Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-induced displacement of a microbead through the thermal expansion of liquid crystals†
Y.
Takenaka
*ab and
T.
Yamamoto
a
aResearch Institute for Sustainable Chemistry, National Institute of Advanced Industrial Science and Technology, Tsukuba, Ibaraki 305-8565, Japan. E-mail: takenaka.yoshiko@aist.go.jp
bPRESTO, JST, Kawaguchi, Saitama 332-0012, Japan
First published on 10th November 2016
Abstract
We found the displacement of a microbead by the oblique irradiation of UV light in pure 4-cyano-4′-pentylbiphenyl (5CB) without photo-responsive molecules. This system is very simple and a complicated experimental setup is not necessary. The displacement is observed in the nematic state of 5CB and not in the isotropic state. The distance of the displacement was proportional to the intensity of the UV light. After the irradiation was stopped, the microbead started to return to the original position. These phenomena are attributed to the thermal expansion of 5CB induced by the photo-thermal effect of polyimide coated on the glass substrate.
1. Introduction
Recently, stimulus responsive soft materials have been studied for the development of future soft actuators. Stimulus responsive soft materials do not need complicated designs of electric circuits because they can change their shapes by themselves or can transport inner materials by converting the external energy, i.e., electric field, magnetic field and light, into kinetic energy.1–13 Actually this characteristic is one of the advantages of the stimulus responsive soft materials to apply to actuators.Among stimulus responsive soft materials, liquid crystals are some of the interesting materials. Stimulus responsive liquid crystals have been studied abundantly as follows. Ikeda et al. reported that a film made of a liquid crystalline polymer containing an azobenzene moiety can bend through the cis–trans photoisomerization induced by the irradiation of light.1 Ikeda et al. also reported that the film of a liquid crystalline polymer can rotate wheels.2 Feringa et al. reported the light driven rotation of a nanomaterial in a liquid–crystal film containing light responsive molecules.3,4 Kurihara et al. reported that a microbead translates in a liquid–crystal film containing azobenzene derivatives by photoirradiation.5,6 In these cases, proper photoisomerization molecules should be mixed to a liquid crystal to induce the motion of liquid crystalline polymer films or the transportation of microbeads in liquid crystals.
In some reports, microbeads are transported in liquid crystals without photoisomerization molecules.7 The effects of heat,8,9 electro-osmosis,10,11 electric convection,12 defect13 and light pressure on the motion of microbeads have been reported. To induce these motions, external power supply or custom design of the system would be necessary. For instance, in case that the heat effect is used, a thermal controller is necessary and the sample should be observed on a thermal stage. When we utilize electro-osmosis or electric convection, we should set electrodes to the samples. Laser is indispensable to translate microbeads by light pressure. Therefore, easy transportation of microbeads in liquid crystals without a complicated setup is really to be achieved. Such systems are expected to be future actuators, sensors and pumps in microfluidics.
In this paper, we show that microbeads can be transferred at room temperature by the irradiation of UV light in common liquid crystals. The microbeads return to the original position after stopping the irradiation. Our system is very easy to set up without complicated architectures and we do not need to prepare a thermal controller, an electrode, or laser to extract the motion of microbeads.
2. Experimental
SiO2 microbeads (Wako Pure Chemical Industries, Ltd, ϕ10 μm) were dispersed in 99.5% ethanol (Wako Pure Chemical Industries, Ltd) solution. The concentration of the dispersion is 20 mg ml−1. 1.25 μl of this dispersion was mixed with 50 μl of 4-cyano-4′-pentylbiphenyl (5CB, Wako Pure Chemical Industries, Ltd). This mixture was kept at 60 °C for 10 min to evaporate the ethanol. Then, the mixture was cooled down to room temperature (ca. 25 °C) and injected into the following 4 kinds of cells. The nematic–isotropic transition temperature of 5CB is 35 °C.(1) Cell A. 90 μl of NMP (N-methylpyrrolidone, Sigma-Aldrich) solution of polyamic acid (poly(pyromellitic dianhydride-co-4,4′-oxydianiline) Sigma-Aldrich) was coated on a cover glass (Matsunami, 22 × 40 mm) using a spin-coater at 4000 rpm for 90 s. The substrate was heated at 180 °C for 3 h to induce the polymerization of polyamic acid to polyimide. After heating, the surfaces of polyimide were rubbed and arranged in an anti-parallel way with a spacer of 80 μm.
(2) Cell B (KSRP-50/A107P1NSS, EHC). The surfaces of the cell were coated with rubbed polyimide and arranged in an anti-parallel way with a spacer of 50 μm.
(3) Cell C (KSRZ-50/A107P1NSS, EHC). The surface of the cell was coated with polyimide without rubbing. The thickness of the cell is 50 μm.
(4) Cell D (KSHH-50/A107P1NSS, EHC). The surface of the cell was coated with cetyltrimethylammonium bromide (CTAB). The thickness of the cell is 50 μm.
B, C and D are commercial cells, and the electrodes made of indium tin oxide (ITO) were applied as a template on the surface of the cells. These ITO electrodes were not used in our experiments and we will show later that ITO electrodes have no effects on the present motion of microbeads. Depending on the characteristics of each cell, liquid crystals exhibit parallel alignment in the cells A and B, random alignment in the cell C and homeotropic alignment in the cell D.
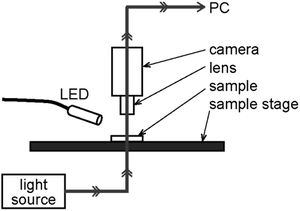
The sample cells were observed using a digital microscope (VW9000, Keyence Corp.) with an obliquely-upward irradiation of 365 nm LED light (150 mW cm−2, Hamamatsu Photonics) as shown in Fig. 1. The irradiation area was like ellipse with 1 cm × 2 cm. Besides the observation at room temperature (25 °C), we also made an observation at 40 °C for comparison by using a thermal plate (Blast, BL-SR15001).
3. Results and discussion
The spatio-temporal plot of the motion of the microbead during the irradiation of UV light for 5 s at 25 °C is shown in Fig. 2. The horizontal and the vertical axes of Fig. 2 represent space and time, respectively. The spatio-temporal plot was obtained by stacking vertically the same size of rectangular areas at the fixed pixel point cut out from each snapshot. The starting and the ending times of the irradiation of UV light are inserted in Fig. 2. In this plot, the light was irradiated from the left-hand side, and therefore the microbead ran away quickly from the irradiation of UV light and returned back gradually toward the original position by the extinction of the light. The focus of a microbead in Fig. 2 was fixed but that of another microbead was shifted as shown in Fig. S1 (see ESI†). This means that the distances between microbeads and substrates are different for different microbeads. In the present experiments, all microbeads moved the distance of the same order. Although we could not confirm whether a microbead adsorbed on the cell surface or not, we can assume that the difference in the distance between microbeads and substrates did not have a large effect on the moving distance of microbeads.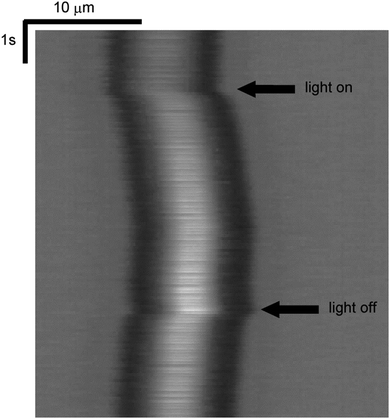 | ||
| Fig. 2 Spatio-temporal plot of the motion of the microbead. The light was irradiated from the left-hand side. The times when the light was on and off are shown. | ||
We made the same experiments for four kinds of cells at 25 and 40 °C. As a result, the phase of the liquid crystal and the alignment of the director seem to affect the motion (Table 1). When the temperature was 40 °C, 5CB exhibits the isotropic state and then the microbead did not move in any cells. On the other hand, when the temperature was 25 °C, 5CB exhibits the nematic state and then the microbead moved in the cells A, B and C. In the cell D, the microbead did not move even when 5CB exhibits the nematic state.
| 25 °C | 40 °C | |
|---|---|---|
| Cell A | Motion | Motionless |
| Cell B | Motion | Motionless |
| Cell C | Motion | Motionless |
| Cell D | Motionless | Motionless |
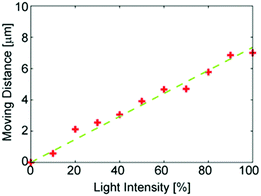
In Fig. 3, the dependence of the moving distance of the microbead on the intensity of light is demonstrated. When the light intensity increases, the moving distance proportionally increases. This indicates that the intensity of the light strongly affects the motion of microbeads.
Up to here, we presented the motion of microbeads in nematic 5CB a parallel alignment induced by the irradiation of UV light of 365 nm. Now we investigate the reason why the microbead moves. As we mentioned in the introduction part, several kinds of external stimuli promoted the motion of microbeads in liquid crystals: i.e., heat, electro-osmosis, electric convection, defect and light pressure. Among these, the effects of electro-osmosis and electric convection are unlikely. The electro-osmosis and electric convection generally occur with DC field or with AC field of low frequency (5 Hz for electro-osmosis and several hundreds of hertz for electric convection), and thus the frequency of light (∼1014 Hz) is too high for the charge distribution to follow. When a defect affects the motion, the change in the alignment of the director is necessary. In the present system, however, a microbead could move even in the nematic 5CB without unidirectional alignment. The effect of light pressure is not attributed to the motion. It should be noted the backward motion of a microbead after ceasing the irradiation of UV light. This backward motion would be induced by the release of energy stored in the liquid crystal field accumulated by the irradiation of UV light. If the microbead is transferred by the effect of the light pressure, no energy is accumulated in the liquid crystal field, because the light pressure works on the irradiated surface of a microbead not on the liquid crystal field. Therefore, we speculate that the photo-thermal effect would cause the present motion. It is consistent with the fact that the moving distance in the present system depends on the intensity of light, because the effect of heat also depends on the intensity of light.
Then, the effect of heat should be examined. According to Weiss et al. 5CB showed drastic volume expansion just below the nematic–isotropic transition temperature.14 Kim et al. reported that microbeads dispersed in 5CB were transferred by the volume expansion that occurred near the nematic–isotropic transition temperature.9 They observed the motion of the microbead dispersed in 5CB followed by the temperature change. They changed the temperature from 20 to 25 °C at a rate of 30 °C min−1. In such experiment, the microbead moved 100 μm in 30 s and the speed was ca. 3.3 μm s−1, which is on the same order of that of our observed motion. Thus, the microbead would be able to move by heat, if the energy of irradiated light changes to heat.
To check that the present motion was able to be caused by the photo-thermal effect, the temperature of the sample surface was measured using an infra-red thermometer. During the irradiation of UV light, the surface temperature quickly increased from 30.4 to 31.2 °C and the temperature gradually returns to 30.4 °C when the light was turned off (Fig. 4). According to Weiss et al. the thermal expansion coefficient α [10−4 K−1] of 5CB increases from 10 to 11, following the temperature increase from 30.4 to 31.2 °C. This change is ten times larger than that of water,15 where the thermal expansion coefficient changes from 3.03 to 3.11 [10−4 K−1] when the temperature changes from 30 to 31 °C. When the volume of 5CB expanded, 5CB would tend to extend in a thin cell. As a result, microbeads dispersed in 5CB would move keeping up with the surrounding 5CB molecules. The thermal expansion coefficient α increases in a divergent way just below the nematic–isotropic transition temperature. Thus the microbeads moved very much just below the nematic–isotropic transition temperature. Above the temperature, however, the situation was similar in water; namely, the microbeads did not move, because α is almost constant.
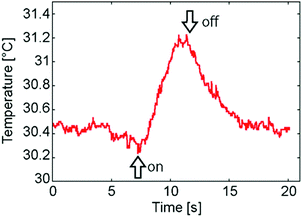 | ||
| Fig. 4 Temperature of the sample surface with the irradiation of light. The on/off moments of the irradiation of UV light are shown as arrows. | ||
All of the above results indicate that the present motion is assisted by the thermal expansion of liquid crystals through photo-thermal conversion. We considered which substance performed the photo-thermal conversion. In the present experiment, we used a light of 365 nm, and thus some of the materials in the system should be able to absorb the light of 365 nm and converted the light energy to heat. One of the possible materials is indium tin oxide (ITO) which is coated on the surface of some cells, because it is well known that ITO absorbs light in the ultra-violet (UV) region. However, we could confirm that ITO was not the substance which performed the photo-thermal conversion in the present case, because Table 1 shows that a microbead was transferred in cell A, the surface of which was not coated with ITO.
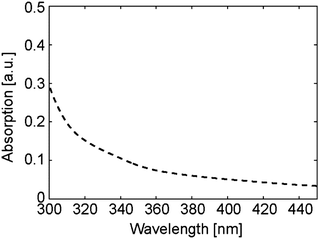
To find out the substance which performed the photo-thermal conversion in our experiments, we measured the UV-vis spectra of the substances included in our system (JASCO V630). As a result, polyimide had subtle absorption around 365 nm (Fig. 5). The absorption of polyimide decreased gradually with the increase in the wavelength. Therefore, microbeads would not be transferred by the light with a long wavelength. Actually, we changed the wavelength of the light with the same light intensity (150 mW cm−2). As we speculated, the motion of the microbead was not observed when the wavelength was 470, 525, 590, 625 nm (data are not shown). In addition, the fact that microbeads were not transferred in the cell D without polyimide coating supports our speculation that the polyimide is the substance which performs the photo-thermal conversion in our system (Table 1). However one may expect that the anisotropy of thermal expansion in the nematic state can affect the displacement. Then we show the results where microbeads moved in the cell D when heated by an infra-red laser (Fig. S2, ESI†). Fig. S2 (ESI†) also shows the increase in the temperature of the cell surface. These results mean that the effects of heat are much larger than that of the alignment of liquid crystal molecules on the displacement of microbeads. Of course, the effects of the anisotropy of thermal expansion should be considered and be explored in future studies.
4. Conclusions
The transfer of microbeads in the liquid crystal (5CB) by light (365 nm) was realized. This motion was induced by the thermal expansion of 5CB through the photo-thermal conversion by polyimide. It is known that the thermal expansion coefficient of 5CB shows a drastic increase just below the nematic–isotropic transition temperature. Thus the subtle increase in sample temperature induced by the photo-thermal conversion of polyimide at 365 nm expanded the volume of 5CB drastically, and microbeads were transferred by this volume expansion. These results present a contactless method to transfer microbeads in liquid crystals by light. In the present experiments, the distances of displacements were limited to ∼50% of diameter of microbeads. In the future, however, we can expect that a long distance can be transferred by tracing the microbeads with light. These systems are expected to be applied to heat sensors when microbeads are used as tracers of thermal expansion of liquid crystals, and also to pumps in microfluidics.Acknowledgements
This work was partly supported by JST, PRESTO on the Molecular Technology and Creation of New Functions and the fundamental research fund of AIST. The authors thank Ms Chikako Sekiguchi (AIST, Japan) for her support with experiments, and Jun-ichi Fukuda (AIST, Japan) for his helpful comments.Notes and references
- T. Ube and T. Ikeda, Angew. Chem., Int. Ed., 2014, 53, 10290 CrossRef CAS PubMed.
- M. Yamada, M. Kondo, J. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986 CrossRef CAS PubMed.
- J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef PubMed.
- R. Eelkema, M. M. Pollard, N. Katsonis, J. Vicario, D. J. Broer and B. L. Feringa, J. Am. Chem. Soc., 2006, 128, 14397 CrossRef CAS PubMed.
- A. Kausar, H. Nagano, T. Ogata, T. Nonaka and S. Kurihara, Angew. Chem., Int. Ed., 2009, 48, 2144 CrossRef CAS PubMed.
- S. Kurihara, K. Ohta, T. Oda, R. Izumi, Y. Kuwahara, T. Ogata and S.-N. Kim, Sci. Rep., 2013, 3, 2167 Search PubMed.
- O. D. Lavrentovich, Soft Matter, 2014, 10, 1264 RSC.
- Y.-K. Kim, M. Majumdar, B. I. Senyuk, L. Tortora, J. Seltmann, M. Lehmann, A. Jakli, J. T. Gleeson, O. D. Lavrentovich and S. Sprunt, Soft Matter, 2012, 8, 8880 RSC.
- Y.-K. Kim, B. Senyuk and O. D. Lavrentovich, Nat. Commun., 2012, 3, 1133 CrossRef PubMed.
- S. Hernandez-Navorro, P. Tierno, J. Ignes-Mullol and F. Sagues, IEEE Trans. Nanobiosci., 2015, 14, 267 CrossRef PubMed.
- I. Lazo, C. Peng, J. Xiang, S. V. Shiyanovskii and O. D. Lavrentovich, Nat. Commun., 2014, 5, 5033 CrossRef CAS PubMed.
- Y. Sasaki, Y. Takikawa, V. S. R. Jampani, H. Hoshikawa, T. Seto, C. Bahr, S. Herminghaus, Y. Hidaka and H. Orihara, Soft Matter, 2014, 10, 8813 RSC.
- A. Eremin, P. Hirankittiwong, N. Chattham, H. Nadasi, R. Stannarius, J. Limtrakul, O. Haba, K. Yonetake and H. Takezoe, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 1716 CrossRef CAS PubMed.
- S. Weiss and G. Ahlers, J. Fluid Mech., 2013, 737, 308 CrossRef CAS.
- G. S. Kell, J. Chem. Eng. Data, 1967, 12, 66 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sm02324j |
| This journal is © The Royal Society of Chemistry 2017 |