Engineering metal organic framework derived 3D nanostructures for high performance hybrid supercapacitors†
Rutao
Wang
ab,
Dongdong
Jin
a,
Yabin
Zhang
a,
Shijie
Wang
a,
Junwei
Lang
b,
Xingbin
Yan
 *b and
Li
Zhang
*a
*b and
Li
Zhang
*a
aDepartment of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Shatin NT, Hong Kong SAR, P. R. China. E-mail: lizhang@mae.cuhk.edu.hk
bLaboratory of Clean Energy Chemistry and Materials, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P. R. China. E-mail: xbyan@licp.cas.cn
First published on 18th November 2016
Abstract
Metal–organic frameworks (MOFs) have demonstrated great promise as a new platform for the synthesis of porous electrode materials for energy storage. Research effort on MOFs and MOF derived nanostructures has focused mainly on tuning the chemical composition at the molecular level and developing highly porous frameworks in which enhancing the capacity and reducing the transport path of ions are favorable. Here we report an approach using the MOF (polyhedral ZIF-8) as a novel precursor to synthesize two electrode materials with different energy-storage mechanisms: the capacitor-like porous carbon polyhedra and the battery-like MoS2–ZIF composite. The porous carbon polyhedra have a continuous 3D porous network with an extremely high surface area of 3680.6 m2 g−1 and a well-controlled pore size distribution, and the MoS2–ZIF composite shows a three-dimensional (3D) nanostructure with an open framework. Furthermore, a novel hybrid supercapacitor is fabricated by employing these two 3D nanostructured MOF-derived electrode materials, which shows the best properties among the current hybrid supercapacitors with respect to energy, power and cycling life. The presented strategy for the controlled design and synthesis of 3D MOF-derived nanostructures provides prospects in developing high-performance active materials in advanced energy storage devices.
1. Introduction
With the rapid development of portable electronics, hybrid electric vehicles, and large-scale grid storage, there has been an urgent need for high-performance energy storage devices with both high energy and power density as well as a long cycle life.1,2 Hybrid lithium-ion capacitors (LICs), a class of hybrid supercapacitors that combine a battery-type/pseudocapacitive electrode (energy source) with a capacitor-type electrode (power source) in the same configuration, provide an attractive alternative to conventional electrochemical double layer capacitors (EDLCs) and lithium-ion batteries.3–5 Hybrid LICs exhibit excellent energy-storage properties due to the faradaic charge-storage mechanisms of the battery-type/pseudocapacitive electrode and maintain a fast charge–discharge response because of the double-layer mechanism of capacitor-type electrodes. Most successful examples of hybrid LIC designs are based on an activated carbon (AC) cathodic electrode paired with a battery-type/pseudocapacitive anodic electrode such as graphite,6 silicon,7,8 or metal oxides/nitride9–20 in a lithium salt containing organic electrolyte. However, the imbalances between the anode and cathode with respect to the kinetics and capacities present the major problems and would be a significant obstacle to further improvement in energy and power density for hybrid LICs. Thus, substantial progress in electrode materials (both anode and cathode) design is still important if the wide-scale usage of hybrid LICs is to be fully realized.Metal organic frameworks (MOFs), a new class of porous crystalline materials, are formed by linking inorganic with organic components via covalent coordination linkages, which have attracted great attention due to their tunable structures, versatile functionalities and multiple applications.21–25 Recently, MOFs have been employed as initial materials for the synthesis of porous carbons and metal/metal oxide nanomaterials.22–31 For example, MOFs can be easily transformed into carbon materials with well-defined 1D, 2D, and 3D structures through the direct calcination or extra chemical activation of various MOFs.25,26,30,32–34 Most MOF-derived carbon materials inherit the porous structure of the parent MOFs and have tunable doping by heteroatoms and acceptable conductivity,22,23,25,26,30,32–34 thus providing a new platform, like 1D CNTs, 2D graphene, and 3D mesoporous carbon or carbon aerogel, for the synthesis of carbon related nanocomposites. Previous studies also showed that MOF derived carbon materials can be considered to be a good supporting matrix for various nanostructures which can effectively reduce the diffusion pathway of Li+ ions to increase the Li-ion storage capacity and rate capability.22,23,27,28,31 In addition, MOFs were previously demonstrated to be directly used as the precursor or as the template for the synthesis of porous carbon materials with a tunable surface area ranging from 800 to 3400 m2 g−1 and a controllable pore-size distribution that exhibited high capacitive performance.22,23,26,33,34 Therefore, MOF-derived nanocomposites or porous carbons exhibit great potential in bridging the kinetics and capacity gaps between the two electrodes of hybrid LICs, if the unique structures of MOFs are properly used.
In this work, we present a strategy to achieve two fundamentally different electrodes (anode and cathode) from a novel precursor of a zeolitic imidazolate framework (ZIF-8, a subclass of MOFs) by adjusting the synthesis process to fully utilize the unique structure of ZIF-8 as shown in Fig. 1. The cathode material (porous carbon polyhedra), achieved by the self-sacrificial and morphology-preserved chemical activation of ZIF-8 derived carbon polyhedra, has a continuous 3D porous network with an extremely high surface area of 3680.6 m2 g−1 and a well-controlled pore size ranging from 1 to 4 nm. This porous carbon polyhedron shows the typical EDLC behaviour with a large specific capacitance and can be used as an ideal positive electrode material to enhance the energy storage of hybrid LICs. The anode material shows a three-dimensional (3D) nanostructure in which the randomly assembled ultrathin MoS2 nanosheets are uniformly coated on the ZIF-8 derived carbon polyhedron. As a result, high performance in specific capacity and rate capability for this 3D composite electrode is achieved, which will greatly benefit bridging of the kinetic gap with the capacitor-type electrode. Thus, a novel hybrid LIC is fabricated by employing these two high-performance electrode materials, which exhibits among the best properties of current hybrid LICs reported so far with respect to energy, power and cycling life.
 | ||
| Fig. 1 Schematic of the synthesis of the 3D MoS2–ZIF composite and ZIF-8 derived porous carbon (ZDPC). | ||
2. Experimental section
2.1. Materials
All the reagents and solvents used for the synthesis were commercially available and used without further purification. Zinc acetate dihydrate (AR, 99.0%), 2-methylimidazole (AR, 99.0%), sodium molybdate dehydrate (AR, 99.0%), thiourea (AR, 99.0%), glucose (AR) and potassium hydroxide (AR, 90.0%) were purchased from Aladdin Chemicals.2.2. Preparation of ZIF-8
ZIF-8 was prepared via a modified literature procedure.24 In detail, zinc acetate dehydrate (7.2 g) was dissolved in deionized water (200 ml) to form a solution. 2-Methylimidazole (30 g) was dissolved in deionized water (200 mL) to generate another clear solution. Then, both solutions were mixed together, and stirred for 2 min. The solution was aged for 48 hours at room temperature. After that, white powders were collected and washed several times with methanol and deionized water, respectively. Finally, the powders were dried in an oven for 8 h at 60 °C.2.3. Carbonization of ZIF-8
ZIF-8 powders (5 g) were put into a tube furnace and carbonized at 800 °C for 1 h under a nitrogen atmosphere using a heating rate of 5 °C min−1. The carbonized product was washed using a 35 wt% concentration of HCl to remove the residual Zn component. After that, the sample was washed several times with deionized water, and then dried in an oven for 8 h at 60 °C. The resulting carbon product is denoted as ZIF-8-800.2.4. Synthesis of the MoS2 nanosheets coated ZIF-8-800 (MoS2–ZIF) composite
MoS2–ZIF is synthesized by a modified literature procedure.35,36 The as-prepared ZIF-8-800 (50 mg) was dispersed into a glucose solution (25 ml, 0.05 M) by ultrasonication for 5 min, and Na2MoO4·2H2O (0.3 g) and thiourea (0.6 g) were added in. After stirring for 30 min, the reaction dispersion was transferred into a 50 mL Teflon-lined stainless steel autoclave and kept in an oven at 200 °C for 24 h. The autoclave was then left to cool down to room temperature in the oven. The black precipitate was collected by centrifugation, washed thoroughly with deionized water, and dried in a freezing dryer. The as-prepared MoS2–ZIF sample was further treated at 500 °C in an argon atmosphere for 4 h with a heating rate of 1 °C min−1 to obtain a highly crystalline MoS2 sample.2.5. Synthesis of ZIF-8-800 derived porous carbon (ZDPC)
Porous carbon (ZDPC) with polyhedron morphology was synthesized by the KOH activation method. Typically, ZIF-8-800 (0.5 g) was impregnated with KOH (1 g) in the aqueous phase with the aid of stirring followed by evaporation at 100 °C in a water bath. After the removal of water, the dried KOH/ZIF-8-800 mixture was heated at 800 °C for 1 h under an argon atmosphere with a heating rate of 5 °C min−1. After being cooled down to room temperature, the product was washed with dilute HCl solution and deionized water until the pH value of the washing water reached 7, and then dried at 60 °C in air. For comparison, the resulting ZDPCs with different KOH/ZIF-8-800 mass ratios were synthesized, and the final ZDPC products are designated as ZDPC-x, where x represents the KOH/ZIF-8-800 mass ratio.2.6. Structural characterization
Field emission scanning electron microscopy (FESEM, JSM 7800F, JEOL, Japan) was employed to investigate the surface morphology of as-prepared samples. Transmission electron microscopy (TEM, TECNAl G2 TF20, FEI) was employed to investigate the microstructure of as-prepared samples. Powder X-ray diffraction (XRD, Rigaku D/Max-2400, Japan) was performed using Cu-Kα radiation to investigate the structure and composition of the samples. Raman spectra of the samples were recorded using a micro-Raman spectroscope (JY-HR800, excitation wavelength of 532 nm). The C mass ratio in MoS2–ZIF was analyzed using a thermogravimetric analyzer (TGA, STA 6000, PerkinElmer). The nitrogen adsorption–desorption isotherm measurements were performed on an ASAP 2020 volumetric adsorption analyzer (Micromeritics, USA) at 77 K.2.7. Fabrication of half cells and the hybrid device
All the supercapacitor devices studied for the material performance in this work were fabricated using the two-electrode standard method. For the cathode material (e.g., ZDPC), 90 wt% ZDPC-x and 10 wt% polytetrafluoroethylene (PTFE) were mixed and then were rolled into a thin sheet. After heating at 110 °C for 10 h, the sheet was pressed and cut into rectangle electrodes. After that, the ZDPC cathode electrode was dried overnight at 160 °C under vacuum and then was transferred into a glove box filled with Ar. The current collector of the cathode electrode was Al foil. For the fabrication of the MoS2–ZIF composite electrode, 80 wt% of the active material MoS2–ZIF, 10 wt% of acetylene black as the conducting filler, and 10 wt% of polyvinylidene fluoride (PVDF) in methyl-2-pyrrolidone (NMP) were mixed well and then coated on the copper foil which served as a current collector. After heating at 110 °C for 10 h under vacuum, the sheet was pressed and punched into 10 mm diameter electrodes with a mass loading of 0.8–1.2 mg. For half cells, both anode and cathode were tested using coin type cells (2032 type), where Li metal foil was used as the counter and reference electrodes, and 1 M LiPF6 dissolved in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of ethylene carbonate/diethyl carbonate (EC/DEC) was employed as the electrolyte. Hybrid LICs were also assembled in coin cells with pre-activated MoS2–ZIF anode and ZDPC cathode in the same electrolyte, and the mass ratio of cathode/anode was optimized to 1
1 v/v mixture of ethylene carbonate/diethyl carbonate (EC/DEC) was employed as the electrolyte. Hybrid LICs were also assembled in coin cells with pre-activated MoS2–ZIF anode and ZDPC cathode in the same electrolyte, and the mass ratio of cathode/anode was optimized to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The MoS2–ZIF electrode was pre-activated under a low current density of 0.1 A g−1 for 10 cycles and then lithiated to 0.5 V.
2. The MoS2–ZIF electrode was pre-activated under a low current density of 0.1 A g−1 for 10 cycles and then lithiated to 0.5 V.
2.8. Electrochemical measurements
All the electrochemical tests were carried out at room temperature. Cyclic voltammetry (CV), galvanostatic charge/discharge measurements and electrical impedance spectroscopy (EIS) studies were carried out using the electrochemical workstations: Chenhua (CHI660E, Shanghai, China) and Autolab (PGSTAT 302N, Metrohm, Switzerland). Cycle-life tests for the half-cell and hybrid cells used a battery test system (Land CT2001A model, Wuhan Land Electronics. Ltd):| C = I/[(dE/dt) × m] ≈ I/[(ΔE/Δt) × m] | (1) |
The energy density (E, W h kg−1) of LICs can be obtained from the specific capacitance (C) and the cell voltage (V1-upper cutoff voltage, V2-lower cutoff voltage) according to the following equation:
| E = 0.5C(V12 − V22) | (2) |
The power density (P, W kg−1) of LICs can be obtained from the energy density (E) and the discharging time (t) according to the following equation:
| P = E/t | (3) |
3. Results and discussion
3.1. Structure, morphology, and electrochemical properties of 3D MoS2–ZIF
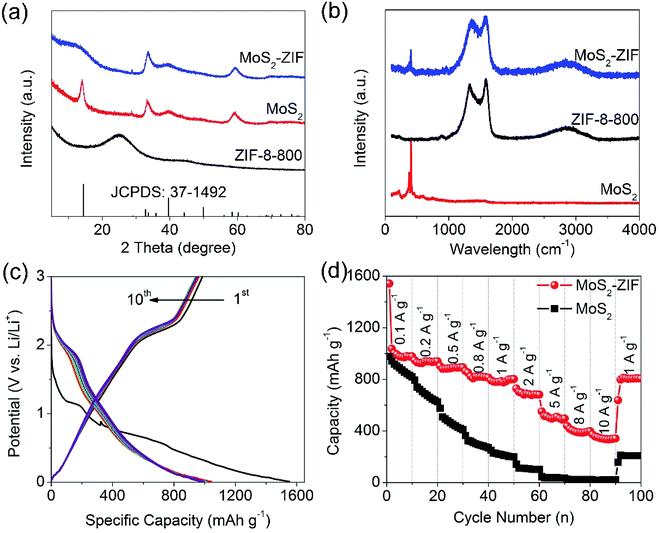
MoS2 is a typical two-dimensional material, which enables Li storage in a two-step process, namely by a typical insertion reaction followed by a conversion reaction to generate Li2S and metallic Mo.37–39 Thus, MoS2 has a large theoretical specific capacity (670 mA h g−1, twice as large as that of graphite) within a wide working range from 0 to 3 V (vs. Li/Li+), showing great potential to boost the operating potential and energy densities of hybrid LICs.40,41 However, MoS2 suffers from the intrinsically low electronic/ion conductivity of the sulfide and the instability of the sulfur species during the lithiation/delithiation process, resulting in unsatisfactory rate performance and poor cycling stability in hybrid LIC applications. So far, the combination of nanostructured MoS2 with conductive carbon has been demonstrated to be an effective strategy to improve the electrochemical performance.35–40,42–45 ZIF-8, a subclass of MOFs that is composed of Zn ions and 2-methylimidazolate linkers, has a three-dimensional (3D) porous structure and polyhedral-like morphology.24,30 The unique structure of ZIF-8 can be used as the base material to obtain a ZIF-8 derived carbon material (denoted as ZIF-8-800) via a simple thermal treatment of white ZIF-8 particles (Fig. S1†) at 800 °C in an Ar atmosphere, followed by an acid treatment to remove the residual Zn component. Fig. 2a and b, and S2† show the typical field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) images of the as-prepared ZIF-8-800, respectively. The ZIF-8-800 sample inherits the polyhedral shape of the ZIF-8 particles after the thermal treatment. Brunauer–Emmett–Teller (BET) characterization (Fig. S3a†) shows that both ZIF-8 and ZIF-8-800 display the typical Type I isotherms [according to the International Union of Pure and Applied Chemistry (IUPAC) classification],46 which indicates that they are typical microporous materials. The pore-size distribution (PSD) curve of ZIF-8-800 (Fig. S3b†) shows that the pores are generally 1 nm in diameter. A high BET surface area of 784 m2 g−1 is obtained for ZIF-8-800, which is consistent with other reports.30,36 The high BET surface area and uniform polyhedral morphology enable ZIF-8-800 to serve as a promising supporting matrix for loading nanostructured MoS2 materials with high Li-ion storage capability. Few studies had reported the combination of MoS2 nanostructures with MOF or MOF derived carbon,36,47–49 however, the fabrication of MOF derived carbon uniformly coated with ultrathin MoS2 nanosheets remains challenging.The 3D MoS2–ZIF was synthesized via a facile hydrothermal approach using glucose. Fig. 2c–g show the representative SEM and TEM images of as-synthesized MoS2–ZIF composites. The randomly assembled ultrathin MoS2 nanosheets are uniformly coated on the ZIF-8-800 polyhedron. The HRTEM images of MoS2–ZIF composites (Fig. 2h and i) indicate that the open space between neighboring MoS2 nanosheets is relatively large, which is consistent with the SEM observation. All of the ultrathin MoS2 nanosheets in the composites are only a few layers (<10) with an interlayer d-spacing of 0.64 nm, which is in good agreement with the spacing of the (002) plane of MoS2.38,50 The elemental mappings of molybdenum, sulfur, and carbon atoms (Fig. 2j) further reveal the core–shell hierarchical structure of the 3D MoS2–ZIF composite. It should be noted that the MoS2 nanosheets cannot grow well on ZIF-8-800 without the addition of glucose in the hydrothermal procedure (Fig. S4†), and the MoS2 nanosheets will agglomerate into bulk morphology without the addition of ZIF-8-800 (Fig. S5†). X-ray diffraction (XRD) patterns in Fig. 3a show the major peaks of the MoS2–ZIF composite and pure MoS2, which can be indexed to the hexagonal crystal structure of MoS2 (JCPDS no. 77-1716). The (002) reflections of the MoS2–ZIF composite centered at 14.4° (2 theta) are broader than those of pure MoS2, which indicates that highly disordered MoS2 nanosheets with a single-layer or few-layer structure are coated on ZIF-8-800 polyhedra.35 The Raman spectrum of the ZIF-MoS2 composite in Fig. 3b exhibits typical peaks of Mo–S atom vibrations located between 380 and 407 cm−1,37 and characteristic peaks of carbon materials with the G-band and D-band at around 1355 and 1597 cm−1, respectively,10,13,20 which is distinguished from those of pure MoS2 with only peaks of Mo–S vibrations and pure ZIF-8-800 with only peaks of the typical D-band and G-band of carbon. The mass loading of MoS2 in the MoS2–ZIF composite can be estimated to be about 83.5 wt% according to the thermo-gravimetric analysis (TGA) as shown in Fig. S6.† The porous structure of the MoS2–ZIF composite was characterized by nitrogen adsorption–desorption isotherm measurements as shown in Fig. S7a.† It should be noted that after the introduction of the ZIF-8-800 polyhedron, the specific BET surface area of the MoS2–ZIF composite increases eight times from 4.02 to 34.89 m2 g−1 as compared with pure MoS2 bulks. The largely increased surface area can increase the electrode/electrolyte interface, which is beneficial for electrolyte access. Moreover, pore size distribution (PSD) curves (Fig. S7b†) show that the pores around 15–70 nm for the MoS2–ZIF composite are largely increased in comparison to the same pore range for pure MoS2, which is mainly associated with the open space between neighbouring MoS2 nanosheets. In short, the aforementioned physical–chemical characterizations indicate that the MoS2–ZIF composite has a hierarchical core–shell and highly porous structure, which may be a favourable feature for enhancement of the electrochemical performance.
The electrochemical performance of MoS2–ZIF composite electrodes was evaluated in a half-cell. Fig. 3c shows the typical discharging/charging curves of MoS2–ZIF composites at a current density of 100 mA g−1. Two voltage plateaus at around 1.2 V and 0.6 V can be observed in the first discharge process, which are related to a typical Li+ insertion reaction to generate LixMoS2 and a reaction to generate Li2S and metallic Mo.35,38 In the following cycles, the charge/discharge plateau is mainly related to Li2S/S reversible redox reactions, while the pseudo-triangular line is attributed to lithium storage on the boundaries or interface of Mo/Li2Sx (1 < x < 8) composites.37 Fig. S8a† shows the typical cyclic voltammograms (CVs) of MoS2–ZIF composites, where two prominent cathodic peaks centered at 1.08 and 0.54 V were observed in the first cycle, corresponding to the above stated phase transformation and further reduced conversion processes, respectively.35,38 In the following cycles, the former cathodic peaks disappear and instead two new peaks centered around 1.86 V and 1.0 V arise. These new cathodic peaks coupled with anodic peaks are associated with the reversible redox reactions of MoS2 derived materials.37 The relatively low initial coulombic efficiency is mainly caused by irreversible processes such as the formation of the solid-electrolyte interface or decomposition of the electrolyte.35,38–40 The MoS2–ZIF composite (Fig. 3d) delivers high specific capacities of 978 and 802 mA h g−1 (taken from the 10th cycle at each current density) at the current densities of 0.1 and 1 A g−1, respectively. Even at the high rate of 5 and 10 A g−1, the specific capacities of the MoS2–ZIF composite remain at 496 and 342 mA h g−1, which are equal to a capacity retention of 50.7% and 35.0%, respectively. The specific capacity and rate capability of the MoS2–ZIF composite are much more attractive when compared to the pure MoS2 sample. Furthermore, the capacity performance of MoS2–ZIF is still comparable to other studies on MoS2–carbon composites.35–45 After 200 discharge/charge cycles at a current density of 1 A g−1, the specific capacity of MoS2–ZIF shows no obvious decay (Fig. S8b†), indicating its good cycling performance. These results clearly demonstrate that the MoS2–ZIF composite with the core–shell architecture possesses high-performance electrochemical properties, which make it a good candidate for anodes in hybrid LICs.
3.2. Structure, morphology, and electrochemical properties of the 3D ZDPC cathode
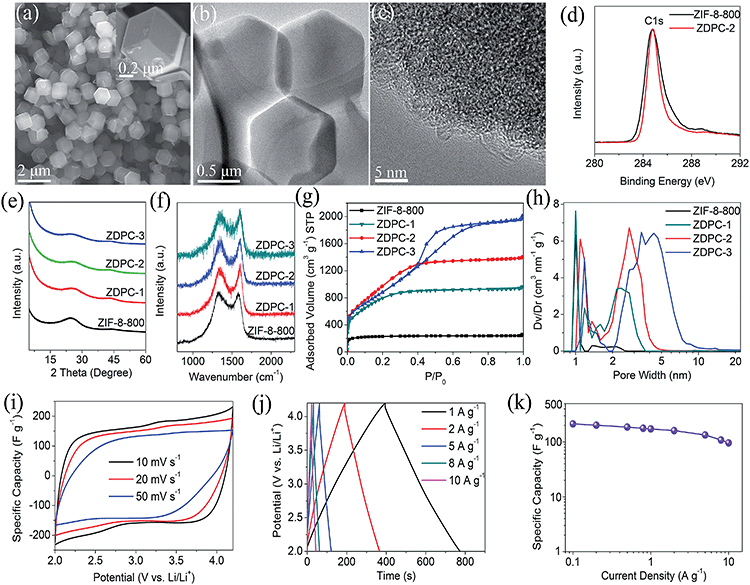
Porous carbon, a typical EDLC material that adsorbs ions on its surface to store charge, has been widely used as the cathode material in hybrid LICs due to its high physical/chemical stability (>4.5 V vs. Li/Li+) and low cost.3,51 According to the charge storage mechanism of porous carbon, the capacitive performance of porous carbon is strongly related to the surface area and pore structure, especially in organic electrolytes.52–54 In this study, ZIF-8 derived polyhedral carbon can be employed as a good supporting matrix for fabricating the MoS2–ZIF hierarchical composite, and can be considered as a desired carbon precursor for preparing porous carbon with a high surface area. Similar to other carbon precursors obtained from polymers and biomass via the hydrothermal or pyrolysis process,55–59 ZIF-8 derived carbon is primarily composed of C and heteroatoms (N and O) after pyrolysis at 800 °C (Table S1†). The corresponding heteroatom functionalities introduce large defects and active sites on the surface and inner part of ZIF-8 derived carbon, which are highly active in KOH activation.55–62 Moreover, the uniform polyhedral morphology and high surface area of ZIF-8 derived carbon ensure sufficient mixing and increase the interface with the activation agent (KOH), and the inherent intercalated pore structure with a predominant pore-size distribution centred around 1 nm may facilitate the infiltration of the activation agent (KOH). Thus, such uniform polyhedral carbon particles are rich in heteroatoms and pores, which may be an ideal precursor to achieve the porous carbon materials with a large surface area and optimal pore-size distribution.The activation of ZIF-8 derived carbon with KOH, which proceeds according to the equation 6KOH + 2C → 2K + 3H2 + 2K2CO3, followed by decomposition of K2CO3 and/or the reaction of K/K2CO3/CO2 with carbon, can generate or broaden the nanoscale pores in the resulting carbon.54,63 The pore structure and morphology of ZIF-8 derived porous carbon (denoted as ZDPC) could be readily controlled by manipulating the mass ratio of KOH/ZIF-8-800. Fig. 4a and b show the typical SEM and TEM images of the as prepared ZDPC-2 sample, respectively. The ZDPC-2 sample inherits the polyhedral shape of the ZIF-8 particles. High-resolution TEM (HRTEM) images in Fig. 4c show a dense porous structure with highly curved carbon nanosheets. Interestingly, a high activation ratio (greater than or equal to 3) will lead to a morphology transformation from the uniform polyhedron carbon to a large bulk with an irregular shape as shown in Fig. S9.† It appears that the chemical activation can not only dig tunnels in ZIF-8-800 but also dramatically restructure ZIF-8-800 at the high activation ratio. The morphology transformation has been observed in other reported studies on porous carbons using KOH activation, which may be associated with over-activation caused by high KOH addition or a high temperature process.55–57
X-ray photoelectron spectroscopy (XPS) characterization was employed to investigate the chemical composition of ZDPC and ZIF-8-800. As shown in Table S1,† the ZIF-8-800 precursor possesses high contents of N (15.83 wt%) and O (8.14 wt%), indicating the presence of abundant heteroatom functionalities on the surface of ZIF-8-800 (as shown in Fig. S10†). It should be noted that the N content in ZDPC decreases significantly with increase of the KOH/ZIF-8-800 mass ratio. For example, only 1.31 wt% of N is preserved for ZDPC-2. A significant decrease in the N heteroatom content in KOH activation is also observed in other similar studies on porous carbon prepared from N heteroatom rich carbon precursors, such as MOF-74,34 polypyrrole55,56 and biomass.51,61,62Fig. 4d and S10b† show the XPS C1s spectra of ZDPC-2 and ZIF-8-800. C–C (sp3) and C–N groups (appearing between 285 and 287 eV) are strongly suppressed after chemical activation, which indicates that carbon atoms in ZDPC-2 mainly show sp2 bonding.10,56Fig. 4e shows the XRD patterns of ZDPC samples. The (002) peaks of ZDPC samples have a significantly reduced intensity and are broadened in comparison with the ZIF-8-800 precursor, demonstrating a highly disordered and porous structure of ZDPC. In order to understand the carbon plane arrangement of ZDPC materials, we employ an empirical parameter (R), which is described as the ratio of the height of the (002) Bragg peak to the background.51,59 It is generally held that the value of R could be used to evaluate the number of carbon sheets arranged as a single layer, with a larger R value meaning a lower percentage of single graphene sheets within a carbon.51,59 In this case, R values are around 0.43–0.46 for ZDPC samples (Table S1†). These R values for ZDPC-x samples are lower than that of ZIF-8-800 (R = 1.3), implying a decrease in crystallinity and low degree of graphitization. Meanwhile, the average graphene domain height (Lc) can be approximately determined via Scherrer's equation, using the full width at half maximum values of (002) peaks.10,51 As shown in Table S1,† the Lc values can be approximately determined to be 1.0–1.1 nm, which indicates that the graphene domains for ZDPC-x and ZIF-8-800 samples are mainly composed of about three layer-stacked (e.g., 1.1/0.34 = 3.2) curved graphene sheets. Raman spectra as shown in Fig. 4f were further employed to estimate the size of the graphene domains of as-prepared ZDPC samples. The average domain size (La) (Table S1†) can be obtained by the equation (La (nm) = (2.4 × 10−10) λ4 (ID/IG)−1).10,51,64 The La value for the ZIF-8-800 precursor is 10.59 nm, whereas the La values reduce to 9.42, 9.42 and 8.84 nm for ZDPC-1, ZDPC-2 and ZDPC-3 after the KOH activation, respectively. These La values of ZDPC samples are close to those of other reports on commercial activated carbon (AC) and KOH activated polymer or biomass derived carbons.10,51,60,65 The decrease in La value may be associated with the breakdown of aligned structural domains in the carbon matrix due to potassium intercalation during the KOH activation.51,60
Fig. 4g shows the nitrogen sorption isotherm curves of ZDPC samples. The shape of isotherms of ZDPC samples is quite different from that of ZIF-8-800, indicating a marked change in the pore structure of the ZDPC samples after the KOH activation. As mentioned before, the nitrogen adsorption isotherm of the ZIF-8-800 sample is typical I, corresponding to the microporous structure, while ZDPC-1 and ZDPC-2 samples reveal features from both type I and IV isotherms,46 indicating micro- and mesoporous structures. A large hysteresis loop over a P/P0 range from 0.38 to 0.78 for ZDPC-3 represents the most characteristic feature of type IV isotherms, indicating a predominately mesoporous structure. The corresponding PSDs of ZDPC samples shown in Fig. 4h demonstrate the co-existence of well-defined micro- and mesopores. For example, ZDPC-2 contains mesopores with a size range of 2–4 nm and narrow micropores centred around 1.1 nm in size, and ZDPC-3 contains primarily mesopores with a size range of 2–8 nm and a small fraction of micropores with a size of around 1.2 nm. In contrast, ZIF-8-800 is mostly composed of narrow micropores (close to 1 nm). It appears that there is an enlargement of micropores and mesopores in ZDPC samples with increase of the KOH activation agent, further confirming the role of KOH activation in generating or broadening the nanoscale pores in the resulting carbon. Table S2† summarizes the textural parameters of these ZDPC samples and ZIF-8-800 precursor. It can be seen that the BET surface area and pore volume largely increase after the KOH activation. ZDPC-2 with the polyhedral morphology has an extremely high surface area of 3680.6 m2 g−1 and a large pore volume of 1.93 cm3 g−1, and ZDPC-3 with irregular morphology has a slightly reduced surface area of 3250 m2 g−1 and an increased pore volume of 3.2 cm3 g−1. Therefore, it is clear that we can introduce sufficient porosity without destroying the polyhedral morphology of the precursor via a fine treatment under a relatively low activation mass ratio (KOH/ZIF-8-800). In contrast, in general, the thermal transformation or chemical activation of the MOF to porous carbon with a high BET surface area is accompanied by a partial or complete collapse of the original morphology.22,23,34 Overall, these results show that ZDPC samples have a continuous 3D porous architecture with abundant micropores and mesopores and ultrahigh surface areas, making them a promising candidate for cathodes with better electrochemical performances in Li-ion based organic electrolytes.
To investigate the electrochemical performance of the as-prepared ZDPC electrodes, a half-cell configuration versus Li metal was employed. Fig. 4i and j show the CV curves and galvanostatic charge/discharge curves of ZIF-8-800 and ZDPC electrodes over 2.0–4.2 V (vs. Li/Li+), respectively. The CV curves for ZDPC samples are relatively rectangular in shape without any redox peak and the charge/discharge curves are nearly straight lines, indicating a typical EDLC behaviour (more information about this can also be found in Fig. S11 and S12†). Fig. 4k shows the specific capacitance of the ZDPC-2 electrode as a function of current density. The ZDPC-2 electrode delivers a specific capacity of 172.4 g−1 (105.1 mA h g−1) at a current density of 1 A g−1. Even at the high rate of 10 A g−1, the specific capacity of the ZDPC-2 composite remains at 96 F g−1 (58.7 mA h g−1), respectively. Furthermore, the same EDLC behaviour is also observed for ZDPC electrodes over 3.0–4.5 V (vs. Li/Li+) and 2.0–4.5 V (vs. Li/Li+) as shown in Fig. S11 and S13.† For example, the specific capacities of ZDPC-2 in the potential range of 2.0–4.5 V (vs. Li/Li+) are calculated to be 183.9 F g−1 (equal to 127.2 mA h g−1) at 0.2 A g−1 and 124.8 F g−1 (equal to 86.7 mA h g−1) at a high current density of 10 A g−1. The ZDPC-2 electrode outperforms other ZDPC electrodes, which may be attributed to its high surface area and optimal PSD. In contrast, ZIF-8-800 has a high surface area of 784 m2 g−1, but exhibits extremely low capacitive performance, which may be associated with the development of a surface area that is inaccessible to solvated electrolyte ions due to the small size of the pores.52–54 Furthermore, as shown in Fig. S11e,† these ZDPC electrodes are also stable. In particular, ZDPC-2 retains 92% of its highest capacitance value after a 2000 cycle span under a constant charge/discharge current density of 5 A g−1 with a high coulombic efficiency close to 100%. Overall, the capacitive performance of ZDPC-2 is better than that of conventional activated carbon or comparable to most of other porous carbons derived from MOFs, biomass and polymers (Table S3†).9–16
3.3. Electrochemical performance of MoS2–ZIF//ZDPC hybrid LICs
Fig. S14a† schematically illustrates the configuration of a hybrid LIC in the working state. In this hybrid cell, the ZDPC-2 and MoS2–ZIF composite serve as the positive and negative electrodes, respectively, due to their different working potential ranges in a Li-ion containing organic electrolyte. The working potential windows for ZDPC-2 and MoS2–ZIF can be analysed using a three-electrode system with a Li metal reference electrode (Fig. S14b†).6Fig. 5a shows the initial galvanostatic charge/discharge profiles of this LIC (with the optimal positive/negative mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ZDPC positive electrode and MoS2–ZIF negative electrode during cycling at a constant current density of 2 A g−1 from 0 to 4 V. The working potential range of the ZDPC-2 positive electrode is not stable in the initial cycles. After several cycles, the upper and lower limits of potential windows for ZDPC-2 become stable, which are close to 4.1 V (vs. Li/Li+) and 2.0 V (vs. Li/Li+), respectively. The mass ratio of ZDPC-2 versus MoS2–ZIF was optimized to 2
1), ZDPC positive electrode and MoS2–ZIF negative electrode during cycling at a constant current density of 2 A g−1 from 0 to 4 V. The working potential range of the ZDPC-2 positive electrode is not stable in the initial cycles. After several cycles, the upper and lower limits of potential windows for ZDPC-2 become stable, which are close to 4.1 V (vs. Li/Li+) and 2.0 V (vs. Li/Li+), respectively. The mass ratio of ZDPC-2 versus MoS2–ZIF was optimized to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in terms of the electrochemical performance and the energy/power density of the as-assembled hybrid cells (more details in Fig. S14c and d†). The voltage window of 0–4 V was chosen to avoid the oxidative decomposition of electrolytes and to achieve a long cycle life.8–11Fig. 5b shows the typical CV curves of the MoS2–ZIF//ZDPC hybrid cell. The shape of the CV curve deviates slightly from the ideal rectangular shape of EDLCs, which is associated with the synergistic effect between the two different energy storage mechanisms at the two electrodes.9–12 As the sweep rate increases, the shape of the CV curves is still retained, implying the fast energy-storage properties of the MoS2–ZIF//ZDPC hybrid cell. The linear relationship between cathodic/anodic current response and the scan rate (Fig. S15†) further confirms that the kinetic behaviour of this hybrid system is not diffusion-limited and thus fast.9,19,57 It appears that the fast kinetics characteristic of this hybrid system is associated with the high-rate capability of the MoS2–ZIF anode and EDLC-type ZDPC-2 cathode. Fig. 5c displays the typical charge–discharge curves with a wide current density range from 0.1 to 5 A g−1. These curves exhibit approximately a linear slope, implying high coulombic efficiency and good reversibility. Electrochemical impedance spectrum (EIS) characterization with the frequency range from 10 kHz to 0.01 Hz yields the Nyquist plot shown in Fig. 5d. The plot features a semicircle in the high frequency area and an approximately vertical curve in the low frequency area, implying a nearly capacitive behaviour of the hybrid cell.52,53 This hybrid LIC is also very stable. After 10
1 in terms of the electrochemical performance and the energy/power density of the as-assembled hybrid cells (more details in Fig. S14c and d†). The voltage window of 0–4 V was chosen to avoid the oxidative decomposition of electrolytes and to achieve a long cycle life.8–11Fig. 5b shows the typical CV curves of the MoS2–ZIF//ZDPC hybrid cell. The shape of the CV curve deviates slightly from the ideal rectangular shape of EDLCs, which is associated with the synergistic effect between the two different energy storage mechanisms at the two electrodes.9–12 As the sweep rate increases, the shape of the CV curves is still retained, implying the fast energy-storage properties of the MoS2–ZIF//ZDPC hybrid cell. The linear relationship between cathodic/anodic current response and the scan rate (Fig. S15†) further confirms that the kinetic behaviour of this hybrid system is not diffusion-limited and thus fast.9,19,57 It appears that the fast kinetics characteristic of this hybrid system is associated with the high-rate capability of the MoS2–ZIF anode and EDLC-type ZDPC-2 cathode. Fig. 5c displays the typical charge–discharge curves with a wide current density range from 0.1 to 5 A g−1. These curves exhibit approximately a linear slope, implying high coulombic efficiency and good reversibility. Electrochemical impedance spectrum (EIS) characterization with the frequency range from 10 kHz to 0.01 Hz yields the Nyquist plot shown in Fig. 5d. The plot features a semicircle in the high frequency area and an approximately vertical curve in the low frequency area, implying a nearly capacitive behaviour of the hybrid cell.52,53 This hybrid LIC is also very stable. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 constant current charge/discharge cycles at a high current density of 2 A g−1, 81% of its initial capacitance is retained (Fig. 5e), giving a very small average capacity decay of 0.0021% per cycle, which is highly competitive with other reported LICs (Table S3†).7–20 Moreover, the coulombic efficiency of the MoS2–ZIF//ZDPC hybrid LIC is nearly 100% during the long-life span cycles.
000 constant current charge/discharge cycles at a high current density of 2 A g−1, 81% of its initial capacitance is retained (Fig. 5e), giving a very small average capacity decay of 0.0021% per cycle, which is highly competitive with other reported LICs (Table S3†).7–20 Moreover, the coulombic efficiency of the MoS2–ZIF//ZDPC hybrid LIC is nearly 100% during the long-life span cycles.
 | ||
| Fig. 5 Electrochemical characterization of MoS2–ZIF//ZDPC hybrid LICs: (a) galvanostatic charge/discharge profiles of this LIC during cycling from a three-electrode test cell with a Li metal reference electrode at a constant current density of 2 A g−1 from 0 to 4 V (black line indicates the charge/discharge profile of the hybrid cell, red line indicates the charge/discharge profile of the positive electrode (ZDPC-2), blue line indicates the charge/discharge profile of the negative electrode (MoS2–ZIF)), and the potential profile of the MoS2–ZIF negative electrode calculated from the equation: V(full cell) = VZDPC-2 (vs. Li/Li+) − VMoS2–ZIF (vs. Li/Li+), (b) CV curves at various scan rates ranging from 2 to 20 mV s−1, (c) galvanostatic charge/discharge curves at different current densities of 0.1–5 A g−1, (d) Nyquist plot showing the imaginary part versus the real part of impedance, (e) cycle stability at a current density of 2 A g−1, (f) Ragone plots of the MoS2–ZIF//ZDPC hybrid LIC as well as the ZDPC//ZDPC symmetric supercapacitor. The energy and power densities of the MoS2–ZIF//ZDPC hybrid LIC are compared with other reported hybrid LICs: graphite//AC,6 Nb2O5–C//AC,12 TiO2–graphene//AC,16 TiN//AC,19 VN–RGO//AC,20 H2Ti6O13//CMK-3,66 Li4Ti5O12–graphene//AC,68 and LiNi0.5Mn1.5O4//AC.69 | ||
The Ragone plot (energy density versus power density) of the MoS2–ZIF//ZDPC hybrid LIC is shown in Fig. 5f. This hybrid cell can deliver a large energy density of 155 W h kg−1 at a power density of 200 W kg−1, as obtained from the discharge curve. Even at an ultra-high power density of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1, this LIC system still maintains an energy density of 44.7 W h kg−1. The energy and power densities of the MoS2–ZIF//ZDPC hybrid system are higher than those of the symmetric ZDPC//ZDPC (ZDPC-2 as the active material) supercapacitor (37.5 W h kg−1 at 270 W kg−1) (more details shown in Fig. S16†). Furthermore, from Fig. 5f and Table S3,† it can be concluded that this hybrid LIC exhibits one of the most promising combinations of energy, power, and cycling life as compared with previously reported hybrid LICs, such as graphite//AC,6 Nb2O5–C//AC,12,13 TiO2–graphene//AC,16 TiN//AC,19 VN–RGO//AC,20 Li4Ti5O12//MOF-DC,32 H2Ti6O13//CMK-3,66 graphene//graphene,67 Li4Ti5O12–graphene//AC,68 LiNi0.5Mn1.5O4//AC,69 graphite//porous graphene,70 B-Si/SiO2/C//AC,8 MnO–C//AC,9,11 MoS2–RGO//AC,40 biomass derived carbon//AC71 and Si/C//nitrogen doped activated carbon hybrid cells.7,72 The superior electrochemical performance of this hybrid LIC is believed to be associated with the following aspects. Firstly, ZIF-8 derived carbon inherits the porous structure and polyhedral morphology of ZIF-8, which is not only a qualified supporting matrix for nanocomposites but also a desired carbon precursor for high-surface-area porous carbon. Secondly, the MoS2–ZIF composite has a hierarchical structure in which ultrathin MoS2 nanosheets are uniformly anchored on the ZIF-8-800 polyhedron with an open structure, effectively shortening the diffusion pathway of Li+ ions to increase the Li-ion storage capacity and rate capability. Thirdly, the extremely high surface area (3680.6 m2 g−1) and the optimal PSD (ranging from 1 to 4 nm) of polyhedral ZDPC-2 with a short pore length are favourable for adsorption/desorption PF6− ions to form a large amount of double layers and helpful for the fast transport of the electrolyte ions, thus providing both remarkably energy and power density.
000 W kg−1, this LIC system still maintains an energy density of 44.7 W h kg−1. The energy and power densities of the MoS2–ZIF//ZDPC hybrid system are higher than those of the symmetric ZDPC//ZDPC (ZDPC-2 as the active material) supercapacitor (37.5 W h kg−1 at 270 W kg−1) (more details shown in Fig. S16†). Furthermore, from Fig. 5f and Table S3,† it can be concluded that this hybrid LIC exhibits one of the most promising combinations of energy, power, and cycling life as compared with previously reported hybrid LICs, such as graphite//AC,6 Nb2O5–C//AC,12,13 TiO2–graphene//AC,16 TiN//AC,19 VN–RGO//AC,20 Li4Ti5O12//MOF-DC,32 H2Ti6O13//CMK-3,66 graphene//graphene,67 Li4Ti5O12–graphene//AC,68 LiNi0.5Mn1.5O4//AC,69 graphite//porous graphene,70 B-Si/SiO2/C//AC,8 MnO–C//AC,9,11 MoS2–RGO//AC,40 biomass derived carbon//AC71 and Si/C//nitrogen doped activated carbon hybrid cells.7,72 The superior electrochemical performance of this hybrid LIC is believed to be associated with the following aspects. Firstly, ZIF-8 derived carbon inherits the porous structure and polyhedral morphology of ZIF-8, which is not only a qualified supporting matrix for nanocomposites but also a desired carbon precursor for high-surface-area porous carbon. Secondly, the MoS2–ZIF composite has a hierarchical structure in which ultrathin MoS2 nanosheets are uniformly anchored on the ZIF-8-800 polyhedron with an open structure, effectively shortening the diffusion pathway of Li+ ions to increase the Li-ion storage capacity and rate capability. Thirdly, the extremely high surface area (3680.6 m2 g−1) and the optimal PSD (ranging from 1 to 4 nm) of polyhedral ZDPC-2 with a short pore length are favourable for adsorption/desorption PF6− ions to form a large amount of double layers and helpful for the fast transport of the electrolyte ions, thus providing both remarkably energy and power density.
4. Conclusions
In summary, we have demonstrated that MOFs (polyhedral ZIF-8) can be used as an ideal precursor to synthesize two types of electrode materials with different energy-storage mechanisms: the EDLC-like ZDPC polyhedra and the battery-like MoS2–ZIF composite. The as-synthesized ZDPC can retain the polyhedral morphology of the original ZIF-8 and has a continuous 3D network with an extremely high surface area of 3680.6 m2 g−1, and a suitable PSD ranging from 1 to 4 nm. The MoS2–ZIF composite with a hierarchical, core–shell structure is able to deliver high reversible specific capacities and good rate capacity. Thus, a high-performance hybrid LIC has been successfully constructed by employing the MoS2–ZIF hierarchical composite as the anode and the highly porous ZDPC polyhedron as the cathode. This hybrid system exhibits a large energy density of 155 W h kg−1, a high power density of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1, and shows a very small capacity decay of 0.0021% per cycle (within 10
000 W kg−1, and shows a very small capacity decay of 0.0021% per cycle (within 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), indicating the possibility of potential applications in portable electronics and the automotive industry.
000 cycles), indicating the possibility of potential applications in portable electronics and the automotive industry.
Acknowledgements
This work was supported by HKSAR Innovation and Technology Commission (ITC) with the Project No. ITS/160/14FP, and the General Research Fund (GRF) from the Research Grants Council (RGC) of Hong Kong with Project No. 14209514 and 14203715, and the National Nature Science Foundation of China (No. 21573265).Notes and references
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P.-L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nature Energy, 2016, 1, 16070 Search PubMed
.
- M. F. El-Kady, Y. Shao and R. B. Kaner, Nature Reviews Materials, 2016, 1, 16033 Search PubMed
.
- K. Naoi, S. Ishimoto, J. Miyamoto and W. Naoi, Energy Environ. Sci., 2012, 5, 9363–9373 Search PubMed
.
- V. Aravindan, J. Gnanaraj, Y.-S. Lee and S. Madhavi, Chem. Rev., 2014, 23, 11619–11635 Search PubMed
.
- Y. Ma, H. Chang, M. Zhang and Y. Chen, Adv. Mater., 2015, 27, 5296–5308 Search PubMed
.
- V. Khomenko, E. Raymundo-Piñero and F. Béguin, J. Power Sources, 2008, 177, 643–651 Search PubMed
; W. J. Cao and J. P. Zheng, J. Power Sources, 2012, 213, 180–185 Search PubMed
; J. Zhang, X. Liu, J. Wang, J. Shi and Z. Shi, Electrochim. Acta, 2016, 187, 134–142 Search PubMed
.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Energy Environ. Sci., 2016, 9, 102–106 Search PubMed
.
- R. Yi, S. Chen, J. Song, M. L. Gordin, A. Manivannan and D. Wang, Adv. Funct. Mater., 2014, 24, 7433–7439 Search PubMed
.
- M. Yang, Y. Zhong, J. Ren, X. Zhou, J. Wei and Z. Zhou, Adv. Energy Mater., 2015, 5, 1500550 Search PubMed
.
- F. Zhang, T. Zhang, X. Yang, L. Zhang, K. Leng, Y. Huang and Y. Chen, Energy Environ. Sci., 2013, 6, 1623–1632 Search PubMed
.
- H. Wang, Z. Xu, Z. Li, K. Cui, J. Ding, A. Kohandehghan, X. Tan, B. Zahiri, B. C. Olsen, C. M. B. Holt and D. Mitlin, Nano Lett., 2014, 14, 1987–1994 Search PubMed
; R. Wang, P. Liu, J. Lang, L. Zhang and X. Yan, Energy Storage Materials, 2017, 6, 53–60 Search PubMed
.
- L. Kong, C. Zhang, J. Wang, W. Qiao, L. Ling and D. Long, ACS Nano, 2015, 9, 11200–11208 Search PubMed
.
- E. Lim, C. Jo, H. Kim, M.-H. Kim, Y. Mun, J. Chun, Y. Ye, J. Hwang, K.-S. Ha, K. C. Roh, K. Kang, S. Yoon and J. Lee, ACS Nano, 2015, 9, 7497–7505 Search PubMed
.
- X. Wang and G. Shen, Nano Energy, 2015, 15, 104–115 Search PubMed
.
- N. Arun, A. Jain, V. Aravindan, S. Jayaraman, W. C. Ling, M. P. Srinivasan and S. Madhavi, Nano Energy, 2015, 12, 69–75 Search PubMed
.
- H. Kim, M.-Y. Cho, M.-H. Kim, K.-Y. Park, H. Gwon, Y. Lee, K. C. Roh and K. Kang, Adv. Energy Mater., 2013, 3, 1500–1506 Search PubMed
.
- Z. Chen, V. Augustyn, J. Wen, Y. Zhang, M. Shen, B. Dunn and Y. Lu, Adv. Mater., 2011, 23, 791–795 Search PubMed
.
- M.-S. Park, Y.-G. Lim, J.-H. Kim, Y.-J. Kim, J. Cho and J.-S. Kim, Adv. Energy Mater., 2011, 1, 1002–1006 CrossRef CAS
.
- H. Wang, Y. Zhang, H. Ang, Y. Zhang, H. T. Tan, Y. Zhang, Y. Guo, J. B. Franklin, X. L. Wu, M. Srinivasan, H. J. Fan and Q. Yan, Adv. Funct. Mater., 2016, 26, 3082–3093 CrossRef CAS
.
- R. Wang, J. Lang, P. Zhang, Z. Lin and X. Yan, Adv. Funct. Mater., 2015, 25, 2270–2278 CAS
; P. Wang, R. Wang, J. Lang, X. Zhang, Z. Chen and X. Yan, J. Mater. Chem. A, 2016, 4, 9760–9766 Search PubMed
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 Search PubMed
.
- J.-K. Sun and Q. Xu, Energy Environ. Sci., 2014, 7, 2071–2100 Search PubMed
.
- W. Xia, A. Mahmood, R. Zou and Q. Xu, Energy Environ. Sci., 2015, 8, 1837–1866 Search PubMed
.
- C. Avci, J. Ariñez-Soriano, A. Carné-Sánchez, V. Guillerm, C. Carbonell, I. Imaz and D. Maspoch, Angew. Chem., Int. Ed., 2015, 54, 14417–14421 Search PubMed
.
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. (David) Lou and X. Wang, Nature Energy, 2016, 1, 15006 Search PubMed
.
- R. R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J. H. Kim and Y. Yamauchi, ACS Nano, 2015, 9, 6288–6296 Search PubMed
.
- R. Wu, D. P. Wang, X. Rui, B. Liu, K. Zhou, A. W. K. Law, Q. Yan, J. Wei and Z. Chen, Adv. Mater., 2015, 27, 3038–3044 Search PubMed
.
- G. Huang, F. Zhang, X. Du, Y. Qin, D. Yin and L. Wang, ACS Nano, 2015, 9, 1592–1599 CrossRef CAS PubMed
.
- X. Cao, B. Zheng, W. Shi, J. Yang, Z. Fan, Z. Luo, X. Rui, B. Chen, Q. Yan and H. Zhang, Adv. Mater., 2015, 27, 4695–4701 Search PubMed
.
- F. Zheng, Y. Yang and Q. Chen, Nat. Commun., 2014, 5, 5261 CrossRef CAS PubMed
.
- G. Zhang, S. Hou, H. Zhang, W. Zeng, F. Yan, C. C. Li and H. Duan, Adv. Mater., 2015, 27, 2400–2405 Search PubMed
.
- A. Banerjee, K. K. Upadhyay, D. Puthusseri, V. Aravindan, S. Madhavi and S. Ogale, Nanoscale, 2014, 6, 4387–4394 Search PubMed
.
- H.-L. Jiang, B. Liu, Y.-Q. Lan, K. Kuratani, T. Akita, H. Shioyama, F. Zong and Q. Xu, J. Am. Chem. Soc., 2011, 133, 11854–11857 CrossRef CAS PubMed
.
- P. Pachfule, D. Shinde, M. Majumder and Q. Xu, Nat. Chem., 2016, 8, 718–724 Search PubMed
.
- X. Xu, Z. Fan, X. Yu, S. Ding, D. Yu and X. W. (David) Lou, Adv. Energy Mater., 2014, 4, 1400902 CrossRef
.
- J. Shao, T. Gao, Q. Qu, Q. Shi, Z. Zuo and H. Zheng, J. Power Sources, 2016, 324, 1–7 Search PubMed
.
- L. Oakes, R. Carter, T. Hanken, A. P. Cohn, K. Share, B. Schmidt and C. L. Pint, Nat. Commun., 2016, 7, 11796 Search PubMed
; X. Fang, C. Hua, X. Guo, Y. Hu, Z. Wang, X. Gao, F. Wu, J. Wang and L. Chen, Electrochim. Acta, 2012, 81, 155–160 Search PubMed
.
- X. Wang, G. Li, M. H. Seo, F. M. Hassan, M. A. Hoque and Z. Chen, Adv. Energy Mater., 2015, 5, 1501106 Search PubMed
.
- J. Wang, J. Liu, D. Chao, J. Yan, J. Lin and Z. X. Shen, Adv. Mater., 2014, 12, 7162–7169 Search PubMed
.
- F. Zhang, Y. Tang, H. Liu, H. Ji, C. Jiang, J. Zhang, X. Zhang and C.-S. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 4691–4699 Search PubMed
.
- J. B. Cook, H.-S. Kim, Y. Yan, J. S. Ko, S. Robbennolt, B. Dunn and S. H. Tolbert, Adv. Energy Mater., 2016, 6, 1501937 CrossRef
.
- P. Wang, H. Sun, Y. Ji, W. Li and X. Wang, Adv. Mater., 2014, 26, 964–969 Search PubMed
.
- D. Su, S. Dou and G. Wang, Adv. Energy Mater., 2014, 4, 1401205 Search PubMed
.
- D. Kong, H. He, Q. Song, B. Wang, W. Lv, Q.-H. Yang and L. Zhi, Energy Environ. Sci., 2014, 7, 3320–3325 CAS
.
- Y. Gong, S. Yang, Z. Liu, L. Ma, R. Vajtai and P. M. Ajayan, Adv. Mater., 2013, 25, 3979–3984 CrossRef CAS PubMed
.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 Search PubMed
.
- X. Huang, B. Zheng, Z. Liu, C. Tan, J. Liu, B. Chen, H. Li, J. Chen, X. Zhang, Z. Fan, W. Zhang, Z. Guo, F. Huo, Y. Yang, L.-H. Xie, W. Huang and H. Zhang, ACS Nano, 2014, 8, 8695–8701 Search PubMed
.
- Q. Weng, X. Wang, X. Wang, C. Zhang, X. Jiang, Y. Bandoa and D. Golberg, J. Mater. Chem. A, 2015, 3, 3097–3102 CAS
.
- B. Ma, P.-Y. Guan, Q.-Y. Li, M. Zhang and S.-Q. Zan, ACS Appl. Mater. Interfaces, 2016, 8, 26794–26800 Search PubMed
.
- Y. Liang, R. Feng, S. Yang, H. Ma, J. Liang and J. Chen, Adv. Mater., 2011, 23, 640–643 CrossRef CAS PubMed
.
- J. Ding, H. Wang, Z. Li, K. Cui, D. Karpuzov, X. Tan, A. Kohandehghan and D. Mitlin, Energy Environ. Sci., 2015, 8, 941–955 CAS
.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 Search PubMed
.
- L. Zhang, X. Yang, F. Zhang, G. Long, T. Zhang, K. Leng, Y. Zhang, Y. Huang, Y. Ma, M. Zhang and Y. Chen, J. Am. Chem. Soc., 2013, 135, 5921–5929 Search PubMed
.
- X. Wang, H. Zhou, E. Sheridan, J. C. Walmsley, D. Ren and D. Chen, Energy Environ. Sci., 2016, 9, 232–239 Search PubMed
; R. Wang, J. Lang and X. Yan, Sci. China: Chem., 2014, 57, 1570–1578 Search PubMed
.
- M. Sevilla, P. Valle-Vigón and A. B. Fuertes, Adv. Funct. Mater., 2011, 21, 2781–2787 Search PubMed
.
- L. Qie, W. Chen, H. Xu, X. Xiong, Y. Jiang, F. Zou, X. Hu, Y. Xin, Z. Zhang and Y. Huang, Energy Environ. Sci., 2013, 6, 2497–2504 Search PubMed
.
- X. Zheng, W. Lv, Y. Tao, J. Shao, C. Zhang, D. Liu, J. Luo, D.-W. Wang and Q.-H. Yang, Chem. Mater., 2014, 26, 6896–6903 CrossRef CAS
.
- J. Tang, R. R. Salunkhe, J. Liu, N. L. Torad, M. Imura, S. Furukawa and Y. Yamauchi, J. Am. Chem. Soc., 2015, 137, 1572–1580 Search PubMed
.
- Y. Liu, J. S. Xue, T. Zheng and J. R. Dahn, Carbon, 1996, 34, 193–200 Search PubMed
.
- H. Wang, Z. Xu, A. Kohandehghan, Z. Li, K. Cui, X. Tan, T. J. Stephenson, C. K. King'ondu, C. M. B. Holt, B. C. Olsen, J. K. Tak, D. Harfield, A. O. Anyia and D. Mitlin, ACS Nano, 2013, 7, 5131–5141 Search PubMed
.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Energy Mater., 2011, 1, 356–361 Search PubMed
.
- C. Chen, D. Yu, G. Zhao, B. Du, W. Tang, L. Sun, Y. Sun, F. Besenbacher and M. Yu, Nano Energy, 2016, 27, 377–389 CrossRef CAS
.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 CrossRef CAS PubMed
.
- L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago and M. A. Pimenta, Appl. Phys. Lett., 2006, 88, 163106 Search PubMed
.
- R. T. Wang and X. B. Yan, Sci. Rep., 2014, 4, 3712 Search PubMed
.
- Y. Wang, Z. Hong, M. Wei and Y. Xia, Adv. Funct. Mater., 2012, 22, 5185–5193 Search PubMed
.
- X.-Y. Shan, Y. Wang, D.-W. Wang, F. Li and H.-M. Cheng, Adv. Energy Mater., 2016, 6, 1502064 Search PubMed
.
- K. Leng, F. Zhang, L. Zhang, T. Zhang, Y. Wu, Y. Lu, Y. Huang and Y. Chen, Nano Res., 2013, 6, 581–592 Search PubMed
.
- H. Wu, C. V. Rao and B. Rambabu, Mater. Chem. Phys., 2009, 116, 532–535 Search PubMed
.
- M. D. Stoller, S. Murali, N. Quarles, Y. Zhu, J. R. Potts, X. Zhu, H.-W. Ha and R. S. Ruoff, Phys. Chem. Chem. Phys., 2012, 14, 3388–3391 Search PubMed
.
- P. Sennu, V. Aravindan, M. Ganesan, Y.-G. Lee and Y.-S. Lee, ChemSusChem, 2016, 9, 849–854 Search PubMed
.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Adv. Energy Mater., 2016, 6, 201600802 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta09143a |
| This journal is © The Royal Society of Chemistry 2017 |