Study of the enhancement on photogalvanics: solar energy conversion and storage in EDTA–safranine O–NaLS system
Prerna
Gangotri
and
Pooran
Koli
 *
*
Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan-342001, India. E-mail: poorankoli@yahoo.com; k.mgangotri@yahoo.co.in; Tel: +91 2912614162
First published on 28th March 2017
Abstract
The study focused on exploiting modified cell fabrication parameters for enhancing the solar power generation and storage capacity of a photogalvanic ethylene diamine tetraacetic acid–safranine O–sodium lauryl sulphate chemical system. This chemical system with changed concentrations, a combination electrode and a very small Pt electrode was used to fabricate a modified photogalvanic cell. The modified cell showed greatly enhanced performance (i.e., that for pre-modified cell) in terms of charging time (40 min), initial generation of photocurrent (260 μA min−1), equilibrium photocurrent (1700 μA), power (364.7 μW), half change time (40 min), and efficiency (8.93%). The effect of various cell fabrication parameters was studied for optimization of the value of fabrication variables for the optimal cell performance based on the proposed mechanism.
1 Introduction
The illumination of two unsymmetrical metallic electrodes shows the flow of electric current.1 Considering this phenomenon, Rideal and Williams,2 for the first time, studied photogalvanic cells for solar energy conversion. Later on, Rabinowitch3,4 performed a systemic study of photogalvanic cells. Since then, numerous scientists have contributed to this field of research and discussed the problems encountered in the development of photogalvanic cells based on various aspects, such as single dye sensitizers,5–14 mixed dye sensitizers,15–19 bipolar photogalvanic cells,20 mesoporous dyes,21 mixed reductants,22 single reductants,23–25 surfactants,26–28 and two metallic electrodes. Gangotri and Gangotri29 have reported the EDTA–safranine O–NaLS system in a photogalvanic cell for solar energy conversion and storage with impressive electrical output, conversion efficiency and storage capacity. Therefore, after some modifications in the cell construction, the EDTA–safranine O–NaLS system can potentially replace existing chemical systems used for harnessing and storing solar power. Therefore, the present research focuses on enhancing the electrical output, conversion efficiency and storage capacity as well as reducing the cost by making changes to the construction of the experimental setup of the same system. The modifications in the experimental setup not only abruptly enhanced the photogalvanics to a remarkable extent, but also reduced the cost of the construction of the photogalvanic cell for its commercial viability. The observed equilibrium photocurrent (1700 μA) for the modified cell was 8.5 times greater than that (200 μA) for the pre-modified cell. The initial generation of photocurrent (260 μA min−1) for the modified cell was 5.2 times greater than that (50 μA min−1) for the pre-modified cell. Power (364.70 μW) for the modified cell was 4.86 times greater than that (75.02 μW) of the pre-modified cell. The conversion efficiency (8.93%) for the modified cell was 12.38 times greater than that (0.7213%) for the pre-modified cell.2 Materials and methods
2.1 Materials
Among the equipment, a digital pH meter (Systronics Model-335), a micro-ammeter (Ruttonsha Simpson), a combination electrode (Toshniwal), solar light intensity meter (Solarimeter model-501 cell), 200 W tungsten lamp as the light source and a carbon pot as the resistance variation device were used. M/10 solution of an anionic surfactant sodium lauryl sulphate (NaLS) or sodium dodecyl sulphate (SDS), M/100 solution of reductant ethylene diamine tetraacetic acid (EDTA), M/500 solution of safranine O (photosensitizer), 1 M solution of sodium hydroxide, 1 M solution of oxalic acid and phenolphthalein were used.Safranine-O is a water soluble azonium compound of symmetrical 2,8-dimethyl-3,7-diamino-phenazine with a chemical formula of C20H19ClN4 and M.W. of 350.84 g mol−1. The aqueous solution of safranine-O has a red color with a maximum wavelength of 530–534 nm.
2.2 Method
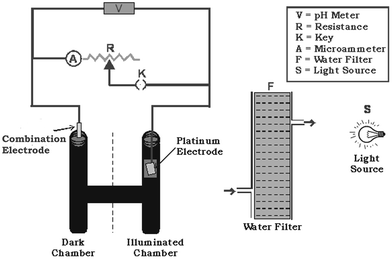
A glass tube with an H-shape was fabricated and darkened keeping a window in one arm of the tube, which was used as the illuminated chamber and other as a dark chamber.30,31 This tube was filled with a known amount of solution of surfactant (anionic micelles), reductant (ethylene diamine tetraacetic acid), photosensitizer (safranine O), and sodium hydroxide. The total volume of the solutions was kept at 25.0 ml by adding doubly distilled water. The dimensions of the platinum electrode area were reduced to (0.2 cm × 0.4 cm), i.e. 12.5 times less than those of the reported system for the purpose of reducing the cost of the cell and dipped in one arm of the H-tube before a window (un-blackened) that is placed just in front of a source of light (a 200 W tungsten lamp). The combination electrode instead of saturated calomel electrode was immersed in another limb of the H tube and used as the reference electrode. The terminals of the electrodes were connected to a digital pH meter and micro-ammeter through a key and resistance to measure the photopotential and photocurrent, respectively, generated in the photogalvanic system [Fig. 1].Initially, the circuit was kept open and cell was placed in dark until it attained a stable potential (dark potential – Vdark). The Pt electrode was then exposed to artificial light emitted from the tungsten bulb. A water filter was put between the cell and the lamp to cut off the infra-red radiation to curb the heating effect of the cell, which otherwise can adversely affect the cell, leading to a lower performance. Upon illumination, the photo potential (V) and photocurrent (i) are generated by the system. After charging the cell, the cell parameters, such as the maximum potential (Vmax), open-circuit potential (Voc), maximum current (imax) and equilibrium current (ieq) or short-circuit current (isc), were measured. A study of i–V characteristics (which has been done by observing the potential at different direct currents by varying a resistance, which was calculated by Ohm law, of the circuit) shows the highest power at which the cell can be used. The cell was operated at the highest power (i.e., power at power point – ppp) at the corresponding external load, current (i.e., current at power point – ipp) and potential (i.e., potential at power point – Vpp) to study its performance by observing the change in current and potential with time.
The i–V characteristics were studied by measuring the potential at different currents by applying an external load. The magnitude of current flowing through the circuit was varied by varying the resistance of the circuit with the help of a carbon pot log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 K. We obtained the power (i × V) of the cell by multiplying the potential and corresponding current values, and the highest product was the power at the power point of the cell (maximum power point – MPP). The resistance corresponding to the MPP is the external load at which the cell can be used to obtain the highest power. The error in the observed values of current (from micro ammeter) and potential (from digital pH meter) was ±10 μA and ±5 mV, respectively.
470 K. We obtained the power (i × V) of the cell by multiplying the potential and corresponding current values, and the highest product was the power at the power point of the cell (maximum power point – MPP). The resistance corresponding to the MPP is the external load at which the cell can be used to obtain the highest power. The error in the observed values of current (from micro ammeter) and potential (from digital pH meter) was ±10 μA and ±5 mV, respectively.
The prototype design of photogalvanic cells will involve assembly of large number of single cells (Fig. 1) linked in parallel/linear series to boost the power output according to the requirement of use.
3 Results
The photogalvanics was studied in a photogalvanic system and effects have been observed on different variations, such as concentration, electrode area, etc. The optimal values of the cell variables for the safranine O–EDTA–NaLS system at 10.4 mW cm−2 light intensity were 4.64 × 10−5 M safranine O, 2.24 × 10−3 M EDTA, 6.00 × 10−3 M NaLS, 13.3096 pH, and 0.4 cm × 0.2 cm Pt electrode area. The optimal cell performance at the optimal values of the cell variable is summarized as the dark potential 677 mV; open-circuit potential 1052 mV; photopotential 375 mV; charging time 40 min; maximum current 2600 μA; short-circuit current 1700 μA; power at power point 364.7 μW; potential at power point 521 mV; current at power point 700 μA; half change time 40 min; conversion efficiency 8.93%; and fill factor 0.2039. The photo decay of dye was responsible for the poor fill factor, but a higher fill factor was not our objective. Instead, a higher electrical output was our main objective that was reached.3.1 Effect of the variation of EDTA concentration
The electrical output increased with increasing EDTA (reductant) concentration and reached the maximum value at a particular concentration (Table 1).| EDTA–safranine O–NaLS system | (EDTA) × 10−3 M | |||||
|---|---|---|---|---|---|---|
| 2.20 | 2.22 | 2.24 | 2.26 | 2.28 | ||
| a [Safranine O] = 4.64 × 10−5 M, [NaLS] = 6.00 × 10−3 M, pH = 13.3096, light intensity = 10.4 mW cm−2, temp. = 303 K, electrode area = 0.2 × 0.4 cm2. | ||||||
| 1 | Dark potential (mV) | 715.0 | 716.0 | 677.0 | 699.0 | 684.0 |
| 2 | Open circuit voltage Voc (mV) | 1060.0 | 1060.0 | 1052.0 | 1045.0 | 1025.0 |
| 3 | Photopotential ΔV (mV) | 345.0 | 352.0 | 375.0 | 346.0 | 341.0 |
| 4 | Maximum photocurrent imax (μA) | 3100.0 | 2600.0 | 2600.0 | 2400.0 | 2400.0 |
| 5 | Short circuit current isc (μA) | 1400.0 | 1650.0 | 1700.0 | 1500.0 | 1450.0 |
| 6 | Potential at power point Vpp (mV) | 500.0 | 515.0 | 521.0 | 514.0 | 464.0 |
| 7 | Current at power point ipp (μA) | 600.0 | 645.0 | 700.0 | 600.0 | 550.0 |
| 8 | Power of cell i × V (μW) | 300.000 | 332.175 | 364.700 | 308.400 | 255.200 |
| 9 | Fill factor (η) | 0.2021 | 0.1958 | 0.2039 | 0.1967 | 0.1717 |
| 10 | Conversion efficiency (%) | 7.28% | 7.81% | 8.93% | 7.29% | 5.26% |
| 11 | Storage capacity t1/2 (min) | 18.0 | 40.0 | 40.0 | 45.0 | 50.0 |
| 12 | Charging time (min) | 45.0 | 40.0 | 40.0 | 45.0 | 65.0 |
The fall in the reductant concentration also resulted in a decrease in power output due to smaller number of molecules available for electron donation to the photosensitizer molecules. On the other hand, large concentration of reductant again resulted in a decrease in photopotential and photocurrent because the large number of reductant molecules prevented the photosensitizer molecules from reaching the electrode in the desired time limit.
3.2 Effect of variation of safranine O concentration
The electrical output, conversion efficiency and storage capacity increased, and reached the maximum at the particular concentration and then decreased (Table 2, Fig. 2).| EDTA–safranine O–NaLS system | (Safranine O) × 10−5 M | |||||
|---|---|---|---|---|---|---|
| 4.48 | 4.56 | 4.64 | 4.72 | 4.80 | ||
| a [EDTA] = 2.24 × 10−3 M, [NaLS] = 6.00 × 10−3 M, pH = 13.3096, light intensity = 10.4 mW cm−2, temp. = 303 K, electrode area = 0.2 × 0.4 cm2. | ||||||
| 1 | Dark potential (mV) | 618.0 | 626.0 | 677.0 | 635.0 | 600.0 |
| 2 | Open circuit voltage Voc (mV) | 1000.0 | 1015.0 | 1052.0 | 1045.0 | 1010.0 |
| 3 | Photopotential ΔV (mV) | 382.0 | 389.0 | 375.0 | 410.0 | 380.0 |
| 4 | Maximum photocurrent imax (μA) | 2400.0 | 2460.0 | 2600.00 | 2510.0 | 2400.0 |
| 5 | Short circuit current isc (μA) | 1200.0 | 1380.0 | 1700.0 | 1400.0 | 1330.0 |
| 6 | Potential at power point Vpp (mV) | 484.0 | 500.0 | 521.0 | 510.0 | 460.0 |
| 7 | Current at power point ipp (μA) | 550.0 | 610.0 | 700.0 | 600.0 | 570.0 |
| 8 | Power of cell i × V (μW) | 266.200 | 305.000 | 364.700 | 306.000 | 262.200 |
| 9 | Fill factor (η) | 0.2218 | 0.2177 | 0.2039 | 0.2091 | 0.1951 |
| 10 | Conversion efficiency (%) | 7.09% | 7.98% | 8.93% | 7.69% | 6.18% |
| 11 | Storage capacity t1/2 (min) | 19.0 | 31.0 | 40.0 | 15.0 | 55.0 |
| 12 | Charging time (min) | 55.0 | 70.0 | 40.0 | 60.0 | 95.0 |
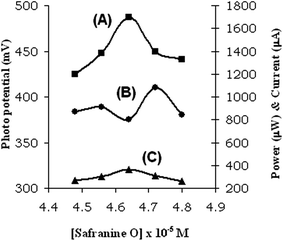 | ||
| Fig. 2 Variation of (A) short circuit current, (B) photo potential, (C) power at the power point and with safranine concentration, the power point and with safranine O concentration. | ||
Upon illumination, safranine O molecules get excited and accept an electron from the reductant and donate it to the electrode and a flow of electron takes place (the generation of electricity). At lower safranine O concentrations, a small number of photosensitizer molecules were available in the system for excitation and consecutive electron transfer, whereas higher concentration of safranine O also resulted in a lowering of the photopotential and photocurrent because the major portion of the light was absorbed by the photosensitizer molecules in the path causing a decrease in the intensity of light reaching the photosensitizer molecule near the Pt electrode.
3.3 Effect of variation of NaLS concentration
Upon variation of sodium lauryl sulphate concentration, the electrical output was the highest around the critical micelles concentration of the surfactant (Table 3, Fig. 3). The photoejection of electron from the dye micelles system suggests the tunneling of the photoelectrons from the micellar phase to the aqueous phase. The photoejection of electrons from the photosensitizer-micelles depends on the charge in the micelles. The negative potential present inside the aggregates of anionic micelles make the ejection of electrons easier. Therefore, the efficiency of the photogalvanic cells also increases, and the observed photogalvanics also confirmed the same.| EDTA–safranine O–NaLS system | (NaLS) × 10−3 M | |||||
|---|---|---|---|---|---|---|
| 5.60 | 5.80 | 6.00 | 6.20 | 6.40 | ||
| a [EDTA] = 2.24 × 10−3 M, [safranine O] = 4.64 × 10−5 M, pH = 13.3096, light intensity = 10.4 mW cm−2, temp. = 303 K, electrode area = 0.2 × 0.4 cm2. | ||||||
| 1 | Dark potential (mV) | 634.0 | 665.0 | 677.0 | 709.0 | 681.0 |
| 2 | Open circuit voltage Voc (mV) | 980.0 | 1022.0 | 1052.0 | 1035.0 | 1005.0 |
| 3 | Photopotential ΔV (mV) | 346.0 | 357.0 | 375.0 | 326.0 | 324.0 |
| 4 | Maximum photocurrent imax (μA) | 2210.0 | 2270.0 | 2600.0 | 2300.0 | 2100.0 |
| 5 | Short circuit current isc (μA) | 1330.0 | 1500.0 | 1700.0 | 1600.0 | 1500.0 |
| 6 | Potential at power point Vpp (mV) | 350.0 | 400.0 | 521.0 | 480.0 | 466.0 |
| 7 | Current at power point ipp (μA) | 620.0 | 700.0 | 700.0 | 680.0 | 600.0 |
| 8 | Power of cell i × V (μW) | 245.000 | 280.000 | 364.700 | 326.400 | 279.600 |
| 9 | Fill factor (η) | 0.1879 | 0.1826 | 0.2039 | 0.1971 | 0.1854 |
| 10 | Conversion efficiency (%) | 5.53% | 6.14% | 8.93% | 7.73% | 6.23% |
| 11 | Storage capacity t1/2 (min) | 10.0 | 14.0 | 40.0 | 25.0 | 20.0 |
| 12 | Charging time (min) | 30.0 | 35.0 | 40.0 | 65.0 | 95.0 |
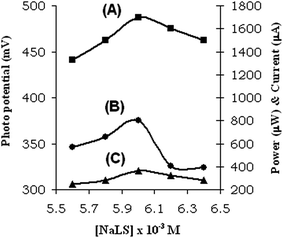 | ||
| Fig. 3 Variation of (A) short circuit current, (B) photo potential, (C) power at the power point and with NaLS concentration, the power point and with NaLS concentration. | ||
3.4 Effect of variation of pH
Upon the variation of pH on the system, it was observed that maximum electrical output was obtained at a particular pH. The determined working range of pH was 13.3062–13.3130 (Table 4). The potential of the system increased with increasing pH. The potential reached a maximum value for a particular pH and then decreased with further increases in pH. The pH for the optimal conditions for the reductant has a relationship with its pKa value, i.e., the desired pH should be slightly higher than its pKa values (pH > pKa). The reason may be the availability of the reductant in its anionic form, which is the better donor form.| EDTA–safranine O–NaLS system | pH | |||||
|---|---|---|---|---|---|---|
| 13.3062 | 13.3079 | 13.3096 | 13.3113 | 13.3130 | ||
| a [Safranine O] = 4.64 × 10−5 M, [NaLS] = 6.00 × 10−3 M, [EDTA] = 2.24 × 10−3, light intensity = 10.4 mW cm−2, temp. = 303 K, electrode area = 0.2 × 0.4 cm2. | ||||||
| 1 | Dark potential (mV) | 650.0 | 670.0 | 677.0 | 645.0 | 640.0 |
| 2 | Open circuit voltage Voc (mV) | 980.0 | 1000.0 | 1052.0 | 1040.0 | 925.0 |
| 3 | Photopotential ΔV (mV) | 330.0 | 321.0 | 375.0 | 395.0 | 285.0 |
| 4 | Maximum photocurrent imax (μA) | 1800.0 | 1900.0 | 2600.0 | 2300.0 | 1800.0 |
| 5 | Short circuit current isc (μA) | 1535.0 | 1650.0 | 1700.0 | 1500.0 | 1425.0 |
| 6 | Potential at power point Vpp (mV) | 471.0 | 480.0 | 521.0 | 490.0 | 400.0 |
| 7 | Current at power point ipp (μA) | 680.0 | 685.0 | 700.0 | 610.0 | 530.0 |
| 8 | Power of cell i × V (μW) | 320.280 | 328.800 | 364.700 | 298.900 | 212.000 |
| 9 | Fill factor (η) | 0.2129 | 0.1992 | 0.2039 | 0.1916 | 0.1608 |
| 10 | Conversion efficiency (%) | 8.19% | 7.87% | 8.93% | 6.88% | 4.09% |
| 11 | Storage capacity t1/2 (min) | 18.0 | 15.0 | 40.0 | 20.0 | 20.0 |
| 12 | Charging time (min) | 35.0 | 30.0 | 40.0 | 95.0 | 125.0 |
3.5 Effect of variation of electrode area
The results upon the variation of the electrode area in the photogalvanic cell containing the EDTA–safranine O–NaLS system show an increasing electric output as the electrode size is decreased (Table 5); this is perhaps because of the increase in the number of strikes of electrons on the smaller-sized electrode and faster transfer of electrons.| EDTA–safranine O–NaLS system | Electrode area (cm2) | |||||
|---|---|---|---|---|---|---|
| 1.0 | 0.40 | 0.20 | 0.08 | 0.06 | ||
| a [Safranine O] = 4.64 × 10−5 M, [NaLS] = 6.00 × 10−3 M, [EDTA] = 2.24 × 10−3, light intensity = 10.4 mW cm−2, temp. = 303 K. | ||||||
| 1 | Dark potential (mV) | 180.0 | 245.0 | 360.0 | 677.0 | 470.0 |
| 2 | Open circuit voltage Voc (mV) | 1051.0 | 1040.0 | 1010.0 | 1052.0 | 1050.0 |
| 3 | Photopotential ΔV (mV) | 871.0 | 795.0 | 650.0 | 375.0 | 580.0 |
| 4 | Maximum photocurrent imax (μA) | 400.0 | 380.0 | 590.0 | 2600.0 | 800.0 |
| 5 | Short circuit current isc (μA) | 200.0 | 180.0 | 390.0 | 1700.0 | 600.0 |
| 6 | Potential at power point Vpp (mV) | 680.0 | 600.0 | 560.0 | 521.0 | 510.0 |
| 7 | Current at power point ipp (μA) | 110.0 | 115.0 | 200.0 | 700.0 | 380.0 |
| 8 | Power of cell i × V (μW) | 74.800 | 69.000 | 112.000 | 364.700 | 193.800 |
| 9 | Fill factor (η) | 0.3558 | 0.3685 | 0.2843 | 0.2039 | 0.3076 |
| 10 | Conversion efficiency (%) | 3.19% | 3.05% | 3.82% | 8.93% | 7.16% |
| 11 | Storage capacity t1/2 (min) | 40.0 | 36.0 | 25.0 | 40.0 | 25.0 |
| 12 | Charging time (min) | 150.0 | 180.0 | 130.0 | 40.0 | 110.0 |
3.6 Current–voltage characteristics of the cell
The observed enhancement in electrical output, conversion efficiency and storage capacity in the EDTA–safranine O–NaLS system of a photogalvanic cell are determined by applying external load on the circuit by a carbon pot (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 K) (Table 6).
470 K) (Table 6).
3.7 Performance of the photogalvanic cell
The overall performance of the photogalvanic cell was studied by determining the i–V characteristics. From the observations, the power at the power point of the cell was determined to be 364.700 μW and the fill factor (FF) was 0.2039, as calculated using the following formulae (eqn (1) and (2)), respectively.| Power at power point = Vpp × ipp | (1) |
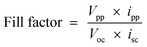 | (2) |
The conversion efficiency32 was determined using the formula (eqn (3))
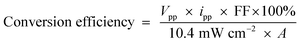 | (3) |
where, ‘A’ is the Pt electrode area. The observed conversion efficiency was 8.93%. The photogalvanic cell developed worked for 40.0 minutes in the dark after illuminating for 40.0 minutes (the charging time). The overall cost of the cell was reduced to around 66% of the reported system.
3.8 Enhancement in the results
The enhancement in electrical output, power at power point of the cell, conversion efficiency and storage capacity were determined in the EDTA–safranine O–NaLS system after modification of the experimental setup. The results are shown in Table 7. The cell fabrication factors, such as chemicals (sensitizer, reductant, surfactant, and alkaline medium), electrodes (Pt, saturated calomel electrode-SCE), diffusion length, illumination intensity, and temperature, affect the cell efficiency for solar energy conversion and storage. Therefore, the efficiency of these cells can be further enhanced by favorably changing all or any of these fabrication parameters. With this background knowledge, the electrode aspect was dealt with in the present study.29 The use of very small Pt with an SCE component of combination electrode in the present study generates a 2600 μA photocurrent, power of 364.7 μW, and efficiency of 8.93%; these values are significantly higher than the 200.0 μA photocurrent, power of 75.2 μW, and efficiency of 0.7213% reported for the EDTA–safranine O–NaLS system.29| EDTA–safranine O–NaLS system | Before modification | After modification | Extent of enhancement | |
|---|---|---|---|---|
| 1 | Dark potential (mV) | 180.0 | 677.0 | |
| 2 | Open circuit voltage Voc (mV) | 1051.0 | 1052.0 | |
| 3 | Photopotential ΔV (mV) | 871.0 | 375.0 | |
| 4 | Maximum photocurrent imax (μA) | 400.0 | 2600.0 | 6.5 times |
| 5 | Short circuit current isc (μA) | 200.0 | 1700.0 | 8.5 times |
| 6 | Potential at power point Vpp (mV) | 682.0 | 521.0 | |
| 7 | Current at power point ipp (μA) | 110 | 700 | 6.36 times |
| 8 | Power of cell i × V (μW) | 75.02 | 364.70 | 4.86 times |
| 9 | Initial generation of current (μA min−1) | 50.0 | 260.0 | 5.2 times |
| 10 | Fill factor (η) | 0.35 | 0.20 | |
| 11 | Conversion efficiency (%) | 0.7213 | 8.93 | 12.38 times |
| 12 | Storage capacity t1/2 (min) | 40 | 40 | |
| 13 | Charging time (min) | 180 | 40 | 4.5 times less |
| 14 | Cost of construction of single cell | Rs. 1596.0 | Rs. 127.6 | <12.5 times less |
The current and power generated in a cell are dependent on the resistance and conductivity of electrodes and test solution. Higher sensitivity and conductivity will enhance the current and power output of the cell, as observed using the reference electrode of combination electrode in photogalvanic cells. The combination electrode incorporates both glass and reference electrode (external and internal) in one body. A glass membrane separates the test solution and inner proton solution contained in combination electrode itself. The glass is tailored for low resistance by varying the amount of alumina (Al2O3) and other common constituents (Na2O, K2O, B2O3, etc.). The observation of a higher result for the combination electrode (with respect to that for single electrode as SCE) may be due to the use of low resistance glass pH electrodes or the use of a reference electrode with a fast, continuous leak rate. When placed in the solution of the cell, these electrodes show improved time response and stability, due to dissolution of the low resistance glass into the low ionic strength solution and the non-quantitative addition of a salt solution from the reference into the solution of cell. Both techniques raise the conductivity. This way, the use of a combination electrode provides tremendous enhancement in current (i.e., 1700 μA in the present study) compared to that (200 μA) in previously reported studies and a drastic cut in the cost of PG based on same chemical photogalvanic system. The use of a combination electrode causes no extra cost as the cost of both SCE and combination electrode is same in the market.33
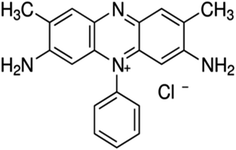
The very high result obtained in the present study may be due to the very small sized Pt with the SCE component of the combination electrode.14,33–36 The photogalvanic cells are diffusion controlled cells; therefore, the small Pt offers less hindrance to the migration of ions leading to a high current and power. The light weight dye molecules (M.W. of 350.84) in this case also facilitate the diffusion. The good power storage capacity (half change time 40 minutes) of the cell in the present study may be due to the presence of a highly extended conjugated pie framework in safranine-O (Fig. 4).
The safranine-O is basically used as a biological stain in histology and cytology, redox indicator in analytical chemistry, and dyeing color in the textile industry. The present study establishes its utility in direct solar conversion and storage in a renewable way through the photogalvanic cells. The photogalvanic cells use a very dilute solution of dye along with other chemicals, such as NaOH, surfactant, and reductant. This means that the dye in an adulterated form is capable of solar power generation and storage. Therefore, the present study also highlights the potential use of industrial effluents (containing safranine-O dye) in solar electricity generation while tackling the problem of pollution caused by dye-based industries.
3.9 Conversion efficiency
With the help of the observed potential and current at power point and the incident power of the illumination, the conversion efficiency of the photogalvanic cell was determined to be 8.93% against the reported value 0.7213%,29 which is enhanced 12.38 fold using eqn (3). The solar conversion data is provided in Table 8.| EDTA–safranine O–NaLS system | In artificial sunlight (10.4 mW cm−2) | In natural sunlight (100 mW cm−2) | |
|---|---|---|---|
| 1 | Open circuit voltage Voc (mV) | 1052.0 | 1060.0 |
| 2 | Maximum photocurrent imax (μA) | 2600.0 | 5720 |
| 3 | Short circuit current isc (μA) | 1700.0 | 3750 |
| 4 | Power of cell i × V (μW) | 364.70 | 1094.1 |
| 5 | Fill factor (η) | 0.20 | 0.20 |
| 6 | Conversion efficiency (%) | 8.93 | 13.67 |
All the output of the photogalvanic cell may be 2–3 times higher in the direct solar radiation depending upon the weather conditions.
The isc does not increase ten times when the irradiance changes about ten times from 10.4 mW cm−2 to 100 mW cm−2. The reason is that the optimal concentration does not increase ten times at ten times intensity as a higher concentration does not allow the light to reach near Pt electrode. A preliminary study on photogalvanics in artificial sun light intensity (10.4 mW cm−2) and natural sun light (∼100 mW cm−2) shows a 2–3 times increase in current and power at 100 mW cm−2 compared to that at 10.4 mW cm−2. On this basis, we estimated solar conversion data (∼2.2 times).
An important point regarding the cell efficiency is the spectrum of the light source used because every wavelength has a spectral irradiance, and reliable conversion efficiencies could be known only at that particular wavelength. In that case, the use of a water filter to cut off the infrared radiation would be more significant.
In this context, we are of the view that the cell should produce a higher electrical output under natural sunlight, irrespective of which wavelength is absorbed. The efficiency at a particular wavelength is insignificant in natural conditions of cell performance because the cell based on dye sensitizers absorbs over a range of wavelengths. The use of filters to obtain a particular wavelength for absorption by cell and to use the water filter in natural sunlight will only complicate the technology and increase the cost of the cell. In view of these facts, the idea of efficiency at average sunlight intensity including all wavelengths is more relevant with special reference to the application of the cell in daily life. We think that the spectral irradiance is basic to know the behavior of PG cell with time. We used the entire electromagnetic sun light spectrum emitted from incandescent bulb as our aim was to obtain higher output with minimum instrumentation.
The photogalvanic cells (PG) reported in this study are similar to photovoltaic cells (PV) as both devices convert sunlight directly to solar power. The PV is a solid phase based device, whereas the PG is a solution phase-based device. The PG has inherent power storage capacity but relatively low efficiency, whereas the PV lacks storage capacity. The PG can be useful as much as the PV with additional advantage of storage capacity if the efficiency of PG is further enhanced.
There are some basic differences between PV and PG cells. In PV, the photo stable semiconductors are used, whereas in PG, photo decaying dye semiconductors are used. Therefore, PV cells give stable electrical output and PG give electrical output that decreases with time.
It should also be noted that very simple PG devices have the potential to supplement other techniques by meeting energy needs in cheap, renewable, and eco-friendly way. The dyes used in PG are already in use in various fields such as tissue staining, and textile. The PG will be relatively eco-friendly (despite use of relatively toxic material like dyes) for the following reasons: (i) very dilute solution (∼10−5 M) of dyes is used; (ii) the dye is photo-decayed during use in the cell and (iii) dye solution inside cell is reusable for recharging during charging–discharging cycles of the cell. Thus, the end disposal of dye in nature after its use and reuse will be relatively safe as its very dilute solution is used and it is already almost completely photo-decayed, leading to the easy and fast degradation in soil. To ensure safer use, these dyes can also be removed from the effluent by an adsorption method.25
3.10 Mechanism
The proposed mechanism for the generation of photocurrent in the photogalvanic cell is described in the following section.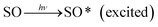 | (4) |
The excited SO molecules accept an electron from the reductant and are converted to the semi or leuco form of SO, and the reductant is converted to its oxidized form:
 | (5) |
At platinum electrode. The semi or leuco form of SO loses an electron and is converted to original SO molecule:
| SO− → SO + e− | (6) |
At counter electrode. The SO molecule accepts an electron from electrode and is converted in semi or leuco form:
| SO + e− → SO− (semi or leuco) | (7) |
Finally the leuco/semi form of SO and oxidized reductant combine to produce original dye and reductant molecule and the cycle will go on:
| SO− + R+ → SO + R | (8) |
4 Conclusion
This study focused on the applied part of a sustainable energy source. The results after the modifications in the experimental set up of the photogalvanic cell were enhanced tremendously compared to those in the previous studies with special reference to the electrical output, maximum power of the cell and conversion efficiency. The cell attained the same storage capacity as its charging time. The initial generation of current in the cell was enhanced fivefold. The cost of cell construction was also reduced to a large extent.Acknowledgements
The authors, particularly Prerna Gangotri is thankful to UGC, New Delhi for award of Post-Doctoral Fellowship with financial assistance and Prof. P. K. Sharma (B), Head, Department of Chemistry for providing all facilities in the Department. Authors are also thankful to Prof. (Dr) Sangeeta Loonkar for fruitful discussion. Dr Gangotri is also thankful to Mentor (Supervisor) Dr Pooran Koli.References
- E. Becquerel, On electron effects under the influence of solar radiation, Comptes Rendues de ‘l’ Academie Sciences, Paris, vol. 9, 1839, pp. 561–567 Search PubMed.
- E. K. Rideal and D. C. Williams, The action of light on the ferrous ferric iodine iodide equilibrium, J. Chem. Soc., 1925, 127, 258–269 RSC.
- E. Rabinowitch, The photogalvanic effect I. The photochemical properties of the thionine-iron system, J. Chem. Phys., 1940, 8, 551–559 CrossRef CAS.
- E. Rabinowitch, The photogalvanic effect II. The photogalvanic properties of the thionine-iron system, J. Phys. Chem., 1940, 8, 560–566 CrossRef CAS.
- M. Kaneko and A. Yamada, Photopotential and photocurrent induced by tolusafranine ethylenediaminetetraacetic acid system, J. Phys. Chem., 1977, 81, 1213–1219 CrossRef CAS.
- A. S. N. Murthy, H. C. Dak and K. S. Reddy, Photogalvanic effect in riboflavin-ethylenediamineteraacetic acid system, Int. J. Energy Res., 1980, 4, 339–343 CrossRef CAS.
- K. K. Rohatgi-Mukherjee, M. Roy and B. B. Bhowmik, Photovoltage generation of the Phenosafranine dye-EDTA Sandwich Cell, Sol. Energy, 1983, 31, 417–418 CrossRef CAS.
- O. Alfredo, P. Georgina and P. J. Sebasteian, Electron transfer via organic dye for solar energy conversion, Sol. Energy Mater. Sol. Cells, 1990, 59, 137–143 Search PubMed.
- S. Dube, S. L. Sharma and S. C. Ameta, Photogalvanic Effect in Azur B-NTA System, Energy Convers. Manage., 1977, 38, 101–106 CrossRef.
- S. Madhwani, J. Vardia, P. B. Punjabi and V. K. Sharma, Use of fuchsine basic: ethylenediaminetetraactic acid system in photogalvanic cell for solar energy conversion, Proc. Inst. Mech. Eng., Part A, 2007, 221, 33–39 CrossRef CAS.
- K. M. Gangotri and M. K. Bhimwal, The Photochemical Conversion of Solar Energy into Electrical Energy: Eosin-D-xylose-System, Energy Sources, Part A, 2011, 33, 2058–2066 CrossRef CAS.
- I. S. Bayer, I. Eroglu and L. Turker, Photogalvanic effect in aqueous methylene blue nickel mesh systems: conversion of light into electricity, Int. J. Energy Res., 2001, 25, 207–222 CrossRef CAS.
- K. K. Bhati and K. M. Gangotri, Photogalvanic Conversion of Solar Energy into Electrical Energy by using NaLS-Xylose-Methylene Blue System, Int. J. Electr. Power Energ. Syst. Eng., 2011, 33, 3028–3035 Search PubMed.
- P. Koli, U. Sharma and K. M. Gangotri, Solar Energy Conversion and Storage: Rhodamine B–Fructose Photogalvanic Cell, Renewable Energy, 2012, 37, 250–258 CrossRef CAS.
- A. K. Jana and B. B. Bhowmik, Enhancement in power output of solar cell consisting of mixed dye, J. Photochem. Photobiol., A, 1977, 110, 41–46 CrossRef.
- K. M. Gangotri and C. Lal, Use of mixed dyes in a photogalvanic cell for solar energy conversion and storage: EDTA-methylene blue-thionine system, Int. J. Energy Syst., 2005, 219, 315–320 CAS.
- C. Lal and S. Yadav, Use of mixed dyes in photogalvanic cell for solar energy conversion and storage: toluidine-Azure-B system, Int. J. Energy Syst., 2007, 164, 926–930 CAS.
- P. Koli, Photogalvanic cells: comparative study of various synthetic dye and natural photo sensitizer present in spinach extract, RSC Adv., 2014, 4, 46194–46202 RSC.
- K. R. Genwa, A. Kumar and A. Sonel, Photogalvanic solar energy conversion: study with photosensitizers toluidine blue and malachite green in presence of NaLS, Appl. Energy, 2009, 86, 1431–1436 CrossRef CAS.
- H. Shiroishi, Y. Kaburagi, M. Seo, T. Hoshi, T. Nomura, S. Tokita and M. Kaneko, Virtual device simulator of bipolar photogalvanic cell, J. Chem. Software, 2002, 8, 47–54 CrossRef CAS.
- Bisquert, D. Cohen, G. Hodes, S. Riiwe and A. Zaben, Physical Chemical Principles of Photovoltaic Conversion with Nanoparticles, Mesoporous Dye-Sensitized Solar Cells, J. Phys. Chem. B, 2004, 108, 8106–8118 CrossRef CAS.
- K. M. Gangotri and V. Indora, Studies in Photogalvanic Effect in Mixed Reductants System for Solar Energy Conversion and Storage: Dextrose and EDTA-Azur A System, Sol. Energy, 2010, 84, 271–276 CrossRef CAS.
- S. Pramila and K. M. Gangotri, Use of anionic micelles in photogalvanic cells for solar energy conversion and storage: DSS-mannitol-safranine system, Energy Sources, Part A, 2007, 29, 1253–1257 CrossRef CAS.
- P. Koli, Solar energy conversion and storage: fast green FCF-fructose photogalvanic cell, Appl. Energy, 2014, 118, 231–237 CrossRef CAS.
- P. Koli and U. Sharma, Photochemical solar power and storage through photogalvanic cells: comparing performance of dye materials, Energy Sources, Part A, 2017, 39, 555–561 CrossRef CAS.
- P. Gangotri and K. M. Gangotri, Studies of the micellar effect on photogalvanics: solar energy conversion and storage in EDTA-safranine O-DSS system, Int. J. Energy Res., 2010, 34, 1155–1163 CrossRef CAS.
- P. Gangotri and K. M. Gangotri, Studies of the micellar effect on photogalvanics: solar energy conversion and storage in EDTA-safranine O-CTAB system, Arabian J. Sci. Eng., 2010, 35, 19–28 CAS.
- P. Gangotri and K. M. Gangotri, Studies of the micellar effect on photogalvanics: solar energy conversion and storage in EDTA-safranine O-Tween-80 system, Energy Fuels, 2009, 23, 2767–2772 CrossRef CAS.
- P. Gangotri and K. M. Gangotri, Studies of the micellar effect on photogalvanics: solar energy conversion and storage in EDTA-safranine O-NaLS system, Energy Sources, Part A, 2013, 35, 1007–1016 CrossRef CAS.
- N. R. Neniwal and K. M. Gangotri, A Study of the Effect of a Surfactant in a Photosensitizer for Solar Energy Conversion and Storage, Energy Sources, Part A, 2014, 36, 1484–1490 CrossRef.
- S. C. Ameta, S. Khamesra, A. K. Chittora and K. M. Gangotri, Use of Sodium of Lauryl Sulphate in a Photogalvanic Cell for Solar Energy Conversion and Storage Methylene Blue–EDTA System, Int. J. Energy Res., 1989, 13, 643–647 CrossRef CAS.
- J. Yu, J. Fan and L. Zhao, Dye Sensitized Solar Cells based on Hollow Anatase TiO2 spheres prepared by Self-Transformation method, Electrochim. Acta, 2010, 55, 597–602 CrossRef CAS.
- https://tools.thermofisher.com/content/sfs/brochures/AN-PHLOWION-E%200914-RevA-WEB.pdf, retrieved on 23 March, 2017.
- P. Koli, “Surfactant and natural sunlight enhanced Photogalvanic effect of Sudan I dye”, Arabian J. Chem., 2014 DOI:10.1016/j.arabjc.2014.11.061.
- P. Koli, Study of enhanced photogalvanic effect of Naphthol Green B in natural sunlight, J. Power Sources, 2015, 285, 310–317 CrossRef CAS.
- P. Koli, Solar energy conversion and storage using Naphthol Green B dye photosensitizer in photogalvanic cells, Appl. Sol. Energy, 2014, 50, 67–73 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |