Catalytic hydrotreatment of bio-crude produced from the hydrothermal liquefaction of aspen wood: a catalyst screening and parameter optimization study
Jinlong
Yu
a,
Patrick
Biller
b,
Aref
Mamahkel
a,
Maika
Klemmer
a,
Jacob
Becker
a,
Marianne
Glasius
b and
Bo Brummerstedt
Iversen
 *a
*a
aCenter for Materials Crystallography, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus, Denmark. E-mail: bo@chem.au.dk
bDepartment of Chemistry, Aarhus University, Langelandsgade 140, 8000 Aarhus, Denmark
First published on 7th March 2017
Abstract
Lignocellulosic plant matter, as a second generation biomass, has potential as a feedstock for the production of liquid bio-fuels providing an alternative to fossil fuels. Herein, we report detailed catalyst screening and parameter optimization for catalytic hydrotreatment of bio-crude produced from the continuous hydrothermal liquefaction (HTL) of aspen wood. Three different commercial metal oxide catalysts, NiW/Al2O3 and NiMo/Al2O3 with a high and low NiMo loading, were examined. Elemental analysis showed significant oxygen expulsion from 10.7 wt% in the bio-crude down to a minimum of 0.7 wt% in the hydrotreated bio-oil. Considering the degrees of hydrodeoxygenation (HDO) along with yields, NiMo/Al2O3 with a high NiMo loading showed the best performance with a yield of 71.9 wt% and an oxygen content of 2.4 wt% for the final bio-oil, followed by low loaded NiMo/Al2O3 (yield 67.4 wt%, oxygen content 3.8 wt%) and NiW/Al2O3 (yield 58.7 wt%, oxygen content 6.9 wt%) under relatively mild conditions. Characterization by gas chromatography coupled with mass spectrometry pointed towards possible conversion pathways of the main bio-crude components during hydrotreatment. These included the conversion of substituted cyclopentenones to cycloalkanes, and oxygen-containing substituted polycyclic aromatic hydrocarbons (PAHs) to polycyclic aromatic hydrocarbons (e.g. anthracene, phenanthrene and naphthalene) and cracking of fatty acids to aliphatic hydrocarbons. The effects of initial hydrogen pressure and reaction time were investigated. The results demonstrate the potential of upgrading the bio-crude produced from the HTL of aspen wood to a hydrocarbon product with properties similar to petroleum derived transportation fuels.
Introduction
On-going efforts to restrict global climate change and provide increased energy security are manifested in the development of alternatives to traditional fossil fuels. Exploitation of energy from biomass offers the potential of a carbon efficient process with little or no additional carbon dioxide being emitted to the atmosphere.1 The general strategy of utilizing biomass, excluding direct combustion, can be separated into two basic categories. Firstly, the thermochemical processes which can be sub-divided into gasification, pyrolysis and hydrothermal liquefaction (HTL). Secondly, the biochemical processes which mainly involve anaerobic digestion, alcoholic fermentation and biodiesel production.1 Due to the tendency of HTL to achieve high biomass conversion yields and bio-crudes with favorable higher-heating-values (HHVs, typically 30–36 MJ kg−1), low oxygen contents (10–20 wt%), reduced presence of corrosive carboxylic acids and a manageable water content (0–5 wt%),2 the process has attracted significant attention.3–5 Particularly its capacity to treat biomasses with high water contents is a clear advantage. These combined properties have led to an estimate of superior performance in technoeconomic and lifecycle assessments.6,7Although the bio-crude derived from HTL is more promising for alternative biofuel production than that from pyrolysis, it still exhibits high heteroatom (N, S, and O) contents which prevent it from being used directly as a transportation fuel. Further upgrading is required in order to improve the quality of the bio-crude via catalytic hydrotreatment. The main purpose of the hydrotreatment of bio-oils is to remove oxygen through hydrodeoxygenation (HDO), while for crude oil the objective mainly lies in hydrodesulphurization (HDS) and hydrodenitrogenation (HDN). This is due to the high oxygen content of bio-crudes (>10 wt%) compared to the sulfur (typically <1 wt%) or nitrogen content (<10 wt%). Not surprisingly, initial research in bio-crude hydrotreatment was based on the know-how on the hydrotreatment of crude oil. The most obvious manifestation is the utilization of typical fossil crude HDS and HDN catalysts such as sulfided NiMo/Al2O3 and CoMo/Al2O3 for the HDO of bio-crude.8,9 Although both sulfided NiMo/Al2O3 and CoMo/Al2O3 performed well in the HDO of bio-crude, the necessity of adding additional sulfur species to the feed in order to maintain the activity of the catalysts could render them less attractive. Moreover, these sulfided catalysts could also cause sulfur contamination in the as-treated bio-oil. By comparison, there are no such plaguing problems when using noble metal catalysts such as Pd/C10 and Ru/C;11 here the obvious disadvantage is their high cost.
Due to these problems, attempts were made to obtain reduced NiMo/Al2O3 or CoMo/Al2O3 catalysts for upgrading of bio-crude where a pretreatment process (reduction of the catalyst with hydrogen) is required.12 Searching for new, cheaper and easy-to-use hydrotreatment catalysts with a high deoxygenation performance remains a challenge.
Generally, biofuels from plant matter are classified into three “generations”.13 First generation biofuels use corn, potatoes, beet, sugarcane, etc., as the feedstock which are mainly for the production of bioethanol, while oils from rapeseed, olive, sunflower, soya or palm provide lipids for biodiesel production. Due to concerns regarding the use of food crops for biofuels, attention has shifted to the conversion of lignocellulosic biomass such as sawdust, switchgrass and wood to fermentable sugars which are further processed to obtain second-generation bio-ethanol through fermentation. Within the biodiesel sphere, the conversion of microalgae has been considered as a third generation pathway due to their fast growth rate, high lipid contents and non-competition for arable land. Due to their wet nature, HTL was quickly identified as a promising technology for the conversion of microalgae to bio-crude. However, high costs associated with the cultivation of microalgae on a large scale for fuel production have hindered their commercialization, particularly since algae can be sold directly as a value-added commodity. Meanwhile, increasing efforts have been directed to the investigation of lignocellulosic biomass via HTL and subsequent upgrading to hydrocarbon based biofuels. Since the first real study on the hydrotreatment of HTL bio-crude in 1984 (ref. 14), very little work has been performed on the upgrading of any type of HTL bio-crude until a recent surge in interest in the hydrotreatment of HTL bio-crude. Several papers have recently investigated the hydrotreatment of bio-crudes from the HTL of microalgae.3,15,16 In contrast, there is an apparent lack of studies investigating the use of lignocellulosic derived bio-crudes. Currently there is only one publication reporting the upgrading of bio-crude produced from the HTL of lignocellulosic biomass17 in which a parametric study of operating conditions was conducted using NiMo catalysts. De-oxygenation of the bio-crude was successful down to a level of 0.1% and increased HHVs and reduced viscosities are demonstrated. Unfortunately, no indication of the upgraded fuel yields and their molecular composition is given so that an assessment for the transportation fuel market is difficult. Another study presents brief results of the upgrading of lignocellulosic derived HTL bio-crude on a trickle bed reactor but no details are given on experimental results as the focus of this study lied on the techno-economic aspects of HTL and bio-crude upgrading.18 While the molecular composition of upgraded fuels derived from microalgae has previously been reported,15,16,19 no such data are available in the literature for upgraded HTL fuels from lignocellulosic sources. Microalgae derived fuels mainly contain alkanes in the range of C8–C20 with very little amounts of aromatics, although quantification of compound classes has not been reported19 (only relative GC-MS peak areas). Due to the different biochemical structure of wood compared to e.g. microalgae, the final composition of HTL derived fuels is expected to vary significantly. This is important to know in order to assess the suitability of HTL fuels for gasoline, diesel and kerosene applications where the hydrocarbon composition is crucial. Gasoline, for example, contains predominantly aromatics while kerosene typically contains less than 25 wt% aromatics and mainly paraffinic hydrocarbons. Despite on-going research, no work has been carried out to quantify such fuel components in upgraded HTL fuels, hindering a further development towards commercialization. The current study seeks to address this lack in knowledge by providing quantitative data on the molecular fuel composition of HTL derived bio-crude, which was produced at an industrially relevant, continuous flow, pilot scale HTL facility.
Catalytic hydrotreatment of the bio-crude of aspen wood using different commercial metal oxide catalysts is presented in this work. Three commercial catalysts were chosen (NiW/Al2O3 and NiMo/Al2O3 with a high and low NiMo loading) which were used without any pre-sulfurization or pre-reduction step with the aim of identifying a hydrotreatment strategy, which is as simple, “mild” and therefore cheap as possible. Detailed results regarding yields, elemental analysis, molecular characterization and boiling point ranges of the hydrotreated bio-oils are provided, allowing their assessment as hydrocarbon fuels. The objective of this research is to explore the possibility of obtaining biofuels compatible for co-processing in existing crude oil refineries or as drop-in liquid transportation fuels from bio-crude produced by HTL.
Experimental
Materials
The bio-crude subject to the hydrotreatment study was obtained from a continuous, pilot scale HTL reactor unit (CBS1), which was designed and constructed at Aalborg University (Aalborg, Denmark) by Steeper Energy Aps (Vedbæk, Denmark) in 2013. Supercritical hydrothermal conditions (∼400 °C, 320 bar) were employed to process the lignocellulosic biomass (aspen wood) into bio-crude.20 Three different transition metal oxide hydrotreatment catalysts, NiW/Al2O3 and NiMo/Al2O3 with high and high loadings of NiMo, (part numbers TK-951, TK-341 and TK-351 respectively) were provided by Haldor Topsøe (Kongens Lyngby, Denmark). From here on the catalysts are referred to as NiW, NiMo-LL, and NiMo-HL (LL = low loading of NiMo and HL = high loading of NiMo). Both the bio-crude and catalysts were used as received. Hydrogen, helium, nitrogen and argon were purchased from AGA (Sweden). A reference gas with a known composition for quantitative gas analysis was supplied by AGA (Denmark). Dichloromethane (DCM) was supplied by Sigma-Aldrich.Hydrotreatment procedure
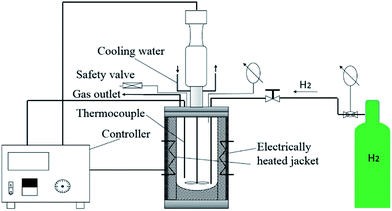
A 500 mL stainless steel batch autoclave (Model 4540, Parr Instrument Company, IL, USA) with the maximum operating temperature and pressure of 350 °C and 260 bar, respectively, was used for hydrotreatment. A schematic representation of the reactor setup is shown in Fig. 1; an electrically heated jacket was used to heat the reactor with a heating rate of ∼6 °C min−1.The experimental plan was composed of initial catalyst screening and subsequent parameter optimization. This approach allowed for the selection of the optimum hydrotreatment catalyst and determination of optimum process parameters. The specific experimental design is shown in Table 1. The catalyst screenings were conducted with a reaction time of 2 h, an initial H2 pressure of 75 bar and a catalyst loading of 10 wt%. The effect of H2 pressure was then further explored by performing three separate hydrotreatment experiments using the NiMo-HL catalyst with an initial H2 pressure of 50 bar, 75 bar and 100 bar, respectively (reaction time 4 h and catalyst loading 10 wt%). To determine the effect of reaction time, another two separate experiments were performed with a reaction time of 1 h and 2 h (initial H2 pressure 100 bar and catalyst loading 10 wt%).
| Run number | Catalyst | Initial hydrogen pressure (bar) | Reaction time (h) | Catalyst loading (wt%) |
|---|---|---|---|---|
| 1 | None | 75 | 2 | 10 |
| 2 | NiW | 75 | 2 | 10 |
| 3 | NiMo-LL | 75 | 2 | 10 |
| 4 | NiMo-HL | 75 | 2 | 10 |
| 5 | NiMo-HL | 50 | 4 | 10 |
| 6 | NiMo-HL | 75 | 4 | 10 |
| 7 | NiMo-HL | 100 | 4 | 10 |
| 8 | NiMo-HL | 100 | 1 | 10 |
| 9 | NiMo-HL | 100 | 2 | 10 |
For each typical run, approx. 100 g of bio-crude and 10 g of catalyst were placed in the autoclave. Subsequently, the autoclave was flushed three times with hydrogen and then pressurized to the intended initial H2 pressure. The reactor was heated to 350 °C and kept at that temperature for the predetermined reaction time (1, 2 or 4 hours). After completion of the reaction, heating was switched off and the reactor was allowed to cool to ambient temperature. Before opening the autoclave, the final pressure was recorded for the mass balance calculation. After hydrotreatment, the gas phase was sampled using a 2 L Teflon, push lock valve, gas bag (Sigma-Aldrich) and analyzed by gas chromatography. The oil phase and aqueous phase were decanted to a pre-weighed glass beaker and separated using a separation funnel. DCM was used to rinse the autoclave and to recover the remaining oil, char and catalyst. After filtration, the DCM was evaporated using a rotary evaporator (200 mbar, 40 °C, 4 hours). Finally, the filter paper was dried overnight at 80 °C and the mixture of char and spent catalyst was recovered. The mass of char was calculated by subtracting the mass of the fresh catalyst. Due to lengthy experimental procedures, all experiments were conducted only once apart from the screening experiment with NiMo-HL, a reaction time of 2 h, H2 pressure of 75 bar and catalyst loading of 10 wt%. This experiment was performed in duplicate and the replicate oil yields were within 2 wt%, pointing towards high reproducibility for all experiments.
Analyses
An Agilent 7890A GC system equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) was used to analyze the gas phase products. Helium (2 mL min−1) and argon (6 mL min−1) served as the carrier gas for the FID and TCD analysis, respectively. The gaseous mixture detected by the FID was separated by a 25 m, 0.32 mm-i.d. and 10 μm-film capillary PoraPLOT Q column. The gaseous mixture detected by the TCD was separated by a 30 m, 0.53 mm i.d. and 15 μm-film capillary CP-Molsieve 5 Å column. Quantitative analysis of gaseous products was performed by analysis of a reference gas with a known composition as described previously.21The hydrotreated bio-oil was characterized by gas chromatography coupled with mass spectrometry (GC-MS), elemental analysis and Fourier transform infrared spectroscopy (FTIR). GC-MS was carried out on an Agilent 7890B gas chromatograph coupled with an Agilent 5977A quadrupole mass spectrometer. Approximately 10 mg of bio-crude or treated bio-oils were prepared with 900 μL DCM and 100 μL of internal standard. The standard was 4-bromotoluene (B82200 Sigma-Aldrich) with a concentration of approximately 200 ppm in DCM to make a final sample concentration of 20 ppm. A 1 μL volume of solution was injected in split mode with a split ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Analytes were separated using a VF-5 ms column (60 m × 0.25 mm × 0.25 μm, 5 m EZ-Guard). The GC inlet temperature was held at 280 °C. The column was initially held at 40 °C for 5 min, ramped to 100 °C at 10 °C min−1, ramped to 280 °C at 4 °C min−1, then ramped to 300 °C at 10 °C min−1 and held for 10 min. Electron impact ionization was employed at 70 eV and data were acquired in scan mode (35–500 m/z). Compound identification was performed by using the NIST mass spectral database. The concentration of aliphatic hydrocarbons from C7 to C24, selected aromatic hydrocarbons and oxygen-containing compounds in the bio-crude and treated bio-oils was quantified by analysis of calibration standards of known concentrations. Using suitable dilutions, calibration curves were plotted for each standard compound using the Agilent MassHunter software package to determine the concentration of the compounds in the oil samples.
1. Analytes were separated using a VF-5 ms column (60 m × 0.25 mm × 0.25 μm, 5 m EZ-Guard). The GC inlet temperature was held at 280 °C. The column was initially held at 40 °C for 5 min, ramped to 100 °C at 10 °C min−1, ramped to 280 °C at 4 °C min−1, then ramped to 300 °C at 10 °C min−1 and held for 10 min. Electron impact ionization was employed at 70 eV and data were acquired in scan mode (35–500 m/z). Compound identification was performed by using the NIST mass spectral database. The concentration of aliphatic hydrocarbons from C7 to C24, selected aromatic hydrocarbons and oxygen-containing compounds in the bio-crude and treated bio-oils was quantified by analysis of calibration standards of known concentrations. Using suitable dilutions, calibration curves were plotted for each standard compound using the Agilent MassHunter software package to determine the concentration of the compounds in the oil samples.
Elemental analysis was performed in triplicate on a Vario MACRO cube (Elementar Analysensysteme, Hanau, Germany). The oxygen content was calculated by difference. The HHV of hydrotreated bio-oil was determined using the Dulong formula:
| HHV = 0.3383C + 1.443(H – (O/8)) + 0.0942S (MJ kg−1) |
FTIR analysis was carried out on a NICOLET 380 FT-IR Spectrometer (Thermo Electron Corporation). Spectra scanned in the range 500–4000 cm−1 were recorded to identify functional groups in the hydrotreated bio-oils.
Simulated distillation (Sim-Dis) of the bio-crude and treated bio-oils was carried out following the ASTM® D2887 procedure. It was performed on an Agilent G1530A GC with a FID. Calibration was performed using the SUPELCO ASTM® D2887 Calibration Mix and verified using ASTM® D2887 Reference Gas Oil. Solutions of 1 wt% bio-crude in DCM were prepared due to the better solubility of bio-crude in DCM than in carbon disulfide.
The water content in bio-crude and hydrotreated bio-oils was determined using Karl Fischer titration with a TitraLab KF 1000 volumetric Karl Fisher titration instrument. The viscosities of bio-crude and hydrotreated bio-oils were determined using a Brookfield RVDV-II/Pro Viscometer with spindle SC-18, speed (rpm) 50 and shear rate 66 at room temperature (RT).
Results and discussion
Oil yields and mass balance
Catalyst screening experiments were carried out using NiW and NiMo catalysts with a high (HL) and low loading (LL) of NiMo; the resulting mass balances are presented in Fig. 2. The initial H2 pressure for these experiments was 75 bar with a final pressure ranging from 150 bar to 165 bar. The yield of upgraded bio-oil (based on the sum of the mass of the bio-crude and initial hydrogen) ranged from 57.5 to 70.3 wt% for catalytic experiments, while decreasing sharply to 17.0 wt% for the non-catalytic experiment. In that experiment, 60.1 wt% solid char was obtained, indicating pronounced condensation reactions of small molecules to char. This also explains the apparent difference in the final appearance of the products, which for the catalyzed runs consisted of a flowable oil and a solid phase while that of the non-catalytic experiment was mainly a tar-like mixture of char and highly viscous oil.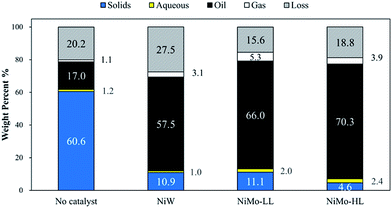 | ||
| Fig. 2 Mass balances for hydrotreatment experiments using different catalysts (initial H2 pressure: 75 bar, reaction time: 2 h, and catalyst loading: 10 wt%). | ||
The mass of the aqueous phase for three different catalytic experiments was 1.0 g (NiW), 2.1 g (NiMo-LL) and 2.4 g (NiMo-HL) respectively, compared to the theoretical yield of approximately 12 g given the assumption that oxygen was removed exclusively as H2O. The discrepancy can be ascribed to the incomplete removal of oxygen, expulsion of oxygen as CO2 and CO (observed by GC analysis), a reminiscence of water in the hydrotreated bio-oils indicated by Karl Fischer analysis (4.5, 5.8 and 2.1 wt% for the catalysts NiW, NiMo-LL and NiMo-HL respectively) and losses during product recovery. Particularly for the NiMo-HL hydrotreatment, a noticeable presence of droplets was observed on the autoclave wall upon opening; this indicates losses during aqueous phase recovery after hydrotreatment. Fig. 3 depicts oxygen contents and yields of hydrotreated bio-oils calculated as the mass ratio between the treated bio-oil and bio-crude for the different catalytic hydrotreatments. The oxygen contents ranged from 2.4 wt% to 8.1 wt% with the highest degree of deoxygenation obtained by NiMo-HL and lowest for the non-catalytic experiment. In order to obtain a bio-oil with a high yield and low oxygen content, NiMo-HL is thus the best choice.
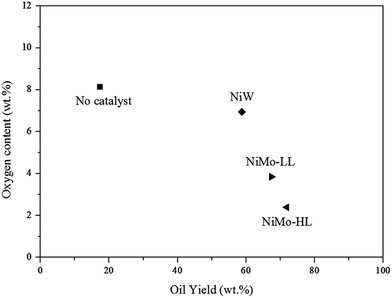 | ||
| Fig. 3 Yields and oxygen contents of hydrotreated bio-oils by using different catalysts (initial H2 pressure: 75 bar, reaction time: 2 h, and catalyst loading: 10 wt%). | ||
The hydrotreated bio-oil yields, carbon recoveries and energy recoveries for the parameter optimization study using NiMo-HL are listed in Table 2. The bio-oil yield increased from 66.5 wt% to 77.6 wt% when the hydrotreatment reaction time was increased from 1 h to 2 h but the yield then decreased to 62.6 wt% when the reaction time was further increased to 4 h. This observation could be due to polymerization reactions promoted by the long reaction time. However, increasing the reaction time up to a certain point will clearly enhance the hydrotreatment reaction. A similar trend of bio-oil yields vs. the reaction time was found in a previous study22 on catalytic hydrotreatment of HTL bio-crude from microalgae. The Sim-Dis results (discussed in detail later) support that longer reaction times lead to heavier oil fractions at the expense of lighter fractions. It is possible that longer reaction times also result in more hydrocracking forming small and volatile molecules, further lowering the final bio-oil yields.
![[Y with combining low line]](https://www.rsc.org/images/entities/char_0059_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif) elds (wt%) and carbon and energy recoveries (%) of hydrotreated bio-oils produced with the NiMo-HL catalyst
elds (wt%) and carbon and energy recoveries (%) of hydrotreated bio-oils produced with the NiMo-HL catalyst
| NiMo-HL, 4 h, 50 bar, 10% | NiMo-HL, 4 h, 75 bar, 10% | NiMo-HL, 4 h, 100 bar, 10% | NiMo-HL, 1 h, 100 bar, 10% | NiMo-HL, 2 h, 100 bar, 10% | |
|---|---|---|---|---|---|
| Oil yield (wt%) | 56.2 | 69.8 | 62.6 | 66.5 | 77.6 |
| Carbon recovery (%) | 61.3 | 77.3 | 69.6 | 73.5 | 86.0 |
| Energy recovery (%) | 64.5 | 81.4 | 73.1 | 76.9 | 90.3 |
The bio-oil yields as a function of the initial H2 pressure exhibited a trend similar to that of the reaction time. The highest yield of 69.8 wt% was obtained with an initial H2 pressure of 75 bar compared to 56.2 wt% and 62.6 wt% for the initial pressures of 50 bar and 100 bar, respectively, associated with the observation of a decreased gaseous product yield. A possible explanation is that with the other variables fixed, lower pressure may enhance the diffusion of gaseous products produced by hydrocracking, leading to more drastic conversion of bio-crude to gaseous products and lighter molecules. This is also supported by the Sim-Dis results. Moreover, Cheng et al.23 proposed that too high pressure could lead to drastic polymerization of small molecules and consequently to a decrease of the treated bio-oil yields.
Elemental analysis and physical properties
The results of elemental analysis, molar atomic ratio, HHV and physical properties for bio-crude and hydrotreated bio-oils are presented in Table 3. The HHV of the raw bio-crude was 37.4 MJ kg−1, similar to e.g. a bio-crude from the HTL of microalgae with a HHV of 36.1 MJ kg−1 (ref. 15) but higher than that of a bio-oil from the pyrolysis of beech wood (20.3 MJ kg−1).24 It is worth noting that despite the relatively high HHV of the bio-crude used in this study, its oxygen content, high viscosity (2.10 × 105 cP at RT) and high density (1.076 g mL−1) all make it unpractical for direct use as a transportation fuel.| Experimental conditions | Elemental analysis, wt% | Molar H/C | Molar O/C | HHV, MJ kg−1 | Viscosity, cP at RT | Water content (wt%) | Density, g mL−1 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | Oa | |||||||
| a By difference. b n.d.—not detected. c n.a.—not available. | |||||||||||
| Bio-crude | 80.5 | 8.4 | 0.4 | 0.08 | 10.7 | 1.25 | 0.10 | 37.4 | 2.10 × 105 | 1.4 | 1.076 |
| Effect of catalyst | |||||||||||
| No catalyst | 82.7 | 8.6 | 0.5 | n.d.b | 8.1 | 1.25 | 0.07 | 39.0 | n.a.c | 2.4 | n.a. |
| NiW, 2 h, 75 bar, 10% | 84.1 | 8.4 | 0.6 | n.d.b | 6.9 | 1.20 | 0.06 | 39.3 | 981.0 | 4.5 | 0.939 |
| NiMo-LL, 2 h, 75 bar, 10% | 86.5 | 9.1 | 0.5 | n.d.b | 3.9 | 1.27 | 0.03 | 41.8 | 33.0 | 5.8 | 0.889 |
| NiMo-HL, 2 h, 75 bar, 10% | 87.8 | 9.2 | 0.6 | n.d.b | 2.4 | 1.26 | 0.02 | 42.6 | 62.4 | 2.1 | 0.907 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Effect of hydrogen pressure | |||||||||||
| NiMo-HL, 4 h, 50 bar, 10% | 87.7 | 9.5 | 0.5 | n.d. | 2.4 | 1.29 | 0.02 | 42.9 | <15.0 | 0.4 | 0.906 |
| NiMo-HL, 4 h, 75 bar, 10% | 89.1 | 9.4 | 0.5 | 0.02 | 1.05 | 1.27 | 0.01 | 43.6 | 29.4 | 0.3 | 0.905 |
| NiMo-HL, 4 h, 100 bar, 10% | 89.5 | 9.4 | 0.5 | n.d. | 0.7 | 1.25 | 0.01 | 43.7 | 16.0 | 0.2 | 0.889 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Effect of reaction time | |||||||||||
| NiMo-HL, 1 h, 100 bar, 10% | 89.0 | 9.2 | 0.4 | 0.01 | 1.4 | 1.25 | 0.01 | 43.2 | <15.0 | 0.3 | 0.893 |
| NiMo-HL, 2 h, 100 bar, 10% | 89.2 | 9.3 | 0.5 | n.d.b | 1.0 | 1.26 | 0.01 | 43.5 | 31.4 | 0.3 | 0.907 |
| NiMo-HL, 4 h, 100 bar, 10% | 89.5 | 9.4 | 0.5 | n.d.b | 0.7 | 1.25 | 0.01 | 43.7 | 16.0 | 0.2 | 0.889 |
As expected, the contents of C and H and the HHV all increased as a result of the hydrotreatment while the O and S contents, viscosity and density significantly decreased. The N contents of treated bio-oils ranged from 0.4 wt% to 0.6 wt% compared to just 0.4 wt% for the bio-crude. Taking the nitrogen concentrations and overall yields into account shows that little HDN is taking place. Despite this, analysis of the hydrotreatment aqueous phase for total nitrogen content suggests that the catalysts indeed do perform at least some HDN. The nitrogen content of aqueous phase samples ranged from 250–870 mg L−1. Generally, the naturally low nitrogen content of this bio-crude compared to those derived from the HTL of other biomasses, e.g. microalgae (∼6 wt%), obscures the extent of HDN and makes comparison with other studies difficult. The experimental uncertainty within the nitrogen measurements is around ±0.2%. Overall, the metal oxide catalysts used in the current study clearly have a stronger impact on HDO than HDN.
Regarding the effect of catalyst type, non-catalytic hydrotreatment increased the C and H contents of bio-crude to some degree (+2.3 wt% and +0.3 wt%, respectively) with the O content remaining high at 8.1 wt%. Visually this bio-oil was found to be an inhomogeneous, semi-solidified liquid product; it was not possible to determine its viscosity and density. As expected, using catalysts led to significant improvements. The NiW catalyst produced a bio-oil with an oxygen content of 6.9 wt%, a viscosity of 981 cP (at RT) and a density of 0.939 g mL−1. NiMo-LL and NiMo-HL catalytic hydrotreatment led to bio-oils with even lower oxygen contents of 3.9 wt% and 2.4 wt% with viscosities of 33.0 cP and 62.4 cP, respectively. As will be discussed, the higher loading of NiMo may enhance the HDO ability of NiMo-HL at the expense of some hydrocracking capability. Comparing the bio-oils produced in this study to e.g. a Northern Sea crude, some parameters are on par while others still require improvement. The density of the Northern Sea crude for example has been quoted as 846 g mL−1, with an oxygen content of 0.3 wt% and a H/C ratio of 1.80.17
Higher initial hydrogen pressure and longer reaction times further promoted the removal of oxygen and therefore improved the HHV, as expected. Hydrotreatment over NiMo-HL at an initial H2 pressure of 100 bar and a reaction time of 4 h produced bio-oil with the lowest oxygen content of 0.7 wt% and the highest HHV of 43.7 MJ kg−1, even higher than that of some petroleum-derived fuels (42 MJ kg−1) and comparable to that of petrodiesel (43 MJ kg−1).25 The molar H/C and O/C ratios for the bio-oil both decreased with increasing initial H2 pressure and reaction time.
Overall, the results clearly show that hydrotreatment with these metal oxide catalysts strongly improves the elemental composition and physical properties of bio-crude from HTL, making them good candidates for HTL bio-crude upgrading. An important factor to consider when choosing hydrotreating catalysts is their re-usability and poisoning potential upon prolonged use. The assessment of the re-usability is beyond the scope of the current screening study but is the focus of our future work. Within the present parametric matrix, optimum conditions (NiMo-HL catalyst, 4 h, 100 bar) produced an oil with 89.5 wt% C; 9.4 wt% H; 0.5 wt% N; 0.7 wt% O and a diesel-comparable HHV of 43.7 MJ kg−1. However, in achieving this, a slight trade-off against the bio-oil yield has to be accepted; therefore a shorter residence time can be favorable overall as shown in Table 1.
Molecular characterization by GC-MS
This section presents the results from molecular characterization for bio-crude and hydrotreated bio-oils. Fig. 4(a and b) show the total ion chromatograms for the bio-crude and treated bio-oil produced with the NiMo-HL catalyst. The difference in volatility is obvious with most compounds in the bio-crude eluting at 45–55 min whereas most compounds in the hydrotreated bio-oils elute earlier at <40 min. It is also worth noting that the total ion count (normalized to the sample mass on the Y axis) is much higher for the hydrotreated bio-oil, further depicting the increased GC amenable volatile fraction. No nitrogen containing compounds were identified in the chromatograms of the bio-crude or upgraded bio-oils. The only identified sulfur-containing compound in the bio-crude, i.e. 4-isopropylthiophenol, was not found in the hydrotreated oils. Generally, four groups of compounds are identified by GC-MS analysis in hydrotreated bio-oils: aliphatic hydrocarbon (C7–C24), substituted cycloalkanes (e.g. cyclohexane), alkyl-substituted aromatic hydrocarbons (e.g. toluene) and substituted PAHs.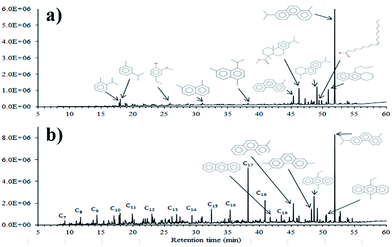 | ||
| Fig. 4 GC-MS total ion chromatograms of (a) bio-crude and (b) hydrotreated bio-oil produced with the NiMo-HL catalyst (initial H2 pressure: 75 bar, reaction time: 2 h, and catalyst loading: 10 wt%). | ||
During the HTL process, the lignin fraction is mostly converted to oxygen-containing substituted polycyclic aromatic hydrocarbons (PAHs) through depolymerisation, condensation, or breakdown reactions, whereas carbohydrate and lipid fractions are mostly converted to substituted cyclopentenones and fatty acids, respectively.26,27 GC-MS analysis of the bio-crude showed the presence of oxygen-containing substituted PAHs and substituted cyclopentenones with small peaks from fatty acids.
Regarding the conversion pathway of substituted cyclopentenones, one possibility is hydrogenation followed by hydrogenolysis wherein they are first hydrogenated to cyclopentanols and then further converted to cycloalkanes. Moreover, the carbonyl group in cyclopentenones may also be removed directly by decarbonylation, which then promotes the conversion of cyclopentenones to alkanes. Decarboxylation of higher fatty acids may lead to the formation of aliphatic hydrocarbons with a carbon number above fifteen, which may in turn be further converted to lighter aliphatics with carbon numbers ranging from C5 to C15.28 The decreased contents of oxygen-containing substituted PAHs (C > 20) in the treated bio-oils and the appearance of large amounts of substituted anthracene, phenanthrene and naphthalene indicate possible hydrodeoxygenation followed by alkyl rearrangement or cracking reactions.
In order to better understand the hydrotreatment conversion, quantitative GC-MS analysis was performed with a selection of oxygen-containing compounds (substituted cyclopentenones, substituted phenols and fatty acids), nitrogen-containing compounds, aliphatic hydrocarbons (C7 to C24) and several aromatic hydrocarbons, using standards and calibration curves for each compound. The results are listed in Tables 4 and 5. Seven nitrogen containing compounds (pyrazines, indoles and pyrroles) were included in the calibration but not detected in any of the samples and are therefore not included in the tables.
| Retention time (min) | Compound identity | Bio-crude (μg g−1) | NiW 2 h, 75 bar (μg g−1) | NiMo-LL 2 h, 75 bar (μg g−1) | NiMo-HL 2 h, 75 bar (μg g−1) | NiMo-HL 4 h,100 bar (μg g−1) |
|---|---|---|---|---|---|---|
| Oxygen-containing compounds | ||||||
| 11.889 | 2-Cyclopenten-1-one | — | — | — | — | — |
| 13.576 | Cyclohexanone | — | — | — | — | — |
| 13.757 | 2-Cyclopenten-1-one, 2-methyl- | 312 | — | — | — | — |
| 15.346 | 2-Cyclopenten-1-one, 3-methyl- | 187 | — | — | — | — |
| 15.527 | Phenol | 418 | 3058 | 2910 | 2574 | 2498 |
| 17.345 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 1083 | 70 | 58 | — | — |
| 18.251 | p-Cresol | 679 | 8112 | 7227 | 6348 | 6162 |
| 21.024 | Phenol, 4-ethyl- | 547 | 3807 | 4287 | 3722 | 3001 |
| 21.444 | Phenol, 2,3-dimethyl- | 179 | 1527 | 1513 | 1291 | 1087 |
| 26.950 | Phenol, 2,6-dimethoxy- | 439 | 459 | 489 | 499 | 426 |
| 46.930 | Palmitic acid | 5229 | — | — | — | — |
| 50.839 | Oleic acid | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862 862 |
— | — | — | — |
| Retention time (min) | Compound identity | Bio-crude (μg g−1) | NiW 2 h,75 bar (μg g−1) | NiMo-LL 2 h,75 bar (μg g−1) | NiMo-HL 2 h,75 bar (μg g−1) | NiMo-HL 4 h,100 bar (μg g−1) |
|---|---|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||||
| 9.255 | Heptane | — | 295 | 909 | 1080 | 671 |
| 11.741 | Octane | 13 | 822 | 2124 | 1986 | 1652 |
| 14.317 | Nonane | 37 | 1272 | 2960 | 2460 | 2430 |
| 17.016 | Decane | 15 | 1410 | 3215 | 2392 | 2665 |
| 19.921 | Undecane | 26 | 1750 | 3457 | 2646 | 2873 |
| 23.016 | Dodecane | — | 1477 | 2841 | 2025 | 2293 |
| 26.192 | Tridecane | — | 1469 | 2586 | 1815 | 2016 |
| 29.353 | Tetradecane | 9 | 1609 | 2662 | 1961 | 2036 |
| 32.431 | Pentadecane | 44 | 2915 | 3836 | 3348 | 2802 |
| 35.385 | Hexadecane | 452 | 2445 | 3277 | 3221 | 2994 |
| 38.200 | Heptadecane | 223 | 8374 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 287 287 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 554 554 |
8456 |
| 40.891 | Octadecane | 29 | 1079 | 2665 | 4332 | 4630 |
| 43.458 | Nonadecane | 35 | 968 | 1221 | 1399 | 1092 |
| 45.903 | Eicosane | 60 | 603 | 734 | 912 | 762 |
| 48.240 | Heneicosane | 146 | 1119 | 1071 | 1395 | 949 |
| 50.470 | Docosane | 110 | 777 | 733 | 1189 | 919 |
| 52.610 | Tricosane | 195 | 829 | 702 | 1052 | 706 |
| 54.659 | Tetracosane | 133 | 519 | 440 | 793 | 636 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Aromatic hydrocarbons | ||||||
| 10.005 | Cyclohexane, methyl- | 160 | 458 | 398 | 500 | 640 |
| 11.066 | Toluene | 416 | 1323 | 1069 | 1200 | 1631 |
| 13.494 | Ethylbenzene | 568 | 1183 | 1041 | 1063 | 1549 |
| 13.766 | o-Xylene | 743 | 2788 | 1929 | 2148 | 3399 |
| 16.416 | Mesitylene | 595 | 1129 | 721 | 766 | 926 |
| 23.419 | Naphthalene | 1281 | 2638 | 3210 | 2268 | 3472 |
| 27.032 | Naphthalene, 2-methyl- | 1630 | 5521 | 5578 | 3914 | 6181 |
| 41.714 | Anthracene | 1759 | 3530 | 3603 | 2665 | 4297 |
| 42.043 | Phenanthrene | 1175 | 3788 | 3955 | 2510 | 4951 |
| 51.977 | Retene | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 666 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166 166 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 323 |
As mentioned above, the bio-crude exhibits a large presence of substituted cyclopentenones such as 2-methyl-2-cyclopenten-1-one (312 μg g−1) and 2,3-dimethyl-2-cyclopenten-1-one (1083 μg g−1), substituted phenols such as 2,6-dimethoxy-phenol, (439 μg g−1), 4-ethyl-phenol (547 μg g−1) and p-cresol (679 μg g−1), phenol (418 μg g−1), and fatty acids, e.g. palmitic acid (5229 μg g−1) and oleic acid (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862 μg g−1). Hydrotreatment with NiW, NiMo-LL and NiMo-HL3 catalysts all resulted in a significant reduction of selected cyclopentenones and fatty acids while the concentration of certain phenols increased. This demonstrates the persistence of phenols which are known for their obstinate characteristics during hydrotreatment.17
862 μg g−1). Hydrotreatment with NiW, NiMo-LL and NiMo-HL3 catalysts all resulted in a significant reduction of selected cyclopentenones and fatty acids while the concentration of certain phenols increased. This demonstrates the persistence of phenols which are known for their obstinate characteristics during hydrotreatment.17
The increase in the content of phenolics in hydrotreated bio-oils further suggests that the heavy non-GC amenable fraction of bio-crude is comprised of a complex, high molecular weight, lignin derived fraction, which upon hydrotreating undergoes cracking to produce more volatile phenolics. The results in Table 3 also point to a trend in the HDO ability of the three catalysts, with NiMo-HL > NiMo-LL > NiW, as also indicated by the elemental analysis results. A higher initial hydrogen pressure and longer reaction time led to more pronounced HDO as revealed by the quantitative analysis results in Table 4, showing the decrease in the concentration of phenolics. However, there is still a significant presence of persistent phenols left in the bio-oil even when treated under these comparatively severe conditions.
Table 5 shows the quantitative analysis results for alkanes and aromatic hydrocarbons. A very small amount of aliphatic hydrocarbons (0.2 wt%) was identified for the bio-crude with the maximum content of 452 μg g−1 for hexadecane. It is known that the HTL process leads to the formation of some small amounts of these alkanes.29 High concentrations of substituted cycloalkanes, aliphatic hydrocarbons and substituted PAHs such as substituted anthracene, phenanthrene and naphthalene were determined for the three different treated bio-oils. It is interesting to compare the content of different aliphatic hydrocarbons between the bio-oils treated by NiMo-LL and NiMo-HL. The former clearly contains more light aliphatic alkanes (C8–C15) than the latter, whereas its content of larger aliphatic hydrocarbons was lower. In combination with the results from elemental analysis on the HDO ability of these two catalysts, it is reasonable to assume that the higher loading of NiMo promoted the HDO process to some degree at the expense of cracking heavy aliphatic hydrocarbons into lighter ones. In total, the quantitative contents of C7–C24 aliphatic alkanes for the different hydrotreated bio-oils over NiW, NiMo-LL and NiMo-HL are 3.0, 4.6 and 4.6 wt% respectively. This quantification only includes linear, saturated alkanes; while high abundances of branched (e.g. 6-metyl-tridencane, 2,5-dimethyl-heptane) and cyclic alkanes (e.g. cyclohexane and cyclopentane) were also observed but not quantified. It has been shown that hydrotreated bio-oil derived from the HTL of microalgae is mainly comprised of alkanes, although the concentrations have not been determined;15,30 indeed the current study is the first to quantify bio-oil compounds from hydrotreated bio-crude. This work shows that hydrotreated bio-oils from wood exhibit a higher concentration of aromatics compared to algae derived bio-oils, which is not surprising given the lignin contents of wood. Nevertheless, bio-crude produced from the HTL of lignocellulosic feedstocks has clear potential as an alternative hydrocarbon source to current fossil crude derived transportation fuels. The combination of analyses points to fuel properties viable for renewable diesel and jet fuel applications after further refining.
In summary, GC-MS molecular characterization results showed that mild hydrotreatment (350 °C, 75 bar initial H2 pressure and 2 h reaction time) over NiW, NiMo-LL and NiMo-HL catalysts sharply reduced the content of heteroatoms (O, N, and S) in the bio-crude. Moreover, severe hydrotreatment (100 bar initial H2 pressure and 4 h reaction time) led to better deoxygenation. The combined results from molecular and elemental characterization demonstrate the possibility of removing heteroatom (O, N, and S) contents of bio-crude from the HTL of aspen wood by hydrotreatment with metal oxide catalysts.
FT-IR analysis
Fig. 5 compares the relative transmittance of FT-IR spectra for the bio-crude and hydrotreated bio-oils produced with the three catalysts. The treated bio-oils exhibit an obvious decrease in the absorbance between 1650 and 1750 cm−1, which is the typical region of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching (e.g. aldehydes, ketones, carboxylic acids and esters). This indicates a drastic elimination of C
O bond stretching (e.g. aldehydes, ketones, carboxylic acids and esters). This indicates a drastic elimination of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Similar reductions in the absorbance intensities at both 3400 and 1700 cm−1 can be found, demonstrating that a majority of C–O and O–H groups have been removed. From the perspective of deoxygenation, the FT-IR analysis thereby shows a trend identical to the elemental analysis and GC-MS characterization, again pointing to the order of activity NiMo-HL > NiMo-LL > NiW. Additionally, in all the spectra, strong absorbance appears around 2800 to 2950 cm−1, which can be attributed to the presence of C–H bond stretching, e.g. alkanes. All FT-IR spectra likewise showed stronger absorbance between 700 and 800 cm−1, the region of phenyl ring substitution bond stretching, e.g. anthracene, phenanthrene and naphthalene. This is consistent with the GC-MS results.
O groups. Similar reductions in the absorbance intensities at both 3400 and 1700 cm−1 can be found, demonstrating that a majority of C–O and O–H groups have been removed. From the perspective of deoxygenation, the FT-IR analysis thereby shows a trend identical to the elemental analysis and GC-MS characterization, again pointing to the order of activity NiMo-HL > NiMo-LL > NiW. Additionally, in all the spectra, strong absorbance appears around 2800 to 2950 cm−1, which can be attributed to the presence of C–H bond stretching, e.g. alkanes. All FT-IR spectra likewise showed stronger absorbance between 700 and 800 cm−1, the region of phenyl ring substitution bond stretching, e.g. anthracene, phenanthrene and naphthalene. This is consistent with the GC-MS results.
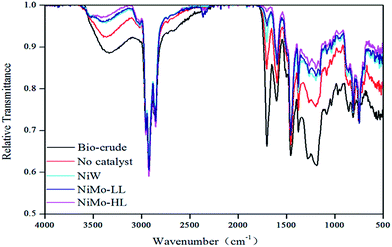 | ||
| Fig. 5 FT-IR spectra of bio-crude and hydrotreated bio-oils obtained by using different catalysts (initial H2 pressure: 75 bar, reaction time: 2 h, and catalyst loading: 10 wt%). | ||
As expected, increasing the initial H2 pressure or prolonging the reaction time both led to increased deoxygenation and therefore produced treated bio-oils with lower oxygen content, which also matches the elemental analysis result in Section 3.2. However, it should be pointed out that increasing the H2 pressure beyond 75 bar did not promote the deoxygenation as effectively as increasing it from 50 bar to 75 bar. As discussed earlier, it may be a better choice to employ a moderate initial H2 pressure and longer reaction time in order to reach a balance between high yield and high quality of treated bio-oil, as both these factors have an effect on the process economy.
Simulated distillation
Sim-Dis was carried out according to ASTM D2887 in order to determine the boiling point distribution of the bio-crude and hydrotreated bio-oils. It should be stressed that ASTM D2887 is designed to determine the boiling range distribution of petroleum fractions with a final boiling point of 538 °C. So for the present Sim-Dis analysis, only compounds with a boiling point lower than that were detected and their weight percentages are presented. According to different boiling point ranges, four distillate fractions are defined: gasoline (b.p. < 190 °C), diesel cut 1 (b.p. 190–290 °C), diesel cut 2 (b.p. 290–340 °C) and vacuum gas oil (b.p. 340–538 °C).Fig. 6 shows the Sim-Dis results of bio-crude and hydrotreated bio-oils obtained over different catalysts. Only 3.8 wt% of HTL bio-crude was fractionated in the gasoline range, 18.2 wt% in diesel cut 1 and 12.9 wt% in diesel cut 2, while 65.2 wt% was fractioned in the vacuum gas oil range, indicating that the bio-crude contains a large amount of compounds with heavy molecular weight and high boiling points. These heavy-fraction compounds are not volatile enough to be identified via GC-MS analysis and therefore not included in Fig. 4. For non-catalytic hydrotreatment, despite an obvious increase in the gasoline fraction the vacuum gas oil is still as high as 52.9 wt%. This is not surprising as 350 °C is not hot enough for severe cracking.15 Hydrotreatment using catalysts led to a sharp decrease in the vacuum gas oil fraction and a significant increase in the gasoline and diesel cuts. The NiMo-LL catalyst showed the most favorable result with 14.2 wt% gasoline, 27.8 wt% diesel cut 1, 18.7 wt% diesel cut 2 and 39.4 wt% vacuum gas oil. This reveals that more hydrocracking took place during hydrotreatment with NiMo-LL, as supported by the GC-MS characterization.
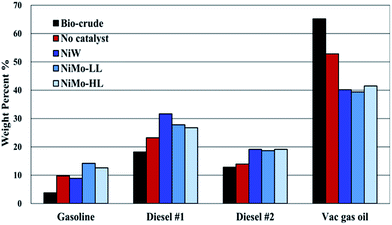 | ||
| Fig. 6 Sim-Dis analysis of bio-crude and hydrotreated bio-oils obtained by using different catalysts (initial H2 pressure: 75 bar, reaction time: 2 h, and catalyst loading: 10 wt%). | ||
Sim-Dis results for different initial H2 pressures and reaction times are compared in Table 6. For the effect of initial H2 pressure, the present study indicated that the catalytic hydrotreatment over the NiMo-HL catalyst with an initial H2 pressure of 50 bar showed the largest gasoline fraction and lowest vacuum gas oil fraction compared with hydrotreatments with initial H2 pressures of 75 bar and 100 bar. A possible explanation may be that higher pressure suppressed the diffusion of the cracked gaseous product from the oil phase to the gas phase, which then further inhibited the whole cracking process and the formation of lighter oil fractions. Also, higher pressure may promote condensation of small molecules to heavier ones as mentioned previously. A similar trend was found for the effect of reaction time, i.e., the longer the reaction the more vacuum gas oil and less gasoline fraction is produced. One possible explanation for this result is that a longer reaction time boosts polymerization reactions, such as esterification,31 of low boiling point compounds to higher boiling point compounds, under the constraint that 350 °C would not be hot enough to break down these heavier compounds completely.
| Experimental conditions | <190 °C (Gasoline) | 190–290 °C (Diesel #1) | 290–340 °C (Diesel #2) | 340–538 °C (Vac gas oil) |
|---|---|---|---|---|
| NiMoB, 4 h, 50 bar, 10% | 19.1 | 30.3 | 16.5 | 34.3 |
| NiMoB, 4 h, 75 bar, 10% | 12.1 | 30.5 | 20.4 | 37.1 |
| NiMoB, 4 h, 100 bar, 10% | 12.8 | 29.3 | 18.6 | 39.5 |
| NiMoB, 1 h, 100 bar, 10% | 13.7 | 29.2 | 18.3 | 39.0 |
| NiMoB, 2 h, 100 bar, 10% | 13.0 | 28.1 | 18.1 | 40.9 |
Conclusions
Catalytic hydrotreatment of bio-crude produced from the HTL of aspen wood with three metal oxide catalysts removes the majority of heteroatoms (O, N, and S) and improves the physical properties of bio-oils. Catalyst screening results indicate that the performance of these three catalysts in HDO is in the order of NiMo-HL > NiMo-LL > NiW. NiMo with a high NiMo loading catalytic hydrotreatment produced a treated bio-oil with a yield of 71.9 wt%, oxygen content of 2.4 wt% and HHV of 42.6 MJ kg−1. Both higher initial hydrogen pressure and longer reaction times are beneficial for deeper HDO and improvement of the physical properties of the bio-oil at the expense of lowering the yield. Under the optimal deoxygenation conditions (NiMo-HL, 100 bar H2 pressure and 4 h reaction time) a bio-oil with the lowest oxygen content of 0.7 wt% and the highest HHV of 43.7 MJ kg−1 was obtained. This compares well with a conventional diesel and highlights the potential of HTL and catalytic hydrotreatment as a technology to replace petroleum-derived fuels.GC-MS molecular characterization results showed three main compound classes in hydrotreated bio-oils: substituted cycloalkanes, aliphatic hydrocarbons (C7–C24) and aromatic hydrocarbons. Their possible formation and conversion pathways are provided as follows: substituted cycloalkanes form via hydrogenation followed by hydrogenolysis of substituted cyclopentenones, aliphatic hydrocarbons (C7–C24) originate from decarboxylation of higher fatty acids and decarbonylation of cyclopentenones, while hydrodeoxygenation and alkyl rearrangement or cracking reaction of oxygen-containing substituted PAHs lead to the formation of PAHs such as anthracene, phenanthrene and naphthalene.
Overall, the current study shows the potential of producing biofuels compatible with the existing fuel infrastructure via the HTL of aspen wood through a simpler hydrotreating strategy.
Acknowledgements
We gratefully acknowledge Steeper Energy Aps and Haldor Topsøe for providing the bio-crude and hydrotreatment catalysts respectively. We further gratefully acknowledge the support of this work by the Danish National Research Foundation (DNRF93) and the Innovation Fund Denmark (Grant No. 1305-00030B).Notes and references
- A. Demirbas, Energy Convers. Manage., 2001, 42, 1357–1378 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Froeling, M. J. Antal Jr and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 CAS.
- P. Duan and P. E. Savage, Energy Environ. Sci., 2011, 4, 1447–1456 CAS.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- A. J. Morup, J. Becker, P. S. Christensen, K. Houlberg, E. Lappa, M. Klemmer, R. B. Madsen, M. Glasius and B. B. Iversen, Ind. Eng. Chem. Res., 2015, 54, 5935–5947 CrossRef.
- I. J. Tews, Y. Zhu, C. V. Drennan, D. C. Elliott, L. J. Snowden-Swan, K. Onarheim, Y. Solantausta and D. Beckman, Biomass Direct Liquefaction Options: Technoeconomic and Life Cycle Assessment, Technol. Rep. Prepared for U.S. Department of Energy, Pacific Northwest Lab, Richland, WA, USA, 2014 Search PubMed.
- S. de Jong, R. Hoefnagels, A. Faaij, R. Slade, R. Mawhood and M. Junginger, Biofuels, Bioprod. Biorefin., 2015, 9, 778–800 CrossRef CAS.
- E. Laurent and B. Delmon, Appl. Catal., A, 1994, 109, 77–96 CrossRef CAS.
- A. Centeno, E. Laurent and B. Delmon, J. Catal., 1995, 154, 288–298 CrossRef CAS.
- D. C. Elliott, T. R. Hart, G. G. Neuenschwander, L. J. Rotness and A. H. Zacher, Environ. Prog. Sustainable Energy, 2009, 28, 441–449 CrossRef CAS.
- R. H. Venderbosch, A. R. Ardiyanti, J. Wildschut, A. Oasmaa and H. J. Heeresb, J. Chem. Technol. Biotechnol., 2010, 85, 674–686 CrossRef CAS.
- A. Y. Bunch, X. Wang and U. S. Ozkan, Appl. Catal., A, 2008, 346, 96–103 CrossRef CAS.
- E. Furimsky, Catal. Today, 2013, 217, 13–56 CrossRef CAS.
- D. C. Elliott and E. Baker, Upgrading Biomass Liquefaction Products through Hydrodeoxygenation, Pacific Northwest Lab., Richland, WA, USA, 1984 Search PubMed.
- P. Biller, B. K. Sharma, B. Kunwar and A. B. Ross, Fuel, 2015, 159, 197–205 CrossRef CAS.
- D. López Barreiro, B. R. Gómez, F. Ronsse, U. Hornung, A. Kruse and W. Prins, Fuel Process. Technol., 2016, 148, 117–127 CrossRef.
- C. U. Jensen, J. Hoffmann and L. A. Rosendahl, Fuel, 2016, 165, 536–543 CrossRef CAS.
- Y. Zhu, M. J. Biddy, S. B. Jones, D. C. Elliott and A. J. Schmidt, Appl. Energy, 2014, 129, 384–394 CrossRef CAS.
- K. O. Albrecht, Y. Zhu, A. J. Schmidt, J. M. Billing, T. R. Hart, S. B. Jones, G. Maupin, R. Hallen, T. Ahrens and D. Anderson, Algal Res., 2016, 14, 17–27 CrossRef.
- T. H. Pedersen, I. F. Grigoras, J. Hoffmann, S. S. Toor, I. M. Daraban, C. U. Jensen, S. B. Iversen, R. B. Madsen, M. Glasius, K. R. Arturi, R. P. Nielsen, E. G. Sogaard and L. A. Rosendahl, Appl. Energy, 2016, 162, 1034–1041 CrossRef CAS.
- R. B. Madsen, P. S. Christensen, K. Houlberg, E. Lappa, A. J. Morup, M. Klemmer, E. M. Olsen, M. M. Jensen, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2015, 192, 826–830 CrossRef CAS PubMed.
- P. Duan and P. E. Savage, Appl. Catal., B, 2011, 104, 136–143 CrossRef CAS.
- D. Cheng, L. Wang, A. Shahbazi, S. Xiu and B. Zhang, Energy Convers. Manage., 2014, 87, 378–384 CrossRef CAS.
- J. Wildschut, F. H. Mahfud, R. H. Venderbosch and H. J. Heeres, Ind. Eng. Chem. Res., 2009, 48, 10324–10334 CrossRef CAS.
- A. Demirbas, Energy Policy, 2007, 35, 4661–4670 CrossRef.
- T. H. Pedersen and L. A. Rosendahl, Biomass Bioenergy, 2015, 83, 206–215 CrossRef CAS.
- J. Akhtar and N. A. S. Amin, Renewable Sustainable Energy Rev., 2011, 15, 1615–1624 CrossRef CAS.
- P. Duan and P. E. Savage, Bioresour. Technol., 2011, 102, 1899–1906 CrossRef CAS PubMed.
- C. Torri, L. G. Alba, C. Samori, D. Fabbri and D. W. F. Brilman, Energy Fuels, 2012, 26, 658–671 CrossRef CAS.
- K. O. Albrecht, Y. H. Zhu, A. J. Schmidt, J. M. Billing, T. R. Hart, S. B. Jones, G. Maupin, R. Hallen, T. Ahrens and D. Anderson, Algal Research-Biomass Biofuels and Bioproducts, 2016, 14, 17–27 Search PubMed.
- Y. Xu, T. Wang, L. Ma, Q. Zhang and W. Liang, Appl. Energy, 2010, 87, 2886–2891 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |