An ultra-absorbent alkyne-rich porous covalent polycalix[4]arene for water purification†
Dinesh
Shetty
a,
Ilma
Jahovic
 a,
Jesus
Raya
b,
Florent
Ravaux
c,
Mustapha
Jouiad
a,
Jesus
Raya
b,
Florent
Ravaux
c,
Mustapha
Jouiad
 c,
John-Carl
Olsen
d and
Ali
Trabolsi
c,
John-Carl
Olsen
d and
Ali
Trabolsi
 *a
*a
aNew York University Abu Dhabi (NYUAD), The Experimental Research Building (C1), Saadiyat Island, Abu Dhabi, United Arab Emirates. E-mail: ali.trabolsi@nyu.edu
bCNRS/Université de Strasbourg 1, Rue Blaise Pascal, Strasbourg, France 67000
cMasdar Institute of Science and Technology, Department of Mechanical and Materials Engineering, Abu Dhabi, United Arab Emirates
dDepartment of Chemistry, University of Rochester, RC Box 270216, Rochester, NY 14607-0216, USA
First published on 15th November 2016
Abstract
The development of novel materials for removal of organic contaminants from water is of global importance for the preservation of the environment. Here, we describe the synthesis and characterization of the first porous covalent calix[4]arene-based polymer (CalP) and its use for water purification. CalP has a high surface area, large pore volume and excellent sorption capacity for a range of organic solvents, oils, and toxic dyes. The polymer can selectively absorb up to seven times its weight of oil from oil/water mixtures. From aqueous solutions, it can adsorb both anionic and cationic dyes in under 15 minutes. Its uptake capacity is significantly higher than those of the most adsorbent materials reported to date, including commercial activated carbon. Additionally, the polymer can be easily regenerated using mild washing procedures and reused several times with no loss of absorption efficiency.
Petroleum products, organic solvents, and textile dyes are primary water pollutants.1,2 For example, in 2015 (and due largely to marine spills near Singapore and Turkey) 7000 tons of oil were accidentally released into the environment.3 Also, about 15% of the total world production of textile dyes is released in industrial effluents yearly during the dyeing process.4 Such dyes are designed to withstand physicochemical and biological degradation and hence generally escape conventional wastewater treatment methods. Their presence in the hydrosphere is toxic and causes harm to many organisms.5,6 Effective methods for the removal of organic contaminants from the environment are, therefore, in high demand. Of several available decontamination methods, sorption presents several advantages for wastewater treatment. This is due to ease of process design, low cost, high efficiency and the ability to treat high concentrations of pollutants.7–11 Recently, because of the additional benefits of low density (which leads to high gravimetric capacity) and reliable regeneration under mild conditions, advanced organic materials have emerged as potential alternatives to traditional inorganic adsorbents.7,11–19 Porous covalent organic polymers (COPs) composed of light elements are attractive candidates in this regard due to their thermal stability and high-yielding syntheses.18,20–22 Herein, we describe the preparation of a porous covalent polycalix[4]arene (CalP) and its use in the removal of organic solvents, oil and toxic dyes from aqueous mixtures.
Calix[n]arenes (n = 4, 6, 8) are cyclic phenolic oligomers that have long been recognized as versatile supramolecular scaffolds.23–26 They possess a hydrophobic cavity that has both polar and non-polar rims for guest recognition. Also, they can be selectively modified to provide analyte-selectivity or to facilitate incorporation into larger structures. Because of their favorable recognition properties, we hypothesized that fixing calixarenes within the backbone of a polymeric network would create an absorbent material. Although many previous studies report the syntheses and properties of monomeric calixarenes, fewer describe their incorporation into polymers, and in most of these, the macrocycles serve only as side-chain pendants.27–29
We chose the Sonogashira–Hagira reaction as a convenient means for cross-linking brominated calixarenes. This method brought with it the additional benefits of alkyne functionality. Alkynes are known to endow materials with a high surface area.21,30 They also increase absorption capacity and provide superhydrophobicity, which, we anticipated, would facilitate recovery of saturated polymer samples from water. We found that the prepared polymer does show excellent sorption properties for oils and other organic pollutants, likely as a result of (i) dipolar interactions, including H-bonding, that involve the hydrophilic rim of the calixarene and (ii) nonpolar interactions involving the hydrophobic rim of the calixarene and the polymer's constituent aromatic rings and alkynes. To the best of our knowledge, CalP is the first example of a porous calixarene-based covalent polymer, though we believe that this architecture has a more general potential to serve as a platform for the development of materials for sorption, toxic contaminant removal, and hydrocarbon separations.
The synthesis of CalP was accomplished by a palladium catalysed Sonogashira–Hagihara cross-coupling of 1,4-diethynylbenzene and tetrabromo-calix[4]arene-tetrol (Fig. 1). The latter compound was synthesized by following a previously reported literature procedure.31 The reaction was carried out in dry THF at 65 °C for 60 h. After centrifugation and multiple washings of the reaction precipitate (see ESI†), the polymer was obtained as a brown powder that was completely insoluble in all organic solvents tested, including THF, acetone, CHCl3, DMF and DMSO, indicating the formation of a covalently cross-linked, reticular structure. Maintaining the optimum dilution of the reaction mixture was critical for obtaining a high-porosity polymer in good chemical yield (60 mol% versus the brominated precursor). The resulting polymer, CalP, exhibited chemical stability in acidic or basic aqueous solutions, as indicated by a lack of dissolution or decomposition (Fig. S1†).
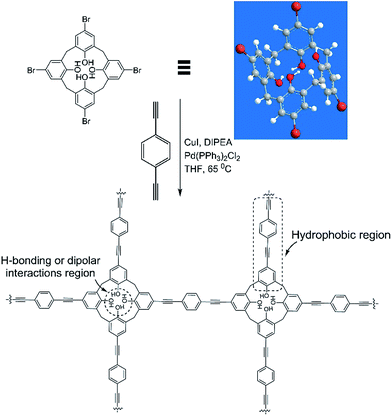 | ||
| Fig. 1 Synthetic route to porous calix[4]arene polymer CalP by Sonogashira–Hagihara cross-coupling. Plausible sites on the polymer for interaction with pollutants are marked by dotted lines. | ||
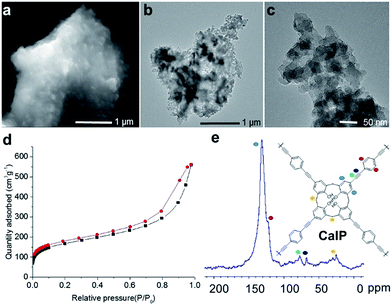
In order to investigate the bulk morphology of CalP, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses were performed. The SEM images show that CalP is composed of fused amorphous clumps (Fig. 2a and S2 in the ESI†). TEM images reveal the same morphology, as well as the presence of pores (Fig. 2b and c). The powder X-ray diffraction (PXRD) pattern of CalP (Fig. S3 in the ESI†) shows a broad signal that is consistent with an amorphous nature. Thermogravimetric analysis (TGA) of the polymer indicates excellent thermal stability over 600 °C (Fig. S4 in the ESI†). Molecular-level characterization of the porous polymer was achieved by FTIR spectroscopy and solid state NMR spectroscopy. An absorption band near 3300 cm−1, consistent with the terminal alkyne –C–H stretching vibration of the 1,4-diethynylbenzene monomer, is conspicuously absent from the spectrum of the polymer (Fig. S5 in the ESI†), whereas broad bands near 3000 cm−1, which indicate calixarene tetrol functionality, are prominent. Furthermore, a new peak near 2200 cm−1, consistent with an asymmetric –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– stretching vibration, is apparent. A solid-state cross-polarization magic angle spinning (CP/MAS) 13C NMR spectrum is shown in Fig. 2e. The chemical shift values in the range of 120–140 ppm correspond to the aromatic carbons of the phenylene groups that constitute the polymeric framework. The broad peak at 25 ppm is associated with the aliphatic methylene moieties (–CH2–) of the calix[4]arene macrocycle. The resonance of alkyne (–C
C– stretching vibration, is apparent. A solid-state cross-polarization magic angle spinning (CP/MAS) 13C NMR spectrum is shown in Fig. 2e. The chemical shift values in the range of 120–140 ppm correspond to the aromatic carbons of the phenylene groups that constitute the polymeric framework. The broad peak at 25 ppm is associated with the aliphatic methylene moieties (–CH2–) of the calix[4]arene macrocycle. The resonance of alkyne (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) carbons appears near 90 ppm. We measured the ratio of methylene carbons to alkyne carbons by solid state direct polarization magic angle spinning (DP/MAS) 13C NMR spectroscopy. Signal integrations reveal (Fig. S6 in the ESI†) a 1
C–) carbons appears near 90 ppm. We measured the ratio of methylene carbons to alkyne carbons by solid state direct polarization magic angle spinning (DP/MAS) 13C NMR spectroscopy. Signal integrations reveal (Fig. S6 in the ESI†) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, which confirms the presence of four conjugated ethylene linkers for every calix[4]arene unit, and therefore a hyper-crosslinked structure. Together, the IR and NMR spectroscopic data confirm the successful formation of the porous covalent calix[4]arene polymer depicted in Fig. 1.
2 ratio, which confirms the presence of four conjugated ethylene linkers for every calix[4]arene unit, and therefore a hyper-crosslinked structure. Together, the IR and NMR spectroscopic data confirm the successful formation of the porous covalent calix[4]arene polymer depicted in Fig. 1.
The porosity of CalP was characterized by N2 adsorption/desorption measurements. To remove the solvent and trapped gas, the polymer was activated at 358 K for 24 h prior to the measurements. Based on the IUPAC classification system, the observed N2 sorption isotherm (Fig. 2d) can be categorized as type II with a H4 type hysteresis loop corresponding to desorption. The data suggest a complex polymeric network that contains both micropores and mesopores and that swells with gas intake. The polymer has a pore size distribution mainly in the mesopore region, a Barrett–Joyner–Halenda (BJH) average pore diameter of 95 Å and a cumulative pore volume of 0.73 cm3 g−1 (Fig. S7 in the ESI†). Calculation using the Brunauer–Emmett–Teller (BET) model gives a specific surface area of 596 m2 g−1. This high value is likely due to the accessibility of both the calixarene cavities and inter-molecular spaces. Notably, CalP was found to be superhydrophobic and to float in aqueous solutions. The polymer's hydrophobicity was investigated by contact angle measurements. The manually ground CalP powder fixed on Scotch tape had an average contact angle of 155.4 ± 4.01 degrees (Fig. S8 in the ESI†). The polymer's hydrophobicity is consistent with the hydrophobic character of its constituent aromatic rings and alkyne-rich backbone. To our knowledge, CalP is the first porous calixarene-based covalent polymer to be reported, and its high surface area suggests that further investigation of calixarene-based polymers could be fruitful.
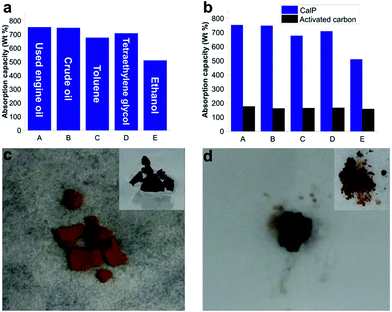
The synthesized polymer, with its superhydrophobicity and relatively high surface area (SBET = 596 m2 g−1) compared to other adsorbent polymeric materials having a macrocyclic backbone (for example, cyclodextrin polymers11 with SBET = 263 m2 g−1 and pillarene-based polymers22 with SBET = 400 m2 g−1), demonstrated a pronounced ability to absorb and remove toxic pollutants from aqueous mixtures. CalP was added to oil/water or organic solvent/water mixtures in a Petri dish and allowed to absorb. The polymer quickly absorbed the crude oil, engine oil or organic solvent (toluene, EtOH, tetraethylene glycol) while repelling the water. The polymer was weighed before and after absorption in order to determine uptake capacity. As shown in Fig. 3, CalP shows high uptake capacities ranging from 500 to 780 wt%. It can absorb up to eight times its own weight of ethylene glycol and seven times its weight of used engine oil. These uptake values are much higher than those reported for commercial activated carbon (Fig. 3b) and other recently described porous materials.15–17 Two types of oil were tested: used engine oil and commercial crude oil (ATSM D5307). After being placed on top of an oil–water mixture, the light brown polymer quickly absorbed the oil and turned dark brown (Fig. 3c and d). Complete absorption occurred in about five minutes, at which point the floating, saturated polymer was removed from the water surface (see the ESI Video file†). The absorbed oil was washed (2 times, 5 min each) from the polymer with diethyl ether (Fig. 3d). The polymer was then dried and could be re-used up to three times with negligible decrease of its absorption capacity (Fig. S9 in the ESI†), a very practical result considering the ease of the recovery process.
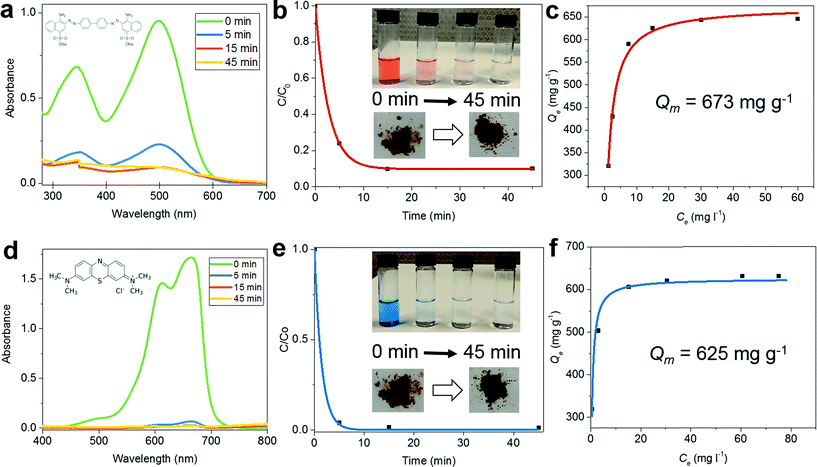
Because of their ability to adsorb large quantities of pollutants with high efficiency, porous materials with high specific surface areas are used for wastewater treatment. To demonstrate the potential of CalP to be effective for such purposes, we determined the polymer's ability to remove Congo red (CR), methylene blue (MB) and rhodamine B (RB) from water. Congo red, an anionic dye, is toxic and considered to be a primary pollutant of water resources.32 Ultra-violet/visible (UV/Vis) absorption spectroscopy was used to estimate the rate of dye adsorption on CalP in an aqueous solution. A 5 mg sample of CalP was suspended in 10 ml of a 0.5 mM aqueous solution of Congo red, and the decrease in the absorbance of 496 nm light was monitored over time (Fig. 4a and b). About 80% of the dye was adsorbed within the first five minutes and complete removal was observed within 15 minutes. The adsorption isotherm fitted by the Langmuir model gives a maximum adsorption capacity Qm of 673 mg g−1, corresponding to complete monolayer coverage (Fig. 4c). This capacity is significantly higher than those of most adsorbent materials reported previously.33–35 The generality of porous CalP's ability to remove organic waste was assessed, preliminarily, by testing other dyes, with different physical properties, commonly used in the textile industry, specifically, methylene blue and rhodamine B (RhB), both cationic dyes. In the case of MB (H2O, 0.5 mM), ∼95% was removed within 5 min whereas in the case of RhB (H2O, 0.5 mM) the removal efficiency was ∼50% after the same amount of time (Fig. 4e and f and S10 in the ESI†) and by the same amount of polymer (5 mg). In both cases complete dye removal was achieved within 120 min, indicating very fast dye adsorption kinetics. The maximum adsorption capacities of MB and RhB are 625 and 484 mg g−1, respectively. These values are greater than those of most adsorbent materials reported.10,36,37 In addition, we carried out the adsorption of a mixture of dyes (CR and MB) in 1 N NaCl solution and found that CalP is as effective (Fig. S11†) as in pure water. The high efficiency and fast kinetics of the adsorption of both anionic and cationic dyes are likely due to several phenomena including (i) hydrophobic interactions (between the aromatic cores of the dyes and the hydrophobic surface of the polymer), (ii) monopole–dipole interactions (between the quaternary ammonium groups of the dyes and the polymer's hydroxyl groups), (iii) the amorphous nature of the polymer which allows swelling of the polymeric network,38,39 and (iv) capillary effects due to the polymer's pores. Furthermore, both MB and RhB were easily removed by simply washing the polymer with ethanol at 50 °C for 3 hours. Again, the polymer could be re-used for dye-adsorption with no loss of its original efficiency. On the other hand, removal of CR failed with both polar and non-polar organic solvent rinses but was finally achieved by treating the dye-loaded polymer with a 0.1 N HNO3 solution at 50 °C for 6 hours. After this acidic treatment, the adsorption capacity of the polymer remained intact. The unusually strong interaction between CR and CalP is consistent (Fig. S12 in the ESI†) with the known affinity of diazo compounds for calixarenes.40
In summary, we have successfully synthesized an alkyne-rich, calix[4]arene-containing polymer that is chemically and thermally stable, superhydrophobic and highly porous. It displays high absorption capacity for organic solvents, oils and dyes and can be used for removing these contaminants from water. We believe that the macrocycles of the polymer, as well as its alkynes, serve as effective sorption sites. Furthermore, the polymer can be easily regenerated upon treatment with organic solvents or slightly acidic aqueous solutions and reused many times. The high efficiency and ease of implementation of the polymer as an adsorbent demonstrate the advantages of incorporating the calixarene moiety within a functional material and bode well for the development of calixarene-based materials for water purification, separations and other applications.
Acknowledgements
This work was supported by New York University Abu Dhabi. We thank NYUAD for their generous support for the research program. We also thank the Core Technology Platforms at NYUAD. The authors also thank Miss Khulood Alawadi for the video assistance.Notes and references
- J. Aurell and B. K. Gullett, Environ. Sci. Technol., 2010, 44, 9431–9437 CrossRef CAS PubMed.
- T. Dalton and D. Jin, Mar. Pollut. Bull., 2010, 60, 1939–1945 CrossRef CAS PubMed.
- Oil tanker spill statistics, http://www.itopf.com/fileadmin/data/Documents/Company_Lit/Oil_Spill_Stats_2016.pdf, 2016.
- R. Kant, Nat Sci, 2012, 4, 22 CAS.
- S. Kiran, S. Ali and M. Asgher, Bull. Environ. Contam. Toxicol., 2013, 90, 208–215 CrossRef CAS PubMed.
- N. M. Mahmoodi, J. Environ. Eng., 2013, 139, 1368–1374 CrossRef CAS.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS PubMed.
- B. Pan, B. Pan, W. Zhang, L. Lv, Q. Zhang and S. Zheng, Chem. Eng. J., 2009, 151, 19–29 CrossRef CAS.
- V. Ponnusami, V. Gunasekar and S. Srivastava, J. Hazard. Mater., 2009, 169, 119–127 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling and W. R. Dichtel, Nature, 2015, 529, 190–194 CrossRef PubMed.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS.
- A. Bayat, S. F. Aghamiri, A. Moheb and G. R. Vakili-Nezhaad, Chem. Eng. Technol., 2005, 28, 1525–1528 CrossRef CAS.
- B. Li, X. Huang, L. Liang and B. Tan, J. Mater. Chem., 2010, 20, 7444–7450 RSC.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413–5419 CrossRef CAS PubMed.
- K. Sohn, Y. J. Na, H. Chang, K.-M. Roh, H. D. Jang and J. Huang, Chem. Commun., 2012, 48, 5968–5970 RSC.
- P. Thanikaivelan, N. T. Narayanan, B. K. Pradhan and P. M. Ajayan, Sci. Rep., 2012, 2, 230 Search PubMed.
- D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS PubMed.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 988–998 CrossRef CAS PubMed.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- W. Lu, D. Yuan, D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Bräse, J. Guenther, J. Blümel and R. Krishna, Chem. Mater., 2010, 22, 5964–5972 CrossRef CAS.
- S. N. Talapaneni, D. Kim, G. Barin, O. Buyukcakir, S. H. Je and A. Coskun, Chem. Mater., 2016, 28, 4460–4466 CrossRef CAS.
- J. L. Atwood, L. J. Barbour and A. Jerga, Science, 2002, 296, 2367–2369 CrossRef CAS PubMed.
- F. Corbellini, A. Mulder, A. Sartori, M. J. Ludden, A. Casnati, R. Ungaro, J. Huskens, M. Crego-Calama and D. N. Reinhoudt, J. Am. Chem. Soc., 2004, 126, 17050–17058 CrossRef CAS PubMed.
- Y. Ishii, Y. Takenaka and K. Konishi, Angew. Chem., Int. Ed., 2004, 43, 2702–2705 CrossRef CAS.
- Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS PubMed.
- S. Memon, O. Oguz, A. Yilmaz, M. Tabakci, M. Yilmaz and Ş. Ertul, J. Polym. Environ., 2001, 9, 97–101 CrossRef CAS.
- L. Wang, X. Shi, P. Jia and Y. Yang, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 6259–6266 CrossRef CAS.
- Y. Yang and T. M. Swager, Macromolecules, 2006, 39, 2013–2015 CrossRef CAS.
- Z. Xiang, D. Cao, W. Wang, W. Yang, B. Han and J. Lu, J. Phys. Chem. C, 2012, 116, 5974–5980 CAS.
- T. E. Clark, M. Makha, A. N. Sobolev, H. Rohrs, J. L. Atwood and C. L. Raston, Chem.–Eur. J., 2008, 14, 3931–3938 CrossRef CAS PubMed.
- O. Anjaneya, S. Y. Souche, M. Santoshkumar and T. Karegoudar, J. Hazard. Mater., 2011, 190, 351–358 CrossRef CAS PubMed.
- J. Fei, Y. Cui, X. Yan, W. Qi, Y. Yang, K. Wang, Q. He and J. Li, Adv. Mater., 2008, 20, 452–456 CrossRef.
- X. Wang, Y. Zhong, T. Zhai, Y. Guo, S. Chen, Y. Ma, J. Yao, Y. Bando and D. Golberg, J. Mater. Chem., 2011, 21, 17680–17687 RSC.
- D. Yuan, W. Lu, D. Zhao and H. C. Zhou, Adv. Mater., 2011, 23, 3723–3725 CrossRef CAS PubMed.
- X. Zhang, G. Lian, S. Zhang, D. Cui and Q. Wang, CrystEngComm, 2012, 14, 4670–4676 RSC.
- Y. Han, W. Li, J. Zhang, H. Meng, Y. Xu and X. Zhang, RSC Adv., 2015, 5, 104915–104922 RSC.
- V. Davankov and M. Tsyurupa, Comprehensive Analytical Chemistry, Elsevier, 2011, vol. 56, pp. 315–358 Search PubMed.
- L. H. Li, J. Xiao, P. Liu and G. W. Yang, Sci. Rep., 2015, 5, 9028 CrossRef CAS PubMed.
- M. A. Kamboh, I. B. Solangi, S. Sherazi and S. Memon, J. Hazard. Mater., 2009, 172, 234–239 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta08388a |
| This journal is © The Royal Society of Chemistry 2017 |