covered with Pt ink (ChemPur C3605) and fired at 1000 °C for 30 min.
The temperature dependence of the total (electronic and ionic) conductivity of La5.5MoO11.25 in dry and wet air was extracted from impedance spectra obtained using a Solartron 1260 frequency response analyzer. The spectra were recorded in the frequency range of 0.1 Hz to 1 MHz on cooling from 900 °C to 150 °C; the root mean square ac voltage amplitude was set to 150 mV. Depending on the atmosphere and temperature it took from 2 to 5 h for the samples to reach the equilibrium conductivity values. The relative humidity of the air fed into the sample stage was controlled by passing it over freshly dehydrated silica gel (designated “dry”) or through a water saturator maintained at 20 °C (designated “wet”), which ensured a constant water content of about 2%.
Electrical conductivity measurements of the two polymorphs, Yb6MoO12 bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and Yb6MoO12 rhombohedral (R
) and Yb6MoO12 rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), were performed using a P-5X potentiostat/galvanostat combined with a frequency response analyzer module (Elins Ltd, Russia) over the frequency range of 500 kHz to 0.1 Hz at a signal amplitude of 150 mV in the temperature range of 100–900 °C. A dry atmosphere was created by passing air through KOH and a wet atmosphere through a water saturator held at 20 °C. The air flow rate is 130 ml min−1.
), were performed using a P-5X potentiostat/galvanostat combined with a frequency response analyzer module (Elins Ltd, Russia) over the frequency range of 500 kHz to 0.1 Hz at a signal amplitude of 150 mV in the temperature range of 100–900 °C. A dry atmosphere was created by passing air through KOH and a wet atmosphere through a water saturator held at 20 °C. The air flow rate is 130 ml min−1.
Results and discussion
Room-temperature synthesis of heavy rare-earth molybdate Yb6MoO12 and intermediate rare-earth molybdate Ho6MoO12 from oxides
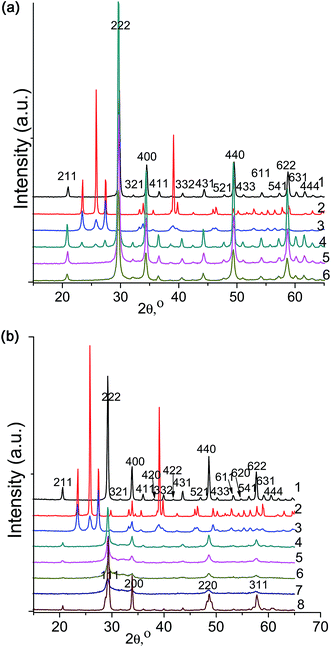
Fig. 1a shows XRD patterns illustrating the mechanochemical synthesis of Yb6MoO12 from its constituent oxides. Scan 1 in Fig. 1a represents the XRD pattern of unmilled Yb2O3, which has the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) (ICDD PDF 18-1463). Its lattice parameter was determined to be a = 10.402(2) Å. Scans 2 and 3 in Fig. 1a represent XRD patterns of MoO3 before and after grinding for 4 min in an Aronov vibrating ball mill. It is seen that the layered oxide MoO3 readily amorphizes during grinding, and its lines become markedly broader. Also shown in Fig. 1a (scan 4) is the XRD pattern of a mixture of 3Yb2O3 + MoO3 (after 4 min of grinding (Aronov mill)). Since the mixture contains a considerable amount of ytterbium oxide, the relative amount of MoO3 is not very large. After 40 min of subsequent grinding in a SPEX 8000 mill, we observed complete dissolution of the molybdenum oxide in Yb2O3 with the bixbyite structure (Ia
) (ICDD PDF 18-1463). Its lattice parameter was determined to be a = 10.402(2) Å. Scans 2 and 3 in Fig. 1a represent XRD patterns of MoO3 before and after grinding for 4 min in an Aronov vibrating ball mill. It is seen that the layered oxide MoO3 readily amorphizes during grinding, and its lines become markedly broader. Also shown in Fig. 1a (scan 4) is the XRD pattern of a mixture of 3Yb2O3 + MoO3 (after 4 min of grinding (Aronov mill)). Since the mixture contains a considerable amount of ytterbium oxide, the relative amount of MoO3 is not very large. After 40 min of subsequent grinding in a SPEX 8000 mill, we observed complete dissolution of the molybdenum oxide in Yb2O3 with the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ). The resultant solid solution also had the bixbyite structure (Ia
). The resultant solid solution also had the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), and its lattice parameter was a = 10.423(2) Å, i.e. it considerably exceeded that of the parent Yb2O3. Thus, it is reasonable to believe that an Yb2O3-based solid solution (Yb6MoO12) forms even during grinding, so this process can be thought of as the room-temperature mechanochemical synthesis of Yb6MoO12 with the bixbyite structure (Ia
), and its lattice parameter was a = 10.423(2) Å, i.e. it considerably exceeded that of the parent Yb2O3. Thus, it is reasonable to believe that an Yb2O3-based solid solution (Yb6MoO12) forms even during grinding, so this process can be thought of as the room-temperature mechanochemical synthesis of Yb6MoO12 with the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ). Note also that subsequent annealing at 1600 °C for 3 h had no significant effect on its lattice parameter a = 10.421(5) Å (Table 1). A similar situation was observed for Tm6MoO12 with parameter a = 10.465(2) Å, which exceeded that of the parent Tm2O3 (a = 10.459(1) Å).
). Note also that subsequent annealing at 1600 °C for 3 h had no significant effect on its lattice parameter a = 10.421(5) Å (Table 1). A similar situation was observed for Tm6MoO12 with parameter a = 10.465(2) Å, which exceeded that of the parent Tm2O3 (a = 10.459(1) Å).
Fig. 1b shows the XRD pattern of unmilled Ho2O3 with the bixbyite structure ((Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ); a = 10.583(2) Å) after annealing at 1000 °C for 1 h (scan 1) and the XRD patterns of MoO3 before and after grinding for 4 min in the Aronov vibrating ball mill (scans 2 and 3). Also shown in Fig. 1b is the XRD pattern of a 3Ho2O3 + MoO3 mixture after 10 min of grinding in the SPEX mill (scan 4). It is seen that MoO3 almost completely disappeared and that the reflections from Ho2O3 became markedly weaker and broader. Scans 5–7 in Fig. 1b represent the XRD patterns of the 3Ho2O3 + MoO3 mixture after 20, 40, and 60 min of grinding. It is seen that the superstructure reflections from the parent Ho2O3 (bixbyite structure, (Ia
); a = 10.583(2) Å) after annealing at 1000 °C for 1 h (scan 1) and the XRD patterns of MoO3 before and after grinding for 4 min in the Aronov vibrating ball mill (scans 2 and 3). Also shown in Fig. 1b is the XRD pattern of a 3Ho2O3 + MoO3 mixture after 10 min of grinding in the SPEX mill (scan 4). It is seen that MoO3 almost completely disappeared and that the reflections from Ho2O3 became markedly weaker and broader. Scans 5–7 in Fig. 1b represent the XRD patterns of the 3Ho2O3 + MoO3 mixture after 20, 40, and 60 min of grinding. It is seen that the superstructure reflections from the parent Ho2O3 (bixbyite structure, (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )) (Fig. 1b, scan 7) completely disappeared and that the strongest reflections remaining in the XRD pattern are due to a fluorite-like phase with a = 5.301(2) Å and are markedly broadened.
)) (Fig. 1b, scan 7) completely disappeared and that the strongest reflections remaining in the XRD pattern are due to a fluorite-like phase with a = 5.301(2) Å and are markedly broadened.
Thus, the mechanochemical synthesis of heavy rare-earth molybdates (Yb6MoO12) differs from that of intermediate rare-earth molybdates (Ho6MoO12), which can be accounted for in terms of the nature of the starting oxide Ln2O3 (Ln = Yb, Ho).
La6−xMoO12−δ (x = 0, 0.5) formation from mechanically activated precursors
The ICDD PDF database of La6MoO12 presents data on low-temperature phases, with broad diffraction lines,26 whereas data on well-crystallized fluorite La6MoO12 at high temperatures, T ∼ 1500–1600 °C, are missing. Previously, rare-earth molybdates were studied as a rule at temperatures no higher than 1400 °C. However, the qualitative transpiration tests27 indicated that the compounds R6WO12 (R = Y, Tm, Lu) and Ln6MoO12 (Ln = Tm–Lu) with a rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) structure demonstrate high thermal stability. After annealing for 2 h at 1750 °C in air, pellets of pure oxides, R2O3 (R = Y, Tm–Lu), had completely volatilized while both of the rhombohedral (R
) structure demonstrate high thermal stability. After annealing for 2 h at 1750 °C in air, pellets of pure oxides, R2O3 (R = Y, Tm–Lu), had completely volatilized while both of the rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Y6MO12 (M = Mo, W) compounds showed only a small loss (0.1–0.3%) in weight.
) Y6MO12 (M = Mo, W) compounds showed only a small loss (0.1–0.3%) in weight.
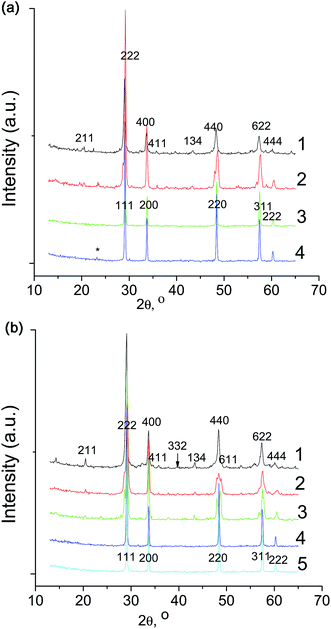
Fig. 2 shows XRD patterns illustrating the phase formation process in La5.5MoO11.25 at 900, 1200, 1600, and 1650 °C (scans 1–4). When comparing the XRD patterns of La5.5MoO11.25 synthesized at 1600 and 1650 °C (Fig. 2, scans 3 and 4) to that of La6MoO12 prepared at 1600 °C (Fig. 2, scan 5), it is important to note that, like La6WO12,8 La6MoO12 does not exist as a distinct compound but is a mixture of La6−xMoO12−δ and La2O3 (ICDD PDF-2, no. 74-144) (*) as also are La(OH)3 (ICDD PDF-2, no. 83-2034) (^), and La2O(CO3)2 × H2O (ICDD PDF-2, no. 28-512) (v), which are La2O3 hydration and carbonation products.
Given this, we synthesized not only Ln6MoO12 but also Ln5.5MoO11.25 (Ln = La, Gd, Dy, Ho, Er, Yb) in order to prevent the final material from being multiphasic.
When analyzing the La5.5MoO11.25 formation process, it is worth noting the formation of a metastable, low-temperature fluorite phase at 900 °C, which then transforms into a rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) phase at 1200 °C (Fig. 2, scans 1 and 2). The formation of a metastable, low-temperature fluorite phase was also observed in lutetium molybdate (Lu6MoO12) synthesis6 and was reported for Y6WO12 and Ln6WO12 (Ln = Nd, Eu, Er, Lu) tungstates14,16 prepared using wet-chemical methods and low temperature annealing at ∼700–1000 °C.
) phase at 1200 °C (Fig. 2, scans 1 and 2). The formation of a metastable, low-temperature fluorite phase was also observed in lutetium molybdate (Lu6MoO12) synthesis6 and was reported for Y6WO12 and Ln6WO12 (Ln = Nd, Eu, Er, Lu) tungstates14,16 prepared using wet-chemical methods and low temperature annealing at ∼700–1000 °C.
Increasing the heat treatment temperature of La5.5MoO11.25 from 1200 to 1600 and 1650 °C led to the formation of a new phase that was also rhombohedral to a first approximation and had an increased unit-cell volume (increased unit-cell parameter) (Fig. 2, scans 2–4). This phase was formed at 1600 °C and prevailed in multiphase La6MoO12 (Fig. 2, scan 5). Comparison of XRD data of the zirconium-substituted molybdate La5.8Zr0.2MoO12.1,10 the La5.5MoO11.25 synthesized in this study, and a molybdenum-rich La28−y(W1−xMox)4+yO54+δ tungstate28 indicates that these solid solutions are identical in structure. Based on SAED results, Amsif et al.28 proposed a rhombohedral cell with cells parameters (∼28 × 28 × 9.8 Å), and with an unusually large volume of 6600 Å3. Thus, further research is needed, with the use of neutron and synchrotron X-ray diffraction, to accurately determine the complex structure of La5.5MoO11.25.
Gd6−xMoO12−δ (x = 0, 0.5) formation from mechanically activated precursors
Fig. 3a and b show XRD patterns illustrating the phase formation process in Gd6MoO12 and Gd5.5MoO11.25, respectively, at 900, 1200, 1500, and 1600 °C. For Gd6MoO12 XRD of the sample after heat treatment at 1100 °C is presented also (Fig. 3a, scan 2). Heat treatment at 900 °C leads to the formation of a tetragonal phase (with all of its diffraction lines markedly broadened) (Fig. 3a and b, scans 1). A similar tetragonal phase was observed for Eu6WO12 in the temperature interval 1200–1480 °C.16 At 1200 °C, a tetragonal phase prevails (Fig. 3a and b, scans 3 and 2 respectively). At high temperatures, 1500–1600 °C, the Gd5.5MoO11.25−δ sample consists of a pure fluorite (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) phase (Fig. 3b, scans 3 and 4), whereas the Gd6MoO12−δ sample contains impurity phases (Fig. 3a, scans 4 and 5). After annealing in the range 1500–1600 °C, the latter sample has a non-uniform coloration, with black inclusions. It seems likely that it is difficult to obtain impurity-free fluorite Gd6MoO12−δ by annealing at 1600 °C because of the partial reduction of the material.
m) phase (Fig. 3b, scans 3 and 4), whereas the Gd6MoO12−δ sample contains impurity phases (Fig. 3a, scans 4 and 5). After annealing in the range 1500–1600 °C, the latter sample has a non-uniform coloration, with black inclusions. It seems likely that it is difficult to obtain impurity-free fluorite Gd6MoO12−δ by annealing at 1600 °C because of the partial reduction of the material.
Ln6−xMoO12−δ (Ln = Dy, Ho, Er, Tm, Yb, Lu; x = 0, 0.5) formation from mechanically activated precursors
Fig. 4a and b show XRD patterns illustrating the phase formation process in Dy6MoO12 and Dy5.5MoO11.25. Annealing at 900 °C leads to the formation of metastable bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), tetragonal, and rhombohedral (tracks) phases (Fig. 4a and b, scans 1). Annealing at 1200 °C also yields a mixture of a bixbyite, a tetragonal, and a rhombohedral phase (tracks) (Fig. 4a and b, scans 2 and 3, respectively). High-temperature annealing, at 1500 °C of Dy6MoO12−δ (Fig. 4a, scan 3) and at 1600 °C of Dy5.5MoO11.25−δ (Fig. 4b, scan 5), leads to the formation of a pure fluorite (Fm
), tetragonal, and rhombohedral (tracks) phases (Fig. 4a and b, scans 1). Annealing at 1200 °C also yields a mixture of a bixbyite, a tetragonal, and a rhombohedral phase (tracks) (Fig. 4a and b, scans 2 and 3, respectively). High-temperature annealing, at 1500 °C of Dy6MoO12−δ (Fig. 4a, scan 3) and at 1600 °C of Dy5.5MoO11.25−δ (Fig. 4b, scan 5), leads to the formation of a pure fluorite (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) phase. After annealing at 1600 °C, the Dy6MoO12−δ sample contains impurities (Fig. 4a, scan 4), in contrast to Dy5.5MoO11.25−δ (Fig. 4b, scan 5). The Dy-containing samples with the fluorite structure have a non-uniform coloration (with black inclusions). The samples with the bixbyite structure have a more uniform coloration.
m) phase. After annealing at 1600 °C, the Dy6MoO12−δ sample contains impurities (Fig. 4a, scan 4), in contrast to Dy5.5MoO11.25−δ (Fig. 4b, scan 5). The Dy-containing samples with the fluorite structure have a non-uniform coloration (with black inclusions). The samples with the bixbyite structure have a more uniform coloration.
Fig. 5a and b present XRD patterns illustrating the phase formation process in Ho6MoO12 and Ho5.5MoO11.25. Here, low-temperature (∼900–1100 °C) annealing also leads to the formation of a mixture of a metastable bixbyite phase (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), a tetragonal, and a rhombohedral phase (tracks) (Fig. 5a and b, scan 1; Fig. 1b, scan 8). After heat treatment at 1600 °C, Ho6MoO12−δ has the bixbyite structure (Ia
), a tetragonal, and a rhombohedral phase (tracks) (Fig. 5a and b, scan 1; Fig. 1b, scan 8). After heat treatment at 1600 °C, Ho6MoO12−δ has the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and Ho5.5MoO11.25−δ has the fluorite structure (Fm
) and Ho5.5MoO11.25−δ has the fluorite structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The Ho-containing samples with the fluorite structure also have a non-uniform coloration, which seems to be evidence of partial reduction.
m). The Ho-containing samples with the fluorite structure also have a non-uniform coloration, which seems to be evidence of partial reduction.
In the case of Er6MoO12 (Fig. 6a), at 900 °C we observe the formation of a metastable bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) phase mixed with a rhombohedral phase (tracks) (Fig. 6a, scan 1). Above 1200 °C, we also obtain a mixture of a bixbyite and a rhombohedral phase (tracks) (Fig. 6a, scan 2). Heat treatment at 1400 °C yields well-crystallized rhombohedral Er6MoO12 (Fig. 6a, scan 3), which transforms almost completely into a bixbyite phase at 1500 °C (Fig. 6a, scan 4). At 1600 °C, we obtain a well-crystallized high-temperature bixbyite (Ia
) phase mixed with a rhombohedral phase (tracks) (Fig. 6a, scan 1). Above 1200 °C, we also obtain a mixture of a bixbyite and a rhombohedral phase (tracks) (Fig. 6a, scan 2). Heat treatment at 1400 °C yields well-crystallized rhombohedral Er6MoO12 (Fig. 6a, scan 3), which transforms almost completely into a bixbyite phase at 1500 °C (Fig. 6a, scan 4). At 1600 °C, we obtain a well-crystallized high-temperature bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) phase Er6MoO12−δ (Fig. 6a, scan 5). Fig. 6b compares the XRD patterns of Er6MoO12−δ and Er5.5MoO11.25−δ after annealing at 1600 °C. The former material has the bixbyite structure (Ia
) phase Er6MoO12−δ (Fig. 6a, scan 5). Fig. 6b compares the XRD patterns of Er6MoO12−δ and Er5.5MoO11.25−δ after annealing at 1600 °C. The former material has the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and the latter has the fluorite (Fm
) and the latter has the fluorite (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) structure. To examine the influence of annealing time on the phase formation process at a low temperature (1200 °C), the mechanically activated mixture of the oxides 3Er2O3 + MoO3 was sintered at 1200 °C for 4 and 40 h, respectively (Fig. 6c). We observed the formation of an Er6MoO12−δ bixbyite cubic phase (metastable) (Fig. 6c, scan 1) in the thermodynamic stability region of the rhombohedral (R
m) structure. To examine the influence of annealing time on the phase formation process at a low temperature (1200 °C), the mechanically activated mixture of the oxides 3Er2O3 + MoO3 was sintered at 1200 °C for 4 and 40 h, respectively (Fig. 6c). We observed the formation of an Er6MoO12−δ bixbyite cubic phase (metastable) (Fig. 6c, scan 1) in the thermodynamic stability region of the rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Er6MoO12 phase with the same composition under the effect of kinetic factors.19 The transformation into the stable rhombohedral (R
) Er6MoO12 phase with the same composition under the effect of kinetic factors.19 The transformation into the stable rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Er6MoO12 phase can be induced by a longer heating duration (Fig. 6c, scan 2). This is a kinetic (growth-related) transition from metastable bixbyite to the stable rhombohedral (R
) Er6MoO12 phase can be induced by a longer heating duration (Fig. 6c, scan 2). This is a kinetic (growth-related) transition from metastable bixbyite to the stable rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Er6MoO12 at 1200 °C. A similar process (from metastable fluorite to the stable rhombohedral (R
) Er6MoO12 at 1200 °C. A similar process (from metastable fluorite to the stable rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )) was recently reported as an irreversible, first order diffusional ordering process for the Ln6WO12 (Ln = Y, Ho, Er, Yb).29
)) was recently reported as an irreversible, first order diffusional ordering process for the Ln6WO12 (Ln = Y, Ho, Er, Yb).29
Yb6MoO12 and Yb5.5MoO11.25 have identical phase formation sequences (Fig. 7a and b). A metastable bixbyite phase (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) is formed at 900 °C (Fig. 7a and b, scans 1). The stability range of the rhombohedral (R
) is formed at 900 °C (Fig. 7a and b, scans 1). The stability range of the rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) phase, 1200 to 1500 °C, is broader in comparison with that of Er6MoO12 (Fig. 7a, scans 2–4; Fig. 7b, scans 3–5). After annealing at 1600 °C, both Yb6MoO12−δ and Yb5.5MoO11.25−δ have the high-temperature bixbyite structure (Ia
) phase, 1200 to 1500 °C, is broader in comparison with that of Er6MoO12 (Fig. 7a, scans 2–4; Fig. 7b, scans 3–5). After annealing at 1600 °C, both Yb6MoO12−δ and Yb5.5MoO11.25−δ have the high-temperature bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) (Fig. 7a and b, scans 5 and 6, respectively). Thus, Ho, Er, and Yb molybdates are characterized by the formation of a metastable bixbyite phase below 1000 °C, and at high temperatures we observe order–disorder (rhombohedral–bixbyite) phase transitions for Ln6MoO12 (Ln = Er, Yb).
) (Fig. 7a and b, scans 5 and 6, respectively). Thus, Ho, Er, and Yb molybdates are characterized by the formation of a metastable bixbyite phase below 1000 °C, and at high temperatures we observe order–disorder (rhombohedral–bixbyite) phase transitions for Ln6MoO12 (Ln = Er, Yb).
Tm6MoO12 was also found to undergo an order–disorder (rhombohedral–bixbyite) phase transition, like Ln6MoO12 (Ln = Er, Yb) (Fig. 8, scans 1 and 2). At the same time, Lu6MoO12 seems to undergo an order–disorder transition at higher temperatures, like La5.5MoO11.25, as evidenced by the fact that, in the range 1400–1600 °C, Lu6MoO12 retains the rhombohedral structure (Fig. 8, scans 3 and 4). A metastable fluorite phase (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) was obtained previously for Lu6MoO12 in the range 800–1000 °C using a wet-chemical method.6 It seems likely that above 1600 °C this compound exists as a high-temperature fluorite phase, rather than as a bixbyite phase.
m) was obtained previously for Lu6MoO12 in the range 800–1000 °C using a wet-chemical method.6 It seems likely that above 1600 °C this compound exists as a high-temperature fluorite phase, rather than as a bixbyite phase.
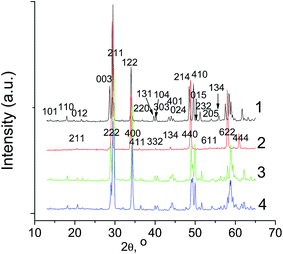 | ||
| Fig. 8 XRD patterns of Tm6MoO12 and Lu6MoO12, synthesized (1 and 3) at 1400 °C-3 h and (2 and 4) at 1600 °C-3 h. | ||
Thus, in this study, Ln6MoO12 (Ln = Er, Yb, Tm) and solid solutions based on these compounds were shown for the first time to undergo high-temperature order–disorder (rhombohedral–bixbyite) phase transitions (TPT ≥ 1500 °C), and we identified metastable bixbyite phases forming below 1000 °C.
Morphotropic and thermodynamic order–disorder (fluorite–rhombohedral phase–bixbyite) transitions
Fig. 9 shows the unit-cell parameter a as a function of the Ln ionic radius of the Ln5.5MoO11.25−δ (Ln = Er–Gd) molybdates with the fluorite structure after annealing at 1600 °C. The a cell parameter is seen to rise linearly with the Ln ionic radius. Also shown in Fig. 9 is the a cell parameter of the metastable fluorite phase La5.5MoO11.25−δ synthesized at 900 °C. Fitting the dependence of a on the Ln ionic radius of the Ln5.5MoO11.25−δ (Ln = Er–Gd) molybdates at 1600 °C by a straight line, we find that the a of high-temperature La5.5MoO11.25 markedly exceeds that of metastable fluorite La5.5MoO11.25. | ||
| Fig. 9 Unit-cell parameter as a function of the Ln3+ ionic radius of fluorite Ln5.5MoO11.25 (Ln = Er–Gd) after annealing at 1600 °C for 3 h. | ||
The data in Fig. 10 illustrate the morphotropic fluorite–bixbyite phase transition in the Ln6MoO12−δ molybdates after annealing at 1600 °C for 3 h. Fig. 10 and Table 1 present the unit-cell parameter as a function of the Ln3+ ionic radius of Ln6MoO12−δ (Ln = Yb, Tm, Er, Ho, Dy, Gd). The heavy rare-earth molybdates Ln6MoO12−δ (Ln = Ho, Er, Yb, Tm) crystallize in the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and the intermediate rare-earth molybdates Ln6MoO12−δ (Ln = Dy, Gd) crystallize in the fluorite structure (Fm
) and the intermediate rare-earth molybdates Ln6MoO12−δ (Ln = Dy, Gd) crystallize in the fluorite structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The Ho6−xMoO12−δ (x = 0, 0.5) molybdates were shown to exist in two phases, with the fluorite and bixbyite structures, and the heavy rare-earth molybdates Ln6MoO12 (Ln = Er, Tm, Yb) exist as rhombohedral and bixbyite phases.
m). The Ho6−xMoO12−δ (x = 0, 0.5) molybdates were shown to exist in two phases, with the fluorite and bixbyite structures, and the heavy rare-earth molybdates Ln6MoO12 (Ln = Er, Tm, Yb) exist as rhombohedral and bixbyite phases.
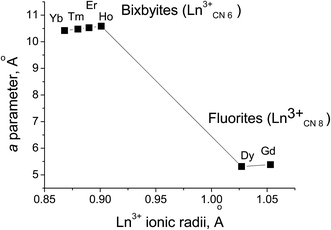 | ||
| Fig. 10 Fluorite–bixbyite morphotropic transition in Ln6MoO12−δ after annealing at 1600 °C for 3 h: unit-cell parameter as a function of the Ln3+ ionic radius of Ln6MoO12−δ (Ln = Yb, Er, Ho, Dy, Gd). | ||
Fig. 11 plots the unit-cell parameters against the Ln3+ ionic radius of rhombohedral Ln6MoO12 (Ln = Er, Tm, Yb, Lu) prepared at 1400 °C (3 h). Both a and c increase with the Ln3+ ionic radius. Note that the temperature stability range of the rhombohedral phase increases with decreasing Ln3+ ionic radius. In particular, the rhombohedral phase of Er6MoO12 exists only after a short 3 h annealing at 1400 °C, whereas that of Yb6−xMoO12−δ (x = 0, 0.5) exists after annealing in the range 1200–1500 °C (Table 1; Fig. 7a and b). However the true stability region of the Er6MoO12 rhombohedral phase starts from 1200 °C as shown by long temperature annealing (40 h) at 1200 °C (Fig. 6c, scan 2). Rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Tm6MoO12 also exists below 1600 °C (Table 1; Fig. 8). The rhombohedral phase of Lu6MoO12 persists at 1600 °C, and it seems to undergo disordering at a higher temperature.
) Tm6MoO12 also exists below 1600 °C (Table 1; Fig. 8). The rhombohedral phase of Lu6MoO12 persists at 1600 °C, and it seems to undergo disordering at a higher temperature.
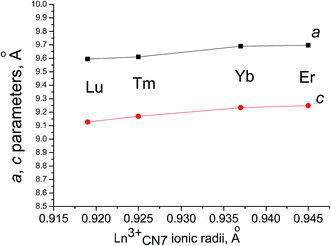 | ||
Fig. 11 Unit-cell parameters as functions of the Ln3+ ionic radius of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Ln6MoO12 (Ln = Er, Tm, Yb, Lu) prepared at 1400 °C (3 h). ) Ln6MoO12 (Ln = Er, Tm, Yb, Lu) prepared at 1400 °C (3 h). | ||
In this study, using brief annealing (3 h) of mechanically activated oxide mixtures, we were able to obtain high-temperature bixbyite Ln6MoO12−δ (Ln = Ho, Er, Tm, Yb) in the range 1400 to 1600 °C. The stability of the heaviest rare-earth molybdates and tungstates, Ln6Mo(W)O12 (Ln = Tm, Yb, Lu), was studied qualitatively by Aitken et al.27 and was shown to be even higher than that of Ln2O3 (Ln = Tm, Yb, Lu).
Microanalysis data of the Ln and Mo-containing samples synthesized in this work are presented in Table 2. These results suggest that no considerable molybdenum loss occurred in the samples during short high-temperature annealing in the range 1200–1600 °C. These results agree with data of rhombohedral R6MoO12 (R = Tm–Lu, Y).27 It is worth noting, however, that the accuracy of determining the Ln/Mo ratio by EDX spectroscopy decreases with increasing synthesis temperature and is lower for the heavy rare-earth molybdates in comparison with the light and intermediate rare-earth molybdates. Note also that the accuracy of determining the Ln/Mo ratio is not very high in the case of the mixed-phase samples synthesized at 1200 °C (Table 2, samples A and B). The data in Fig. 12 illustrate the Mo distribution over the Yb6MoO12−δ (Table 2, no. 23) (Fig. 12a and b) and Ho6MoO12−δ (Table 2, no. 10) (Fig. 12c and d) samples synthesized at 1600 °C. The Mo is seen to be evenly distributed throughout the samples. Similar data were obtained for all of the samples, independent of the synthesis temperature.
 | ||
| Fig. 12 (a and c) SEM image and (b and d) Mo X-ray map of Yb6MoO12−δ and Ho6MoO12−δ, respectively, synthesized at 1600 °C. | ||
Given this, using mechanochemical synthesis, we were able to produce rather dense ceramics with fluorite and related (rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and bixbyite (Ia
) and bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )) structures, and it is of obvious interest to study not only the structure but also the electrical properties of these polymorphs. Indeed, as shown earlier, La5.8Zr0.2MoO12.1 and Ln6−xZrxMoO12+δ (Ln = Nd, Sm, Dy; x = 0.6) zirconium-doped materials have oxygen ion conductivity in a dry atmosphere and proton conductivity in a wet atmosphere below 700, 500, and 425 °C.10,11 These materials have ionic conductivity at low and medium temperatures, whereas at higher temperatures electronic conductivity prevails. Thus, it is important to evaluate the conductivity of the zirconium-free Ln6−xMoO12−δ (Ln = La, Yb; x = 0, 0.5) rare-earth molybdates with the rhombohedral and bixbyite structures.
)) structures, and it is of obvious interest to study not only the structure but also the electrical properties of these polymorphs. Indeed, as shown earlier, La5.8Zr0.2MoO12.1 and Ln6−xZrxMoO12+δ (Ln = Nd, Sm, Dy; x = 0.6) zirconium-doped materials have oxygen ion conductivity in a dry atmosphere and proton conductivity in a wet atmosphere below 700, 500, and 425 °C.10,11 These materials have ionic conductivity at low and medium temperatures, whereas at higher temperatures electronic conductivity prevails. Thus, it is important to evaluate the conductivity of the zirconium-free Ln6−xMoO12−δ (Ln = La, Yb; x = 0, 0.5) rare-earth molybdates with the rhombohedral and bixbyite structures.
Total conductivity of La5.5MoO11.25 in dry and wet air
The impedance spectra obtained for the rhombohedral La5.5MoO11.25 (Tsyn. = 1600 °C) in dry and wet air are given in Fig. 13. In the low-temperature range the spectra showed two separate semicircles that were attributed to the sample bulk and electrode polarization. The values of the equivalent specific bulk and electrode capacitances extracted via non-linear least squares fitting (Cb ∼ 10−12 F cm−1 and Cel ∼ 10−5 F cm−2) are consistent with the aforementioned interpretation. Fig. 14 presents the total conductivity of La5.5MoO11.25 (Tsyn. = 1600 °C) in dry and wet air extracted from these data (Fig. 13). An increase of total conductivity in wet air as compared to the conductivity in dry air is indicative of hydration of this sample resulting in proton conductivity. The Arrhenius plot of the conductivity of La5.5MoO11.25 shows an inflection point at ∼600 °C in a wet atmosphere. A shift from predominant proton conduction at low temperature to predominant electron conduction in the high temperature range takes place. As far as the conductivity of La5.5MoO11.25 in dry air is concerned, we believe that a small amount of residual water might have caused a slight increase in conductivity at the lowest temperatures (below 200 °C). The marginal change of slope at ∼600 °C is probably related to the onset of oxygen ion conductivity as the concentration of protons is very low under dry air. The activation energies for conduction in this sample below and above 600 °C are indicated in Table 3. | ||
| Fig. 13 Impedance spectra of rhombohedral La5.5MoO11.25 (Tsyn. = 1600 °C) in dry air and wet air at difference temperatures. | ||
| Composition | Activation energy of bulk conductivity Ea, eV in dry air | Activation energy of bulk conductivity Ea, eV in wet air | ||
|---|---|---|---|---|
| Below 600 °C | Above 600 °C | Below 600 °C | Above 600 °C | |
| La5.5MoO11.25 | 0.71 | 1.3 | 0.68 | 1.15 |
Yb6MoO12−δ bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) ) |
1.16 | 1.37 | 1.07 | 1.32 |
Yb6MoO12 rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) ) |
0.53 | 0.98 | 0.58 | 0.98 |
Even though the conductivity of La5.5MoO11.25 (2 × 10−4 at 600 °C) is lower than that of La6−xWO12−δ,7–9 the more complex structure of La5.5MoO11.25 seems to ensure higher stability of this compound in a reducing atmosphere.30 Particular features of its microstructure may also play a role.31
Total conductivity of two Yb6MoO12 polymorphs: bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) ) and rhombohedral (R
) and rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/h3_char_0033_0304.gif) ) in dry and wet air
) in dry and wet air
Fig. 15 shows the typical impedance spectra of bixbyite Yb6MoO12−δ (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) at low temperatures (480 and 525 °C, Fig. 15a) and at higher temperatures (650 and 700 °C, Fig. 15b). The spectra each consist of two arcs of circles and can be described using an equivalent circuit consisting of two series connected elements, each composed of a parallel connected resistance (R) and constant phase element (CPE). The impedance of the constant phase element can be represented as
) at low temperatures (480 and 525 °C, Fig. 15a) and at higher temperatures (650 and 700 °C, Fig. 15b). The spectra each consist of two arcs of circles and can be described using an equivalent circuit consisting of two series connected elements, each composed of a parallel connected resistance (R) and constant phase element (CPE). The impedance of the constant phase element can be represented aswhere A is a proportionality factor and the exponent P is related to the phase angle.
The real-axis intercept of the high-frequency arc is the bulk resistance of the material, Rbulk. Note that the center of the high-frequency arc is slightly depressed relative to the real axis (the exponent P is near 0.9). This behavior may be caused by inductive interferences in the tubular furnace used in our measurements. The apparent capacitance (A) is ∼10−11 F cm−1, which corresponds to the geometric capacitance of the material. The other arc (at medium and low frequencies) represents the contribution of electrode polarization at the electrode/electrolyte interface to the impedance response of the system. Note that Rbulk decreases with increasing air humidity (Fig. 15a). This points to proton conductivity, whose contribution to the total conductivity of the material increases with decreasing temperature.
Fig. 16 shows the typical impedance spectra of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 at low (525 and 570 °C) (Fig. 16a) and higher (650 and 700 °C) (Fig. 16b) temperatures. In contrast to the spectrum of Yb6MoO12−δ with the bixbyite structure (Ia
) Yb6MoO12 at low (525 and 570 °C) (Fig. 16a) and higher (650 and 700 °C) (Fig. 16b) temperatures. In contrast to the spectrum of Yb6MoO12−δ with the bixbyite structure (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), the impedance response of this system consists of one arc of a circle, which can be represented by an equivalent circuit composed of a parallel connected resistance (R) and constant phase element (CPE). The real-axis intercept is the bulk resistance of the material. Note that the center of the arc is also slightly depressed relative to the real axis (the exponent P is near 0.8). The apparent capacitance (A) of the material is also ∼10−11 F cm−1.
), the impedance response of this system consists of one arc of a circle, which can be represented by an equivalent circuit composed of a parallel connected resistance (R) and constant phase element (CPE). The real-axis intercept is the bulk resistance of the material. Note that the center of the arc is also slightly depressed relative to the real axis (the exponent P is near 0.8). The apparent capacitance (A) of the material is also ∼10−11 F cm−1.
Fig. 17 presents the temperature dependency of the total conductivity of Yb6MoO12−δ (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and Yb6MoO12 (R
) and Yb6MoO12 (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) in dry and wet air. An increase of total conductivity in wet air as compared to the conductivity in dry air is indicative of hydration of Yb6MoO12−δ bixbyite at T < 600 °C resulting in proton conductivity (Fig. 17, curves 1 and 2). Above 600 °C, the electronic conductivity increases, which is accompanied by changes in activation energy (Table 3). Above 600 °C, bixbyite Yb6MoO12−δ has mixed conductivity. Rhombohedral (R
) in dry and wet air. An increase of total conductivity in wet air as compared to the conductivity in dry air is indicative of hydration of Yb6MoO12−δ bixbyite at T < 600 °C resulting in proton conductivity (Fig. 17, curves 1 and 2). Above 600 °C, the electronic conductivity increases, which is accompanied by changes in activation energy (Table 3). Above 600 °C, bixbyite Yb6MoO12−δ has mixed conductivity. Rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 has no proton conductivity below 600 °C and seems to be an oxygen ion conductor in dry air (Ea = 0.53–0.58 eV) (Table 3). The activation energy for oxygen ion conduction typically falls in the range 0.6–1 eV. However, a number of oxygen ion conductors have lower activation energies. For example, as shown by Kiruthika et al.32 some pyrochlore-structured compounds have lower activation energies for bulk conduction. In particular, the activation energy for bulk conduction in Gd1.8Sr0.2Hf2O6.9 and Nd1.9Sr0.1Hf2O6.95 is 0.57 and 0.42 eV, respectively.
) Yb6MoO12 has no proton conductivity below 600 °C and seems to be an oxygen ion conductor in dry air (Ea = 0.53–0.58 eV) (Table 3). The activation energy for oxygen ion conduction typically falls in the range 0.6–1 eV. However, a number of oxygen ion conductors have lower activation energies. For example, as shown by Kiruthika et al.32 some pyrochlore-structured compounds have lower activation energies for bulk conduction. In particular, the activation energy for bulk conduction in Gd1.8Sr0.2Hf2O6.9 and Nd1.9Sr0.1Hf2O6.95 is 0.57 and 0.42 eV, respectively.
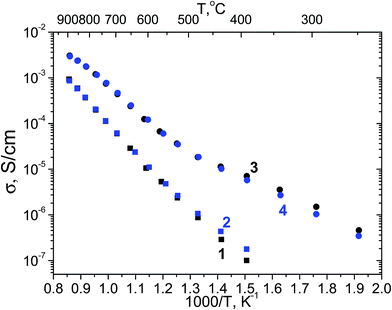 | ||
Fig. 17 Arrhenius plots of the total conductivity in (1 and 3) dry and (2 and 4) wet air of two Yb6MoO12 polymorphs: (1 and 2) bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12−δ and (3 and 4) rhombohedral (R ) Yb6MoO12−δ and (3 and 4) rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12. ) Yb6MoO12. | ||
At T > 600 °C the activation energy of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 conductivity is Ea = 0.98 eV (Table 3) and we can suppose that ionic conductivity contribution prevails in rhombohedral (R
) Yb6MoO12 conductivity is Ea = 0.98 eV (Table 3) and we can suppose that ionic conductivity contribution prevails in rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 at these temperatures.
) Yb6MoO12 at these temperatures.
As shown earlier, the proton conductivity of zirconium-doped molybdates decreases markedly with decreasing Ln ionic radius.10 The highest proton conductivity is offered by the La- and Nd-containing solid solutions. With decreasing ionic radius across the lanthanide series, the proton contribution to the total conductivity decreases, and Dy5.4Zr0.6MoO12.3 has insignificant proton conductivity.10 The Arrhenius plot of the conductivity of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 has a characteristic break at 550 °C (Fig. 17, curves 3 and 4). In previous studies of zirconium-doped rare-earth molybdates and tungstates, such breaks were interpreted as evidence of a change in the dominant carrier type.7,10 In a dry atmosphere at low temperatures (below 550 °C), charge transport is mainly due to oxygen ions, whereas at high temperatures electrons and holes prevail at low and high oxygen partial pressures, respectively. Rhombohedral (R
) Yb6MoO12 has a characteristic break at 550 °C (Fig. 17, curves 3 and 4). In previous studies of zirconium-doped rare-earth molybdates and tungstates, such breaks were interpreted as evidence of a change in the dominant carrier type.7,10 In a dry atmosphere at low temperatures (below 550 °C), charge transport is mainly due to oxygen ions, whereas at high temperatures electrons and holes prevail at low and high oxygen partial pressures, respectively. Rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 seems to have purely oxygen ion conductivity in wet air below 550 °C (Table 3), whereas the conductivity of La5.5MoO11.25 has a significant proton contribution (Fig. 14). Above 600 °C, both rhombohedral (R
) Yb6MoO12 seems to have purely oxygen ion conductivity in wet air below 550 °C (Table 3), whereas the conductivity of La5.5MoO11.25 has a significant proton contribution (Fig. 14). Above 600 °C, both rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and bixbyite Yb6MoO12−δ have growing electronic conductivity contribution, which increases with temperature (Table 3).
) and bixbyite Yb6MoO12−δ have growing electronic conductivity contribution, which increases with temperature (Table 3).
The total conductivity of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 is more than an order of magnitude higher than the conductivity of Yb6MoO12−δ bixbyite, and is 3 × 10−5 S cm−1 at 500 °C.
) Yb6MoO12 is more than an order of magnitude higher than the conductivity of Yb6MoO12−δ bixbyite, and is 3 × 10−5 S cm−1 at 500 °C.
Conclusions
We have investigated the phase formation processes of Ln6−xMoO12−δ (Ln = La, Gd, Dy, Ho, Er, Tm, Yb, Lu; x = 0, 0.5) rare-earth molybdates in the range 900–1600 °C. The materials have been synthesized via mechanical activation of oxide mixtures, which ensured the formation of molybdates even during milling. Our data on low-temperature phase formation and crystallization processes in the range 900–1100 °C demonstrate the formation of a metastable fluorite, bixbyite, and tetragonal or rhombohedral phase, depending on the Ln ionic radius.High-temperature synthesis in the range 1400–1600 °C allowed us to detect for the first time thermodynamic order–disorder (rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )–bixbyite (Ia
)–bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )) phase transitions in the heavy rare-earth molybdates Ln6−xMoO12−δ (Ln = Er, Tm, Yb; x = 0, 0.5). The stability range of the rhombohedral phase increases with decreasing Ln ionic radius.
)) phase transitions in the heavy rare-earth molybdates Ln6−xMoO12−δ (Ln = Er, Tm, Yb; x = 0, 0.5). The stability range of the rhombohedral phase increases with decreasing Ln ionic radius.
The total conductivity of La5.5MoO11.2 and two Yb6MoO12 polymorphs, with rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and bixbyite (Ia
) and bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) structures, has been determined in dry and wet air using impedance spectroscopy. Below 600 °C, the conductivity of La5.5MoO11.25 and bixbyite Yb6MoO12−δ (Ia
) structures, has been determined in dry and wet air using impedance spectroscopy. Below 600 °C, the conductivity of La5.5MoO11.25 and bixbyite Yb6MoO12−δ (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) has a significant proton contribution. Rhombohedral (R
) has a significant proton contribution. Rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 has oxygen ion conductivity under these conditions (T < 550 °C). At 600 °C in wet air, the conductivity of undoped La5.5MoO11.25, which has a complex structure derived from the rhombohedral one, is 2 × 10−4 S cm−1. The conductivity of rhombohedral (R
) Yb6MoO12 has oxygen ion conductivity under these conditions (T < 550 °C). At 600 °C in wet air, the conductivity of undoped La5.5MoO11.25, which has a complex structure derived from the rhombohedral one, is 2 × 10−4 S cm−1. The conductivity of rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) Yb6MoO12 in air at 600 °C is 1 × 10−4 S cm−1 and that of bixbyite Yb6MoO12−δ at 600 °C is 1 × 10−5 S cm−1. So we have obtained and investigated the first members of oxygen ion- and/or proton-conducting materials with the bixbyite (Ia
) Yb6MoO12 in air at 600 °C is 1 × 10−4 S cm−1 and that of bixbyite Yb6MoO12−δ at 600 °C is 1 × 10−5 S cm−1. So we have obtained and investigated the first members of oxygen ion- and/or proton-conducting materials with the bixbyite (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) and rhombohedral (R
) and rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) structure in the family of Ln6−xMoO12−δ (Ln = Ho, Er, Tm, Yb, Lu; x = 0, 0.5) molybdates.
) structure in the family of Ln6−xMoO12−δ (Ln = Ho, Er, Tm, Yb, Lu; x = 0, 0.5) molybdates.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant no. 16-03-00143). We are grateful to Dr Maxim Avdeev from the Bragg Institute, Australian Nuclear Science and Technology Organization for useful discussions in preparing the manuscript.References
- J. Wang, X. Jing, C. Yan, J. Lin and F. Liao, J. Lumin., 2006, 121, 57 CrossRef CAS.
- S. Vishnu, S. Jose and M. L. Reddy, J. Am. Ceram. Soc., 2011, 94, 997 CrossRef.
- J. Kumar Kar, R. Stevens and C. R. Bowen, J. Alloys Compd., 2008, 461, 77 CrossRef.
- J. Sun, B. Xue and H. Du, Infrared Phys. Technol., 2013, 60, 10 CAS.
- J. Sun, B. Xue and H. Du, Opt. Commun., 2013, 298–299, 37 CrossRef CAS.
- H. Li, H. K. Yang, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, H. S. Lee and S. S. Yi, Inorg. Chem., 2011, 50(24), 12522 CrossRef CAS PubMed.
- C. Solís, S. Escolástico, R. Haugsrud and J. M. Serra, J. Phys. Chem. C, 2011, 115, 11124 Search PubMed.
- A. Magrasó and R. Haugsrud, J. Mater. Chem. A, 2014, 2, 12630 Search PubMed.
- G. S. Partin, D. V. Korona, A. Ya. Neiman and K. G. Belova, Russ. J. Electrochem., 2015, 51, 381 CrossRef CAS.
- S. N. Savvin, A. V. Shlyakhtina, A. B. Borunova, L. G. Shcherbakova, J. C. Ruiz-Morales and P. Núñez, Solid State Ionics, 2015, 271, 91 CrossRef CAS.
- S. N. Savvin, A. V. Shlyakhtina, I. V. Kolbanev, A. V. Knotko, D. A. Belov, L. G. Shcherbakova and P. Nuñez, Solid State Ionics, 2014, 262, 713 CrossRef CAS.
- K. Kuribayashi, M. Yoshimura, T. Ohta and T. Sata, J. Am. Ceram. Soc., 1980, 63, 644 CrossRef CAS.
- M. Foex, Bull. Soc. Chim. Fr., 1967, 10, 3696 CAS.
- M. Yoshimura, J. Ma and M. Kakihana, J. Am. Ceram. Soc., 1998, 10, 2721 Search PubMed.
- D. Schildhammer, G. Fuhrmann, L. L. Petschnig, S. Penner, M. Kogler, T. Gotsch, A. Scaur, N. Weinberger, A. Saxer, H. Schottenberger and H. Huppertz, Chem. Mater., 2016, 28(20), 7487 CrossRef CAS.
- S. Escolastico, V. B. Vert and J. M. Serra, Chem. Mater., 2009, 21, 3079 CrossRef CAS.
- V. M. Goldschmidt, Usp. Fiz. Nauk, 1929, 6, 811 CrossRef.
- G. M. Kuz'micheva, Basic Crystal-Chemical Concepts, MITKhT, Moscow, 2001 Search PubMed.
- A. G. Shtukenberg, Yu. O. Punin and O. V. Frank-Kamenetskaya, Usp. Khim., 2006, 75(12), 1212 Search PubMed.
- A. A. Chernov, Usp. Fiz. Nauk, 1970, 100(2), 277 CrossRef CAS.
- A. A. Chernov, Kinetic phase transitions, Soviet Physics–JETP, 1968, 26(6), 1182–1186 Search PubMed.
- A. V. Shlyakhtina, Crystallogr. Rep., 2013, 58, 545 CrossRef.
- V. V. Popov, A. P. Menushenkov, A. A. Yaroslavtsev, Ya. V. Zubavichus, B. R. Gaynanov, A. A. Yastrebtsev, D. S. Leshchev and R. V. Chernikov, J. Alloys Compd., 2016, 689, 669 CrossRef CAS.
- V. M. Vidojikovic, A. R. Brankovic and R. B. Petronijevic, Mater. Lett., 1996, 28, 59 CrossRef.
- A. F. Fuentes, K. Boulahya, M. Maczka, J. Hanuza and U. Amador, Solid State Sci., 2005, 7, 343 CrossRef CAS.
- ICDD PDF 24-1087. Mcllvried, McCarthy, Penn State University, University Park, Pennsylvania, USA. 1972.
- E. A. Aitken, S. F. Bartram and E. F. Juenke, Inorg. Chem., 1964, 3, 949 CrossRef CAS.
- M. Amsif, A. Magraso, D. Marrero-Lopez, J. C. Ruiz-Morales, J. Canales-Vazquez and P. Nuñez, Chem. Mater., 2012, 24, 3868 CrossRef CAS.
- Z. D. Apostolov, P. Sarin, R. P. Haggerty and W. M. Kriven, J. Am. Ceram. Soc., 2013, 96, 987 CrossRef CAS.
- J. S. Mohammed, The Degree Master of Science in Materials Science and Engineering, Georgia Institute of Technology, 2004.
- A. V. Shlyakhtina, S. N. Savvin, A. V. Knotko, L. G. Shcherbakova and P. Nuñez, Inorg. Mater., 2016, 52, 1055 CrossRef CAS.
- G. V. M. Kiruthika, K. V. Gavindan Kutty and U. V. Varadarju, Solid State Ionics, 1998, 110, 335 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |