Polymer-assisted formation of 3D Pd nanoassemblies: highly active catalysts for formic acid electrooxidation†
Tan
Huang
ac,
Seung Ki
Moon
b and
Jong-Min
Lee
 *c
*c
aEnergy Research Institute@NTU, Interdisciplinary Graduate School, Nanyang Technological University, Singapore
bSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore
cSchool of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore. E-mail: jmlee@ntu.edu.sg
First published on 27th March 2017
Abstract
Three-dimensional (3D) Pd nanoassemblies were synthesized by a facile hydrothermal procedure, in which polyallylamine hydrochloride (PAH) was simultaneously used as a cross-linking and scaffold molecule in order to construct the 3D interconnected nanoassemblies. The formation process and mechanism of the Pd nanoassemblies were investigated using temporal TEM images. XRD was used to probe their crystal structure, which showed a clear appearance of metallic nanocrystallites with high index crystal facets. XPS was conducted to study surface elemental composition, which confirmed the physical and chemical integration of Pd and PAH. Finally, the 3D Pd nanoassemblies were used as catalysts for formic acid electro-oxidation. Compared to Pd bulk produced without PAH and commercial Pd black, the Pd nanostructure assemblies towards the electro-oxidation of formic acid exhibited higher electrochemical activity. This behavior is mainly due to their porous structure, high specific surface area which offers more catalytic sites, and their sufficient cavity space which enables facile charge transport of electrochemical reactions.
1 Introduction
Low-temperature polymer electrolyte membrane fuel cells, including direct formic acid fuel cells and direct alcohol fuel cells, have been intensively studied for transportation and portable electronics.1,2 However, their commercialization is impeded by the low electrocatalytic activity of electrocatalysts.3 As important catalytic electrode materials, Pd catalysts possess high intrinsic electrocatalytic properties for the electro-oxidation of an array of organic molecules, including methanol, formic acid and dimethyl ether,3,4 which make them ideal candidates as the anode materials for fuel cells. However, current superior electrocatalysts have been composed of Pd nanoparticles and carbon materials, and the Pd nanoparticles on the carbon supports would aggregate easily due to their high surface energy,5 which would cause low electrocatalytic activity.Constructing three-dimensional (3D) Pd nanoassemblies could improve the electrocatalytic activity of Pd electrode materials, because 3D nanoassemblies have many superior structural properties such as a large surface area, high porosity, and interconnected cavity space, which contribute to enhanced electrocatalytic performance.6,7 For example, Park et al. reported the synthesis of chestnut-bur-like Pd nanoassemblies, which show higher catalytic activity for electro-oxidation of ethanol, compared to cubic and octahedral Pd nanostructures;8 Han et al. fabricated Pd–Au alloy nanodendrites with improved electrocatalytic activity towards ethanol oxidation.9 Previously many studies adopted complicated protocols for constructing 3D nanoassemblies, which usually require multi-step reactions and sophisticated experimental skill.8,9 Therefore, it is necessary to design a facile synthesis approach for 3D nanoassemblies.
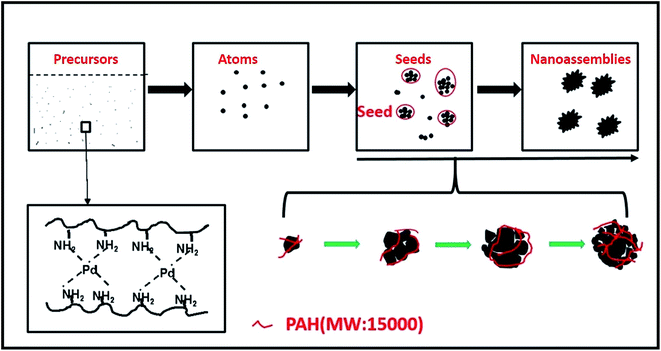
Herein, we used PAH acting as a macromolecular cross-linking unit in order to create uniform 3D Pd nanoassemblies through a simple and green method (Scheme 1). To the best of our knowledge, the observations of the growth process of 3D Pd nanoassemblies have rarely been reported. In this work, we observed the growth process of nanoassemblies during 0–5 hours by temporal TEM in order to study their formation mechanism, and such knowledge could be beneficial for more intricate nanoassembly design. As a proof of concept, we then investigated the electrocatalytic activity of the as-fabricated Pd nanoassemblies for formic acid electro-oxidation using cyclic voltammetry and transient I–t techniques, which demonstrated that Pd nanoassemblies had greater catalytic activity for formic acid electro-oxidation than commercial Pd black, because of their advantageous properties originating from their interconnected 3D nanostructures.
2 Experimental section
2.1 Synthesis of the Pd nanoassemblies
Experimentally, 2 mL of 0.025 M PdCl2 was added into 7.0 mL of water solution. 1.0 mL of 0.50 M PAH was added into the above-mentioned PdCl2 aqueous solution, generating a light brown flocculent precipitate (i.e. PAH–PdII complex solution). After sonication for 10 min, the precipitate flocculent was completely dissolved and a clear PAH–PdII complex solution was obtained. a 0.01 mol reducing agent (CH3OH) was added into the PAH–PdII complex solution, and after heating the mixture at 150 °C for 5 h, the products were collected by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 15 min and washed twice using water before characterization.
500 rpm for 15 min and washed twice using water before characterization.
In order to observe the growth process of our nanoparticles, 10 parallel above reactions were performed simultaneously, and they were labeled 1–10 respectively, from reaction 1–10, and they were terminated at 0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h, 3.0 h, 3.5 h, 4.0 h, 4.5 h, and 5.0 h. After all reactions were finished, the products were observed one by one under a TEM.
2.2 Physical characterization
The particle size distribution, morphology and growth process were studied using a transmission electron microscope (TEM, JEOL 2010) at 300 kV. Powder X-ray diffraction measurements were conducted using a Bruker D8 Advance X-ray Diffractometer, with Cu Kα radiation (λ = 0.15406 nm). X-ray photoelectron spectroscopy (XPS) tests were performed on a Thermo ESCALAB 250 with an Al anode. The nitrogen adsorption–desorption spectra of the samples were obtained by Brunauer–Emmett–Teller (BET) measurements using an Autosorb 6B at −196 °C. UV-vis measurements were carried out on a Prodigy column.2.3 Electrochemical measurements
Electrochemical measurements were conducted on a CHI 600C electrochemical analyzer using the standard three-electrode configuration, including a saturated calomel reference electrode (SCE) and a Pt plate auxiliary electrode. A modified glassy carbon electrode was prepared as follows: after 30 min of sonication of a mixture of 3 mg as-prepared catalysts and 6 mL H2O for generating distributed ink, 20 μL of the as-prepared ink was drop-cast on a pre-cleaned glassy carbon electrode (3 mm diameter, 0.07 cm2), which was dried at room temperature in air. Afterwards, a 5 μL Nafion 5.0 wt% solution was drop-cast on the modified electrode, and the electrode was dried again (28 μg cm2 loading of catalysts). Before the electrochemical measurements, N2 was flowed through the solution for 15 min to remove the dissolved O2. The electrochemically active surface area (ECSA) was determined from the integrated reduction charge of surface palladium oxide which was measured by cyclic voltammetry.3 Result and discussion
3.1 The Pd nanoassemblies' morphology and their growth mechanism
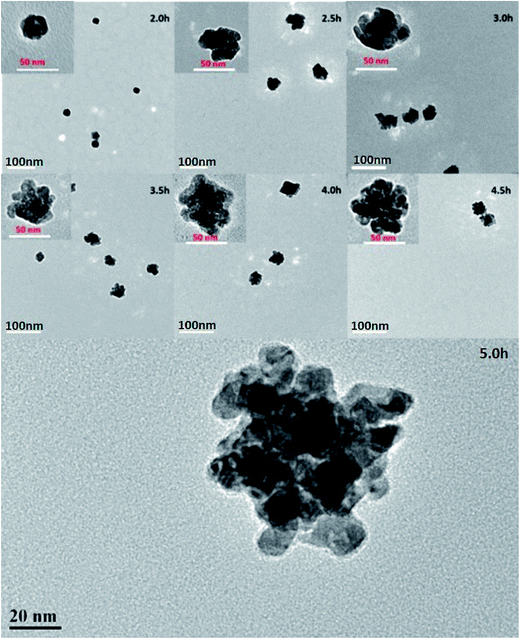
To investigate the morphology and nanoassembly size distribution of the as-prepared catalysts, electron microscopy TEM was employed to observe morphological changes, and Fig. 1 shows representative TEM images of the as-fabricated catalysts. In these images, the catalysts are relatively monodispersed nanoassemblies with a mean diameter of 56.12 nm (Fig. S1†), and their surfaces are rough with a high porosity. Fig. 1C shows that one Pd nanoassembly is a three-dimensional nanostructure, which is composed of a group of building units of Pd nanoparticles whose diameters are about 10 nm. In addition, at different Pd building units the lattice fringes spread across in a consistent way, which was demonstrated by the arrow in Fig. 1D. This could show that the Pd building units grow along the direction of the crystal facet of (111).10–12 Thus, we assume that the Pd seeds were formed first, and then the Pd nanoassemblies grow in a homoepitaxial way on the preformed Pd seeds (Scheme 2). Previous studies show that amine groups contribute to the formation of abundant Pd (111) facets in Pd nanoassemblies.13 Therefore, in the present work it was the coordination of amine that helped to stabilize the facet of (111), leading to the homoepitaxial growth along the (111) crystal facet.In order to elucidate the growth process of the Pd nanoassemblies, Pd nanocrystals at different reaction times were collected and observed by TEM (Fig. 2). Correspondingly, the initiate Pd nanocrystals (ca. 20 nm) were formed at 2.5–3.0 h. At t = 3.5 h, the nanocrystals were transformed into complete Pd nanoassemblies with ca. 50 nm size. From 4.0–4.5 h, the nanoparticles' diameter increased to ca. 60 nm. From 4.5–5.0 h, no obvious variation was observed for the Pd nanoassemblies in terms of size and shape, indicating the formation of fully mature Pd nanoassemblies. Based on these observations, the formation mechanism was proposed as shown in Scheme 2. Specifically, at the beginning, some polyhedron nanoparticles were formed and subsequently they acted as a nucleus; second Pd was reduced and deposited on the nucleus Pd surface to gain a three dimensional shape and finally Pd nanoassemblies grew larger at the end of 5 hours of reaction time.
To study the effect of PAH on the formation of Pd nanoassemblies, the synthesis reaction without PAH was conducted as a control. As demonstrated by Fig. S2A,† the Pd product had irregular shapes and displayed severe aggregation. This demonstrated that PAH, as a cross-linking and scaffold molecule, was essential for synthesizing Pd nanoparticles with a well-controlled morphology.14 The molecular basis could be that the PAH's nature of hydrophilicity and bulky molecular size make it as a good linker and scaffold molecule.15 Therefore, in the process with polyallylamine hydrochloride as a cross-linking unit, due to the unique physical and chemical properties of PAH, the formation process of Pd nanoassemblies can be described as follows: first, the complex of PdII–PAH was well dispersed in a water solution; second, the reaction solution was heated at 150 °C, the PdII was reduced to Pd0, and subsequently the Pd atoms nucleated to seeds; finally, the preformed seeds acted as the core to recruit the building blocks assisted by PAH to form well-defined Pd nanoassemblies due to the aforementioned physical and chemical properties of PAH. Therefore, the Pd nanoassemblies were monodispersed in a water solution, while Pd bulk and Pd black were precipitated (Fig. S5†). Furthermore, compared with that of Pd bulk produced without PAH, the binding energy of Pd 3d in the Pd nanoassemblies negatively shift ca. 0.3 eV, which should prove the interaction between Pd and PAH (Fig. 4C and D).16
3.2 Physical characterization of Pd nanoassemblies
Powder X-ray diffraction was conducted to probe the crystal structure of the Pd nanoassemblies. We identified the Pd nanoassembly crystal structure by comparing the XRD results of the as-fabricated products with a reference database. According to Fig. 3A, the XRD spectra of Pd nanoassemblies displayed four typical characteristic peaks (JCPDS standard 5-681): Pd(111), Pd(200), Pd(220) and Pd(311).10,12 Thus, Pd nanoassemblies have a classic face centered cubic structure with Pd(111) being the dominant face. In order to study the specific surface area and pore size distribution, we conducted nitrogen adsorption and desorption isotherm tests. Fig. 3B and C show the nitrogen adsorption–desorption isotherm and pore size distribution of the Pd nanoassemblies, respectively. The hysteresis loop in Fig. 3B showed the adsorption–desorption features of porous materials. Fig. 3C demonstrates that the Pd nanoassemblies were dominated by pores with sizes ca. 5–10 nm. The Brunauer–Emmett–Teller surface area of the Pd nanoassemblies was measured to be 68.5 m2 g−1, which is 7.7 times the value of 8.9 m2 g−1, which was calculated for the surface area of Pd dense spheres. To sum up, the results of XRD (Fig. 3A) and HRTEM (Fig. 2B) show that Pd nanoassemblies have abundant high index crystal facet, edge and corner atoms. The nitrogen adsorption–desorption isotherm is consistent with the TEM images, showing their porous nanostructure. All these results demonstrate the structural basis of the high electrocatalytic activity of Pd nanoassemblies. | ||
| Fig. 3 (A) XRD pattern of Pd nanoassemblies, (B) nitrogen adsorption–desorption isotherm, and (C) pore size distribution. | ||
In order to analyze the surface chemical composition of the Pd nanoassemblies, XPS tests were performed (Fig. 4). The Pd 3d spectrum of the Pd nanoassemblies corresponds to two pairs of doublets: Pd 3d3/2 (340.74 eV) and Pd 3d5/2 (335.47 eV), and Pd 3d3/2 (342.89 eV) and Pd 3d5/2 (337.88 eV), which can be attributed to the presence of Pd0 and PdIIO species, respectively (Fig. 4B).16 Moreover, by calculating and comparing the respective peak area, the atomic ratio of Pd0 and PdII in the Pd nanoassemblies could be confirmed to be 8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can prove that most of the PdII precursor had already been reduced to metallic Pd in the synthesis reaction. More notably, the presence of the N 1s peak also confirms the interaction of PAH molecules with the Pd (Fig. 4A).17 Specifically, the N 1s spectrum is composed of two peaks (Fig. 4A). The peak at 400.17 eV is assigned to the N 1s peak of –NH2 groups, the peak at 398.74 eV corresponds to the N atom integrated with the Pd. On the other hand, the binding energy values of Pd0 and PdIIO species in the Pd nanoassemblies negatively shift 0.3 eV compared to that of Pd bulk produced without PAH (Fig. 4C and D), which proves the chemical interaction of Pd and N.16
1, which can prove that most of the PdII precursor had already been reduced to metallic Pd in the synthesis reaction. More notably, the presence of the N 1s peak also confirms the interaction of PAH molecules with the Pd (Fig. 4A).17 Specifically, the N 1s spectrum is composed of two peaks (Fig. 4A). The peak at 400.17 eV is assigned to the N 1s peak of –NH2 groups, the peak at 398.74 eV corresponds to the N atom integrated with the Pd. On the other hand, the binding energy values of Pd0 and PdIIO species in the Pd nanoassemblies negatively shift 0.3 eV compared to that of Pd bulk produced without PAH (Fig. 4C and D), which proves the chemical interaction of Pd and N.16
 | ||
| Fig. 4 XPS figure of Pd nanoassemblies in the area of N 1s (A) and Pd 3d (B), and Pd nanoassemblies (C) and Pd bulk (D) in the Pd 3d region. | ||
3.3 Electrocatalytic activity of Pd catalysts for formic acid oxidation
Fig. S6–S8† show the cyclic voltammograms of (A) Pd nanoassemblies, (B) Pd bulk and (C) Pd black in the background solution (0.5 M H2SO4). The multiple peaks in the range from −0.25 to 0 V are attributed to oxidation of absorbed hydrogen and adsorbed hydrogen. Correspondingly, CVs in 0.5 M H2SO4 exhibit an oxidation reaction with their anodic peaks corresponding to A, B and C, along with the reduction peak of the oxide species at 0.491 V, 0.515 V and 0.507 V, respectively, during the reverse cathodic scan.We then investigated Pd nanostructure electrodes in the presence of HCOOH at room temperature. All the electrodes used were put in a mixed HCOOH and H2SO4 solution for 10 min to let the 3-electrode configuration achieve a stable state prior to electrochemical measurement.18Fig. 5A shows the cyclic voltammograms of 3 different Pd catalysts in a 0.5 M HCOOH + 0.5 M H2SO4 solution. For Pd nanoassemblies, the two sharp peaks in the anodic direction at 0.028 V and 0.200 V correspond to the hydrogen desorption and direct oxidation of formic acid.19–21 During the anodic scan, the oxidative peaks of the formic acid are located at 0.200 V for Pd nanoassemblies, 0.225 V for Pd bulk, 0.350 V for commercial black (Fig. 5D).22 More importantly, the formic acid oxidation mass activity peaks in the direct oxidation pathway for Pd nanoassemblies, Pd bulk and Pd black are 158 A g−1, 42 A g−1 and 35 A g−1 (Fig. 5C), respectively. The results show that the as-constructed Pd nanoassembly catalysts displayed the lowest peak potential (0.200 V vs. the SCE) and possessed the highest electrocatalytic activity (158 A g−1) among the three catalysts. I–t tests were conducted to further study the long term electrocatalytic performance of the Pd catalysts. Accordingly, Fig. 5B shows the I–t curves of the three catalysts at a constant potential (0.200 V vs. the SCE). During the whole testing period, the mass activity of Pd nanoassembly catalysts always outperformed the mass activity of Pd bulk and Pd black. In the end, the formic acid oxidation mass activity of the Pd nanoassembly, Pd bulk and Pd black at 2000 s is 7.41 A g−1, 0.218 A g−1 and 2.95 A g−1, proving that the Pd nanoassembly catalysts have the best electrocatalytic long term electrocatalytic performance. More notably, the Pd bulk's mass activity nearly decreased to zero, which in turn demonstrates the superiority of the Pd nanoassemblies constructed with PAH; while the commercial Pd black's mass activity showed a severe serrated curve in the I–t test, which indicates its unstable long term electrocatalytic performance and unsuitability for formic acid oxidation applications. To sum up, our well-constructed Pd nanoassemblies show substantial improvement compared to Pd bulk and commercial Pd black towards formic acid electro-oxidation.
The aforementioned superior electrochemical performance should arise from the three dimensional porous structure of Pd nanoassemblies.23 On one hand, the Pd nanoassemblies possess a three-dimensionally interconnected nanostructure which has more corner and edge sites, which is evidenced by Fig. S2B.† The increase of these atoms exhibits more open coordination sites which leads to boosting of the catalytic activity of the electrocatalysts.24 On the other hand, the three-dimensionally interconnected nanoassembly configuration has sufficient cavity space which facilitates charge transfer, thus enhancing catalytic reaction kinetics.25
4 Conclusion
In summary, we report a facile hydrothermal synthesis of Pd nanoassemblies, utilizing PAH as a cross-linking and scaffold molecule. We observed the growth process of the Pd nanoassemblies using temporal TEM, and proposed their formation mechanism based on the observing results and the synthesis protocol. The Pd nanoassemblies possess abundant cavity space and corner and edge atoms as revealed by HRTEM, and have a high specific surface area as exemplified by nitrogen adsorption–desorption isotherm tests. Therefore, we can safely deduce that the 3D porous nanostructure of Pd nanoassemblies leads to significantly enhanced catalytic activity for electro-oxidation of formic acid compared to commercial Pd black. This work developed a polymer-assisted method to construct 3D Pd nanoassemblies, where we proposed their formation mechanism, and such knowledge would be useful for constructing more intricate assemblies for energy conversion applications.Acknowledgements
This work is supported by Academic Research Fund (RG17/16) of Ministry of Education in Singapore.References
- J. M. Song, S. Y. Cha and W. M. Lee, J. Power Sources, 2001, 94, 78–84 CrossRef CAS.
- J.-H. Wee, Renewable Sustainable Energy Rev., 2007, 11, 1720–1738 CrossRef CAS.
- J.-H. Choi, K.-J. Jeong, Y. Dong, J. Han, T.-H. Lim, J.-S. Lee and Y.-E. Sung, J. Power Sources, 2006, 163, 71–75 CrossRef CAS.
- J.-H. Yu, H.-G. Choi and S. M. Cho, Electrochem. Commun., 2005, 7, 1385–1388 CrossRef CAS.
- Z. Jin, D. Nackashi, W. Lu, C. Kittrell and J. M. Tour, Chem. Mater., 2010, 22, 5695–5699 CrossRef CAS.
- S.-W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642–7643 CrossRef CAS PubMed.
- Y. Piao, Y. Jang, M. Shokouhimehr, I. S. Lee and T. Hyeon, Small, 2007, 3, 255–260 CrossRef CAS PubMed.
- S. J. Ye, D. Y. Kim, S. W. Kang, K. W. Choi, S. W. Han and O. O. Park, Nanoscale, 2014, 6, 4182–4187 RSC.
- Y. W. Lee, M. Kim, Y. Kim, S. W. Kang, J.-H. Lee and S. W. Han, J. Phys. Chem. C, 2010, 114, 7689–7693 CAS.
- S. Kim, D.-W. Lee and K.-Y. Lee, J. Mol. Catal. A: Chem., 2014, 383–384, 64–69 CrossRef CAS.
- H. Yoon, S. Ko and J. Jang, Chem. Commun., 2007, 1468–1470, 10.1039/b616660a.
- S. W. Kim, J. Park, Y. Jang, Y. Chung, S. Hwang and T. Hyeon, Nano Lett., 2003, 3, 1289–1291 CrossRef CAS.
- X. Huang, Z. Zhao, J. Fan, Y. Tan and N. Zheng, J. Am. Chem. Soc., 2011, 133, 4718–4721 CrossRef CAS PubMed.
- L. M. Rossi, I. M. Nangoi and N. J. Costa, Inorg. Chem., 2009, 48, 4640–4642 CrossRef CAS PubMed.
- T. Okubo, S. Kanayama, H. Ogawa, M. Hibino and K. Kimura, Colloid Polym. Sci., 2004, 282, 230–235 CAS.
- A. Drelinkiewicz, M. Hasik and M. Choczyński, Mater. Res. Bull., 1998, 33, 739–762 CrossRef CAS.
- J. R. Kitchin, J. K. Norskov, M. A. Barteau and J. G. Chen, Phys. Rev. Lett., 2004, 93, 156801 CrossRef CAS PubMed.
- Y. J. Gu and W. T. Wong, Langmuir, 2006, 22, 11447–11452 CrossRef CAS PubMed.
- Y. Zhou, J. Liu, J. Ye, Z. Zou, J. Ye, J. Gu, T. Yu and A. Yang, Electrochim. Acta, 2010, 55, 5024–5027 CrossRef CAS.
- W. Pan, X. Zhang, H. Ma and J. Zhang, J. Phys. Chem. C, 2008, 112, 2456–2461 CAS.
- J. Zhang, C. Qiu, H. Ma and X. Liu, J. Phys. Chem. C, 2008, 112, 13970–13975 CAS.
- R. Larsen, S. Ha, J. Zakzeski and R. I. Masel, J. Power Sources, 2006, 157, 78–84 CrossRef CAS.
- T. Sun, Z. Zhang, J. Xiao, C. Chen, F. Xiao, S. Wang and Y. Liu, Sci. Rep., 2013, 3, 2527 Search PubMed.
- D. Kim, Y. W. Lee, S. B. Lee and S. W. Han, Angew. Chem., 2012, 51, 159–163 CrossRef CAS PubMed.
- Y.-W. Lee and K.-W. Park, Catal. Commun., 2014, 55, 24–28 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00031f |
| This journal is © The Royal Society of Chemistry 2017 |