Recent developments in tetrathiafulvalene and dithiafulvalene based metal-free organic sensitizers for dye-sensitized solar cells: a mini-review†
Naresh
Duvva
,
Ushasri
Chilakamarthi
and
Lingamallu
Giribabu
 *
*
Inorganic & Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, CSIR-Network Institutes for Solar Energy (CSIR-NISE), Tarnaka, Hyderabad 500007, India. E-mail: giribabu@iict.res.in; Fax: +91-40-27160921
First published on 13th April 2017
Abstract
Currently, there is a great need for green and clean energy sources to meet the needs of the ever-increasing population in the world. Consequently, scientists across the globe are paying much attention to the development of low cost and high performance renewable energy devices. Dye-sensitized solar cells (DSSCs) are the front-runners among new solar cell technologies owing to their low production cost and high efficiency. Dyes (sensitizers) are one of the essential components of DSSC devices in which the widely used sensitizers are Ru(II) polypyridyl complexes. Despite the fact that Ru(II) polypyridyl complexes show high efficiency, they have limitations due to technical constrains. Hence, research has been accelerated in the design and synthesis of various dyes based on non-ruthenium metal complexes, porphyrins, phthalocyanines and metal-free organic compounds. In recent years, tetrathiafulvalenes (TTFs), dithiafulvalenes (DTFs) and their derivatives have been found to be best alternatives to Ru(II) polypyridyl complexes based on their easy synthesis, and electronic and thermal properties. In this review, we summarize the recent progress in metal-free organic dyes using TTF and DTF scaffolds for dye-sensitized solar cells. The physical properties of devices can be tuned via the strategic design of sensitizers, which in turn help in increasing the performance of the devices. Herein, special attention is paid to correlate the structure activity relationship of the components of D–π–A systems to gain insight into the efficient design strategies.
1. Introduction
Currently, the ever-increasing global energy demand is one of the biggest challenges in the world. The International Energy Agency (IEA) has also identified this problem and hence framed policies on renewable sources and energy efficiency. The World Energy Outlook 2015 released by the IEA tracks the energy use and efficiency policies around the world and the ways in which product design, recycling and reuse (material efficiency) can contribute to saving energy. Therefore, it is imperative to develop new technologies that are economically feasible and environmentally friendly.1 In the present scenario, sun light is the most important renewable energy source on earth among many other renewable energy sources. The energy from the sun is vast and exploiting solar energy is one of the best solutions to meet the global energy demands. Solar cells (also called photovoltaic cells) are solid-state electrical devices that convert solar energy into a usable energy form. Therefore, solar cells have become the focus of attention of many industries and research for developing high efficiency photovoltaic devices has been accelerated.Solar cells are broadly divided into three generations.2 The first and second generation photovoltaic cells are based on silicon and thin film technology using amorphous silicon (a-Si), CdeTe, GaAs, GaInAs, etc.3–5 The first two generations of solar cells are highly efficient, but their applications are limited due to the expensive or hazardous nature of the materials used. Therefore, considering the necessity for trapping solar energy and converting it into electric energy, excitonic solar cells, which are known as third generation solar cells, have been paid immense attention. Dye-sensitized solar cells. (DSSC) and organic/polymer solar cells belong to the third generation solar cells. Of these, DSSC technology is at the verge of commercialization. DSSC devices are easy to fabricate, optically transparent and are potentially much cheaper per watt output compared to SiO2 based photovoltaics. Grätzel et al.7 first reported a DSSC in 1991 whose concept was similar to the natural photosynthetic process with an efficiency of 7.1%. Since then, research in this area has been intensified for the development of competent materials with improved durability and efficiency for the device.6 The working principle and device architecture are shown in Fig. 1.7,8 Among the various components of the device, the sensitizer plays a vital role in achieving high efficiency and durability of the device. The most successful charge transfer sensitizers employed thus far in DSSC are cis-dithiocyanatobis-(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) (together with its various protonated forms), its modified forms (N3 and N719) and trithiocyanato-4,4′4′′-tricaboxy-2,2′,6′,2′′-terpyridine ruthenium(II) (the black dye) with certified conversion efficiencies greater than 11% under air mass (AM) 1.5 solar conditions using liquid redox electrolytes.6–10 Although, Ru(II) polypyridyl complexes have shown very good efficiency, their commercial use is not feasible due to their cost, which is mainly because of the rarity of ruthenium in the earth's crust, and also tedious synthetic protocols. In order to improve the efficiency of the device, the absorption of the sensitizer has to be improved in the red region of the visible spectrum with high molar extinction coefficients above 600 nm. However, Ru(II) polypyridyl complexes lack these optical properties particularly in near-IR region of the absorption spectrum. For this reason, great efforts have been devoted to replace ruthenium dyes with organic dye molecules owing to their advantages such as cost effectiveness, high absorption coefficients, and thermal and photochemical stability.9–13 Among the approaches used for organic dye based sensitizers, the most efficient approach was based on an electron-donor (D) and an electron-acceptor (A) unit linked through a π-bridge, which resulted in a broad and intense optical absorption band in the visible spectral region due to effective intramolecular charge transfer (ICT) from the D to A units. Several organic dyes have been developed based on the D–π–A approach for DSSCs with good efficiencies using coumarin,14,15 indoline,16–19 triarylamine,20–24 carbazole,25–27 and diketopyrrolopyrrole (DPP)28,29 scaffolds. To date, the best DSSC based on metal-free organic sensitizers showed an efficiency of ca. 14%.30 Among all the scaffolds, dithiafulvalene (DTF), tetrathiafulvalene (TTF) and their derivatives as donor–π–acceptor metal-free organic sensitizers for DSSCs are particularly interesting. Both DTF and TTF scaffolds have been widely used in materials chemistry and are predominantly used for optoelectronic applications.31 Only a few reports are available in the literature with the use of dithiafulvalene (DTF) and tetrathiafulvalene (TTF) moieties as electron donors in DSSCs and further research in this regard would definitely yield high efficiency solar cells.
2. Design of metal-free organic dyes based on the donor–π–acceptor system
Compared with the other classes of dyes, organic dyes have many advantages such as low cost, absorption in the visible to near-IR region of the absorption spectrum, straightforward synthetic protocols which are often well established procedures, environmental compatibility and transparent solar cell-window. Moreover, the optical and electrochemical properties of organic dyes can be tuned in the desired manner through suitable molecular design strategies. In addition to this, the high molar extinction coefficients (up to 105 M−1 cm−1) of these metal-free organic sensitizers are particularly attractive because they enhance the conversion efficiency significantly due to their high ε values. In order to obtain a broad absorption spectrum, some of these dyes can be used as sensitizers as well as co-sensitizers.32–34 The use of these dyes in DSSC also faces some limitations such as short emission lifetimes compared to metal complexes35,36 and the need to use liquid electrolytes. Liquid electrolytes are not stable at extreme temperatures and at low temperatures they freeze, which ends power production and potentially leads to physical damage and at high temperatures. Also, they expand creating problems with the sealing of panels.In the past decade, organic dyes have been reported with a structural arrangement of donor–π–acceptor (D–π–A), as shown in Fig. 2. Many researchers focused on organic materials containing D–π–A conjugation systems since they have high polarizability. This type of molecular structural arrangement facilitates the photoinduced intramolecular electron transfer reaction between donor and acceptor through the π-electron bridge with a strong polarity effect and may produce considerable photoelectric conversion (PCE).
The first report on metal-free organic dyes was by Kamat and co-workers who used squaraine based scaffolds.37,38 They observed that the devices constructed using these dyes showed a very low incident photon-to-current conversion efficiency (IPCE) in the range of 0.05–0.7%. The poor conversion efficiencies were attributed to the back electron transfer and very low-singlet fluorescence quantum yields of the symmetrical squaraine sensitizers. Since then many metal-free organic sensitizers based on different scaffolds have been reported and tested for DSSC application. In this review, we summarize the recent progress in metal-free organic dye-sensitized solar cells using the thermally stable strong electron donors dithiafulvalenes (DTFs) and tetrathiafulvalenes (TTFs) as sensitizers. Tetrathiafulvalene (TTF) is a well-known electron-donating group, and its derivatives are widely applied as optoelectronic materials.39,40 Recently, Grätzel et al. introduced the use of extended π-conjugated tetrathiafulvalene (exTTF) as a donor unit in a sensitizer for the construction of DSSCs and showed its efficient photovoltaic conversion.41 Subsequently, dithiafulvalene (DTF) was also used in DSSCs due to its terminal electron-donating group, ease of synthesis and effective charge separation.42,43
3. Tetrathiafulvalene (TTF) and dithiafulvalene (DTF) sensitizers for DSSCs
Tetrathiafulvalene (TTF), which is a sulfur-containing compound, acts as strong electron donor, and is mainly used in molecular electronics. Its planarity and high symmetry favours charge delocalization to give a stable radical cation upon oxidation reactions. In 1970 for the first time, Wudl et al. synthesized and reported TTF as a strong electron donor.44 In this system, two 1,3-dithiolidine rings are covalently connected with an alkenyl bridge,45,46 a combination that resulted in novel and exciting electrochemical properties. TTF undergoes two reversible one-electron oxidations at mild potentials yielding a stable radical cation (TTF˙+) first and there upon further oxidation resulting a dication (TTF2+) (Fig. 3). The favourable electronic and conductive properties of TTF have sparked intense interest in the use of TTFs and modified extended TTFs in various applications47 such as donor–acceptor systems in DSSCs, nonlinear optics, superconductors, C60-complexes, sensors and supramolecular chemistry. TTFs have been featured in an astounding number of publications (>30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and also several reviews48 and books.49 exTTF-based dyes have been synthesized and optimized for their incorporation in dye sensitized solar cells (DSSCs). Particularly, TTF-based D–π–A systems have found numerous potential applications since their electrochemical and optical properties can be finely tuned through molecular engineering.50,51 Despite this, only few examples have been reported in the literature on extended π-conjugated tetrathiafulvalene (exTTF) based sensitizers for DSSCs.52,53 The photovoltaic parameters of TTF-based sensitizers are shown in Table 1.
000) and also several reviews48 and books.49 exTTF-based dyes have been synthesized and optimized for their incorporation in dye sensitized solar cells (DSSCs). Particularly, TTF-based D–π–A systems have found numerous potential applications since their electrochemical and optical properties can be finely tuned through molecular engineering.50,51 Despite this, only few examples have been reported in the literature on extended π-conjugated tetrathiafulvalene (exTTF) based sensitizers for DSSCs.52,53 The photovoltaic parameters of TTF-based sensitizers are shown in Table 1.
| Dye | J sc [mA cm−2] | V oc [mV] | FF | η (%) | λ abs (nm) | Ref. |
|---|---|---|---|---|---|---|
| T1 | 7.2 | 555 | 0.72 | 2.9 | ∼390 | 54 |
| T2 | 7.7 | 578 | 0.73 | 3.2 | ∼415 | 54 |
| T3 | 8.6 | 579 | 0.76 | 3.8 | 419 | 54 |
| T4 | 13.76 | 617 | 0.75 | 6.47 | 526 | 55 |
| T5 | 1.35 | 426 | 0.62 | 0.37 | 527 | 59 |
| T6 | 2.69 | 463 | 0.68 | 0.86 | 507 | 59 |
| T7 | 2.67 | 443 | 0.66 | 0.79 | 512 | 59 |
| T8 | 0.66 | 487 | 0.61 | 0.16 | 600 | 59 |
| T9 | 3.94 | 539 | 0.74 | 1.59 | 372 | 60 |
| T10 | 2.06 | 477 | 0.72 | 0.72 | 430 | 60 |
| T11 | 1.85 | 477 | 0.76 | 0.68 | 435 | 60 |
| T12 | 15.48 | 601 | 0.71 | 6.60 | 421 | 56 |
| T13 | 15.09 | 650 | 0.73 | 7.15 | 417 | 56 |
| T14 | 11.42 | 659 | 0.72 | 5.45 | 445 | 56 |
| T15 | 13.03 | 671 | 0.73 | 6.36 | 455 | 56 |
| T16 | 12.71 | 549 | 0.72 | 5.04 | 440 | 56 |
| T17 | 10.92 | 576 | 0.72 | 4.55 | 385 | 56 |
Grätzel et al. first reported exTTF based DSSCs and achieved a power conversion efficiency (PCE) of 3.8% (T3).54 Later, Liu and co-workers reported quinoxaline fused TTF sensitizers using chenodeoxycholic acid as a co-adsorbent (T4) which achieved an efficiency of 6.47%.55 Recently, our group reported exTTF based dyes (T12–T17) having thioalkyl substituted TTF as a donor, thiophene or ethynylthiophene as a π bridge and cyanoacrylic acid as an acceptor and achieved 7.15% (T13) efficiency without using any co-absorbent, which is the highest efficiency reported to date using TTF based sensitizers.56
Along with TTF, dithiafulvalene (DTF) is also considered a good electron donor due to its unique charge transport characteristics. Its co-planar molecular structures with strong π–π* and S–S interfaces, aggregation resistant property and non-planar molecular configuration make DTF a good choice in D–π–A systems for DSSC applications. Dye-sensitized solar cells using DTFs as electron-donating groups occupy the top position among metal free organic solar cells57,58 because of their high efficiencies. DTF being a strong electron donor facilitates ultrafast interfacial electron injection from excited state dye molecules to the conduction band of semiconductors. In addition, the recombination of injected electrons with the redox couple can be suppressed due to the propeller-shaped DTF molecular structure. Moreover, the oxidized DTF unit is conveniently placed spatially to support the movement of electrons towards the redox couple species, thus ensuring fast dye regeneration. A schematic diagram of the DTF based dye is shown in Fig. 4 and the photovoltaic parameters of DTF-based sensitizers are given in Table 2.
| Dye | J sc [mA cm−2] | V oc [mV] | FF | η (%) | λ abs (nm) | Ref. |
|---|---|---|---|---|---|---|
| D1 | 6.51 | 670 | 0.72 | 3.15 | 428 | 61 |
| D2 | 8.20 | 730 | 0.70 | 4.18 | 406 | 61 |
| D3 | 14.35 | 830 | 0.69 | 8.29 | 427 | 61 |
| D4 | 9.58 | 648 | 0.71 | 4.41 | 398 | 62 |
| D5 | 8.27 | 634 | 0.72 | 3.78 | 413 | 63 |
| D6 | 8.82 | 645 | 0.72 | 4.09 | 433 | 63 |
| D7 | 10.77 | 718 | 0.76 | 5.87 | 432 | 64 |
| D8 | 12.01 | 746 | 0.74 | 6.63 | 435 | 64 |
| D9 | 6.43 | 540 | 0.68 | 2.36 | 448 | 65 |
| D10 | 6.94 | 540 | 0.62 | 2.34 | 461 | 65 |
| D11 | 11.33 | 650 | 0.71 | 5.24 | 438 | 65 |
| D12 | 6.76 | 550 | 0.57 | 2.11 | 488 | 65 |
| D13 | 6.66 | 590 | 0.70 | 2.75 | 447 | 65 |
| D14 | 6.95 | 670 | 0.75 | 3.48 | 436 | 65 |
| D15 | 5.02 | 630 | 0.72 | 2.28 | 439 | 65 |
| D16 | 3.97 | 632 | 0.78 | 2.18 | 577 | 66 |
| 9.26 | 485 | 0.72 | 3.19 | |||
| D17 | 7.27 | 687 | 0.77 | 4.12 | 584 | 66 |
| 12.26 | 493 | 0.69 | 4.13 | |||
| D18 | 10.02 | 680 | 0.73 | 4.97 | 378 | 67 |
| D19 | 11.82 | 780 | 0.73 | 6.75 | 383 | 67 |
| D20 | 11.51 | 720 | 0.73 | 6.05 | 387 | 67 |
| D21 | 13.84 | 780 | 0.71 | 7.66 | 382 | 67 |
3.1. TTF based sensitizers
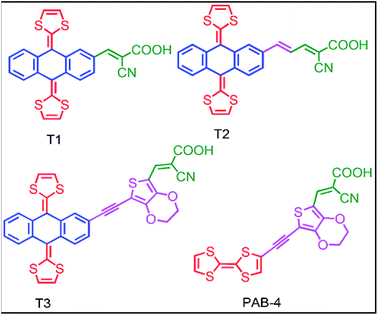
TTF with extended π-conjugation (exTTF) was reported as an efficient electron donor system for DSSC applications. The first report of using exTTF as donors in DSSCs appeared in 2010 by Grätzel and co-workers.54 They designed three (T1–T3) dyes with exTTF as the donor and cyanoacrylic acid as the acceptor. The change in molecular structure of these sensitizers was the extent of π-conjugation of the spacer. The donor and acceptor are directly linked without a spacer in T1 and vinyl spacer in T2 using the D–π–A approach. In contrast, the auxiliary acceptor 3,4-ethylenedioxythiophene (EDOT) unit in T3 and PAB-4 resulted in the D–A–π–A approach.59 The molecular structures of these dyes are shown in Fig. 5. The absorption maxima red-shifted as the π-conjugation extended from sensitizers T1 to T3 (λmax of T1, T2 and T3 are 390, 415 and 419 nm, respectively). This group also synthesized an analogue of T3 named PAB-4 with TTF as the donor. The sensitizer PAB-4 showed a very poor performance due to more aggregation on the TiO2 surface. In all three sensitizers, the incident photon-to-current conversion efficiency (IPCE) was broad which extended up to the 700 nm region. The current–voltage (J–V) characteristics indicated that T1 showed a short-circuit current (Jsc) of 7.2 mA cm−2, open-circuit voltage (Voc) of 555 mV and fill factor (FF) of 0.72, with an overall efficiency (η) of 2.9%. In contrast, in the case of T2 and T3 both Jsc and Voc were enhanced resulting in a conversion efficiency of 3.2 and 3.8%, respectively. The low efficiency of these exTTF-based sensitizers is probably due to absorption only in the UV region. They also have an energetically high-lying HOMO compared to the conduction band of TiO2 due to which electrons undergo recombination with tri-iodide and possibly with the sensitizer radical cation or even the dication at the TiO2/dye/electrolyte interface. Therefore, dye regeneration after electron-injection is thermodynamically unfavourable.Based on the above report, in an attempt to tune the HOMO level and increase intramolecular charge transfer (ICT) absorbance, a quinoxaline-fused TTF-based sensitizer (T4) (Fig. 6) was reported by Amacher et al.55
This new strategy employed quinoxaline-fused TTF as the donor, two carboxylic acid groups as acceptor molecules with an ethynylphenyl moiety as the π-bridge. This alignment keeps the donor and acceptor molecules in a rigid and planar configuration. The ICT absorption spectrum of T4 in THF solution showed λmax at 526 nm with a molar extinction coefficient close to 2 × 104 M−1 cm−1 with a substantially stabilized HOMO level. The IPCE spectrum is broad and extends up to 750 nm with more than 70% of the spectrum between the 500 to 600 nm region. The J–V characteristics showed that the T4 sensitizer has an efficiency 5.19% with a Jsc of 12.56 mA cm−2, Voc of 580 mV and FF of 0.70. The presence of the co-absorbent chenodeoxycholic acid (CDCA) minimizes the aggregation with an enhanced Jsc of 13.76 mA cm−2, Voc of 617 mV and FF of 0.75 resulting in the improved efficiency of 6.47%. These results indicate the device performances are to some extent affected by dye aggregation and the presence of CDCA prevents dye aggregation. In addition, the presence of alkyl chains in the dye molecular structure not only enhances its solubility but also effectively increases the charge injection efficiency and retards back electron transfer.
In an another study by Y. Geng et al., they designed four TTF-based D–π–A sensitizers using hexyl substituted TTF as the common donor and cyanoacrylic acid as the acceptor with varying π-conjugated spacers (Fig. 7) to improve the efficiency of the PAB-4 sensitizer.60 The change of the π-linkers between the donor and acceptor units of the sensitizers tailor their frontier orbital energy levels which leads to changes in their optical properties. The UV-Vis absorption spectra of the dyes (T5, T6, T7 and T8) in THF solution showed the absorption maxima of 527, 507, 512 and 600 nm, respectively. The red-shifted absorption maximum of T8 is probably due to the presence of the electron deficient benzothiazole group in its molecular structure. Photovoltaic studies indicate that T5 showed an efficiency, η, of 0.37% with a Jsc of 1.35 mA cm−2, Voc of 420 mV and FF of 0.628. The η values for T6–T8 are 0.86% (Jsc = 2.69 mA cm−2, Voc = 463 mV and FF = 0.686), 0.79% (Jsc = 2.67 mA cm−2, Voc = 443 mV and FF = 0.662) and 0.16% (Jsc = 0.66 mA cm−2, Voc = 387 mV and FF = 0.617), respectively. For all the four sensitizes both the Jsc and Voc values increase gradually in the order of T8 < T5 < T7 < T6, which does not completely match the order of electron donating ability (T5 < T6 < T8 < T7) of the combined donor spacer region. These dyes also have the problem of recombination due to their high-energy HOMO levels similar to the earlier reported T1–T3 dyes based on exTTF dyes. In case of the T5–T8 dyes, fast recombination from the electronically exited state to the ground state is possible and as a result poor power conversion efficiencies were observed.
Echeverry et al. re-designed the T1–T3 sensitizers by the incorporation of rhodanine-3-carboxylic acid as an acceptor, using different π-bridge spacers (vinyl or vinyl-thiophene spacers) (Fig. 8).61 In these sensitizers by increasing the length of π-conjugated systems, the band gap between LUMO and TiO2 conduction band decreased and hence T11 showed a poor photovoltaic performance compared to T9. The absorption spectra of T9, T10 and T11 showed λmax at 372 nm (ε = 5449 M−1 cm−1), 430 nm (ε = 6613 M−1 cm−1) and 435 nm (ε = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1), respectively. The absorption maxima were red-shifted in the order of T11 > T10 > T9, with an increase of π-conjugation (vinyl or vinyl-thiophene).
000 M−1 cm−1), respectively. The absorption maxima were red-shifted in the order of T11 > T10 > T9, with an increase of π-conjugation (vinyl or vinyl-thiophene).
The resulting photovoltaic energy conversion efficiency showed the order of T11 < T10 < T9 which was found to be 0.68%, 0.72% and 1.59%, respectively. The low efficiency may be attributed to the low band gaps between the HOMO of the dyes and the I−/I3− redox couple on one side and the LUMO level of the dyes and the conduction band of TiO2 on the other side preventing the proper functioning of the DSSCs.
Giribabu et al. recently designed a series of novel exTTF based sensitizers (T12–T17) with thioalkyl substituted TTF-anthracene as the donor, either cyanoacrylic acid or rhodanine acetic acid as the acceptor and different π-spacers (thiophene, 3-enthynyl thiophene, phenyl or 3-ethynyl phenyl). The incorporation of thioalkyl was done to decrease aggregation and also to tune the HOMO–LUMO levels. The efficiency results were very encouraging and indicated that the problems associated with the earlier designs might be resolved. The molecular structures of these dyes are shown in Fig. 9.56 The optimized structures of all these sensitizers show flying bird-like structures consisting of hexyl substitutions at the 9 and 10th positions and different anchoring groups at the 2nd position of anthracene. These dyes show enhanced internal charge transfer compared to the previously reported exTTF sensitizers. The absorption maximum of T15 is at 421 nm (ε = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1) and as the π-conjugation increases it is red-shifted to 455 nm (ε = 28
700 M−1 cm−1) and as the π-conjugation increases it is red-shifted to 455 nm (ε = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 M−1 cm−1). This shift is attributed to the π–π* charge transfer transitions in the exTTF chromophores. The photovoltaic properties, i.e. IPCE of T16, are extended up to the 900 nm region with a maximum of 85% in the case of the T13 sensitizer. The detailed photovoltaic parameters of all the sensitizers are as follows. The T12 device showed a Jsc of 15.48 mA cm−2, Voc of 601 mV and FF of 0.709 with an overall conversion efficiency of 6.60%. On the other hand, T13 showed an enhanced Jsc of 15.09 mA cm−2, Voc of 650 mV and FF of 0.729, resulting in an efficiency of 7.15%. The power conversion efficiency of T15 is lower than that of T13, which might be due to the presence of benzene as the conjugated bridge in T15 as opposed to thiophene in T13. Thiophene is known to keep the donor in better planarity than that of six member-rings such as benzene. Compared to all the previously reported exTTF metal-free organic dyes, this class of sensitizers showed enhanced efficiency due to the small changes in their design. In this design, the HOMO level of the sensitizers was stabilized through extended π-conjugation as well as reduction in aggregation due to the presence of the thioalkyl group on tetrathiafulvalene. The thioalkyl group acts as a wrapper over the TTF moiety which minimizes the recombination of electrons in the TiO2 conduction band with the oxidized sensitizers. These dyes are thermally stable which increases their commercial application value and possible roof-top applications in DSSCs. T12 is stable up to 250 °C, whereas T13 is stable up to 200 °C. Among all the metal-free organic exTTF based sensitizers reported to date, these dyes have shown the highest efficiency.
973 M−1 cm−1). This shift is attributed to the π–π* charge transfer transitions in the exTTF chromophores. The photovoltaic properties, i.e. IPCE of T16, are extended up to the 900 nm region with a maximum of 85% in the case of the T13 sensitizer. The detailed photovoltaic parameters of all the sensitizers are as follows. The T12 device showed a Jsc of 15.48 mA cm−2, Voc of 601 mV and FF of 0.709 with an overall conversion efficiency of 6.60%. On the other hand, T13 showed an enhanced Jsc of 15.09 mA cm−2, Voc of 650 mV and FF of 0.729, resulting in an efficiency of 7.15%. The power conversion efficiency of T15 is lower than that of T13, which might be due to the presence of benzene as the conjugated bridge in T15 as opposed to thiophene in T13. Thiophene is known to keep the donor in better planarity than that of six member-rings such as benzene. Compared to all the previously reported exTTF metal-free organic dyes, this class of sensitizers showed enhanced efficiency due to the small changes in their design. In this design, the HOMO level of the sensitizers was stabilized through extended π-conjugation as well as reduction in aggregation due to the presence of the thioalkyl group on tetrathiafulvalene. The thioalkyl group acts as a wrapper over the TTF moiety which minimizes the recombination of electrons in the TiO2 conduction band with the oxidized sensitizers. These dyes are thermally stable which increases their commercial application value and possible roof-top applications in DSSCs. T12 is stable up to 250 °C, whereas T13 is stable up to 200 °C. Among all the metal-free organic exTTF based sensitizers reported to date, these dyes have shown the highest efficiency.
3.2. DTF based sensitizers
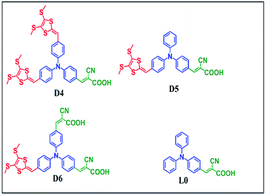
Shihe Yang and co-workers for the first time investigated the potential of dithiafulvalene (DTF) as an electron donor in metal-free organic dyes. They synthesized three sensitizers D1, D2 and D3 keeping the same donor (DTF) and acceptors (cyanoacrylic acid), but changing the π-bridges. The π-bridges are phenyl, biphenyl and phenyl-thiophene-phenyl moieties for D1, D2 and D3 (Fig. 10),62 respectively, using the D–A–π–A approach. The presence of two n-hexyl groups, which are attached symmetrically on the dithiafulvenyl donor unit, not only prevent aggregation but also enhance the solubility of the sensitizer. Photovoltaic properties showed that the D1 sensitized device gave a Jsc of 6.51 mA cm−2, Voc of 670 mV, and FF of 0.72, which correspond to an η value of 3.15%. On the other hand, the η value of 4.18% (Jsc = 8.20 mA cm−2, Voc = 730 mV and FF = 0.70) was obtained for the D2 sensitizer and 8.29% (Jsc = 14.35 mA cm−2, Voc = 830 mV and FF = 0.69) for the D3 sensitizer. Among the three dyes, D3 showed the maximum IPCE of 85% with an overall conversion efficiency of 8.29%. The absorption maxima of the compounds are in the order of D1 (λmax = 428 nm) > D3 (λmax = 427 nm) > D2 (λmax = 406 nm). The introduction of electron rich thiophene between the two phenyl groups of D2 led to D3 which resulted in an increase in ε value with a red-shift of the absorption maximum (from 406 nm to 427 nm) and also broadening of the spectrum. This improved light harvesting property of D3 resulted in the efficiency of 8.29%, which is comparable with the standard N719 sensitizer (η = 8.76%) under similar test cell conditions. However, the FF values of the three sensitizers are similar, but efficiency is enhanced due to the increase in both Jsc and Voc with the increase in auxiliary acceptor π-bridge length from D1 to D3. These results indicate that device performance can be enhanced by increasing the π-bridge length with electron rich species.Based on the above report, the use of DTF as donor in D–A–π–A systems in the design of sensitizers for DSSCs has been accelerated. In a study to increase the electron donating capacity of the donor TPA (in L0), two DTF units were introduced into TPA to form D4 (Fig. 11) and its efficacy compared with the standard dye L0.63 The absorption maximum of L0 is 407 nm (ε = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 M−1 cm−1), whereas in the case of D4 a broad absorption was observed with three absorption peaks at 335 nm (ε = 17
230 M−1 cm−1), whereas in the case of D4 a broad absorption was observed with three absorption peaks at 335 nm (ε = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 M−1 cm−1), 398 nm (ε = 36
950 M−1 cm−1), 398 nm (ε = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 M−1 cm−1) and 443 nm (ε = 20
840 M−1 cm−1) and 443 nm (ε = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 M−1 cm−1). The shoulder peaks in the spectra of D4 can be ascribed to the substituted DTF unit, which are red-shifted relative to that of L0. The absorption spectra of these dyes on 3 μm thick TiO2 films after 12 h adsorption showed maxima at 435 nm and 410 nm for L0 and D4, respectively. The broad absorption spectra observed for both dyes on the TiO2 surface can be ascribed to the formation of J-type aggregates which benefits the photoelectrical conversion efficiencies of DSSCs.
350 M−1 cm−1). The shoulder peaks in the spectra of D4 can be ascribed to the substituted DTF unit, which are red-shifted relative to that of L0. The absorption spectra of these dyes on 3 μm thick TiO2 films after 12 h adsorption showed maxima at 435 nm and 410 nm for L0 and D4, respectively. The broad absorption spectra observed for both dyes on the TiO2 surface can be ascribed to the formation of J-type aggregates which benefits the photoelectrical conversion efficiencies of DSSCs.
The detailed photovoltaic parameters of the L0 device showed a Jsc of 5.48 mA cm−2, Voc of 6.17 mV, FF of 0.73 and resulting efficiency η of 2.47%. The efficiency of the D4 dye device is 4.41%. The introduction of two DTF units in TPA finally resulted in an increase in η value to about 79%. Under standard global Air Mass 1.5 solar irradiation (100 mW cm−2) they also compared the D4 dye with the standard N3 dye. The N3 generates an η of 7.30% (Jsc = 16.63 mA cm−2, Voc = 665 mV and FF = 0.66). The IPCE spectra confirmed the trend of Jsc variation in the order of N3 > D4 > L0. The introduction of the DTF unit in L0 resulted in an enhancement of both the Jsc and Voc values (D4) because of the more efficient light harvesting capacity and suppression of dark current. These results indicate that the introduction of the DTF unit into the simple TPA dye improves the performance of TPA-based DSSCs.
The same group conducted another study keeping DTF substituted TPA as the donor and using 2-cyanoacetic acid as the acceptor (D5) or two cyanoacetic acid molecules as acceptors (D6) (Fig. 11).64 The absorption spectra of L0, D5 and D6 show (λmax) at 407, 413 and 433 nm with an ε of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230, 26
230, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and 27
300 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1, respectively. The light harvesting capacity and power conversion efficiencies were increased in the order of L0 < D5 < D6 due to the double donor (DTF–TPA) moiety in these dyes. The introduction of the DTF unit not only enhanced the electron injection into the TiO2 conduction band but also inhibited aggregation between dye molecules which prevented I3− in the electrolyte from recombining with injected electrons on TiO2. D5 showed an η of 3.78% with a Jsc of 8.27 mA cm−2, Voc of 634 mV and FF of 0.72. D6 showed a Jsc of 8.82 mA cm−2, Voc of 645 mV and FF of 0.72 with an η of 4.09%, under standard global Air Mass 1.5 solar irradiation. Compared to the standard dye L0, D5 and D6 showed a higher Jsc and Voc due to the reduced aggregation and recombination because of the two acceptor groups. D6 showed a power conversion efficiency of 4.09% which is far better than that of the DTF free dye L0 (2.47%).
400 M−1 cm−1, respectively. The light harvesting capacity and power conversion efficiencies were increased in the order of L0 < D5 < D6 due to the double donor (DTF–TPA) moiety in these dyes. The introduction of the DTF unit not only enhanced the electron injection into the TiO2 conduction band but also inhibited aggregation between dye molecules which prevented I3− in the electrolyte from recombining with injected electrons on TiO2. D5 showed an η of 3.78% with a Jsc of 8.27 mA cm−2, Voc of 634 mV and FF of 0.72. D6 showed a Jsc of 8.82 mA cm−2, Voc of 645 mV and FF of 0.72 with an η of 4.09%, under standard global Air Mass 1.5 solar irradiation. Compared to the standard dye L0, D5 and D6 showed a higher Jsc and Voc due to the reduced aggregation and recombination because of the two acceptor groups. D6 showed a power conversion efficiency of 4.09% which is far better than that of the DTF free dye L0 (2.47%).
Similarly, in another study, the DTF moiety without alkyl chains or with alkyl chains was introduced into the phenothiazine organic dye (C6PTZ) forming D7 and D8, respectively (Fig. 12). The absorption maxima of C6PTZ, D7 and D8 are at 420 nm (ε = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1 cm−1), 432 nm (ε = 19
100 M−1 cm−1), 432 nm (ε = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1) and 435 nm (ε = 20
200 M−1 cm−1) and 435 nm (ε = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1), respectively.65 The power conversion efficiencies increased significantly compared to the simple phenothiazine dye C6PTZ (η = 4.16%, Jsc = 8.33 mA cm−2, Voc = 703 mV and FF = 0.71) for D7 (η = 5.87%, Jsc = 10.77 mA cm−2, Voc = 718 mV and FF = 0.76) and D8 (η = 6.63%, Jsc = 12.01 mA cm−2, Voc = 746 mV and FF = 0.74). The increased efficiency is due to their increased light harvesting capacity (because of the electron rich DTF), and reduced aggregation and recombination (because of the hexyl chains). The efficiency increased from C6PTZ to D8, but could not reach the efficiency of the standard reference N719 dye (7.45%) under similar test cell conditions. These results demonstrate that the incorporation of the DTF group into organic dyes is an effective approach to develop high-performance metal-free organic dyes.
600 M−1 cm−1), respectively.65 The power conversion efficiencies increased significantly compared to the simple phenothiazine dye C6PTZ (η = 4.16%, Jsc = 8.33 mA cm−2, Voc = 703 mV and FF = 0.71) for D7 (η = 5.87%, Jsc = 10.77 mA cm−2, Voc = 718 mV and FF = 0.76) and D8 (η = 6.63%, Jsc = 12.01 mA cm−2, Voc = 746 mV and FF = 0.74). The increased efficiency is due to their increased light harvesting capacity (because of the electron rich DTF), and reduced aggregation and recombination (because of the hexyl chains). The efficiency increased from C6PTZ to D8, but could not reach the efficiency of the standard reference N719 dye (7.45%) under similar test cell conditions. These results demonstrate that the incorporation of the DTF group into organic dyes is an effective approach to develop high-performance metal-free organic dyes.
Ting-Hui Lee et al. in a systematic study synthesized seven dyes keeping DTF as the donor and cyanoacrylic acid as the acceptor, and varying the π-conjugated spacer. They introduced thiophene (D9) or bithiophene (D10) or phenyl-thiophene-phenyl (D11) as π-bridges. They also synthesize sensitizers containing dimers of D–π–A units (D12, D13, D14 and D15) through the iodine-induced dimerization of an appropriate DTF-containing segment. These dimers also have DTF as the donor and cyanoacrylic acid as the acceptor but differ in π-bridges. The π-bridge moieties are bithiophene in D12, phenyl-thiophene in D13, phenyl-thiophene-phenyl in D14 and fluorine in D15 (Fig. 13).66 Under global Air Mass 1.5 solar conditions the monomer (D–π–A) dyes D9 and D10 showed an η of 2.36% and 2.34%, respectively. D11 exhibited a better cell performance (η = 5.24%, Jsc = 11.33 mA cm−2, Voc = 650 mV and FF = 0.71) compared to D9 and D10 due to its extended π-conjugation spacer (phenyl-thiophene-phenyl). It should be noted that D11 is similar to D3 except that it has butyl substitution on the DTF unit instead of the hexyl group, which caused a huge difference in efficiency. Although higher efficiency is expected from (D–π–A)2 type sensitizers, they showed lower efficiencies (ranging from 2.11% to 3.48%) than D11. In spite of their better light harvesting abilities, the (D–π–A)2 dyes did not show high efficiency because of their less efficient dye regeneration. Therefore, more appropriate designs taking into consideration the HOMO levels of the dye may yield better sensitizers.
The effect of the presence of a phenyl spacer between the donor and acceptor on the power conversion efficiencies of metal free organic sensitizers was studied using a hybrid DTF as the donor. The two dyes D16 and D17 (Fig. 14)67 were designed keeping a hybrid electron donor comprising cyclopentadithiophene and dithiafulvenyl and cyanoacrylic acid as the acceptor. In D16, the donor and acceptor are directly linked and in D17, a phenyl group separates them. These two sensitizers showed two reversible oxidations and absorb strongly in the visible region. The absorption maxima of D16 and D17 are 577 nm (ε = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1) and 548 nm (ε = 56
500 M−1 cm−1) and 548 nm (ε = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1), respectively. The direct linkage of D and A units resulted in effective π-conjugation in D16, thus lowering the HOMO–LUMO gap. Upon insertion of the phenyl spacer, the π-conjugation was partially broken and hence D17 showed a hypsochromic shift which indicated an increased HOMO–LUMO gap. Device studies showed the power conversion efficiencies of these two dyes in the presence of iodine as well as cobalt electrolytes.
700 M−1 cm−1), respectively. The direct linkage of D and A units resulted in effective π-conjugation in D16, thus lowering the HOMO–LUMO gap. Upon insertion of the phenyl spacer, the π-conjugation was partially broken and hence D17 showed a hypsochromic shift which indicated an increased HOMO–LUMO gap. Device studies showed the power conversion efficiencies of these two dyes in the presence of iodine as well as cobalt electrolytes.
In presence of I−/I3− electrolyte D16 performed with a Jsc of 9.26 mA cm−2, Voc of 485 mV and FF of 0.72, resulting in an efficiency of 3.19%. Dye D17 showed a Jsc of 12.26 mA cm−2, Voc of 493 mV and FF of 0.69, giving an overall efficiency of 4.13%. In contrast, using the Co(II/III) redox electrolyte the dye D16 device gave a Jsc of 3.97 mA cm−2, Voc of 632 mV and FF of 0.78, which correspond to an overall conversion efficiency of 2.18%. Dye D17 showed a Jsc of 7.27 mA cm−2, Voc of 687 mV and FF of 0.77 with an η of 4.12%. Of the two electrolytes the iodide redox couple gave better conversion efficiencies than the cobalt electrolyte. These results indicate that the presence of a phenyl ring enhances the efficiency of the device.
In a recent report a slightly different version of the D–π–A approach, the D–π–D–A concept, was explored in an attempt to increase the efficiency of DTF and phenothiazine based DSSCs and significant increase in η value was achieved. In the study, the effect of different π-spacers between DTF and phenothiazine on η value was explored. The four newly designed dyes (D18, D19, D20 and D21) were slightly modified compared to the previously reported D8 by the introduction of varying π-spacers (phenyl, fluorophenyl, alkoxy phenyl and biphenyl, respectively) between DTF and phenothiazine. The DSSCs with dyes having phenyl and substituted phenyl spacers displayed higher photovoltaic conversion compared to D8, where DTF and phenothiazine are directly linked. This can be attributed to more efficient charge separation due to the interruption of conjugation between DTF and PTZ (Fig. 15).68D19 showed a marked increase in Jsc and Voc due to the presence of the electron withdrawing fluorine atom which facilitated intramolecular electron transfer from DTF to the anchoring group.
The performance of D20 is not up to expectation when compared to D19 due to the presence of the electron donating hexyloxy group. The best photovoltaic performance (η of 7.66%, Voc of 0.78 V and Jsc of 13.84 mA cm−2) was achieved when the biphenyl ring was inserted as a spacer. Furthermore, quasi solid-state DSSCs based on D21 display an η of 6.59%, and their η values retain 99% of the initial efficiency value after continuous light soaking for 500 h. DFT calculation results indicate that the molecular structure of D21 favours effective charge separation and prevents aggregation. The two torsion angles of the biphenyl ring also prevent the back reaction and contribute to the high performance. This report paves a way for new types of molecular design strategies to be employed in order to improve the performance of DSSCs.
4. Conclusion
This review focuses on the recent advances in molecular design and technological features of tetrathiafulvalene (TTF) and dithiafulvalene (DTF) based metal-free organic dyes for applications in dye-sensitized solar cells. In the recent years, many research groups have investigated a variety of different donors and multi-anchoring groups to increase the performance of metal-free dyes in DSSCs and quite a few groups explored the potential of tetrathiafulvalene (TTF) and dithiafulvalene (DTF) in dye-sensitized solar cells. DTF and TTF have many advantages such as ease of synthesis at low cost, high absorption coefficients, thermal stability, and environmentally friendliness. DSSCs based on organic dyes have achieved η values as high as 14%, which is comparable to that of Ru-complexes (η = 11%). The highest η values reported with DTF and TTF based DSSCs to date are 8.29% and 7.15%, respectively. This has been achieved by adopting the donor–π–acceptor approach by tuning the HOMO–LUMO levels of the sensitizers. More efficient sensitizers can be obtained by applying sensible design strategies on the DTF or TTF core such as extended π-conjugation to increase the absorption coefficient, effective positioning of anchoring groups and substitution of alkyl groups to reduce aggregation, and proper alignment of the donor and acceptor positions to enhance intramolecular charge transfer. In our opinion, this review furnishes useful directions to develop design strategies for DTF and TTF based DSSCs with high efficiency. In the future, further careful manipulations in molecular design and device fabrication would make DTF and TTF moieties promising electron donors in this broad and quickly growing area of metal free organic dye solar sensitizers. For example the introduction of appropriate auxiliary acceptors between the donor and π-spacer leads to D–A–π–A as well as a secondary donor between the π-spacer and acceptor leads to the D–π–D–A approach which may result in efficient sensitizers.Acknowledgements
Authors thanks to CSIR-NISE and Department of Science and Technology (DST), Government of India under major project DST-UK (‘APEX’) for financial support to carry out this work. Author ND thanks to CSIR for junior research fellowship, UC thanks to DST for women scientist fellowship and very special thanks to our research group for the most valuable contributions to our work on dye-sensitized solar cells.Notes and references
- World Energy Outlook 2015, Released on 10 November 2015.
- R. C. Neville, Solar Energy Conversion: The Solar Cell, Elsevier, Amsterdam, 1995 Search PubMed.
- J. Perlin, The Silicon Solar Cell Turns 50, National Renewable Energy Laboratory, 2004 Search PubMed.
- M. A. Green, Third Generation Photovoltaics: Advanced Solar Energy Conversion, Springer Verlag, Berlin, 2005 Search PubMed.
- (a) B. Tian, X. Zheng, T. J. Kempa, Y. Fang, N. Yu, G. Yu, J. Huang and C. M. Lieber, Nature, 2007, 449, 885 CrossRef CAS PubMed; (b) M. A. Green, Prog. Photovoltaics, 2009, 17, 183 CrossRef CAS.
- Solar Energy Handbook, ed. J. F. Krieder and F. Kreith, ch. 24, p. 24 Search PubMed.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49 CrossRef CAS.
- (a) A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 2000, 33, 269 CrossRef CAS PubMed; (b) B. C. O'Regan and J. Durrant, Acc. Chem. Res., 2009, 42, 1799 CrossRef PubMed; (c) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Graetzel, J. Am. Chem. Soc., 1993, 115(14), 6382–6390 CrossRef CAS.
- Z. Chen, F. Li and C. Huang, Curr. Org. Chem., 2007, 11, 1241 CrossRef CAS.
- R. K. Kanaparthi, J. Kandhadi and L. Giribabu, Tetrahedron, 2012, 68, 8383–8393 CrossRef CAS.
- A. Mishra, M. K. R. Fischer and P. Bauerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS PubMed.
- Z. S. Wang, Y. Cui, K. Hara, Y. Dan-Oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138 CrossRef CAS.
- Z. S. Wang, Y. Cui, Y. Dan-Oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2008, 112, 17011 CAS.
- T. Horiuchi, H. Miura and S. Uchida, Chem. Commun., 2003, 3036 RSC.
- T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, J. Am. Chem. Soc., 2004, 126, 12218 CrossRef CAS PubMed.
- S. Ito, S. M. Zakeeruddin, R. Humphry-Baker, P. Liska, R. Charvet, P. Comte, M. K. Nazeeruddin, P. Pechy, M. Takata, H. Miura, S. Uchida and M. Grätzel, Adv. Mater., 2006, 18, 1202 CrossRef CAS.
- S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Pechy and M. Grätzel, Chem. Commun., 2008, 5194 RSC.
- T. Kitamura, M. Ikeda, K. Shigaki, T. Inoue, N. A. Anderson, X. Ai, T. Q. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806 CrossRef CAS.
- D. P. Hagberg, J. H. Yum, H. Lee, F. De Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. C. Sun, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259 CrossRef CAS PubMed.
- J. H. Yum, D. P. Hagberg, S. J. Moon, K. M. Karlsson, T. Marinado, L. C. Sun, A. Hagfeldt, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2009, 48, 1576 CrossRef CAS PubMed.
- H. Choi, C. Baik, S. O. Kang, J. Ko, M. S. Kang, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 327 CrossRef CAS PubMed.
- G. L. Zhang, H. Bala, Y. M. Cheng, D. Shi, X. J. Lv, Q. J. Yu and P. Wang, Chem. Commun., 2009, 2198 RSC.
- (a) N. Koumura, Z. S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2008, 130, 4202 CrossRef CAS; (b) K. Srinivas, Ch. R. Kumar, M. A. Reddy, K. Bhanuprakash, V. J. Rao and L. Giribabu, Synth. Met., 2011, 161, 96–105 CrossRef CAS.
- Z. S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CrossRef CAS.
- D. Naresh, R. K. Kanaparthi, J. Kandhadi, G. Marotta, P. Salvatori, F. De Angelis and L. J. Giribabu, Chem. Sci., 2015, 127(3), 383–394 CrossRef.
- W. H. Thomas, Y. Jun-Ho, K. Yongjoo, R. Kasparas and M. Grätzel, J. Mater. Chem. A, 2013, 1, 13978–13983 Search PubMed.
- D. Naresh, R. Dimitris, V. K. Challuri, N. K. Emmanuel, G. Lingamallu and L. Panagiotis, Dyes Pigm., 2016, 134, 472–479 CrossRef.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. I. Fujisawa and M. Hanaya, Chem. Commun., 2015, 51, 15894–15897 RSC.
- L. Segura and N. Martin, Angew. Chem., Int. Ed., 2001, 40, 1372–1409 CrossRef PubMed.
- K. Hara, K. Sayama, Y. Ohga, A. Shinpo, S. Suga and H. Arakawa, Chem. Commun., 2001, 569 RSC.
- J. Fang, L. Su, J. Wu, Y. Shen and Z. Lu, New J. Chem., 1997, 270, 145 CAS.
- P. Y. Reddy, L. Giribabu, C. Lyness, H. J. Snaith, C. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J.-H. Yum, K. Kalyanasundaram, M. Grätzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2007, 46, 373–376 CrossRef CAS PubMed.
- J. Moser and M. Grätzel, J. Am. Chem. Soc., 1984, 106, 6557 CrossRef CAS.
- (a) P. Y. Reddy, L. Giribabu, C. Lyness, H. J. Snaith, C. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J.-H. Yum, K. Kalyanasundaram, M. Grtzel and M. K. Nazeer, Angew. Chem., 2007, 119, 377–380 CrossRef; (b) L. Giribabu, R. K. Kanaparthi and V. Velkannan, Chem. Rec., 2012, 12, 306–328 CrossRef CAS PubMed; (c) L. Giribabu, V. K. Singh, T. Jella, Y. Soujanya, A. Amat, F. De Angelis, A. Yella, P. Gao and M. K. Nazeeruddin, Dyes Pigm., 2013, 98, 518–559 CrossRef CAS; (d) V. K. Narra, H. Ullah, V. K. Singh, L. Giribabu, S. Senthilarasu, S. Z. Karazhanov, A. A. Tahir, T. K. Mallick and H. M. Upadhyaya, Polyhedron, 2015, 100, 313–320 CrossRef CAS; (e) V. K. Singh, R. K. Kanaparthi and L. Giribabu, RSC Adv., 2014, 4, 6970–6984 RSC.
- S. Hotchandani, S. Das, K. G. Thomas, M. V. George and P. V. Kamat, Res. Chem. Intermed., 1994, 20, 927–938 CrossRef CAS.
- P. V. Kamat, S. Hotchandani, M. De Lind, K. G. Thomas, S. Das and M. V. J. George, J. Chem. Soc., Faraday Trans., 1993, 89, 2397–2402 RSC.
- A. Gorgues, P. M. Hudhomme and M. Sallé, Chem. Rev., 2004, 104, 5151 CrossRef CAS PubMed.
- J. L. Segura and N. Martin, Angew. Chem., Int. Ed., 2001, 40, 1372 CrossRef CAS PubMed.
- S. Wenger, P. A. Bouit, Q. L. Chen, J. Teuscher, D. D. Censo, R. Humphry-Baker, J. E. Moser, J. L. Delgado, N. Martín, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 5164 CrossRef CAS PubMed.
- Dithiafulvene (DTF) compounds (examples) as NLO materials, see: (a) M. Blanchard Desce, I. Ledoux, J. M. Lehn, J. Malthete and J. Zyss, J. Chem. Soc., Chem. Commun., 1998, 737 Search PubMed; (b) F. Meyers, J. L. Bredas and J. Zyss, J. Am. Chem. Soc., 1992, 114, 2914 CrossRef CAS; (c) A. I. DeLucas, N. Martin, L. Sanchez, C. Seoane, J. Garin, J. Orduna, R. Alcala and B. Villacampa, Tetrahedron Lett., 1997, 38, 6107 CrossRef CAS; (d) A. S. Batsanov, M. R. Bryce, M. A. Coffin, A. Green, R. E. Hester, J. A. K. Howard, I. K. Lednev, N. Martín, A. J. Moore, J. N. Moore, E. Ortí, L. Sánchez, M. Savirón, P. M. Viruela, R. Viruela and T. Ye, Chem.–Eur. J., 1998, 4, 2580 CrossRef CAS.
- K. Guo, K. Yan, X. Lu, Y. Qiu, Z. Liu, J. Sun, F. Yan, W. Guo and S. Yang, Org. Lett., 2012, 14, 2214–2217 CrossRef CAS PubMed.
- F. Wudl, G. M. Smith and E. J. Hufnagel, Chem. Commun., 1970, 1453 RSC.
- (a) D. L. Coffen, J. Q. Chamber, D. R. Williams, P. E. Garret and N. D. Canfield, J. Am. Chem. Soc., 1971, 109 Search PubMed; (b) S. Hunig, G. Kiesslich, D. Scheutzow, R. Zahradnik and P. Carsky, Int. J. Sulfur Chem., Part C, 1971, 6, 109 Search PubMed.
- The first synthesis of TTF was reported in 1965 under the name bis-1,3-dithiolium. However, the electron donating ability of this molecule was not discovered until several years later. H. Prinzback, H. Berger and A. Luttringhaus, Angew. Chem., Int. Ed. Engl., 1965, 4, 435 CrossRef.
- J. Echer, Z. T. Li, P. Blanchard, N. Svenstrup, J. Lau, M. B. Nielsen and P. Leriche, Pure Appl. Chem., 1997, 69, 465 Search PubMed.
- (a) M. Salle, D. Canevet, J.-Y. Balandier, J. Lyskawa, G. Trippe, S. Goeb and F. Le Derf, Phosphorus Sulfur Silicon Relat. Elem., 2011, 186, 1153 CrossRef CAS; (b) D. Canevet, M. Salle, G. Zhang, D. Zhang and D. Zhu, Chem. Commun., 2009, 2245 RSC; (c) D. Lorcy, N. Bellec, M. Fourmigue and N. Avarvari, Coord. Chem. Rev., 2009, 253, 1398 CrossRef CAS; (d) H.-Q. Li, Y. X. Song and X. F. Peng, Chem. World, 2008, 49, 306 CAS; (e) R. Pauliukaite, A. Malinauskas, G. Zhylyak and U. E. Spichiger-Keller, Electroanalysis, 2007, 19, 2491 CrossRef CAS; (f) H. Q. Li, Y. K. Song, Q. F. Yin and D. B. Zhu, Org. Chem., 2007, 27, 1220 CAS; (g) Y. L. Zhu, Y. J. Yang, Q. F. Yin and D. B. Zhu, Org. Chem., 2005, 25, 1167 CAS; (h) A. El-Wareth, Tetrahedron, 2005, 61, 3889 CrossRef; (i) J. O. Jeppesen and J. Becher, Eur. J. Org. Chem., 2003, 3245 CrossRef CAS; (j) J. L. Segura and N. Martin, Angew. Chem., Int. Ed., 2001, 40, 1372 CrossRef CAS; (k) M. R. Bryce, J. Mater. Chem., 2000, 10, 589 RSC.
- TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene, ed. J. Yamada and T. Sugimoto, Springer, Berlin, 2004 Search PubMed.
- (a) N. Martin, L. Sanchez, M. A. Herranz, B. Illescas and D. M. Guldi, Acc. Chem. Res., 2007, 40, 1015–1024 CrossRef CAS PubMed; (b) J. L. Segura and N. Martin, Angew. Chem., Int. Ed., 2001, 40, 1372–1409 CrossRef CAS.
- (a) M. A. Herranz, N. Martin, J. Ramey and D. M. Guldi, Chem. Commun., 2002, 2968–2969 RSC; (b) F. Giacalone, J. L. Segura, N. Martin, J. Ramey and D. M. Guldi, Chem.–Eur. J., 2005, 11, 4819–4834 CrossRef CAS PubMed.
- A. Amacher, Ch. Yi, J. Yang, M. P. Bircher, Y. Fu, M. Cascella, M. Grätzel, S. Decurtins and S.-X. Liu, Chem. Commun., 2014, 50, 6540–6542 RSC.
- Y. Geng, F. Pop, Ch. Yi, N. Avarvari, M. Grätzel, S. Decurtins and S. X. Liu, New J. Chem., 2014, 38, 3269–3274 RSC.
- S. Wenger, P. A. Bouit, Q. Chen, J. Teuscher, D. DiCenso, R. Humphry Baker, J. E. Moser, J. L. Delgado, N. Martin, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 5164–5169 CrossRef CAS PubMed.
- A. Amacher, Ch. Yi, J. Yang, M. P. Bircher, Y. Fu, M. Cascella, M. Grätzel, S. Decurtins and S.-X. Liu, Chem. Commun., 2014, 50, 6540–6542 RSC.
- (a) L. Giribabu, N. Duvva, S. P. Singh, L. Han, I. M. Bedja, R. K. Gupta and A. Islam, Mater. Chem. Front., 2017, 1, 460–467 RSC; (b) L. Giribabu, N. Duvva, S. Prasanthkumar, S. P. Singh, L. Han, I. M. Bedja, R. K. Gupta and A. Islam, Sustainable Energy Fuels, 2017, 1, 345–353 RSC.
- (a) K. Guo, K. Yan, X. Lu, Y. Qiu, Z. Liu, J. Sun, F. Yan, W. Guo and S. Yang, Org. Lett., 2012, 14, 2114–2117 CrossRef PubMed; (b) Z. Wan, C. Jia, Y. Duan, X. Chen, Y. Lin and Y. Shi, Org. Electron., 2013, 14, 2132–2138 CrossRef CAS.
- Y. C. Chen, C.-Y. Hsu and J. T. Lin, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2121–2129 CrossRef CAS.
- Y. Wu, W.-H. Zhu, S. M. Zakeeruddin and M. Grätzel, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CAS.
- Y. Geng, F. Pop, C. Yi, N. Avarvari, M. Grätzel, S. Decurtins and S.-X. Liu, New J. Chem., 2014, 38, 3269–3274 RSC.
- C. A. Echeverry, M. A. Herranz, A. Ortiz, B. Insuasty and N. Martín, New J. Chem., 2014, 38, 5801–5807 RSC.
- K. Guo, K. Yan, X. Lu, Y. Qiu, Z. Liu, J. Sun, F. Yan, W. Guo and S. Yang, Org. Lett., 2012, 14, 2214 CrossRef CAS PubMed.
- Z. Wan, C. Jia, Y. Duan, X. Chen, Y. Lin and Y. Shi, Org. Electron., 2013, 14, 2132–2138 CrossRef CAS.
- Z. Wan, C. Jia, Y. Duan, X. Chen, Z. Li and Y. Lin, RSC Adv., 2014, 4, 34896–34903 RSC.
- Z. Wan, C. Jia, Y. Wang, J. Luo and X. Yao, Org. Electron., 2015, 27, 107–113 CrossRef CAS.
- T. H. Lee, C. Y. Hsu, Y. Y. Liao, H. H. Chou, H. Hughes and J. T. Lin, Chem. Asian J., 2014, 9, 1933–1942 CrossRef CAS PubMed.
- G. Sorohhov, C. Yi, M. Grätzel, S. Decurtins and S. Liu, Beilstein J. Org. Chem., 2015, 11, 1052–1059 CrossRef CAS PubMed.
- X. Zhang, F. Gou, D. Zhao, J. Shi, H. Gao, Z. Zhu and H. Jing, J. Power Sources, 2016, 324, 484–491 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00068e |
| This journal is © The Royal Society of Chemistry 2017 |