Enhanced photocatalytic hydrogen generation using carbazole-based sensitizers†
Norberto
Manfredi
 *a,
Matteo
Monai
b,
Tiziano
Montini
b,
Matteo
Salamone
a,
Riccardo
Ruffo
a,
Paolo
Fornasiero
*a,
Matteo
Monai
b,
Tiziano
Montini
b,
Matteo
Salamone
a,
Riccardo
Ruffo
a,
Paolo
Fornasiero
 *b and
Alessandro
Abbotto
*b and
Alessandro
Abbotto
 *a
*a
aDepartment of Materials Science, Solar Energy Research Center MIB-SOLAR, University of Milano-Bicocca, INSTM Milano-Bicocca Research Unit, Via Cozzi 55, I-20125, Milano, Italy. E-mail: norberto.manfredi@unimib.it; alessandro.abbotto@unimib.it
bDepartment of Chemical and Pharmaceutical Sciences, INSTM Trieste Research Unit, ICCOM-CNR Trieste Research Unit, University of Trieste, via L. Giorgieri 1, I-34127, Trieste, Italy. E-mail: pfornasiero@units.it
First published on 6th April 2017
Abstract
Phenothiazine-, phenoxazine- and carbazole-based dyes have been synthesized and used as photosensitizers in Pt/TiO2 films for photocatalytic hydrogen generation. Compared to commonly used phenothiazine dyes, planar and sulphur-free carbazole derivatives showed different molecular and supramolecular features which in turn yielded greatly enhanced (one order of magnitude) H2 production performances.
The growing needs of present and future society concerning energy and sources for industrial chemical intermediates involve the search for sustainable technologies for production of fuels and chemicals.1,2 In this scenario, hydrogen, required for many industrial chemical processes and one of the possible energy vectors of the future, can be generated from renewable and cheap sources. The challenging photocatalytic water splitting3–5 suffers from poor efficiency.6 Ideally, this limitation can be partially overcome by enhancing visible absorption.7 In this respect, many approaches have been rather extensively exploited, ranging from homogeneous catalysis8–10 to new generation semiconductor oxides11 and composite nanomaterials.12–14 Dye-sensitized photocatalysis is an alternatively and less investigated strategy to increase visible light absorption.15–20 The photocatalytic scheme implies the use of a tandem system with two components: a redox storing catalysts (such as the benchmark Pt/TiO2) and a sensitizer capable of absorbing a broad part of the solar spectrum and of transferring electrons to the catalysts. The photosensitizer must also be stable under the working conditions under continuous irradiation over long periods of time. Thus, a rational design of the molecular structure of the dye must be developed to improve the stability and performances of sensitized photocatalysts. Various studies on dye-sensitized hydrogen generation involve the use of more traditional organometallic dyes.21 Only recently, the focus has been moving to metal-free sensitizers, which have the advantages of being cheaper, free of rare and toxic metals, and endowed with a greater variety of structural and optical properties.15,18,22–26 Organic sensitizers are typically constituted by a donor–π–acceptor architecture. In such donor–acceptor dyes the HOMO is located in the donor core and the LUMO in the acceptor end-group. Upon irradiation, an electron is promoted from the HOMO to the LUMO of the dye, corresponding to an intramolecular charge transfer (ICT) transition. Once in its excited state, electron transfer from the LUMO of the sensitizer to the conduction band of TiO2 takes place, thus triggering the photocatalytic cycle.15,27
We have recently reported a series of branched donor–(π–acceptor)2 phenothiazine (PTZ)-based sensitizers functionalized with an alkyl terminal and containing different thiophene-based spacers,28 showing lower activity but improved stability with respect to the reference system without the thiophene spacer. This fact has been related to the electronic and steric effect of the thiophene spacer, favouring charge separation after light excitation of the donor group (phenothiazine) but probably inducing sulphur poisoning phenomena on the surface of the Pt nanoparticles.
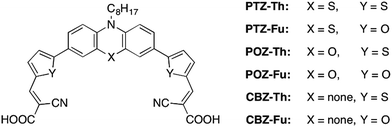
In this work, the effect of molecular design of the dye on the photocatalytic hydrogen generation is investigated by sequentially removing sulphur-based units from the chemical structure of the dye. In particular, new sensitizers have been prepared by replacing the PTZ donor core with a sulphur-free electron-rich heteroaromatic ring and replacing the thiophene spacers with a sulphur-free heteroaromatic analogue. The introduction of the carbazole (CBZ) moiety in the molecular structure afforded a strong enhancement of the efficiency of photocatalytic H2 production compared to both PTZ and phenoxazine (POZ) based dyes, using Pt/TiO2 as the benchmark redox storing catalyst. The amount of produced hydrogen and turnover numbers (TONs) are top-ranked amongst studies on dye-sensitized photocatalytic hydrogen production.
The CBZ building block has been selected as an ideal sulphur-free alternative to PTZ, being a strong electron-rich heteroaromatic ring thanks to the presence of the central five-membered ring containing a pyrrole-like nitrogen atom.29 Despite the fact that CBZ is highly investigated for its applications in materials science,30–33 and in dye-sensitized solar cells (DSSCs),27,34–36 only a very few studies on photocatalysis have been reported.37–39 Remarkably, whereas PTZ is associated with a typical butterfly structure,40,41CBZ is planar, suggesting a strong effect of the charge generation and transport properties on the catalytic efficiency of the tandem system. In order to separate the effects of the replacement of the S atom and of the change in the spatial arrangement, we have also investigated POZ donor groups. Within the scope of this work, POZ has the advantage of maintaining the butterfly structure as in PTZ, permitting better elucidation of the role of the CBZ unit.
To investigate the role of S in the spacer unit, we have designed new dyes by replacing the thiophene (Th) π-bridge with its sulphur-free five-membered analogue furan ring (Fu), which shares with thiophene many electronic and structural properties with the exception of the nature of the ring heteroatom.29 The structures of new dyes are summarized in Fig. 1, PTZ-Th being the reference system.28
PTZ-Th has been synthesized according to the literature.28 The same synthetic scheme (Scheme S1†) has been adopted as a simple and easily extendible procedure for synthesis of the new dyes. The development of a general procedure (see ESI†) is not only relevant for industrial scale-up but also to easily access an extended library of dyes. Although the synthesis of CBZ-Th and a molecule similar to CBZ-Fu (bearing a different N-alkyl functionalization) has been previously reported in the literature,35,42 the CBZ-based sensitizers were synthesized by the aforementioned general approach using 3,6-dibromocarbazole as a starting reagent while POZ derivatives were synthesized from 3,7-dibromo-10-octyl-10H-phenoxazine.43Fu and Th derivatives have been prepared connecting the donor and the spacer units by a Suzuki cross-coupling reaction using the commercially available 2-furan-aldehyde-5-boronic acid and 2-thiophene-aldehyde-5-boronic acid, respectively.
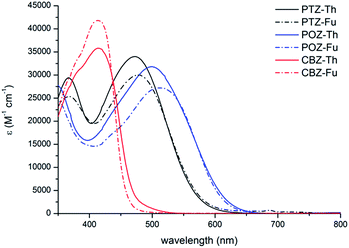
The absorption spectra of the dyes (10−5 M in DMSO) are shown in Fig. 2 and the detailed main optical and energetic parameters (HOMO–LUMO energies) are listed in Table S1.† In general, the three families of PTZ, POZ and CBZ dyes exhibited the typical 2 bands, related to the π–π* absorption in the 300–450 nm range and to the ICT transition in the 400–600 nm range.35,44 The π–π* band shows a progressive red-shift in the order POZ–PTZ–CBZ according to an increased electron delocalization in the core. On the other hand, the ICT transition is subjected to a progressive blue-shift. As a result, π–π* and ICT transitions significantly overlap for the CBZ derivatives. The absorption maxima are centered around ca. 470 nm for PTZ, ca. 410 nm for CBZ and ca. 530 nm for the POZ dyes. Importantly, the maximum molar absorptivity is higher for the CBZ derivatives, whereas marginal differences (less than 10%) are recorded between the PTZ and POZ analogs. Finally, the introduction of the Fu spacer in place of Th did not significantly affect the absorption properties.
The electrochemical properties have been investigated for all the dyes and the main optical and electrochemical parameters are summarized in Table S1 (see ESI†). Cyclic voltammetry (CV) profiles (Fig. S1, ESI†) showed a quasi-reversible behavior for the oxidation process in all the investigated dyes, whereas reduction was irreversible. Differential Pulsed Voltammetry (DPV) results (Fig. S2, ESI†) were used to determine the HOMO energy levels from the current peak.45 The LUMO levels have been derived from electrochemical HOMO values and optical bandgaps, measured by means of Tauc plots.46 Levels are pictorially shown in Fig. S3 (see ESI†) and reported in Table S1 (see ESI†). Even though the HOMO energy levels are quite similar for most of the dyes (∼−5.60 eV), the different bandgaps significantly affect their LUMO energies and, accordingly, the electron injection capabilities to the Pt/TiO2 system. In particular, the LUMO energy of the POZ dyes, as well as that of PTZ-Fu, is very close to the conduction band (CB) of TiO2 (−4.0 eV).47
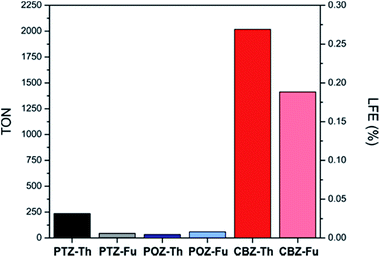
The sensitized Pt/TiO2 photocatalysts were tested for H2 production under visible light irradiation (λ > 420 nm) from a triethanolamine (TEOA)/HCl aqueous buffer solution at pH = 7.0. The experiments have been performed adopting the same conditions previously optimized for PTZ-based photocatalysts.48,49 No H2 production was observed while using the bare Pt/TiO2 under the same experimental conditions. The measured H2 production rates and H2 productivity vs. the irradiation time are presented in Fig. S4 and S5 (see ESI†), respectively. Turnover numbers (TONs) and Light-to-Fuel Efficiency (LFE20) calculated after 20 h of irradiation are presented in Fig. 3 (obtained values are listed in Table S2, ESI†).
All the investigated catalysts showed remarkable stability over a reasonable irradiation time of 20 h (Fig. S4, ESI†). CBZ-sensitized photocatalysts showed by far the highest H2 productivities, TON and LFE values. Namely, performances were at least one order of magnitude higher than those referred to the benchmark PTZ-Th dye. Amongst CBZ based dyes, the photocatalytic activity of CBZ-Th was considerably higher than that of CBZ-Fu. The same relative trend was recorded for the PTZ family. Both POZ-sensitized photocatalysts demonstrated very low activity in H2 production.
In order to evaluate the role of the carbazole core by itself, excluding the effect of the carbazole-based branched donor–(π–acceptor)2 structure in the photocatalytic activity, a control experiment using N-octylcarbazole, and a mixture of N-octylcarbazole and thiophene, has been performed. As expected by their limited π conjugated framework length and low light absorption, because of the lack of an intense ICT transition, no photoinduced hydrogen generation was detected, thus excluding a strategic role of the carbazole, or thiophene, by themselves. Finally, to support electron injection from the dye excited state to the CB of TiO2 we have compared the emission of the CBZ-Th dye in solution (3 × 10−6 M DMSO) and adsorbed onto a film of the semiconductor (5 μm). Whereas the DMSO solution showed an appreciable emission, no signal was recorded in the case of the TiO2 film (as for the bare TiO2 film without dye sensitization), thus suggesting that in this case the electron transfer mechanism is operating (Fig. S6, ESI†).
Such an enhancement can be rationalized in terms of the planar spatial arrangement of the CBZ donor group, which in turn positively affects the charge generation, transport properties, and catalytic efficiency of the tandem system. CBZ-Th showed a red-shifted visible light absorption (up to 600 nm) when adsorbed on the surface of TiO2, as well as a broadening of the absorption spectrum (Fig. S7, ESI†) after a long staining time, according to previous observations for analogous derivatives.35 These data might suggest the occurrence of some kind of self-organization on the surface of the semiconductor induced by the planar structure of the CBZ donor core. A similar behaviour has not been previously reported for corresponding similar phenothiazine and phenoxazine derivatives.50 Such different arrangements of photosensitizer molecules on the surface of the semiconductor film may provide one of the main reasons for the different observed spectroscopic and photocatalytic properties of the CBZ derivatives. To better investigate this phenomenon, the absorption spectrum of CBZ-Th was recorded in the presence of chenodeoxycholic acid (CDCA), a widely used co-adsorbing and disaggregating agent (Fig. S8, ESI†).51 The results revealed that the presence of CDCA, even in a relatively high quantity, does not affect the optical properties of the adsorbed dyes. Accordingly, the photocatalytic activity in the presence of CDCA gave similar results to that without the co-adsorbent (Fig. S9, ESI†). This is a clear suggestion that the role of supramolecular aggregates, if present, is not predominant and thus is not likely the main cause of the recorded enhanced photocatalytic performance.
In addition to the above discussed dye-sensitization mechanism, the improved photocatalytic performance can originate from a ligand-to-metal charge transfer (LMCT) phenomenon.52,53 The LMCT mechanism is observed when charge transfer takes place after formation of complexes between TiO2 and the surface adsorbates. LMCT species absorb in the visible range whereas neither TiO2 nor the organic adsorbates typically absorb visible light. Upon coupling with TiO2, electron-rich organic compounds with linker groups (e.g., catechol, carrying two ortho OH anchoring groups) exhibit a LMCT band in the visible region, as a result of the strong coupling between the molecular orbital (HOMO) of the adsorbate and the energy band of the semiconductor. In this case, the absorption of a photon allows the excitation of an electron directly from the ground state (HOMO level) of the adsorbate (ligand) to the semiconductor CB with mainly metal orbital characters, without involving the excited state of the adsorbate. This mechanism has been scantly investigated in DSSCs in the presence of a simple catechol-based molecule resulting in a modest increase of the power conversion efficiency.54,55 Since the peculiar feature of the LMCT is the formation of a band around 600 nm, in highly π-conjugated molecules it is more difficult to distinguish the presence of the LMCT band in the presence of the visible ICT band of common dyes employed in TiO2 sensitization. In the case of LMCT, the nature of the sensitizers can drastically affect the back electron transfer from TiO2 to the oxidized sensitizer. Furthermore, it was shown that a large portion (>75%) of charge recombination occurs within a few picoseconds in the LMCT sensitization.54 Based on these considerations, it is difficult to unequivocally prove the presence of the LMCT mechanism in the present CBZ dyes, though the presence of an absorption tail in the 600 nm region may suggest that. We argue that the combined effects of different surface molecular arrangements and LMCT are responsible for the increased efficiency of CBZ dyes, as a result of a more efficient light harvesting. To investigate the presence of a similar mechanism in the PTZ and POZ dyes, H2 productivity has been checked using a cut-off filter at 515 nm (Fig. S10, ESI†). After activation of the photocatalysts for 8 h under the usual conditions, CBZ-Th still shows an appreciable photocatalytic activity whereas H2 production completely disappeared for PTZ-Th, despite its intense light absorption up to 600 nm. This fact suggests that in the latter dye the longer wavelength band is very less efficient in promoting the photocatalytic H2 production. Taking this into account, the low activity observed under irradiation with λ > 420 nm using PTZ and POZ dyes as sensitizers (Fig. 3) could be related to the lower efficiency of the π–π* transition in the photocatalytic cycle.
The long term stability of the CBZ-Th based material has been investigated under irradiation with λ > 420 nm (500 W m−2 at 50 °C) while adsorbed on a 3 μm transparent TiO2 film and under photocatalytic conditions. The absorption of the dye-stained film dropped by ca. 6% after 24 h (Fig. S11, ESI†). This result is in agreement with an analogous decrease of the aforementioned gas production rate after 20 h (Fig. S9, ESI†). We point out that such irradiation times should be compared with the much shorter periods (4–5 h) routinely reported in the literature for similar photocatalytic studies.22,23,37,56
In conclusion, a rational design of the sensitizers was shown to be the key to obtain greatly enhanced performances in H2 photogeneration under visible light irradiation. The presence of heteroatoms in the donor group and spacer moieties of the investigated sensitizers dramatically affects the photocatalytic H2 production in the visible range over Pt/TiO2. In particular, the replacement of butterfly-like phenothiazine derivatives with planar, sulphur-free carbazole dyes provided different molecular and supramolecular properties. These features in turn afforded greatly enhanced (one order of magnitude) dye-sensitized hydrogen generation performances, amongst the best ever reported in the literature for photocatalytic systems based on organic photosensitizers.15
Acknowledgements
The University of Milano-Bicocca, University of Trieste (through project FRA 2015), Beneficentia Stiftung, ICCOM-CNR and INSTM are gratefully acknowledged for financial support.Notes and references
- N. Armaroli and V. Balzani, in Energy for a Sustainable World, Wiley-VCH Verlag GmbH & Co. KGaA, 2010 Search PubMed.
- G. A. Ozin, Energy Environ. Sci., 2015, 8, 1682–1684 CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- K. Kalyanasundaram and M. Graetzel, Curr. Opin. Biotechnol., 2010, 21, 298–310 CrossRef CAS PubMed.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Nat. Photonics, 2012, 6, 511–518 CrossRef CAS.
- N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21–36 CrossRef CAS PubMed.
- V. Balzani, A. Credi and M. Venturi, ChemSusChem, 2008, 1, 26–58 CrossRef CAS PubMed.
- T. M. McCormick, B. D. Calitree, A. Orchard, N. D. Kraut, F. V. Bright, M. R. Detty and R. Eisenberg, J. Am. Chem. Soc., 2010, 132, 15480–15483 CrossRef CAS PubMed.
- P. Du, K. Knowles and R. Eisenberg, J. Am. Chem. Soc., 2008, 130, 12576–12577 CrossRef CAS PubMed.
- S. Losse, J. G. Vos and S. Rau, Coord. Chem. Rev., 2010, 254, 2492–2504 CrossRef CAS.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- W. Fan, Q. Zhang and Y. Wang, Phys. Chem. Chem. Phys., 2013, 15, 2632–2649 RSC.
- A. Beltram, M. Melchionna, T. Montini, L. Nasi, P. Fornasiero and M. Prato, Green Chem., 2017 10.1039/c6gc01979j.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- B. Cecconi, N. Manfredi, T. Montini, P. Fornasiero and A. Abbotto, Eur. J. Org. Chem., 2016, 2016, 5194–5215 CrossRef CAS.
- X. H. Zhang, T. Y. Peng and S. S. Song, J. Mater. Chem. A, 2016, 4, 2365–2402 CAS.
- X. Zhang, L. Yu, C. Zhuang, T. Peng, R. Li and X. Li, ACS Catal., 2014, 4, 162–170 CrossRef CAS.
- M. Watanabe, H. Hagiwara, A. Iribe, Y. Ogata, K. Shiomi, A. Staykov, S. Ida, K. Tanaka and T. Ishihara, J. Mater. Chem. A, 2014, 2, 12952–12961 CAS.
- F. Li, K. Fan, B. Xu, E. Gabrielsson, Q. Daniel, L. Li and L. Sun, J. Am. Chem. Soc., 2015, 137, 9153–9159 CrossRef CAS PubMed.
- K. A. Click, D. R. Beauchamp, Z. Huang, W. Chen and Y. Wu, J. Am. Chem. Soc., 2016, 138, 1174–1179 CrossRef CAS PubMed.
- Z. Yu, F. Li and L. Sun, Energy Environ. Sci., 2015, 8, 760–775 CAS.
- X. Li, S. C. Cui, D. Wang, Y. Zhou, H. Zhou, Y. Hu, J. G. Liu, Y. T. Long, W. J. Wu, J. L. Hua and H. Tian, ChemSusChem, 2014, 7, 2879–2888 CrossRef CAS PubMed.
- L. Yu, X. Zhang, C. Zhuang, L. Lin, R. Li and T. Peng, Phys. Chem. Chem. Phys., 2014, 16, 4106–4114 RSC.
- N. Queyriaux, N. Kaeffer, A. Morozan, M. Chavarot-Kerlidou and V. Artero, J. Photochem. Photobiol., C, 2015, 25, 90–105 CrossRef CAS.
- F. Ronconi, Z. Syrgiannis, A. Bonasera, M. Prato, R. Argazzi, S. Caramori, V. Cristino and C. A. Bignozzi, J. Am. Chem. Soc., 2015, 137, 4630–4633 CrossRef CAS PubMed.
- N. Kaeffer, J. Massin, C. Lebrun, O. Renault, M. Chavarot-Kerlidou and V. Artero, J. Am. Chem. Soc., 2016, 138, 12308–12311 CrossRef CAS PubMed.
- N. Manfredi, B. Cecconi and A. Abbotto, Eur. J. Org. Chem., 2014, 2014, 7069–7086 CrossRef CAS.
- B. Cecconi, N. Manfredi, R. Ruffo, T. Montini, I. Romero-Ocana, P. Fornasiero and A. Abbotto, ChemSusChem, 2015, 8, 4216–4228 CrossRef CAS PubMed.
- A. R. Katritzky and C. W. Rees, Comprehensive Heterocyclic Chemistry, Pergamon, Oxford, 1984 Search PubMed.
- A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193–3328 CrossRef CAS PubMed.
- G. Sathiyan, E. K. T. Sivakumar, R. Ganesamoorthy, R. Thangamuthu and P. Sakthivel, Tetrahedron Lett., 2016, 57, 243–252 CrossRef CAS.
- J. Li and A. C. Grimsdale, Chem. Soc. Rev., 2010, 39, 2399–2410 RSC.
- H. Jiang, Asian J. Org. Chem., 2014, 3, 102–112 CrossRef CAS.
- C. Chen, J.-Y. Liao, Z. Chi, B. Xu, X. Zhang, D.-B. Kuang, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem., 2012, 22, 8994–9005 RSC.
- K. S. V. Gupta, T. Suresh, S. P. Singh, A. Islam, L. Han and M. Chandrasekharam, Org. Electron., 2014, 15, 266–275 CrossRef CAS.
- S. Pramjit, U. Eiamprasert, P. Surawatanawong, P. Lertturongchai and S. Kiatisevi, J. Photochem. Photobiol., A, 2015, 296, 1–10 CrossRef CAS.
- R. Abe, K. Shinmei, N. Koumura, K. Hara and B. Ohtani, J. Am. Chem. Soc., 2013, 135, 16872–16884 CrossRef CAS PubMed.
- R. S. Sprick, B. Bonillo, R. Clowes, P. Guiglion, N. J. Brownbill, B. J. Slater, F. Blanc, M. A. Zwijnenburg, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2016, 55, 1792–1796 CrossRef CAS PubMed.
- C. Su, R. Tandiana, B. Tian, A. Sengupta, W. Tang, J. Su and K. P. Loh, ACS Catal., 2016, 6, 3594–3599 CrossRef CAS.
- J. McDowell, Acta Crystallogr., Sect. B: Struct. Sci., 1976, 32, 5–10 CrossRef.
- S. S. Park, Y. S. Won, Y. C. Choi and J. H. Kim, Energy Fuels, 2009, 23, 3732–3736 CrossRef CAS.
- R. Grisorio, L. De Marco, G. Allegretta, R. Giannuzzi, G. P. Suranna, M. Manca, P. Mastrorilli and G. Gigli, Dyes Pigm., 2013, 98, 221–231 CrossRef CAS.
- J. Soloducho, J. Doskocz, A. Nowakowska, J. Cabaj, M. Lapkowski and S. Golba, Pol. J. Chem., 2007, 81, 2001–2012 CAS.
- X. Liu, J. Long, G. Wang, Y. Pei, B. Zhao and S. Tan, Dyes Pigm., 2015, 121, 118–127 CrossRef CAS.
- K. Izutsu, in Electrochemistry in Nonaqueous Solutions, Wiley-VCH Verlag GmbH & Co. KGaA, 2009 Search PubMed.
- J. Tauc, Mater. Res. Bull., 1968, 3, 37–46 CrossRef CAS.
- Dye Sensitized Solar Cells, ed. K. Kalyanasundaram, CRC Press, Boca Raton, FL, USA, 2010 Search PubMed.
- H. Kisch and D. Bahnemann, J. Phys. Chem. Lett., 2015, 6, 1907–1910 CrossRef CAS PubMed.
- N. Manfredi, B. Cecconi, V. Calabrese, A. Minotti, F. Peri, R. Ruffo, M. Monai, I. Romero-Ocana, T. Montini, P. Fornasiero and A. Abbotto, Chem. Commun., 2016, 52, 6977–6980 RSC.
- W.-I. Hung, Y.-Y. Liao, C.-Y. Hsu, H.-H. Chou, T.-H. Lee, W.-S. Kao and J. T. Lin, Chem.–Asian J., 2014, 9, 357–366 CrossRef CAS PubMed.
- K.-M. Lee, C.-Y. Chen, S.-J. Wu, S.-C. Chen and C.-G. Wu, Sol. Energy Mater. Sol. Cells, 2013, 108, 70–77 CrossRef CAS.
- H. Park, H.-I. Kim, G.-h. Moon and W. Choi, Energy Environ. Sci., 2016, 9, 411–433 CAS.
- G. Zhang, G. Kim and W. Choi, Energy Environ. Sci., 2014, 7, 954–966 CAS.
- Y. Ooyama, M. Kanda, K. Uenaka and J. Ohshita, ChemPhysChem, 2015, 16, 3049–3057 CrossRef CAS PubMed.
- E. L. Tae, S. H. Lee, J. K. Lee, S. S. Yoo, E. J. Kang and K. B. Yoon, J. Phys. Chem. B, 2005, 109, 22513–22522 CrossRef CAS PubMed.
- S. H. Lee, Y. Park, K. R. Wee, H. J. Son, D. W. Cho, C. Pac, W. Choi and S. O. Kang, Org. Lett., 2010, 12, 460–463 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic scheme and procedures, optical and electrochemical parameters, CV, DPV, overall activity results, 1H-NMR and 13C-NMR. See DOI: 10.1039/c7se00075h |
| This journal is © The Royal Society of Chemistry 2017 |