Multi-anchored sensitizers for dye-sensitized solar cells
Yung-Chung
Chen
 *a and
Jiann T.
Lin
*a and
Jiann T.
Lin
 b
b
aDepartment of Chemical and Materials Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung City 80778, Taiwan, Republic of China. E-mail: chenyc@kuas.edu.tw
bInstitute of Chemistry, Academia Sinica, Nankang, Taipei 11529, Taiwan, Republic of China
First published on 2nd May 2017
Abstract
In this review we cover the current progress on multi-anchored sensitizers for dye-sensitized solar cells, including the variation of the molecular structure, type of anchor (cyanoacrylic acid, carboxylic acid, rhodanine-3-acetic acid and pyridyl anchors via a type I indirect electron injection mechanism; catechol anchors via a type II indirect electron injection mechanism; and hybrid anchors), and variation of the number of anchors. The influence of these structural variations on the cell performance is discussed.
1. Introduction
Due to limited natural resource reserves and continual rise in fossil fuel consumption and environmental concern accompanying fuel use, the search for green and renewable energy has been deemed as a top priority task globally. Among various sustainable energies, DSSCs (Dye-Sensitized Solar Cells) have attracted considerable interest from both academic and industrial communities due to their lower cost in cell fabrication compared to silicon solar cells and high molecular design flexibility of sensitizers.1 To date, DSSCs based on a single ruthenium dye2 and Zn–porphyrin dye3 have reached high cell efficiencies of up to 10% under AM 1.5 G irradiation.Ruthenium is a rare metal and not environmentally harmless. Moreover, the metal-to-ligand charge transfer bands of ruthenium-based sensitizers normally have extinction coefficients below 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. In comparison, metal–porphyrin complexes normally need complicated synthesis and purification. Metal-free sensitizers are therefore strong competitors of the above two types of sensitizers considering their high electronic absorption intensity, flexibility in molecular design and low cost. It is worth noting that there has been significant improvement of the cell efficiency for metal-free dyes during the past two years, and efficiencies up to 13% and >14% have been achieved for single dye-based DSSCs and co-dyed systems,4 respectively. Furthermore, high molar extinction coefficients characteristic of metal-free dyes should render them good candidates for solid-state or co-sensitized cells.
000 M−1 cm−1. In comparison, metal–porphyrin complexes normally need complicated synthesis and purification. Metal-free sensitizers are therefore strong competitors of the above two types of sensitizers considering their high electronic absorption intensity, flexibility in molecular design and low cost. It is worth noting that there has been significant improvement of the cell efficiency for metal-free dyes during the past two years, and efficiencies up to 13% and >14% have been achieved for single dye-based DSSCs and co-dyed systems,4 respectively. Furthermore, high molar extinction coefficients characteristic of metal-free dyes should render them good candidates for solid-state or co-sensitized cells.
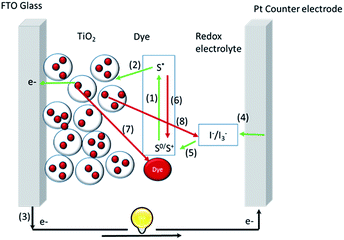
Prototype metal-free DSSCs comprise a transparent photoanode, a mesoporous oxide layer, dye sensitizers, a redox mediator (or an electrolyte) and a counter electrode. Fig. 1 illustrates the working principles of a traditional n-type DSSC: (1) the dye was converted from the ground state to the excited state upon light irradiation; (2) the excited dye molecule injected electrons into the conduction band of TiO2; then the electrons flowed from the TiO2 layer to the transparent conducting electrode (such as FTO); (3) the electrons flowed through an external circuit to the counter electrode; (4) the oxidized dyes were regenerated by the redox mediator; and (5) the oxidized electrolyte mediators were regenerated by the electrons from the counter electrode obtained from external circuit regeneration of the oxidized redox mediator at the counter electrode by the electrons from the external circuit. There also exist several negative operation processes which deteriorate the cell performance: (6) relaxation of the dyes from the excited state to the ground state; (7) recombination of the oxidized dyes with the injected electrons; (8) recombination of the oxidized electrolyte with the injected electrons (also known as the dark current). Mitigation of processes 6 to 8 will definitely lead to better power conversion efficiency.1c
The overall DSSC conversion efficiency (PCE; η) depends on the short-circuit current density (Jsc), open-circuit voltage (Voc) and fill factor (FF) according to the equation, η = Jsc × Voc × FF. The FF value depends on the total series resistance and therefore engineering device fabrication is the most critical factor for the FF. In contrast, Jsc and Voc values are strongly affected by the sensitizers. In quest of high Jsc values, the dye needs to have intense and broad absorption, suitable energy levels allowing facile electron injection into TiO2, dye regeneration, and a high dye loading density without dye aggregation. On the other hand, the Voc value is determined by the difference between the quasi-Fermi level of TiO2 and the Nernstian potential of the electrolyte (such as I−/I3−). Charge recombination with the oxidized redox mediator will result in a downward shift of the quasi-Fermi level of TiO2. Therefore, efficient suppression of dark current is also important during dye design to assure high Voc values of DSSCs. Certainly a more positive Nernstian potential of the electrolyte is beneficial to high Voc values.1c
Traditional metal-free organic dyes have a common structural motif, D–π–A, as shown in Fig. 2, where D is the electron donor, π is the conjugated linker and A is the electron acceptor (and anchor as well).5 Upon absorption of the light, induced intra-molecular charge transfer transition from the donor to the acceptor will drive electron injection to the conduction band of the photoanode (such as TiO2) via the anchor.
The anchoring group (A) has a significant influence on the binding energy of the dye on TiO2, which is relevant to the long-term stability and the rate of electron injection from the dye to TiO2. Besides, the LUMO level of the dye will be affected when the anchoring group also functions as the acceptor.5 There has been increasing attention on multi-anchored sensitizers in recent years.6 Compared to dyes with a single anchor, these dyes have several potential advantages: (1) better light-harvesting and more electron pathways if the anchors are conjugated with the donor; (2) higher dye loading when the independent D–π–A units are connected by a flexible spacer; (3) more effective suppression of intermolecular interaction and charge recombination; (4) better temporal stability of the dye molecule on the surface of the photoanode, similar to multi-dentate vs. mono-dentate chelation.6
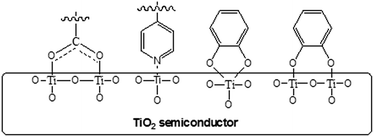
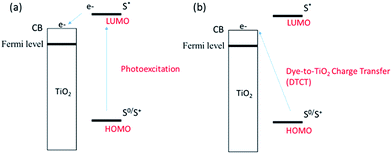
Popularly used anchoring groups for DSSCs so far include cyanoacrylic acid, carboxylic acid and rhodanine-3-acetic acid.7 These anchors are bonded to a TiO2 semiconductor through a bidentate bridging linkage (Fig. 3) and they undergo electron injection via the type-I mechanism i.e., “two-step” or “indirect” electron-injection (Fig. 4a). Phosphonic acid also received considerable attention due to its higher stability against dye leaching compared with cyanoacrylic or carboxylic acid.8 Other anchors with type-I electron injection include heterocyclic anchors, perylene dicarboxylic acid anhydride and anhydride tetracyanate.7c,9 Another interesting anchor undergoing type-I electron injection is the electron withdrawing pyridyl group. A unique feature of the pyridyl group is its high tendency to bind to the Lewis acid site of the TiO2 surface (Fig. 3).10
There exists a second electron injection mechanism for DSSCs (Fig. 4b), which is also called the type-II mechanism. This type of electron injection pathway follows a “one-step” process: the photoexcited dye injects electrons directly from the HOMO of the dye to the CB of the TiO2 semiconductor, i.e. dye-to-TiO2 charge transfer (DTCT). The catechol dyes are well-known type-II sensitizers (Fig. 3).11 Although currently the type-II sensitizers have relatively lower PCE than the type-I sensitizers, they deserve further study due to their broad DTCT band and their ability to mitigate the inappropriate LUMO level between the dye and TiO2. An interesting hybrid sensitizer bearing type I (pyridyl) and type II (catechol) anchors was reported to have good light-harvesting and efficient electron injection.12
The sensitizers covered in this review article concerning multi-anchoring include: (1) dyes using cyanoacrylic acid, carboxylic acid, rhodanine-3-acetic acid or a pyridyl group as the anchor with electron injection via the type-I working mechanism; (2) dyes using a catechol (Cat) moiety as the anchor with electron injection via the type-II working mechanism; and (3) dyes with hybrid anchors. As there has been a recent review on multi-branched multi-anchored metal-free sensitizers for DSSCs,13 in this review we will mainly focus on new reports after 2014 unless the sensitizers published earlier were not highlighted in ref. 13.
2. Recent progress on multi-anchored organic dyes of DSSCs
2.1 Sensitizers based on the type I working mechanism
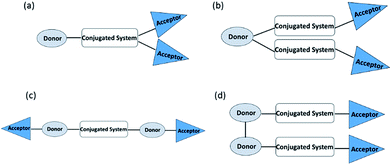 | ||
| Fig. 5 Branched acceptor architectures (a) D–π–(A)2 (b) D–(π–A)2, (c) A–D–π–D–A and (d) L(D–π–A)2 of a metal-free organic dye. | ||
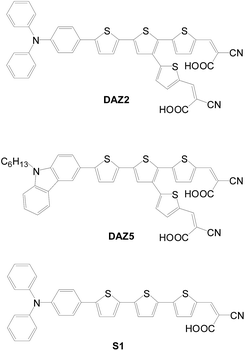
2.1.1.1 Y-Shaped D–π–(A)2 structure. Y-Shaped dyes need an aromatic entity capable of incorporating three conjugated substituents linked to one donor and two acceptors, respectively. For example, 2,3,5-substituted thiophene was used by Lin et al. as the construction motif for DAZ2 and DAZ5 (Fig. 6).15 The dyes exhibit better electron injection and dark current suppression efficiencies than their congener with only one anchor (S1). Accordingly, their DSSCs have significantly higher Voc and Jsc values than S1 (DAZ2: 7.02%, 16.97 mA cm−2, 0.63 V; DAZ5: 7.28%, 16.98 mA cm−2, 0.63 V; S1: 4.45%, 12.43 mA cm−2, 0.59 V). Upon addition of a co-adsorbent, the DAZ2-based cell exhibits the best cell performance (η = 7.87%, Jsc = 17.59 mA cm−2, Voc = 0.65 V, FF = 0.68).
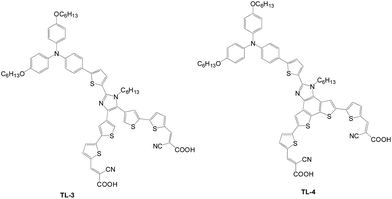
Later, imidazole was used as the construction motif for Y-shape sensitizers (TL-3 and TL-4), as shown in Fig. 7.16 The planar five-membered ring is beneficial to light harvesting and intramolecular charge transfer from the donor to the acceptor. TL-4 with a fused dithieno benzimidazole has the longest wavelength absorption and the highest molar extinction coefficient than other sensitizers, leading to the highest Jsc value.
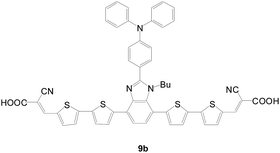
An electron-deficient benzimidazole unit as the π-conjugated spacer of a D–π–(A)2 sensitizer was also reported (Fig. 8). The dye 9b with triphenylamine as the donor and 2,2′-bithiophene as the conjugated linker exhibit the highest Jsc due to the red-shifted and broader absorption. In addition, the 9b-based cell shows a slower charge recombination rate and longer electron lifetime, which leads to a higher Voc value.17
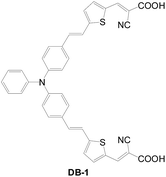
2.1.1.2 D–(π–A)2 structure. In 2009, Grätzel et al. reported the first example of a D–(π–A)2 type di-anchored sensitizer (DB-1) (Fig. 9).6 More protons release from the di-anchored dye onto the TiO2 surface, resulting in a lower Fermi level of TiO2 and consequently lower voltage. It has a relatively higher current due to better light harvesting and better long-term stability, however. The DB-1-based cell with an ionic liquid electrolyte retained 67% of its initial efficiency after 1000 h of light soaking. In comparison, a mono-anchored congener retained only 55% of the cell efficiency under the same condition. The higher temporal stability of the DB-1-based cell is due to the better Jsc sustainability.
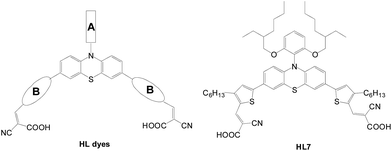
Lin et al. reported D–(π–A)2 type phenothiazine-based sensitizers in which the π-conjugated spacers were linked to the phenothiazine entity at C-3 and C-7 sites (HL series, Fig. 10).18,19 Compared with the congener with a mono-anchor, these dyes exhibit a bathochromic shift of the absorption and can more effectively suppress the dark current. The π-conjugated heteroaromatic rings are found to be influential on the cell performance: (1) increasing the thiophene units leads to better light harvesting; (2) incorporation of a long alkyl chain at the heteroaromatic ring helps with dark current suppression. It is also noteworthy that the N-substituent also affects the light harvesting of the dye and the cell performance. When the substituent changes from a 2-ethylhexyl chain to 2,6-bis(alkoxy)phen-y-l unit, the dye exhibits a bathochromic shift of the absorption and less tendency of dye aggregation, and therefore better cell efficiency. The cell based on HL-7 has the best PCE of 8.32% (Jsc of 16.23 mA cm−2 and Voc of 0.75 V), which surpasses that of N719 (7.35%, 14.96 mA cm−2 and 0.75 V).
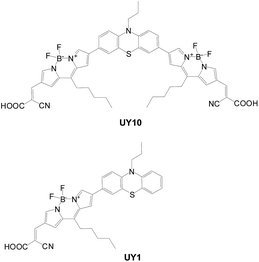
Zhang et al. introduced an electron-deficient “Bodipy” unit as the conjugated spacer between phenothiazine and the acceptor (UY10; Fig. 11).20UY10 exhibits a higher molar extinction coefficient and red-shifted absorption than the mono-anchor congener, UY1, due to a longer conjugation length. The cell of UY10 has the highest Jsc and Voc values. This was attributed to several factors: (1) higher dye loading of UY10 leads to higher current density; (2) UY10 has better light harvesting; (3) more efficient dark current suppression of UY10, i.e. slower electron recombination, leads to a higher voltage. This was also confirmed by EIS measurement (Nyquist plots).
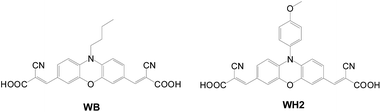
The N-substituent in the phenoxazine-based sensitizer (Fig. 12)21 also affects the cell performance. The WH2 dye shows a higher molar extinction coefficient, but slightly lower current density compared to WB. This is attributed to the decreased donating power of phenoxazine due to poor orbital overlap of the methoxyphenyl entity with the nitrogen atom of the phenoxazine. Therefore, the cell of WH2 has narrower IPCE spectra. On the other hand, it has a higher Voc value as the bulky methoxyphenyl ring can more effectively suppress dye aggregation and charge recombination (WH2: 4.86%, 10.75 mA cm−2, 0.66 V; WB: 5.02%, 11.79 mA cm−2, 0.65 V).
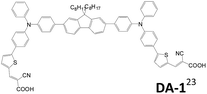
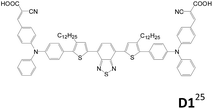
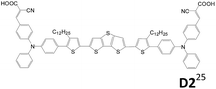
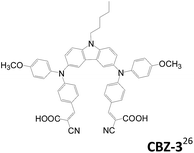
2.1.1.3 A–D–π–D–A structure. Triphenylamine units are popularly used as the donor for traditional linear sensitizers due to their good electron donating and hole transporting capabilities.5c,14,22 A–D–π–D–A type triphenylamine-based sensitizers published after 2014 are collected in Table 1. Different π-conjugated units such as fluorene, biphenyl, dithienothiophene and benzothiadiazole were used to connect the two arylamine donors.23–26 The A–D–π–D–A dyes are demonstrated to be more potent in suppressing the charge recombination and prolonging the electron lifetime than the mono-anchored dye, and consequently a better Voc value is achieved. Though D1 with strong electron-deficient “benzothiadiazole” located at the bridge unit leads to broader absorption due to the ICT effect, the LUMO of D1 is located on benzothiadiazole instead of the anchoring groups, which hampers photoinduced electron transfer from the dye to the TiO2 electrode. Consequently, D1 has lower cell current density than D2.25
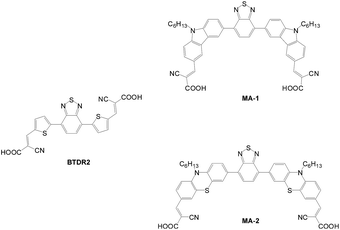
Other A–D–π–D–A sensitizers such as BTDR2, MA-1 and MA-2 based on benzothiadiazole moiety as the π bridge with different donor segments are shown in Fig. 13.27,28 The PCE values of the cells are 2.42%, 4.35 and 6.87% for BTDR2, MA-1 and MA-2, respectively. MA-2 has the best efficiency due to its best light harvesting capability. In addition, the MA-2-based cell exhibits good long-term stability, retaining 98% of its original value after storage for 7 days.
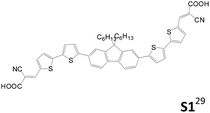
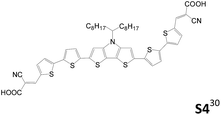
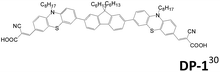
Table 2 lists the cell performance of amine-free dianchored sensitizers with different core structures.29–31 Lin et al. synthesized different types of amine-free acceptor–donor–acceptor sensitizers (S1 and S4).29 They incorporated an electron-rich core unit such as fluorene or an N-alkyl dithieno[3,2-b:20,30-d]pyrrole structure. The cell performance of S1 is higher than that of S4. Although S4 has relative broader absorption than S1, its more serious dye aggregation results in poorer cell efficiency than the latter. In 2015, Cao et al. developed an A–D–π–D–A type sensitizer, DP-1, using phenothiazine as the donor and fluorene as the π-conjugated spacer.30 This cell exhibits a good cell performance of 5.70% (Jsc of 11.73 mA cm−2 and Voc of 0.726 V), which is better than the mono-anchored congener.
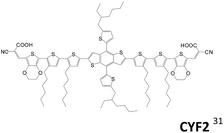
Liu et al. developed sensitizers, CYF2, incorporating benzo[1,2-b:4,5-b′]-dithiophene as the amine-free donor.31 An efficiency of 5.85% is achieved. Despite its relatively lower efficiency than the mono-anchored dye (η = 8.01%), it retains 95% of the initial value after light soaking at 60 °C for 500 h. It is interesting to note that the CYF2 dye exhibited better FF and Jsc values than the mono-anchored dye under the same testing conditions.
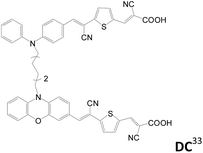
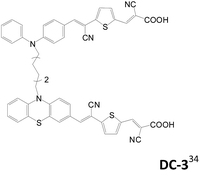
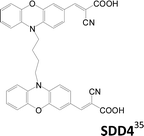
2.1.1.4 L(D–π–A)2 (H-type). Another approach for the preparation of dianchored dyes involves connecting two identical or different D–π–A dyes by a non-conjugated or conjugated linker L, named as H-type sensitizers. In 2011, Meier et al. reported such dyes for application in DSSCs.32 These dyes have a higher molar extinction coefficient of the absorption and lower tendency for dye aggregation. Sensitizers with a non-conjugated linker at the donor are summarized in Table 3. Later, the same group reported a similar branch type of sensitizers, DC, DC3 and SDD4 (Table 3).33–35 Different kinds of donors such as arylamine/phenoxazine, arylamine/phenothiazine and phenoxazine/phenoxazine are selected for better light harvesting, electron injection efficiency and cell performance. All the double-branched D–π–A sensitizers have better solar performance than the mono-anchored D–π–A congeners.
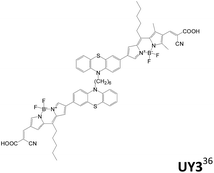
Zhang et al. also developed phenothiazine-based D–π–A branch sensitizers with Bodipy as the π conjugation spacer (UY3, Table 3).36 The double-branched sensitizers possess higher optical density of the UV absorption, resulting in higher current density than the linear congeners.
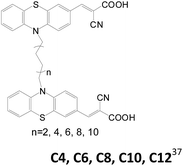
In 2016, Kim et al. also reported double branched D–π–A sensitizers by using phenothiazine as the donor moiety and different alkyl chains (C4–C12, Table 3) as the L linker.37 A longer alkyl chain increases current density and photovoltage due to better electron injection and more efficient suppression of charge recombination (i.e. dark current), respectively. In addition to EIS measurement, stepped light-induced transient measurement of photocurrent and photovoltage (SLIM-PCV) were also carried out to support their argument. Comparable cell efficiency of C10 and C12 with C8 is attributed to the decreased dye loading of the former.
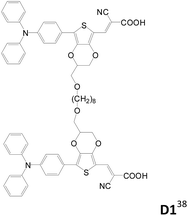
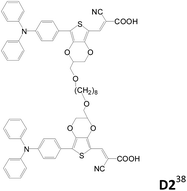
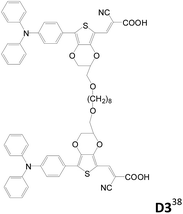
Double-branched D–π–A sensitizers connected by a non-conjugated linker at the conjugated segment of each D–π–A motif instead of the donor were also reported (Table 4).38,39 Wang et al. designed three double-branched dyes (D1–D3, Table 4) composed of two isomeric monoanchored dyes (S1 and S2, Table 4) with different linking sites.38D1–D3 have higher IPCE and Jsc values than S1 and S2. It is suggested that the two D–π–A entities (S1 or S2) in the same D molecule have weaker interaction and therefore less tendency of excited state quenching. Moreover, the D dyes can more effectively suppress the dark current than the S dyes and therefore exhibit higher Voc values. The D1-based DSSC shows the highest efficiency of up to 8.1% as compared to the D3-based cell (η = 6.6%) under liquid electrolyte conditions.
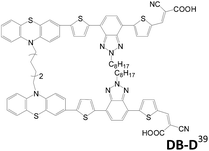
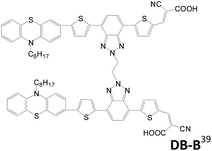
In 2015, Cao et al. reported DB-D and DB-B sensitizers with a hexylene connector linked at the donor or π-conjugated spacer.39 There is only a small difference in physical and electrochemical properties between the two dyes. However, the higher dye loading of DB-B than DB-D indicates that linking mode of double anchored dyes affects the configuration of the dyes on the TiO2 film. In comparison, DB-B shows serious intermolecular aggregation, leading to poor charge injection efficiency. DB-B is also less efficient in suppressing charge recombination and leads to lower voltage. As a result, DB-D shows better performance (η = 6.13%; Voc = 0.72 V; Jsc = 14.39 mA cm−2) than DB-B (η = 3.65%; Voc = 0.66 V; Jsc = 9.40 mA cm−2).
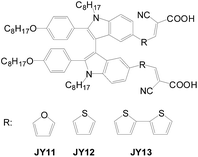
Double anchored dyes with a conjugated linker between the two D–π–A entities have also been developed, and they are expected to have relatively longer conjugation lengths. Zheng et al. developed a series of biindole-based D-A branch sensitizers (JY11, JY12 and JY13) (Fig. 14) for DSSCs.40 The twist conformation of the molecule is beneficial to suppression of intermolecular interaction, mitigation of charge recombination as well as light harvesting. Among the sensitizers, the JY13-based cell exhibits the best cell performance due to the broader light harvesting region (η = 6.54%; Voc = 0.66 V; Jsc = 15.8 mA cm−2).
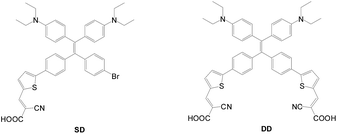
Shao et al. reported another X-shaped dye using a twisted 1,1,2, 2-tetraphenylethene (TPE) entity to connect two anchors with the donor (DD, Fig. 15).41 The dye has significantly higher Voc, Jsc and PCE values than the mono-anchored congener, SD (DD: 6.08%, 13.20 mA cm−2, 0.69 V; SD: 4.56%, 10.95 mA cm−2, 0.65 V).
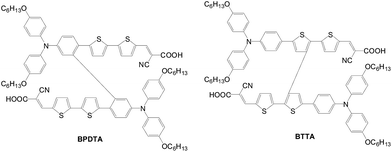
Highly twisted dual D–A unit sensitizers were also developed by Wong (BPDTA and BTTA) (Fig. 16).42 Those two sensitizers are connected directly at different positions for the same structures (i.e., phenylene for BPDTA and thiophene for BTTA) of the π-conjugated backbone. The device fabricated using these dyes yields similar efficiencies of 6.61% for BPDTA and 6.86% for BTTA, although BPDTA shows red-shifted and higher molar extinction coefficients based on photo-physical studies. These dual D-A unit sensitizers all show superior PCE than their linear congeners due to the smaller charge transfer resistance and better light harvesting.
2.1.1.5 Other multi-anchored sensitizers.
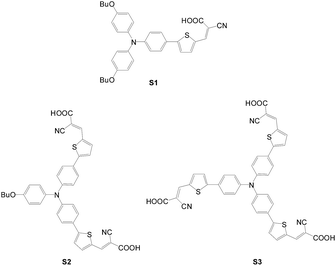
(a) Number of anchors. Compared with dianchored dyes, trianchored dyes are much less studied. In 2010, Jiang synthesized a series of dyes containing a triphenylamine core and thiophene bridge with a different number of anchoring groups (S1, S2 and S3) (Fig. 17).43 Some trends on photophysical properties are observed: (1) the ICT absorption wavelength increases with a decrease in the number of anchoring groups, S1 > S2 > S3; (2) the molar extinction coefficient increases with an increase in the number of anchoring groups, S3 > S2 > S1; (3) the ICT absorption wavelength increases with an increase in the number of electron donating butoxy groups, S3 > S2 > S1. The butoxy group apparently enhances the donating power of the arylamine, leading to a red shift of the ICT band. The current density of the cells shows insignificant difference among the three dyes. The overall PCE of the DSSCs fabricated decreases in the order of S1 > S2 > S3, which is consistent with the trend of the photovoltage (S1: 6.65%, 16.80 mA cm−2, 0.63 V; S2: 5.49%, 16.53 mA cm−2, 0.57 V; S3: 5.34%, 16.77 mA cm−2, 0.565 V). The lower voltage for dyes with more anchors is suggested to be due to more released protons and less efficient dark current suppression due to a smaller number of butoxy groups. However, no firm evidence such as electrochemical impedance spectroscopic data is given.
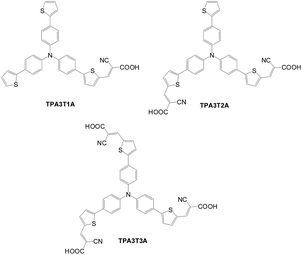
Later, Kim et al. also synthesized three sensitizers (TPA3T1A, TPA3T2A and TPA3T3A) (Fig. 18) with a different number of anchoring groups.44 In contrast to S1–S3, the dye with more anchors exhibits longer wavelength absorption and a higher extinction coefficient. Therefore, increasingly higher current density is found with an increase in the number of anchors. Increasing the number of anchors also results in a more efficient charge injection rate and longer electron lifetime. However, a larger number of protons released from the dyes with a larger number of anchors led to a downward shift of the conduction band edge of TiO2, which offset the voltage rise due to better dark current suppression. Consequently, no significant difference in voltage among the dyes is found. TPA3T3A shows the best PCE of 6.3%, Jsc of 13.6 mA cm−2 and Voc of 0.65 V. The tri-anchored system has better long-term stability than both mono- and di-anchored systems due to the higher binding energy of the dye. Compared with Voc, Jsc was more influential on the efficiency drop of the cell. The instability of the cells was attributed to the formation of unstable radicals and dye desorption under the light soaking condition.
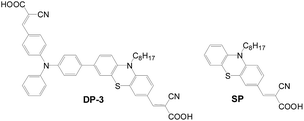
(b) Asymmetry anchors. Another special case was asymmetric di-anchored dyes developed by Cao et al., as shown in Fig. 19.45 Cell performance based on the mono-anchored SP dye (Jsc = 9.01 mA cm−2, Voc = 0.758 V, FF = 0.71, and PCE = 4.87%) exhibits relatively lower efficiency than the asymmetric di-anchored DP-3 dye (Jsc = 11.24 mA cm−2, Voc = 0.796 V, FF = 0.66, and PCE = 5.92%). This is attributed to several factors of DP-3: (1) broader absorption; (2) longer electron lifetime due to better suppression of charge recombination; (3) better suppression of dye aggregation by the bulky triphenylamine entity.
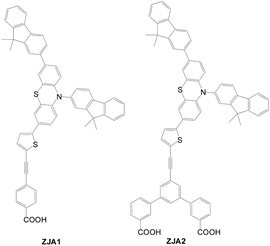
Zhang et al. designed new Y-shaped dyes with two carboxyl anchoring groups (ZJA2) and a mono-anchoring group dye as the reference (ZJA1) (Fig. 21).47 A ZJA2-based cell exhibits better solar performance (η = 4.55%) than ZJA1 (η = 2.72%). This is because of the relatively higher dye loading, longer electron lifetime and higher injection efficiency of the ZJA2-based cell.
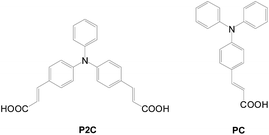
Mono- and di-anchored metal-free dyes using amine as the donor were also synthesized (Fig. 22).48 Higher cell performance of the P2C-based cell is due to better light harvesting and electron injection efficiency, and slower electron recombination (i.e., a longer electron lifetime).
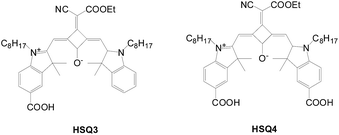
Near-infrared squaraine sensitizer possessing two carboxylic acid anchors (HSQ4) and its congener with a single anchor (HSQ3) were investigated by Han's group (Fig. 23).49 The Jsc value of the cell based on HSQ4 is higher than that based on HSQ3 due to better light harvesting of the former. In contrast, Voc is lower for HSQ4 (0.558 V) than for HSQ3 (0.581 V) because of the substantial charge recombination via the two anchors in the former. The overall efficiency of HSQ4 (5.66%) is significantly higher than that of HSQ3 (4.60%). Besides, the cell efficiency of HSQ4 remains the same after light soaking for 1000 h based on the ionic liquid electrolyte, which outperforms that of HSQ3, as well as the reference squaraine sensitizer, SQ1. In addition to the hydrophobic octyl chain, the two anchors apparently help in suppressing desorption of the HSQ4 dye. The worst stability of the SQ1-based cell was attributed to the desorption and/or decomposition of the sensitizer.
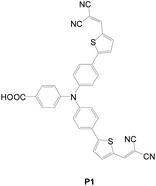
In contrast to n-type DSSCs, p-type DSSCs use a NiO semiconductor for adsorbing the sensitizers and accepting holes from the HOMO of the excited dye.50 The first sensitizer for p-type DSSCs was developed by Sun (P1, Fig. 24). He utilized triphenylamine as the donor and a mono-carboxylic group as the anchoring group.51
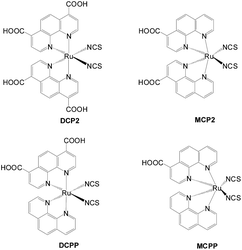
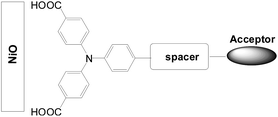
New sensitizers with two carboxylic groups as the anchors for p-type DSSCs also attracted interest. Similar to n-type DSSCs, sensitizers with two anchoring groups show higher Voc and Jsc values because of more efficient hole injection and suppression of the dark current. In addition, the di-anchoring groups should help with stronger dye bonding to the NiO surface and improve the long-term stability (Fig. 25).52–54
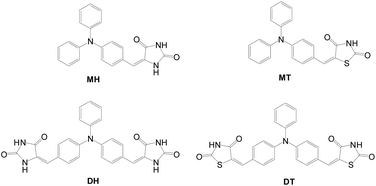
Heterocyclic moieties were also utilized as the anchors of multi-anchored dyes. Sensitizers with 2,4-thiazolidinedione or hydantoin as the anchor and triphenylamine as the donor were developed (Fig. 27).56 The higher molar extinction coefficient for DH and DT sensitizers is due to their extended conjugation length and larger oscillator strength. The cells of both DH and DT exhibit higher Jsc and cell performance than their congeners with a mono-anchor. Compared with the mono-anchored congeners (MH and MT, Fig. 27), DH and DT have more favorable electron injection and therefore higher current density. In addition, di-anchored sensitizers show better light harvesting capability. Though with slightly lower voltages, the di-anchored dyes have better overall cell conversion efficiencies than their mono-anchored congeners (MH: 1.75%, 4.29 mA cm−2, 0.60 V; DH: 2.70%, 7.606 mA cm−2, 0.57 V; MT: 0.99%, 4.29 mA cm−2, 0.60 V; DT: 1.42%, 5.19 mA cm−2, 0.50 V).
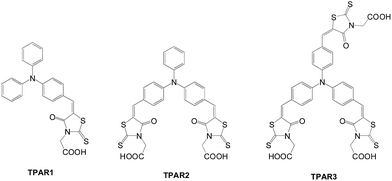
Yang et al. synthesized new dyes with triphenylamine as the donor and multiple rhodanine-3-acetic acids as the acceptor (Fig. 28).57 There is no clear trend of the cell performance vs. the number of anchors. In this study, TPAR2 with two anchoring groups shows better performance (η = 4.77%) than TPAR1 (η = 4.28%) and TPAR3 (η = 2.97%) under the same testing conditions.
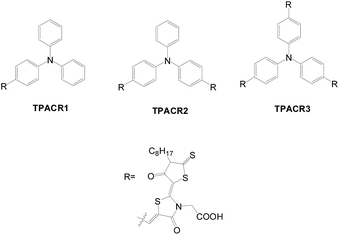
Rhodanine was also used for multi-anchored dyes by Dai et al. (Fig. 29).58 In contrast to TPA3TnA (n = 1–3) which has a longer wavelength and higher extinction coefficient of the ICT band as the number of anchors increases,44TRPCR2 has the largest absorption maximum and intensity among all, resulting in the largest IPCE and Jsc values. Better electron lifetime and more efficient suppression of charge recombination in the dark are in accordance with the higher Voc of the TRPCR2-based cell compared with the other two. Consequently, TRPCR2 has the best cell performance.
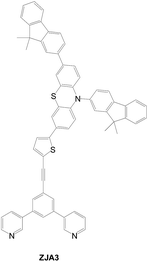
A pyridine anchor is unique as it can anchor to the Lewis acid site of the TiO2 surface. A di-anchored Y-shaped sensitizer based on pyridine, ZAJ3, was investigated (Fig. 30). However, the low dye loading results in poor cell efficiency (0.45%) despite its longer electron lifetime.47
An excellent light harvesting Y-shaped dye with two pyridyl acceptors connecting at 3- and 5-positions of a Bodipy entity was developed by Ooyama et al. (Fig. 31).59 The dye has rather red-shifted and broad absorption in the NIR region. However, the overall conversion efficiency is poor because of low dye loading and serious dye aggregation. Dye aggregation is also confirmed by the increased IPCE of the cell when CDCA is added to the dye solution during dye soaking.
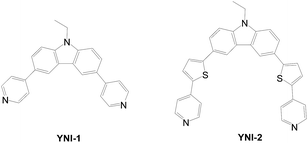
YNI-1 and YNI-2 dyes with carbazole as the donor and two pyridines as the acceptors were also prepared (Fig. 32).12,60 The amount of dye loading for the cell of YNI-2 is two times that of YNI-1. Large planar π-conjugated systems apparently lead to stronger coordinate bonding between pyridine and the TiO2 surface. Accordingly, YNI-2 shows better cell performance than YNI-1.
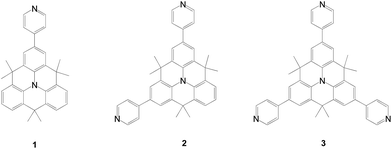
In 2016, Kivala reported multi-pyridine anchored dyes using an N-heterotriangulene core for DSSCs (Fig. 33).61 A time period of 30 h is found to be sufficient for maximum surface coverage of the dyes on TiO2. The lowest PCE for the dye (1) is attributed to the poor surface coverage and thus more serious electron recombination. In contrast, the dye with three anchors (3) has serious dye aggregation, leading to poor current density. As a result, the bi-anchored dye (2) has the highest cell performance due to the most balanced electron injection and charge recombination.
2.2 Sensitizer based on the type II working mechanism
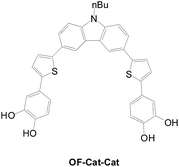
Even though extensive research, especially with respect to sensitization and interfacial charge transfer on TiO2 substrates, has been carried out on dyes with catechol anchors in the early stages of DSSCs, their cell performance still needs to be improved. The effect of substituents on the performance of DSSCs was studied by Ooyama on a series of catechol dyes.62 Some conclusions are summarized: (1) a strongly electron-donating substituent on the Cat unit results in thermodynamically unfavorable regeneration of the oxidized dye by the I3−/I− redox couple due to a high HOMO energy level; (2) a strongly electron withdrawing substituent on the Cat unit leads to a decrease in the dye-to-TiO2 charge transfer efficiency due to the stabilization of the LUMO energy level, facilitating back electron transfer from the TiO2 electrode to the oxidized dye; in turn this results in low photovoltaic performance. They imply that a moderately electron-withdrawing substituent on a catechol dye may have better cell performance due to optimized dye-to-TiO2 charge transfer efficiency and back electron transfer. They also developed a sensitizer (OF-Cat-Cat) with two catechol units (Fig. 34).12 There is very low cell efficiency (0.07%) due to facile back electron transfer and higher electron resistance at the interface.2.3 Sensitizers with hybrid anchors
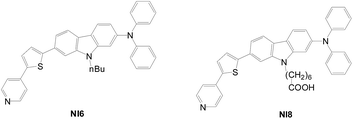
Multi-anchored sensitizers comprising anchors with different bonding modes or injection functionalities were also reported in the literature.63A novel hybrid anchor D–π–A dye (NI8) bearing a pyridine anchor and a carboxylic anchor was prepared, and an NI6 dye with only pyridine anchors was also prepared for comparison (Fig. 35).64 Although there is no insignificant difference in UV absorption spectra, the Jsc, Voc and η values for NI8 (7.04 mA cm−2, 0.568 V, 2.35%) are larger than those of NI6 (5.63 mA cm−2, 0.548 V, 1.84%).
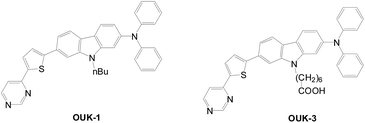
Later, pyrazyl and carboxyl groups were combined as hybrid anchors for DSSCs (Fig. 36).65 The hybrid anchored dye (OUK-3) has higher Jsc and Voc values and therefore better cell performance. Better dye loading, light harvesting efficiency, and electron injection efficiency of OUK-3 is confirmed via UV-vis spectra and electrochemical impedance spectroscopy.
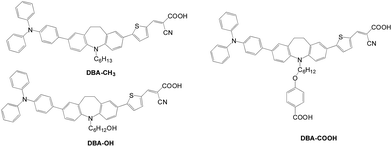
In 2013, Cao et al. also incorporated hydroxyl or carboxyl groups as the additional anchor to form hybrid anchored dyes, DBA-OH and DBA-COOH (Fig. 37).66 A mono-anchored dye DBA-CH3 was also prepared for comparison. The light harvesting, dye loading and performance of the DSSCs are affected by the additional anchor. Among the three dyes, DBA-OH exhibited the best PCE of 4.88% and Jsc of 10.46 mA cm−2. The slightly higher Jsc value for DBA-OH is due to its better light harvesting capability in the solution and film state. The molecular orientation and spatial arrangement of those dyes also influence the electron injection efficiency. On the other hand, DBA-COOH has serious dye aggregation upon adsorption on TiO2, which results in a considerable blue shift of the absorption and leads to the lowest Jsc value.
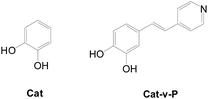
Yoon et al. reported Cat and Cat-v-P dyes and discussed the performance between type II only and type I/type II hybrid systems (Fig. 38).63a With an additional type I anchor, Cat-v-P exhibits a 2-fold increase in cell performance.
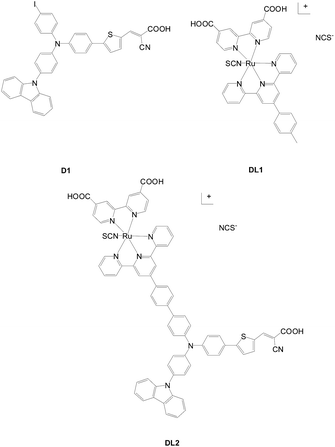
Liu et al. reported a ruthenium complex with hybrid anchors (DL2) and compared the results with analogous mono-anchored dyes (D1, DL1) (Fig. 39).67 Although the DL2-based cell shows a slightly lower voltage than that of D1 due to a more serious charge recombination, it has a significantly higher current density, leading to relatively higher conversion efficiency than the cell with D1 or DL1 alone. This is ascribed to the broader absorption and better light harvesting of DL2 due to the composition of the intramolecular charge transfer band and metal-to-ligand charge transfer band characteristics.
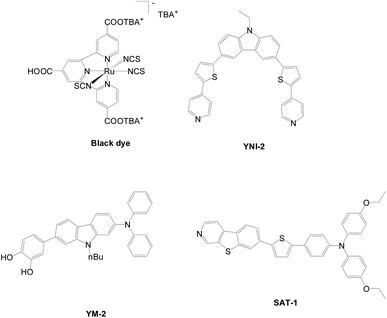
Ooyama et al. utilized a multi-branched acceptor for a co-sensitization approach (Fig. 40),68–70 for example, combining a Black dye and YNI-2 (9.8%, 20.1 mA cm−2, 0.67 V), YNI-2 and YM-2 (0.40%, 1.55 mA cm−2, 0.48 V) or YNI-2 and SAT-1 (1.99%, 5.56 mA cm−2, 0.56 V) systems. Only the Black dye and YNI-2 system exhibits positive results on a panchromatic approach up to 9.8% compared to the original Black dye (9.5% 18.7 mA cm−2, 0.69 V).
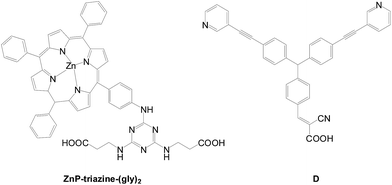
Sharma et al. reported a co-sensitizing system comprising a porphyrin dye (ZnP-triazine-(gly)2) with two carboxylic acid groups and co-sensitized with D containing two ethynyl-pyridine substituents and a terminal cyanoacrylic acid anchor (Fig. 41).71 The final PCE is increased from 4.72% (10.85 mA cm−2, 0.64 V) (ZnP-triazine-(gly)2 based) to 7.34% (14.78 mA cm−2, 0.70 V) (ZnP-triazine-(gly)2 + D based). Both the current density and voltage are increased in the co-sensitization device. The higher current density is attributed to improved light harvesting, and faster electron injection due to less dye aggregations. Efficient suppression of charge recombination also results in a higher voltage.
3. Conclusions and outlook
In this review, we summarized the current progress of multi-anchored sensitizers for dye-sensitized solar cells. A multi-anchored approach, which can provide magnificent characteristics such as better light harvesting, electron injection efficiency and long-term cell stability, has great potential to be effective sensitizers in DSSCs. Recent examples using multi-anchored sensitizers for photoelectrochemical water splitting72 further witness promising future of multi-anchored dyes. To further improve the DSSC cell performance based on multi-anchors, more efforts can be directed to (1) new multi-anchored sensitizers with broader light harvesting capability, such as incorporation of electron deficient units into the conjugated spacer; (2) hybrid anchors with adsorption on both Lewis acid and Bronsted acid sites of the photoanode for better dye loading; (3) partial exchange of protons on the anchors with cations for alleviating the downward shift of the conduction band edge of the photoanode; and (4) more robust anchors to resist dye leaching. Certainly, photophysical studies on the correlation between the molecular structure with the cell performance and ingenious device engineering are equally important.Acknowledgements
Financial support from the Ministry of Science and Technology, Taiwan, ROC is gratefully acknowledged (MOST 105-2218-E-151-002).References
- (a) B. O. Regan and M. Grätzel, Nature, 1991, 343, 737–740 CrossRef; (b) T. W. Hamann, R. A. Jensen, A. B. F. Martinson, H. V. Ryswyk and J. T. Hupp, Energy Environ. Sci., 2008, 1, 66–78 RSC; (c) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed; (d) J. N. Clifford, E. Martinez-Ferrero, A. Viterisi and E. Palomares, Chem. Soc. Rev., 2011, 40, 1635–1646 RSC.
- (a) M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835–16847 CrossRef CAS PubMed; (b) C. Y. Chen, M. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-Le, J. D. Decoppet, J. H. Tsai, C. Grätzel, C. G. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103–3109 CrossRef CAS PubMed; (c) S. W. Wang, C. C. Chou, F. C. Hu, K. L. Wu, Y. Chi, J. N. Clifford, E. Palomares, S. H. Liu, P. T. Chou, T. C. Wei and T. Y. Hsiao, J. Mater. Chem. A, 2014, 2, 17618–17627 RSC; (d) W. C. Chen, F. T. Kong, Z. Q. Li, J. H. Pan, X. P. Liu, F. L. Guo, L. Zhou, Y. Huang, T. Yu and S. Y. Dai, ACS Appl. Mater. Interfaces, 2016, 8, 19410–19417 CrossRef CAS PubMed; (e) W. C. Chen, F. T. Kong, R. Ghadari, Z. Q. Li, F. L. Guo, X. P. Liu, Y. Huang, T. Yu, T. Hayat and S. Y. Dai, J. Power Sources, 2017, 346, 71–79 CrossRef CAS.
- A. Y. Simon Mathew, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef PubMed.
- Z. Yao, H. Wu, Y. Li, J. Wang, J. Zhang, M. Zhang, Y. Guo and P. Wang, Energy Environ. Sci., 2015, 8, 3192–3197 CAS.
- (a) Y. S. Yen, H. H. Chou, Y. C. Chen, C. Y. Hsu and J. T. Lin, J. Mater. Chem., 2012, 22, 8734–8747 RSC; (b) S. Chaurasia and J. T. Lin, Chem. Rec., 2016, 16, 1311–1336 CrossRef CAS PubMed; (c) A. Mahmood, Sol. Energy, 2016, 123, 127–144 CrossRef CAS; (d) Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC; (e) A. Mishra, M. K. R. Fisher and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS PubMed; (f) R. K. Kanaparthi, J. Kandhadi and L. Giribabu, Tetrehadron, 2012, 68, 8383–8393 CrossRef CAS; (g) C. P. Lee, R. Y. Y. Lin, L. Y. Lin, C. T. Li, T. C. Chu, S. S. Sun, J. T. Lin and K. C. Ho, RSC Adv., 2015, 5, 23810–23825 RSC; (h) J. S. Luo, Z. Q. Wan and C. Y. Jia, Chin. Chem. Lett., 2016, 27, 1304–1318 CrossRef CAS.
- A. Abbotto, N. Manfredi, C. Marinzi, F. De Angelis, E. Mosconi, J. H. Yum, Z. Xianxi, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2009, 2, 1094–1101 CAS.
- (a) J. Wiberg, T. Marinado, D. P. Hagberg, L. Sun, A. Hagfeldt and B. Albinsson, J. Phys. Chem. C, 2009, 113, 3881–3886 CrossRef CAS; (b) F. Ambrosio, N. Martsinovich and A. Troisi, J. Phys. Chem. Lett., 2012, 3, 1531–1535 CrossRef CAS PubMed; (c) L. Zhang and J. M. Cole, ACS Appl. Mater. Interfaces, 2015, 7, 3427–3455 CrossRef CAS PubMed.
- (a) E. Bae and W. Choi, J. Phys. Chem. B, 2006, 110, 14792–14799 CrossRef CAS PubMed; (b) I. López-Duarte, M. Wang, R. Humphry-Baker, M. Ince, M. V. Martínez-Díaz, M. K. Nazeeruddin, T. Torres and M. Grätzel, Angew. Chem., Int. Ed., 2012, 124, 1931–1934 CrossRef.
- (a) F. L. Guo, Z. Q. Li, Z. P. Liu, L. Zhou, F. T. Kong, W. C. Chen and S. Y. Dai, Adv. Funct. Mater., 2016, 26, 5733–5740 CrossRef CAS; (b) J. Mao, N. He, Z. Ning, Q. Zhang, F. Guo, L. Chen, W. Wu, J. Hua and H. Tian, Angew. Chem., Int. Ed., 2012, 124, 10011–10014 CrossRef; (c) Z. Wu, X. Li, H. Agren, J. Hua and H. Tian, ACS Appl. Mater. Interfaces, 2015, 7, 26355–26359 CrossRef CAS PubMed; (d) C. Koenigsmann, T. S. Ripolles, B. J. Brennan, C. F. A. Negre, M. Koepf, A. C. Durrell, R. L. Milot, J. A. Torre, R. H. Crabtree, V. S. Batista, G. W. Brudvig, J. Bisquert and C. A. Schmuttenmaer, Phys. Chem. Chem. Phys., 2014, 16, 16629–16641 RSC.
- (a) Y. Ooyama, K. Uenaka, T. Kamimura, S. Ozako, M. Kanda, T. Koide and F. Tani, RSC Adv., 2016, 6, 16150–16158 RSC; (b) N. Shibayama, H. Ozawa, M. Abe, Y. Ooyama and H. Arakawa, Chem. Commun., 2014, 50, 6398–6401 RSC.
- H. Frei, D. J. Fitzmaurice and M. Grätzel, Langmuir, 1990, 6, 198–206 CrossRef CAS.
- Y. Ooyama, K. Furue, T. Enoki, M. Kanda, Y. Adachi and J. Ohshita, Phys. Chem. Chem. Phys., 2016, 18, 30662–30676 RSC.
- N. Manfredi, B. Cecconi and A. Abbotto, Eur. J. Org. Chem., 2014, 2014, 7069–7086 CrossRef CAS.
- B. G. Kim, K. Chung and J. Kim, Chem.–Eur. J., 2013, 19, 5220–5230 CrossRef CAS PubMed.
- R. Y. Y. Lin, F. L. Wu, C. H. Chang, H. H. Chou, T. M. Chuang, T. C. Chu, C. Y. Hsu, P. W. Chen, K. C. Ho, Y. H. Lo and J. T. Lin, J. Mater. Chem. A, 2014, 2, 3092–3101 Search PubMed.
- Y. S. Yen, J. S. Ni, T. Y. Lin, W. I. Hung, J. T. Lin and M. C. P. Yeh, Eur. J. Org. Chem., 2015, 2015, 7367–7377 CrossRef CAS.
- G. Babu Bodedla, K. R. Justin Thomas, M. S. Fan and K. C. Ho, Chem.–Asian J., 2016, 11, 2564–2577 CrossRef PubMed.
- W. I. Hung, Y. Y. Liao, C. Y. Hsu, H. H. Chou, T. H. Lee, W. S. Kao and J. T. Lin, Chem.–Asian J., 2014, 9, 357–366 CrossRef CAS PubMed.
- W. I Hung, Y. Y. Liao, T. H. Lee, Y. C. Ting, J. S. Ni, W. S. Kao, J. T. Lin, T. C. Wei and Y. S. Yen, Chem. Commun., 2015, 51, 2152–2155 RSC.
- M. Mao, X. Zhang, L. Cao, Y. Tong and G. Wu, Dyes Pigm., 2015, 117, 28–36 CrossRef CAS.
- W. Lee, S. B. Yuk, J. Choi, H. J. Kim, H. W. Kim, S. H. Kim, B. Kim, M. J. Ko and J. P. Kim, Dyes Pigm., 2014, 102, 13–21 CrossRef CAS.
- M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453–3488 RSC.
- C. H. Siu, L. T. L. Lee, P. Y. Ho, P. Majumdar, C. L. Ho, T. Chen, J. Zhao, H. Li and W. Y. Wong, J. Mater. Chem. C, 2014, 2, 7086–7095 RSC.
- H. J. Ahn, S. Thogiti, J. M. Cho, B. Y. Jang and J. H. Kim, Electron. Mater. Lett., 2015, 11, 822–827 CrossRef CAS.
- H. Shang, Q. Li, K. Jiang and X. Zhan, J. Energy Chem., 2016, 25, 615–620 CrossRef.
- S. Pramjit, U. Eiamprasert, P. Surawatanawong, P. Lertturongchai and S. Kiatisevi, J. Photochem. Photobiol., A, 2015, 296, 1–10 CrossRef CAS.
- J. A. Mikroyannidis, P. Suresh, M. S. Roy and G. D. Sharma, J. Power Sources, 2010, 195, 3002–3010 CrossRef CAS.
- M. G. Murali, X. Wang, Q. Wang and S. Valiyaveettil, Dyes Pigm., 2016, 134, 375–381 CrossRef CAS.
- D. Sahu, H. Padhy, D. Patra, J. F. Yin, Y. C. Hsu, J. T. Lin, K. L. Lu, K. H. Wei and H. C. Lin, Tetrahedron, 2011, 67, 303–311 CrossRef CAS.
- X. X. Dai, H. L. Feng, Z. S. Huang, M. J. Wang, L. Wang, D. B. Kuang, H. Meier and D. Cao, Dyes Pigm., 2015, 114, 47–54 CrossRef CAS.
- Y. F. Chen, J. M. Liu, J. F. Huang, L. L. Tan, Y. Shen, L. M. Xiao, D. B. Kuang and C. Y. Su, J. Mater. Chem. A, 2015, 3, 8083–8090 CAS.
- D. Cao, J. Peng, Y. Hong, X. Fang, L. Wang and H. Meier, Org. Lett., 2011, 13, 1610–1613 CrossRef CAS PubMed.
- Y. Hong, J. Y. Liao, D. Cao, X. Zang, D. B. Kuang, L. Wang, H. Meier and C. Y. Su, J. Org. Chem., 2011, 76, 8015–8021 CrossRef CAS PubMed.
- Y. Hong, J. Y. Liao, J. Fu, D. B. Kuang, H. Meier, C. Y. Su and D. Cao, Dyes Pigm., 2012, 94, 481–489 CrossRef CAS.
- Y. Hong, Z. Iqbal, X. Yin and D. Cao, Tetrahedron, 2014, 70, 6296–6302 CrossRef CAS.
- M. Mao, X. L. Zhang, X. Q. Fang, G. H. Wu, S. Y. Dai, Q. H. Song and X. X. Zhang, J. Power Sources, 2014, 268, 965–976 CrossRef CAS.
- Y. H. Lee, R. K. Chitumalla, B. Y. Jang, J. Jang, S. Thogiti and J. H. Kim, Dyes Pigm., 2016, 133, 161–172 CrossRef CAS.
- S. Jiang, S. Fan, X. Lu, G. Zhou and Z. S. Wang, J. Mater. Chem. A, 2014, 2, 17153–17164 CAS.
- Z. S. Huang, C. Cai, X. F. Zang, Z. Iqbal, H. Zeng, D. B. Kuang, L. Wang, H. Meier and D. Cao, J. Mater. Chem. A, 2015, 3, 1333–1344 CAS.
- X. Qian, H. H. Gao, Y. Z. Zhu, L. Lu and J. Y. Zheng, RSC Adv., 2015, 5, 4368–4375 RSC.
- F. Zhang, J. Fan, H. Yu, Z. Ke, C. Nie, D. Kuang, G. Shao and C. Su, J. Org. Chem., 2015, 80, 9034–9040 CrossRef CAS PubMed.
- C. Y. Lo, D. Kumar, S. H. Chou, C. H. Chen, C. H. Tsai, S. H. Liu, P. T. Chou and K. T. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 27832–27842 CAS.
- H. Shang, Y. Luo, X. Guo, X. Huang, X. Zhan, K. Jiang and Q. Meng, Dyes Pigm., 2010, 87, 249–256 CrossRef CAS.
- Y. H. Lee, H. J. Yun, S. K. Choi, Y. S. Yang, T. Park, K. S. Ahn, T. Suresh and J. H. Kim, Synth. Met., 2016, 217, 248–255 CrossRef CAS.
- X. X. Dai, H. L. Feng, W. J. Chen, Y. Yang, L. B. Nie, L. Wang, D. B. Kuang, H. Meier and D. Cao, Dyes Pigm., 2015, 122, 13–21 CrossRef CAS.
- (a) K. Hara, H. Sugihara, Y. Tachibana, A. Islam, M. Yanagida, K. Sayama and H. Arakawa, Langmuir, 2001, 17, 5992–5999 CrossRef CAS; (b) K. Hara, H. Horiuchi, R. Katoh, L. P. Singh, H. Sugihara, K. Sayama, S. Murata, M. Tachiya and H. Arakawa, J. Phys. Chem. B, 2002, 106, 374–379 CrossRef CAS.
- H. Jia, K. Shen, X. Ju, M. Zhang and H. Zheng, New J. Chem., 2016, 40, 2799–2805 RSC.
- S. manoharan, A. M. Asiri and S. Anandan, Sol. Energy, 2016, 126, 22–31 CrossRef CAS.
- C. Qin, Y. Numata, S. Zhang, X. Yang, A. Islam, K. Zhang, H. Chen and L. Han, Adv. Funct. Mater., 2014, 24, 3059–3066 CrossRef CAS.
- F. Odobel, L. Le Pleux, Y. Pellegrin and E. Blart, Acc. Chem. Res., 2010, 43, 1063–1071 CrossRef CAS PubMed.
- (a) P. Qin, H. Zhu, T. Edvinsson, G. Boschloo, A. Hagfeldt and L. Sun, J. Am. Chem. Soc., 2008, 130, 8570–8571 CrossRef CAS PubMed; (b) P. Qin, M. Linder, T. Brinck, G. Boschloo, A. Hagfeldt and L. Sun, Adv. Mater., 2009, 21, 2993–2996 CrossRef CAS.
- Y. S. Yen, W. T. Chen, C. Y. Hsu, H. H. Chou, J. T. Lin and M. C. P. Yeh, Org. Lett., 2011, 13, 4930–4933 CrossRef CAS PubMed.
- Z. Ji, G. Natu, Z. Huang and Y. Wu, Energy Environ. Sci., 2011, 4, 2818–2821 CAS.
- C. H. Chang, Y. C. Chen, C. Y. Hsu, H. H. Chou and J. T. Lin, Org. Lett., 2012, 14, 4726–4729 CrossRef CAS PubMed.
- E. Gabrielsson, H. Tian, S. K. Eriksson, J. Gao, H. Chen, F. Li, J. Oscarsson, J. Sun, H. Rensmo, L. Kloo, A. Hagfeldt and L. Sun, Chem. Commun., 2015, 51, 3858–3861 RSC.
- B. Hosseinzadeh, A. Salimi Beni, M. Azari, M. Zarandi and M. Karami, New J. Chem., 2016, 40, 8371–8381 RSC.
- C. H. Yang, H. L. Chen, Y. Y. Chung, C. G. Wu, C. P. Chen, S. H. Liao and T. L. Wang, J. Power Sources, 2009, 188, 627–634 CrossRef CAS.
- G. Wu, F. Kong, Y. Zhang, X. Zhang, J. Li, W. Chen, W. Liu, Y. Ding, C. Zhang, B. Zhang, J. Yao and S. Dai, J. Phys. Chem. C, 2014, 188, 8756–8765 Search PubMed.
- Y. Ooyama, Y. Hagiwara, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2479–2485 RSC.
- Y. Ooyama, N. Yamaguchi, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem. Commun., 2013, 49, 2548–2550 RSC.
- U. Meinhardt, F. Lodermeyer, T. A. Schaub, A. Kunzmann, P. O. Dral, A. Chiara Sale, F. Hampel, D. M. Guldi, R. D. Costa and M. Kivala, RSC Adv., 2016, 6, 67372–67377 RSC.
- Y. Ooyama, M. Kanda, K. Uenaka and J. Ohshita, ChemPhysChem, 2015, 16, 3049–3057 CrossRef CAS PubMed.
- (a) E. L. Tae, S. H. Lee, J. K. Lee, S. S. Yoo, E. J. Kang and K. B. Yoon, J. Phys. Chem. B, 2005, 109, 22513–22522 CrossRef CAS PubMed; (b) F. de Angelis, Chem. Phys. Lett., 2010, 493, 323–327 CrossRef CAS.
- Y. Ooyama, T. Nagano, S. Inoue, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem.–Eur. J., 2011, 17, 14837–14843 CrossRef CAS PubMed.
- Y. Ooyama, K. Uenaka and J. Ohshita, Org. Chem. Front., 2015, 2, 552–559 RSC.
- X. F. Zang, Y. F. Xu, Z. Iqbal, Z. S. Huang, D. B. Kuang, L. Wang, H. Meier, Y. Li and D. Cao, Dyes Pigm., 2013, 99, 1072–1081 CrossRef CAS.
- P. Y. Su, J. M. Liu, X. L. Lin, Y. F. Chen, Y. Shen, L. M. Xiao, D. B. Kuang and C. Y. Su, Inorg. Chem. Front., 2015, 2, 1040–1044 RSC.
- N. Shibayama, H. Ozawa, M. Abe, Y. Ooyama and H. Arakawa, Chem. Commun., 2014, 50, 6398–6401 RSC.
- Y. Ooyama, K. Uenaka, M. Kanda, T. Yamada, N. Shibayama and J. Ohshita, Dyes Pigm., 2015, 122, 40–45 CrossRef CAS.
- Y. Ooyama, K. Uenaka, T. Sato, N. Shibayama and J. Ohshita, RSC Adv., 2015, 5, 2531–2535 RSC.
- G. D. Sharma, P. A. Angaridis, S. Pipou, G. E. Zervaki, V. Nikolaou, R. Misra and A. G. Coutsolelos, Org. Electron., 2015, 25, 295–307 CrossRef CAS.
- (a) B. Cecconi, N. Manfredi, R. Ruffo, T. Montini, I. Romero-Ocaña, P. Fornasiero and A. Abbotto, ChemSusChem, 2015, 8, 4216–4228 CrossRef CAS PubMed; (b) X. Ding, Y. Gao, L. Ye, L. Zhang and L. Sun, ChemSusChem, 2015, 8, 3992–3995 CrossRef CAS PubMed; (c) B. Cecconi, N. Manfredi, T. Montini, P. Fornasiero and A. Abbotto, Eur. J. Org. Chem., 2016, 2016, 5194–5215 CrossRef CAS; (d) J. Fielden, J. M. Sumliner, N. Han, Y. V. Geletii, X. Xiang, D. G. Musaev, T. Lian and C. L. Hill, Chem. Sci., 2015, 6, 5531–5543 RSC; (e) Z. Yu, F. Li and L. Sun, Energy Environ. Sci., 2015, 8, 760–775 RSC; (f) T. E. Rosser, M. A. Gross, Y.-H. Lai and E. Reisner, Chem. Sci., 2016, 7, 4024–4035 RSC.
| This journal is © The Royal Society of Chemistry 2017 |