Laboratory filter paper as a substrate material for flexible supercapacitors†
Leicong
Zhang
ab,
Xuecheng
Yu
ab,
Pengli
Zhu
 *a,
Fengrui
Zhou
a,
Gang
Li
a,
Rong
Sun
*a,
Fengrui
Zhou
a,
Gang
Li
a,
Rong
Sun
 *a and
Ching-ping
Wong
*a and
Ching-ping
Wong
 acd
acd
aShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: pl.zhu@siat.ac.cn
bShenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen 518055, China
cSchool of Materials Science and Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA
dDepartment of Electronics Engineering, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
First published on 6th October 2017
Abstract
In this study, a flexible asymmetrical solid-state filter paper-based supercapacitor was fabricated. Common laboratory filter paper (FP) provides toughness and flexibility to the whole device. Simply, traditional electroless Ni plating and electro-deposition were introduced to change the electrical conductivity of FP as a flexible and conductive substrate material. Then, the electrochemical active material Co(OH)2 and active carbon (AC) were respectively coated on the conductive Ni paper through electro-deposition and physical coating methods to prepare Ni/Co(OH)2-FP as a positive electrode and Ni/AC-FP as a negative electrode. Moreover, they were separated by a piece of FP coated with an ionic gel electrolyte to assemble the flexible asymmetrical solid-state supercapacitors. This supercapacitor exhibits superior flexibility, large volume energy density (0.64 mW h cm−3), and good bending cycle performance (95.89% capacitance retention after 500 bending cycles). It has been demonstrated that the assembled supercapacitor can still power a red light-emitting diode (LED) indicator normally regardless of the bending state and bending frequency. This study may provide promising insights for the design and fabrication of a flexible asymmetrical solid-state supercapacitor in a flexible power supply for wearable and portable applications.
1. Introduction
As one of the most promising technologies in the 21st century, flexible electronic technology has changed human life to some extent and met the rapid demand for personalized electronic products.1,2 Unlike traditional electronic devices prepared by rigid materials, flexible electronic devices possess exciting characteristics, such as stretchability, flexibility, bendability, shape diversity, etc.,3–5 which enable the possibility of various practical applications such as in roll-up displays,6 biomedical devices,7 and wearable devices.8 The flexible power supply of these flexible electronic devices is also an important part of flexible electronic research that has been intensively studied in the past few years.9–11 Among them, flexible supercapacitors have gradually drawn attention from many researchers because of their advantages of fast charge–discharge rate, high power density, much longer cycling life, and so on,12–14 which are promising for special applications such as for embedding them in movable and arbitrarily shaped objects or even for directly attaching them to the body.7,15,16 More importantly, flexible supercapacitors safely and robustly ensure excellent electronic performance, and the base requirements of mechanical deformability can be simultaneously supported, which are unachievable by conventional power sources.17–19However, the technical and practical challenges, such as selection and design of highly conductive current collectors with good mechanical deformability and discovery and fabrication of low-cost electrode materials with outstanding electrochemical performance, facing the development of flexible supercapacitors cannot be reasonably met; this restricts the practical applications of flexible supercapacitors.17 Over the past decade, tremendous efforts have been devoted towards addressing these issues. At present, the methods used to prepare flexible supercapacitors are concentrated on the following aspects: (1) direct growth of non-conductive or poorly conductive active materials on conductive substrates, e.g. nickel foam,20,21 carbon cloth,10 conductive paper,22 and so on, or use of the same materials, such as pure graphene-based supercapacitors fabricated by chemical vapor deposition (CVD), as both the current collectors and electrochemical materials;23 (2) changing the electrical properties of commonly flexible non-conductive substrates via physical or chemical methods, such as sputtering deposition,24 atomic layer deposition,25 photoetching,26etc., followed by the active materials' growth. They all have accelerated the development and progress of the flexible supercapacitors' preparation technology. However, the complicated production processes, high equipment requirements, and expensive raw materials limit the range of applications for flexible supercapacitor.26 Paper, which is flexible, widely available, lightweight, and commonly used in daily life, is a truly ideal substrate applied to flexible supercapacitors.27–29 Moreover, to date, paper is the cheapest substrate and environmentally friendly since it is made from renewable raw materials.27 Therefore, paper-based supercapacitors are considered to be one of the most competitive flexible power sources of the future.29 However, the poor electrical conductivity of paper is one of the most significant obstacles in its direct employment in flexible supercapacitors.28 In an effort to overcome this defect, researchers keep trying various methods to fabricate functional hybrid paper-based electrodes. For example, Yuan et al. prepared the composite paper/Au/polyaniline (PANI) electrode through the electron beam evaporation and constant potential deposition method;30 Zheng et al. developed a paper electrode by directly drawing the graphite layer on a piece of cellulose paper;31 Weng et al. fabricated a graphene-cellulose paper electrode by vacuum filtration;32 and Feng et al. prepared the sandwiched-like graphite/Ni/Co2NiO4-cellulose paper electrode through a simple drawing-electrodeposition-anodic oxide combined method.33 Although the preparations outlined in these research studies are simple, poor adhesion between active materials and the paper substrate seriously damages the performance of the as-fabricated composite paper-based electrodes. To improve the adhesion and fully exhibit the advantages of paper, novel approaches need to be developed to design a new composite paper electrode.
Herein, a flexible Ni/Co(OH)2-filter paper (FP) electrode with a multilayered structure was designed via an electroless plating and electrodeposition combined method, which was proposed according to the characteristics of filter paper. In the ordinary laboratory filter paper, both rough and porous surfaces provide convenient conditions for electroless nickel plating; this eliminates the surface roughing treatment in chemical plating reactions. Additionally, due to the abundant hydroxyl groups on the cellulose chains, filter paper is highly water-absorbing, and nickel ions in water can easily diffuse into the filter paper when water molecules attach to the hydroxyl groups. Once reduced, the cellulose fibers, whether macrofibrils or microfibrils, are fully coated by the homogeneous metal Ni layer. Therefore, not only the strong adhesion between the Ni layer and filter paper, but also the fast electron transport can improve the electrochemical performance of paper-based electrodes and supercapacitors. The second Ni layer was deposited through electrodeposition to further improve the electrical conductivity, and this could overcome the extremely poor conductivity of the Co(OH)2 nanosheets deposited later; this would further enhance the conductivity of the composite electrode. The electrochemical data showed that the as-designed Ni/Co(OH)2-FP electrode exhibited excellent performance. Finally, a flexible asymmetrical solid-state supercapacitor was assembled using Ni/Co(OH)2-FP, Ni/active carbon (AC)-FP, and ionic gel as the positive electrode, negative electrode, and electrolyte, respectively, and it presented superior flexibility, large volume specific capacitance (1.15 F cm−3), large volume energy density (0.64 mW h cm−3), and stable bending cycle performance (95.89% capacitance retention after 500 bending cycles). These research results are technologically promising for the development of flexible power supplies.
2. Experimental
2.1 Materials
All chemical reagents were of analytical grade and directly used in the chemical reactions. Tin dichloride (SnCl2), palladium dichloride (PdCl2), and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4) were purchased from Aladdin Industrial Co., Ltd. Nickel(II) sulfate hexahydrate (NiSO4·6H2O), citric acid monohydrate (C6H8O7·H2O), ammonium chloride (NH4Cl), sodium hypophosphite monohydrate (NaH2PO2·H2O), cobalt(II) nitrate hexahydrate [Co(NO3)2·6H2O], sodium sulfate (Na2SO4), ammonia water (NH3·H2O), and hydrochloric acid (HCl) were purchased from Sinopharm Chemical Reagent Co., Ltd. Fumed silica (FS) was purchased from Evonik Industries AG. Deionized water (DIW) was used for the whole experimental process.2.2 Fabrication of the electroless Ni plating filter paper [Ni(I)-FP]
Conventional and mature electroless Ni plating technology was employed to change the insulation of the FP according to our previous study.34 Moreover, since Ni2+ ions were directly reduced to metal Ni and then Ni particles stacked together to form the Ni layer to be coated on the surface of cellulose fibers, the adhesion strength between the metal Ni layer and cellulose fibers was very strong. The detailed process was introduced as follows: (1) sensitizing treatment: a piece of FP was put in a sensitizing solution (0.05 M SnCl2 and 0.12 M HCl) at 30 °C for 15 min, then taken out, and completely cleaned by DIW; (2) activating treatment: the sensitized FP was immersed in an activating solution (100 μg mL−1 PdCl2 and 0.03 M HCl) at 30 °C for 15 min, then taken out, and well rinsed by DIW three times; (3) electroless Ni plating reaction: the activated FP was added to the electroless plating solution A (0.05 M NiSO4·6H2O and 0.10 M C6H8O7·H2O), and the solution A was then heated to a temperature of 70 °C. Ammonia water was added to A until the pH was 10.0, and the electroless plating solution B (0.10 M NaH2PO2·H2O) was subsequently added to A. The temperature and pH of the reaction system were maintained at 70 °C and 10.0, respectively. After 30 min, the electroless Ni-plated FP was taken out, thoroughly cleaned by DIW and alcohol, and dried at 60 °C in a vacuum oven. The electroless Ni-plated FP was named Ni(I)-FP.2.3 Preparation of the porous electrodeposition Ni filter paper [Ni(II)-FP]
To further improve the electric conductivity of the FP-based current collectors, a porous Ni layer was deposited again by electroplating technology. The Ni(I)-FP and Pt plate were inserted into the electrolyte (0.15 M NiSO4·6H2O and 0.12 M NH4Cl) and separately employed as the cathode and the anode. Moreover, Ni2+ ions were reduced at a constant potential of 2 V, and the deposition time was 8 min. The as-prepared electroplated Ni FP was dried at 60 °C in a vacuum oven and named Ni(II)-FP.2.4 Preparation of the flexible Ni/Co(OH)2-FP electrode
The porous Ni(II)-FP substrate (0.2 × 2.0 cm2) and Pt plate were separately used as the cathode and anode, and the electrolyte, deposition time, applied potential, and depth of Ni(II)-FP in the electrolyte were 0.1 M Co(NO3)2, 15 min, 0.75 V, and 1.5 cm, respectively.2.5 Preparation of the flexible Ni/active carbon (AC)-FP electrode
The flexible Ni/AC-FP electrode was prepared by coating the slurry mixed with 90 wt% of active carbon powder and 10 wt% of PVDF in N-methyl-pyrrolidinone (NMP). After being dried in air, the flexible Ni/AC-FP electrode was obtained.2.6 Preparation of the flexible asymmetrical solid-state supercapacitor
Herein, 0.4 g FS was added to 0.1 mL EMIMBF4, and the mixture was intensely rotated for 3 min at a speed of 2000 rpm in a mixer (SpeedMixer™ DAC 400.1 VAC-P, FlackTek, Inc.) to form a homogeneous white slurry, which was used as the ionic gel electrolyte. Subsequently, the white slurry was applied on Ni/Co(OH)2-FP, Ni/AC-FP, and filter paper. Ni/Co(OH)2-FP and Ni/AC-FP were, respectively, employed as the positive electrode and the negative electrode and separated by the treated filter paper to assemble the flexible asymmetrical supercapacitor. After being packaged by the PET films, the flexible asymmetrical supercapacitor was obtained.2.7 Materials and electrochemical characterization
Morphology analysis was performed via a field-emission scanning electron microscope (FE-SEM, FEI Nova Nano SEM 450). Phase and structure analysis were investigated by X-ray diffraction measurements (XRD, Rigaku D/Max 2500). The electrochemical performance of the prepared electrode characterized via cyclic voltammetry (CV) and galvanostatic charging/discharging (GCD) was measured by an electrochemical station (Zennium, Zahner, Germany) that used a three-electrode configuration in a 1 M KOH aqueous electrolyte. Moreover, the as-prepared electrode, Pt plate, and saturated calomel electrode (SCE) were, respectively, used as the working, counter, and reference electrode. The potential windows applied to CV and GCD for the Ni/Co(OH)2-FP electrode were −0.5 to 0.7 V and 0–0.44 V, respectively.3. Results and discussion
The facile structure of the flexible composite Ni/Co(OH)2-FP electrode is shown in Fig. 1. The initially uniform metal Ni layer was coated on the surface of bare filter paper by the electroless Ni plating method, and the basic conductive framework was formed. The electrical conductivity (κ) of the Ni(I)-FP was 1.2 × 103 S m−1 after the electroless plating process. Then, the metal Ni layer was coated again through electrodeposition to fabricate the conductive Ni paper; this further enhanced the conductivity of the FP, and provided the well foundation for the deposition of the electrochemical active materials, the poor electrical conductivity of which could affect the final electrochemical performance of the as-prepared electrode. After electroplating the second Ni layer, the electrical conductivity of the Ni(II)-FP was improved to 1.5 × 105 S m−1. Finally, the electrochemical active material of Co(OH)2 nanosheets was deposited on the surface of Ni(II)-FP via the constant potential deposition method to fabricate the Ni/Co(OH)2-FP electrode (details in the Experimental section).SEM characterization reveals the microstructure of the pristine FP, Ni(I)-FP, Ni(II)-FP, and Ni/Co(OH)2-FP electrode, as shown in Fig. 2. For the pristine filter paper, Fig. 2a exhibits that both cellulose fibers intersect each other; this builds the basic network of a cellulose paper. Moreover, there are a large number of pores, voids, and microfibrils between cellulose fibers, creating the rough surface structure of the filter papers. Specifically, both the macrofibrils and microfibrils have abundant hydroxyl functional groups on their surfaces, all of which provide the fairly hygroscopic feature to the filter paper and favour the electroless Ni plating technique applied to the filter paper. As revealed in Fig. 2b, for the Ni(I)-FP, the diameters of cellulose fibers become larger than before because all exposed cellulose fibers are coated by a metal Ni layer after the electroless Ni plating process; this changes the electrical conductivity of the filter paper. Then, the most basic conductive network forms and provides the possibility to conduct the electroplating technique. To further increase the electrical conductivity of Ni(I)-FP, Ni was again deposited, as shown in Fig. 2c. All the fibers are covered by a porous lunar crater-like Ni layer, and the pores and voids between fibers are completely filled by Ni particles that connect tightly. The good electrical conductivity and the porous surface of Ni(II)-FP would facilitate the mass loading of Co(OH)2 and would not seriously damage the electrical conductivity of the whole electrode. Fig. 2d presents the microscopic morphology of the surface of the prepared Ni/Co(OH)2-FP electrode. A thin, uniform, and continuous electrochemical active Co(OH)2 layer can be easily deposited on the surface of the Ni(II) layer due to good electrical conductivity. And the former formed potholes on the surface of the Ni layer and is gradually filled by Co(OH)2 if additional time to deposit, as provided in the inset of Fig. 2d. Moreover, it can be clearly seen that Co(OH)2 nanosheets vertically stand and tightly stack with each other, which would more fully contact the electrolyte and promote the electrochemical reactions.
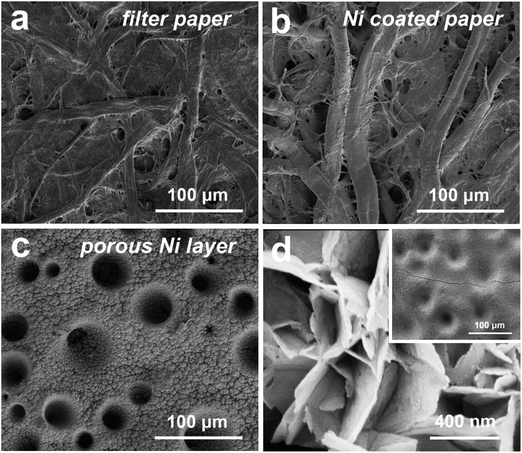 | ||
| Fig. 2 SEM images of the (a) bare FP, (b) Ni(I)-FP, (c) Ni(II)-FP with a porous Ni layer, and (d) Ni/Co(OH)2-FP electrode with the Co(OH)2 nanosheet-constructed layer. | ||
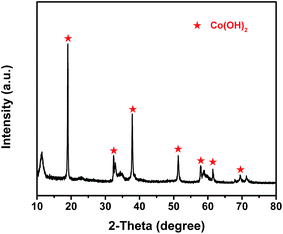
The composition of the electrochemical active material, prepared by the electroplating method, was determined by X-ray diffraction (XRD), as shown in Fig. 3. The XRD pattern obviously reveals that the Co(OH)2 phase really generates on the cathode [Ni(II)-FP] after the electroplating process, which is consistent with the electrochemical reaction in which H+ ions around the Ni(II)-FP are gradually reduced to H2 when an electric current passes through water; moreover, Co2+ ions in the aqueous electrolyte are combined with free OH− to form Co(OH)2 that is deposited on the surface of Ni(II)-FP. In addition, in the pattern analysis, the peaks (marked in red) of the prepared electrochemical active material is in good agreement with that of Co(OH)2 (PDF#30-0443).
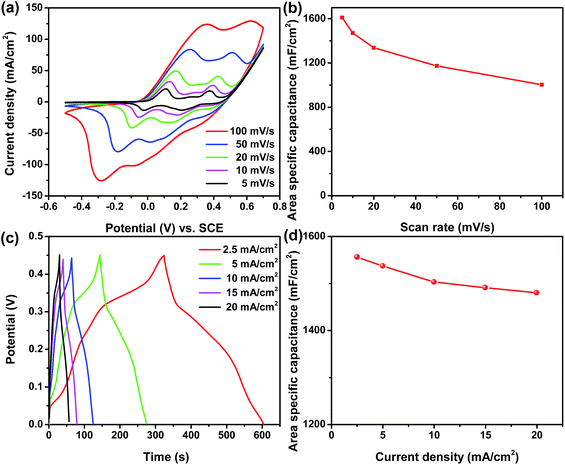
The electrochemical performance of the as-prepared Ni/Co(OH)2-FP positive electrode was tested by a three-electrode system using 1 M KOH as the aqueous electrolyte, and the results are shown in Fig. 4. All CV curves of the as-prepared Ni/Co(OH)2-FP electrode, as shown in Fig. 4a, exhibit a nearly similar shape that has double-redox peaks due to three valance states of elemental Co and show the typical characteristics of faradaic pseudocapacitance. Even when the scan rate increases to 100 mV s−1, the shape of the curve does not change, and the integral area of the closed curve is larger; this indicates remarkable rate capability and a highly reversible electrochemical reaction of the prepared paper electrode. The area specific capacitance calculated from the CV curves versus scan rates of 5, 10, 20, 50, and 100 mV s−1 is described in Fig. 4b, which presents the large area specific capacitance of 1610, 1470, 1337, 1173, and 1003 mF cm−2, respectively, resulting in a high capacitance retention of 62%, which benefits from the good electrical conductivity and the fast ion diffusion rate of the Ni/Co(OH)2-FP electrode. Fig. 4c shows the GCD curves of the Ni/Co(OH)2-FP electrode obtained at different current densities, all of which are almost symmetrical, once again demonstrating the highly reversible redox reaction performance of the electrode. Along with the increase of current density, the discharge time gradually decreases. However, the discharge time is still up to 22 s even at a high current density of 20 mA cm−2. The area specific capacitance calculated from the GCD curves versus current density is plotted in Fig. 4d, and a high area specific capacitance of 1556 mF cm−2 at a current density of 2.5 mA cm−2 and good rate capability are obtained. The electrochemical performance of the negative Ni/AC-FP electrode is detailed in the ESI (Fig. S1†), which shows the good capacitance and superior rate capability, and the Ni/AC-FP electrode can be directly used for supercapacitors.
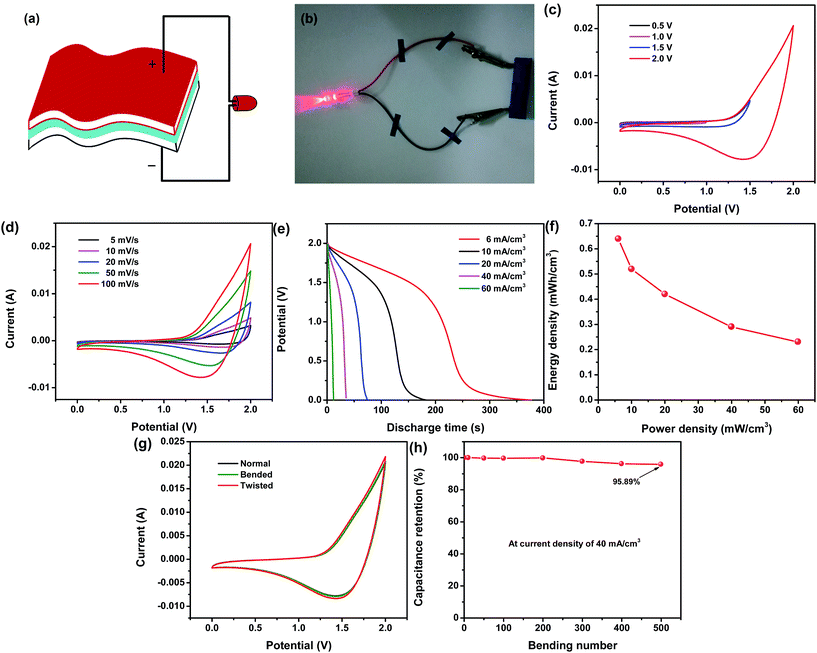
Due to the outstanding electrochemical properties of both the Ni/Co(OH)2-FP and Ni/AC-FP electrodes, a flexible asymmetrical solid-state supercapacitor was fabricated, as depicted in Fig. 5a. The fumed silica and EMIMBF4 were mixed together to fabricate the solid ionic gel electrolyte to be applied to the solid-state supercapacitor. The Ni/Co(OH)2-FP and Ni/AC-FP electrodes were severally used as the positive and negative electrode and separated by one filter paper coated with an ion gel electrolyte (details are provided in the Experimental section). In this way, both the faradaic process and the ion adsorption process are respectively realized on the positive [Co(OH)2] and negative (AC) electrode; therefore, the optimal energy and power density are anticipated. The real assembled supercapacitor is shown in Fig. 5b, which exhibits that a red LED indicator has been powered by the as-assembled supercapacitor. Moreover, the supercapacitor could still work normally regardless of the bending state and bending frequency. The red LED was continuously lit, as shown in Fig. S2 (ESI) and Movie S1;† this indicated excellent flexibility and good mechanical capability, all of which was derived from the pliability and toughness of the conductive filter paper and the good adhesion and stability of the deposit. To confirm the maximum potential window in which the supercapacitor can properly function, it is best to apply a lower voltage to the supercapacitor at the beginning and then slowly increase the voltage until a spike appears at the boundary of the potential window where a disordered curve appears, indicating that the applied maximum voltage is beyond the scope a supercapacitor can endure.35 In Fig. 5c, the potential window increases gradually from 0.5 V to 2.0 V, there are no spikes at the boundary of the CV curves, and all the curves have the same shape, but the spike occurs when the applied voltage is larger than 2.0 V. Therefore, the as-assembled supercapacitor can work in a maximum window of 2 V. With an increase in the potential window, the capacitance of the device also increases (Fig. S3a, ESI†), which agrees with the hypothesis that extension of the working voltage helps to improve the capacitance of the supercapacitors. The detailed CV curves of the supercapacitor in the potential window of 0–2.0 V at different scan rates are plotted in Fig. 5d; they all possess the same characteristic even at a higher scan rate of 100 mV s−1, and the integral area of the curves increases with an increase in the scan rate. The capacitance calculated from the abovementioned CV curves is shown in Fig. S3b (ESI†). The large volume specific capacitance reaches up to 2126.7 mF cm−3, and even when the scan rate increases to 100 mV s−1, the volume specific capacitance can also achieve a value of 815.1 mF cm−3 (38.3% of the original value); this indicates the superior rate capability of the assembled supercapacitor due to the fast ion diffusion rate and electron mobility. The GCD test was also conducted to characterize the electrochemical performance of the supercapacitor, and the constant-current discharge curves at various volume current densities are plotted in Fig. 5e. The long discharge time is 377 s at 6 mA cm−3, revealing good capacity behavior. The energy and power density of the supercapacitor are calculated from the capacitance of the discharge process, and the results are shown in Fig. 5f. It exhibits that the maximum energy density can reach up to 0.64 mW h cm−3 as the power density is 6 mW cm−3, which is higher than that of most of the flexible supercapacitors in the same category11,33,36–43 (more details are shown in Table S1, ESI†). The cycling test was conducted to characterize the cycling life of the as-prepared flexible supercapacitor. With an increase in the cycling number, the capacitance of the supercapacitor decreases, but the capacitance retention is as high as 77.37% after 5000 cycles, as shown in Fig. S4 (ESI†); this reveals the long cycling life of the supercapacitor. To further test and verify the electrochemical properties and working state of the assembled supercapacitor when it was under a mechanical deformation, such as a bended and twisted state, the CV test was carried out, and the results are shown in Fig. 5g. There was no significant difference in the three CV curves whether the supercapacitor was in a normal, bended, or twisted state; this indicated that mechanical deformation had almost no effect on the supercapacitor when it worked. Additionally, the bending cycle life measurement was conducted to demonstrate the flexibility and fatigue bending resistance of the assembled supercapacitor. The approach is detailedly introduced as follows: tracing and obtaining the capacitance of a supercapacitor that is continuously bended to 180° several times with hands. All the capacitance was calculated from the GCD curves at the current density of 40 mA cm−3. The initial capacitance was obtained before conducting the bending process, and then, a series of capacitance values were measured after bending 10, 100, 200, 300, 400, and 500 times. Capacitance retention changes as a function of bending number are plotted in Fig. 5h, which clearly shows that it maintains 95.89% retention according to the initial capacitance after a total number of 500 bending times; this indicates the good flexibility and fatigue bending resistance of the supercapacitor due to the superior toughness of the filter paper and the good adhesion between the active material, conductive layer, and filter paper.
Conclusions
In summary, traditional electroless Ni plating was applied to common laboratory filter paper to change the electrical property of the filter paper such that it could be used a highly conductive and flexible substrate, which laid the foundation for the excellent electrochemical and mechanical flexibility performance of electrodes and assembled paper-based supercapacitors. The as-prepared Ni/Co(OH)2-FP and Ni/AC-FP electrodes exhibit outstanding area specific capacitance of 1610 mF cm−2 and 404 mF cm−2 at the scan rate of 5 mV s−1, respectively, which are applied to assemble a flexible supercapacitor with high performance. The assembled asymmetrical solid-state Ni/Co(OH)2-FP//Ni/AC-FP supercapacitor gathers both pseudocapacitance and electrical double-layer capacitance, which help to broaden the potential window (2 V), and as a larger operating voltage is achieved, more energy is stored. Large volume energy density (0.64 mW h cm−3) and good bending cycle performance (95.89% capacitance retention after 500 bending cycles) are both realized. Moreover, it can be used as a flexible power source to lighten a red LED indicator whether it is in a normal or mechanical deformation state. Owing to the excellent electrochemical and mechanical flexibility performance achieved through it, our strategy may provide a new method to design and fabricate flexible electrodes and supercapacitors for wearable and portable applications.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Key R&D Project from the Minister of Science and Technology of China (2016YFA0202702), the National Natural Science Foundation of China (21571186), the Chinese Academy of Sciences Key Research Projects of Frontier Science (QYZDY-SSW-JSC010), the Guangdong Provincial Key Laboratory (2014B030301014), the Youth Innovation Promotion Association (2017411), the Guangdong TeZhi plan youth talent of science and technology (2014TQ01C102), and the Shenzhen basic research plan (JSGG20150512145714246).References
- B. D. Gates, Science, 2009, 323, 1566–1567 CrossRef CAS PubMed.
- J. H. Robert, Nature, 2001, 412, 489–490 CrossRef PubMed.
- Y. Huang, M. S. Zhu, Z. X. Pei, Q. Xue, Y. Huang and C. Y. Zhi, J. Mater. Chem. A, 2016, 4, 1290–1297 CAS.
- B. Li, P. Gu, Y. C. Feng, G. X. Zhang, K. S. Huang, H. G. Xue and H. Pang, Adv. Funct. Mater., 2017, 27, 1605784 CrossRef.
- M. S. Zhu, Y. Huang, Y. Huang, H. F. Li, Z. F. Wang, Z. X. Pei, Q. Xue, H. Y. Geng and C. Y. Zhi, Adv. Mater., 2017, 29, 1605137 CrossRef PubMed.
- G. A. Ferrero, M. Sevilla and A. B. Fuertes, Sustainable Energy Fuels, 2017, 1, 127–137 CAS.
- L. F. Chen, Z. H. Huang, H. W. Liang, H. L. Gao and S. H. Yu, Adv. Funct. Mater., 2014, 24, 5104–5111 CrossRef CAS.
- Y. L. Shao, M. F. El-Kady, L. J. Wang, Q. H. Zhang, Y. G. Li, H. Z. Wang, M. F. Mousaviae and R. B. Kaner, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC.
- X. R. Li, S. Y. Ding, X. Xiao, J. Y. Shao, J. L. Wei, H. Pang and Y. Yu, J. Mater. Chem. A, 2017, 5, 12774–12781 CAS.
- L. F. Chen, Z. Y. Yu, X. Ma, Z. Y. Li and S. H. Yu, Nano Energy, 2014, 9, 345–354 CrossRef CAS.
- Z. J. Su, C. Yang, B. H. Xie, Z. Y. Lin, Z. X. Zhang, J. P. Liu, B. H. Li, F. Y. Kang and C. P. Wong, Energy Environ. Sci., 2014, 7, 2652–2659 CAS.
- S. S. Zheng, H. G. Xue and H. Pang, Coord. Chem. Rev., 2017 DOI:10.1016/j.ccr.2017.07.002.
- L. F. Chen, Z. Y. Yu, J. J. Wang, Q. X. Li, Z. Q. Tan, Y. W. Zhu and S. H. Yu, Nano Energy, 2015, 11, 119–128 CrossRef CAS.
- Y. Huang, M. S. Zhu, Y. Huang, Z. X. Pei, H. F. Li, Z. F. Wang, Q. Xue and C. Y. Zhi, Adv. Mater., 2016, 28, 8344–8364 CrossRef CAS PubMed.
- Y. Khan, M. Garg, Q. Gui, M. Schadt, A. Gaikwad, D. Han, N. A. D. Yamamoto, P. Hart, R. Welte and W. Wilson, et al. , Adv. Funct. Mater., 2016, 26, 8764–8775 CrossRef CAS.
- C. Z. Meng, C. H. Liu, L. Z. Chen, C. H. Hu and S. S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- M. Koo, K. I. Park, S. H. Lee, M. Suh, D. Y. Jeon, J. W. Choi, K. Kang and K. J. Lee, Nano Lett., 2012, 12, 4810–4816 CrossRef CAS PubMed.
- L. F. Chen, Y. Feng, H. W. Liang, Z. Y. Wu and S. H. Yu, Adv. Energy Mater., 2017, 1700826 CrossRef.
- Y. Huang, Y. Huang, W. J. Meng, M. S. Zhu, H. T. Xue, C. S. Lee and C. Y. Zhi, ACS Appl. Mater. Interfaces, 2015, 7, 2569–2574 CAS.
- J. Hu, M. C. Li, F. C. Lv, M. Y. Yang, P. P. Tao, Y. G. Tang, H. T. Liu and Z. G. Lu, J. Power Sources, 2015, 294, 120–127 CrossRef CAS.
- M. Y. Yang, H. Cheng, Y. Y. Gu, Z. F. Sun, J. Hu, L. J. Cao, F. C. Lv, M. C. Li, W. X. Wang and Z. Y. Wang, et al. , Nano Res., 2015, 8, 2744–2754 CrossRef CAS.
- L. B. Hu and Y. Cui, Energy Environ. Sci., 2012, 5, 6423–6435 Search PubMed.
- N. Li, G. Z. Yang, Y. Sun, H. W. Song, H. Cui, G. W. Yang and C. X. Wang, Nano Lett., 2015, 15, 3195–3203 CrossRef CAS PubMed.
- J. Busom, A. Schreiber, A. Tolosa, N. Jäckel, I. Grobelsek, N. J. Peter and V. Presser, J. Power Sources, 2016, 329, 432–440 CrossRef CAS.
- X. R. Wang and G. Yushin, Energy Environ. Sci., 2015, 8, 1889–1904 CAS.
- W. P. Si, C. L. Yan, Y. Chen, S. Oswald, L. Y. Han and O. G. Schmidt, Energy Environ. Sci., 2013, 6, 3218–3223 CAS.
- Z. Gui, H. L. Zhu, E. Gillette, X. G. Han, G. W. Rubloff, L. B. Hu and S. B. Lee, ACS Nano, 2013, 7, 6037–6046 CrossRef CAS PubMed.
- D. Tobjörk and R. Österbacka, Adv. Mater., 2011, 23, 1935–1961 CrossRef PubMed.
- Y. Z. Zhang, Y. Wang, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Chem. Soc. Rev., 2015, 44, 5181–5199 RSC.
- L. Y. Yuan, X. Xiao, T. P. Ding, J. W. Zhong, X. H. Zhang, Y. Shen, B. Hu, Y. H. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 51, 4934–4938 CrossRef CAS PubMed.
- G. Y. Zheng, L. B. Hu, H. Wu, X. Xie and Y. Cui, Energy Environ. Sci., 2011, 4, 3368–3373 CAS.
- Z. Weng, Y. Su, D. W. Wang, F. Li, J. H. Du and H. M. Cheng, Adv. Energy Mater., 2011, 1, 917–922 CrossRef CAS.
- J. X. Feng, S. H. Ye, A. L. Wang, X. F. Lu, Y. X. Tong and G. R. Li, Adv. Funct. Mater., 2014, 24, 7093–7101 CrossRef CAS.
- L. C. Zhang, P. L. Zhu, F. R. Zhou, W. J. Zeng, H. B. Su, G. Li, J. H. Gao, R. Sun and C. P. Wong, ACS Nano, 2016, 10, 1273–1282 CrossRef CAS PubMed.
- S. L. Zhang and N. Pan, Adv. Energy Mater., 2015, 5, 1401401 CrossRef.
- J. X. Feng, S. H. Ye, X. F. Lu, Y. X. Tong and G. R. Li, ACS Appl. Mater. Interfaces, 2015, 7, 11444–11451 CAS.
- P. H. Yang, X. Xiao, Y. Z. Li, Y. Ding, P. F. Qiang, X. H. Tan, W. J. Mai, Z. Y. Lin, W. Z. Wu, T. Q. Li, H. Y. Jin, P. Y. Liu, J. Zhou, C. P. Wong and Z. L. Wang, ACS Nano, 2013, 7, 2617–2626 CrossRef CAS PubMed.
- B. Yao, L. Y. Yuan, X. Xiao, J. Zhang, Y. Y. Qi, J. Zhou, J. Zhou, B. Hu and W. Chen, Nano Energy, 2013, 2, 1071–1078 CrossRef CAS.
- S. Z. Wang, C. L. Sun, Y. L. Shao, Y. Z. Wu, L. Zhang and X. P. Hao, Small, 2017, 13, 1603330 CrossRef PubMed.
- J. F. Sun, Y. Huang, C. X. Fu, Z. Y. Wang, Y. Huang, M. S. Zhu, C. Y. Zhi and H. Hu, Nano Energy, 2016, 27, 230–237 CrossRef CAS.
- Z. L. Wang, Z. L. Zhu, J. H. Qiu and S. H. Yang, J. Mater. Chem. C, 2014, 2, 1331–1336 RSC.
- X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, S. L. Xie, Y. C. Ling, C. L. Liang, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 5376–5381 CrossRef CAS PubMed.
- X. Xiao, X. Peng, H. Y. Jin, T. Q. Li, C. C. Zhang, B. Gao, B. Hu, K. F. Huo and J. Zhou, Adv. Mater., 2013, 25, 5091–5097 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00411g |
| This journal is © The Royal Society of Chemistry 2018 |