TOPO mediated rapid hydrothermal synthesis and study of electrochemical performance of nano-structured copper oxide thin films†
A. M.
Teli
 a,
G. J.
Navathe
a,
D. S.
Patil
b,
P. R.
Jadhav
a,
G. J.
Navathe
a,
D. S.
Patil
b,
P. R.
Jadhav
 ae,
S. B.
Patil
d,
T. D.
Dongale
e,
M. M.
Karanjkar
c,
Jae Cheol
Shin
b and
P. S.
Patil
*a
ae,
S. B.
Patil
d,
T. D.
Dongale
e,
M. M.
Karanjkar
c,
Jae Cheol
Shin
b and
P. S.
Patil
*a
aThin Film Materials Laboratory, Department of Physics, Shivaji University, Kolhapur 416004, Maharastra, India. E-mail: patilps_2000@yahoo.com
bDepartment of Physics, Yeungnam University, Gyeongsan, Gyeonbuk 38541, South Korea. E-mail: dipali.patilphy@gmail.com
cDepartment of Physics, Vivekanand College, Kolhapur – 416004, Maharashtra, India
dDepartment of Physics, Gogate Jogalekar College, Ratnagiri – 415612, Maharashtra, India
eDepartment of School of Nanoscience and Technology, Shivaji University, Kolhapur – 416004, Maharashtra, India
First published on 9th January 2017
Abstract
This paper reports the synthesis of trioctylphosphine oxide (TOPO) assisted copper oxide (CuO) electrodes by a rapid hydrothermal technique. The effects of the capping agent (TOPO) on structural, morphological and electrochemical properties for different concentrations of the Cu precursor were investigated. Surface morphology was examined by using scanning electron microscopy (SEM) which showed a yarn ball-like morphological structure for a lower concentration of the Cu precursor and rising of leaves onto the yarn balls was observed for higher concentrations. The cyclic voltammogram (CV) study showed the highest specific capacitance of about 143 F g−1 for CuOT3 at 10 mV s−1 and an energy density of 18.1 W h kg−1 at 1 mA cm−2 with about 82% capacitance retention after 1000 CV cycles at 100 mV s−1 in 1 M aq. KOH. Also the statistical model for capacitance retention and specific capacitance using the fitted exponential model was studied. Therefore, the specific capacitance can be tailored by appropriate selection of concentrations of the Cu precursor and TOPO.
Introduction
Nowadays, demand for energy storage is important due to the requirement of portable electronic devices and hybrid electric vehicles.1 Thus, the recent trend towards next-generation energy storage devices is electrochemical capacitors (ECs). ECs have higher power densities than batteries and higher energy densities than conventional capacitors, which has the ability to bridge the gap between batteries and conventional capacitors.2 ECs are used as a back-up memory for cameras, computers, clocks, clock radios, telephones, electronic toys, and office equipment. According to charge storage mechanisms, ECs are classified as Electric Double Layer Capacitors (EDLCs) and pseudocapacitors. Electric charges are stored in the interface between the active electrode and the electrolyte in the case of EDLCs, while in pseudocapacitors, charges are stored by the redox reaction followed by fast, reversible faradaic charge transfer between the active electrode and the electrolyte.3–5Materials like carbon based,6–9 conducting polymers10,11 and transition metal oxides12–15 with nanostructure designs were investigated for supercapacitor applications. Carbon-based materials have a high surface area (∼2500 m2 g−1) when used as an active electrode, however have a capacitance of only a few hundred F g−1.16 Compared to carbon-based materials, conducting polymers and transition metal oxides have higher energy density and higher theoretical (in thousands F g−1) specific capacitance, achieved by faradaic redox reactions. So research has been on the way to achieve high power and energy densities of transition metal oxides arising from multiple oxidation states as well as a multi-electron transfer during redox reactions.17
Metal oxides such as ruthenium oxide (RuO2) have a high theoretical specific capacitance (2000 F g−1) in 1.4 V wider potential window, good conductivity, and high reversible redox reaction, which make them an ideal electrode material for ECs.18,19 Due to its high cost and toxic nature, RuO2 cannot be commercialized.20 Here, the study of cupric oxide based supercapacitors is in focus because they indicate promising specific capacitance, good electrochemical properties, environmental harmlessness and low cost.20,21 However, the drawback of CuO as an electrode material is that it has much lower specific capacitance and low conductivity. It is, therefore, important to use the capping agent, trioctylphosphine oxide (TOPO), to overcome this problem. The presence of capping molecules facilitates controlled growth of nanocrystals in solution, which passivates the “bare” surface atoms with protecting groups. The capping agents protect the particle and control the growth.22 The bonding between the capping molecules and the precursors is noticeable; this bond is neither too weak nor too strong. If bonding between capping molecules and nanocrystals is very weak, then their particle growth is fast, forming bigger crystallites. On the other hand, growth of nanoparticles was hindered because of strong bonding between capping molecules and precursors.23 The size (growth rate) of nanoparticles is influenced by the attaching and detaching of capping molecules to the surface. It limits the crystal growth and provides the stability of the structure in solution.24 The stability of TOPO-assisted CuO nanostructures is due to the high affinity of TOPO for the Cu2+ ions. Bulky coating of capping agents on the surface of nanocrystals reduces the growth rate of crystals.25 A capping agent that binds a metal ion also provides an easy path for flow of electrons. Generally, TOPO is used for the preparation of quantum dots.26 Capping agents affect the fundamentals of growth kinetics, structural control, morphology, dimensionality and protective barrier to prevent degradation.27,28 Of these ways, we used a capping agent to control the shape of the nanostructure for tuning properties and the overall functionality of their proposed application, using a low-cost chemical method.29,30
Recently, we have reported CuO nanobuds despite a specific capacitance of about 60 F g−1 in [HPMIM][Cl] ionic liquid as the electrolyte.31 CuO was fabricated by different methods to investigate the changes in structures, morphologies, and electrochemical properties. Shaikh et al.32 reported Ru-doped CuO nanocrystals, which were prepared by the colloidal solution method and achieved a specific capacitance of about 131 F g−1 in −0.2 and +0.5 V vs. SCE at 20 mV s−1. In addition, CuO flower-like nanostructures and CuO nano belts displayed a specific capacitance of 133.6 F g−1 in 6 M KOH electrolyte and 62 F g−1 1 M LiPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC electrolyte, respectively.33
DEC electrolyte, respectively.33
Earlier some successful efforts have been made to synthesize CuO thin films using microwave irradiation,34 chemical bath deposition,35 electrodeposition36 and solvothermal methods at high temperatures or via complex and template-assisted methods to generate bulk nanostructures of CuO.37,38 Usually, the powdered form of metal oxides was obtained by the hydrothermal method and further, by using additives and binders, the powder is applied onto a current collector in order to make an electrode.
In this paper, we report a novel and rapid synthesis of capping agent (TOPO) assisted CuO thin films directly on stainless steel by a hydrothermal method. The shape, size and crystalline nature of the CuO nanostructure can be changed by using TOPO. Also, we examine the change in specific capacitance by varying the concentration of the Cu precursor. Here we use 1 M aqueous KOH for electrochemical measurements within a wide potential window of 0.55 V (0 to +0.55 V vs. SCE).
Experimental details
The capping agent (TOPO) assisted nanostructured CuO thin films were deposited on stainless steel substrates by a hydrothermal technique. Analytical grade copper sulfate (CuSO4) was used as a precursor for Cu, ammonia (NH3) as a reducing agent and trioctylphosphine oxide as a capping agent. All chemicals were purchased from SD Fine Pvt. Ltd., India and used without further purification for the synthesis of CuO thin films. Double-distilled water (DDW) and ethanol were used as solvents for the chemical synthesis.Preparation of solution
The experimental procedure was the same as our previous paper.31 Here we study the effects of the capping agent on the concentration of the Cu precursor to tune the morphology of CuO thin film electrodes. The typical procedure contains following steps. TOPO solution was prepared by dissolving 0.05 M TOPO in 10 mL of ethanol, with constant stirring. Then 0.1 M CuSO4 was dissolved in 50 mL DDW. Ammonia (NH3) solution was added to the CuSO4 solution until the solution became transparent blue. 2 mL of the as-prepared TOPO solution was added into CuSO4 solution with constant stirring, then a polished stainless steel substrate was immersed in the final solution by inclining it to the wall of the beaker and then kept in an autoclave at 90 °C for 5 min. After cooling the autoclave gradually to room temperature, the stainless steel substrate was removed. The substrate was dipped in ethanol for 1 h to remove the TOPO content from the CuO electrode and rinsed thoroughly using DDW. The sample was dried at room temperature. The thus obtained CuO thin film is abbreviated as CuOT1.The deposited mass on the substrate was calculated by using weight difference method. We repeated the above procedure for the concentrations of the Cu precursor as 0.2, 0.3, 0.4 and 0.5, respectively, but the concentration of TOPO for all concentrations of CuSO4 was 0.05 M. These thin films were abbreviated as CuOT2, CuOT3, CuOT4, and CuOT5, respectively. The comparative results of the bare CuO electrode with the CuSO4 concentration of 0.3 M have been illustrated in the ESI.† For all depositions, the temperature and time of the autoclave were kept at 90 °C and 5 min, respectively.
Characterization of the prepared samples
The X-ray diffraction (XRD) patterns of the prepared samples were recorded using a X-ray powder diffractometer (Bruker AXS Analytical Instruments Pvt. Ltd., Germany, Model: D2 phaser). The morphology was examined using a scanning electron microscope (SEM) (Model JEOL-JSM-6360, Japan), operated at 18 kV. The specific surface area of the powder was measured using the Brunauer–Emmett–Teller (BET) method, at liquid nitrogen temperatures, using N2 gas as an absorbent (Quantachrome NOVA 1000e, USA). The electrochemical measurements were performed in an electrolyte of 1 M KOH in a conventional three-electrode arrangement comprising a graphite counter electrode and Saturated Calomel Electrode (SCE) serving as the reference electrode, by using a potentiostat (PGSTAT302N Metrohmautolab, Netherland).Results and discussion
Possible formation mechanism of the CuO nanostructure
The overall chemical reactions for the preparation of CuO nanostructures can be explained as the reaction between CuSO4 and NH3 solution resulting in the formation of copper hydroxide Cu(OH)2 and ammonium sulphate (NH4)2SO4. Initially, six water molecules (ligands) surrounded copper. When aqueous ammonia is added, the water molecules are replaced by ammonia molecules. There is a significant color change observed from pale blue to a deep blue color.39 The possible reaction mechanism is as below:| CuSO4 + 2H2O → [Cu(H2O)6]2+ + SO42− | (1) |
With the addition of a small amount of aq. (NH3), precipitation occurs,
| [Cu(H2O)6]2+ + SO42− + 2NH4OH → [Cu(H2O)4(OH)2] + (NH4)2SO4 | (2) |
With the addition of excess amount of aq. (NH3), the precipitates dissolve and the solution becomes transparent blue,
| [Cu(H2O)4(OH)2] + (NH4)2SO4 + 2NH4OH → [Cu(NH3)4(H2O)2]2+ + SO42− + 8H2O | (3) |
The solution is then subjected to hydrothermal treatment,
 | (4) |
All these deposited samples were subjected to structural, morphological and electrochemical characterization.
Fig. 1A shows the XRD patterns of TOPO-assisted nanostructured CuO electrodes (CuO-3, CuOT1, CuOT2, CuOT3, CuOT4 and CuOT5) on the stainless steel substrate at various concentrations of the Cu precursor. The diffraction angle 2θ was varied between 20° and 90°. The peaks observed at 35.5° and 38.1° with dhkl planes corresponding to (−111) and (111) are in good agreement with the XRD patterns of the standard ICDD card (96-101-1149), confirming the formation of the polycrystalline CuO material. The peaks at 43.19°, 44.12°, 50.30° and 74.32° are observed for all samples due to the stainless steel substrate which is marked as ‘Δ’. It was observed that the peak intensity (crystallinity) increases with an increase in the concentration of the Cu precursor (CuOT1 to CuOT5).40 The absence of any other peak indicates that there is no formation of impurities such as Cu2O, indicating the formation of single-phase CuO with a monoclinic structure.40
To investigate the effects of the capping agent (TOPO) on the morphology of the CuO electrode, the samples were characterized by SEM. Fig. 1B shows the surface morphologies of all the samples (CuOT1–CuOT5) at ×15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnifications. The SEM image of the CuOT1 sample [Fig. 1B(a)] revealed a yarn ball-like microstructure with a compact arrangement. As the concentration of the Cu precursor increases, petal-like nanostructures are grown on the (yarn ball-like) microstructure [Fig. 1B(b–e)]. For sample CuOT3, the SEM image resembles a porous nature with the presence of nano-petals on the yarn ball-like microstructure. Further increase in the concentration of the Cu precursor (CuOT4 and CuOT5) causes agglomeration of nano-petals on the microstructure, which decreases the active surface area and porosity.41
000 magnifications. The SEM image of the CuOT1 sample [Fig. 1B(a)] revealed a yarn ball-like microstructure with a compact arrangement. As the concentration of the Cu precursor increases, petal-like nanostructures are grown on the (yarn ball-like) microstructure [Fig. 1B(b–e)]. For sample CuOT3, the SEM image resembles a porous nature with the presence of nano-petals on the yarn ball-like microstructure. Further increase in the concentration of the Cu precursor (CuOT4 and CuOT5) causes agglomeration of nano-petals on the microstructure, which decreases the active surface area and porosity.41
The growth mechanism of CuO with and without TOPO is mentioned schematically in Fig. 1C. Initially Cu2+ and a small amount of NH3 from aqueous solution react to generate the Cu(OH)2 precipitate. After the excess addition of NH3, there is dissolution of the Cu(OH)2 precipitate forming ([Cu(NH3)4(H2O2)]2+), a dark blue coloured CuO nano-particle solution.42–44 CuO nano-particles tend to self-assemble to decrease the energy of the system, forming eventually the CuO nano-particles. The van der Waals force and hydrogen bonds between the molecules make the CuO nano-petals assemble into bundles. The Ostwald-ripening mechanism called dissolution/recrystallization takes place during the hydrothermal processing.45 Thus, the thermodynamically-driven spontaneous process occurs because larger particles are more energetically favored than smaller ones. Therefore, the heating caused aggregation of CuO nano-petals by van der Waals forces, which is consistent with the intrinsic anisotropic properties of monoclinic CuO.46,47
The capping agent TOPO plays an important role in the nuclei and growth of metal oxide crystals through the selective and kinetic adsorption on the surface of particles.48,49 The TOPO molecules kinetically control the growth rates of crystallographic planes of monoclinic CuO, and also increase the solubility of Cu(OH)2 and CuO.50 The bundles are transformed into petals by introducing TOPO and thermal dehydration, which results in the development of nano-petals on the CuO microstructure and recrystallization of CuO under hydrothermal conditions.51,52 After that, by increasing the concentration of CuSO4, there is growth and recrystallization, leading to the aggregation of nano-petals on CuO microstructures. The electrochemical measurements of TOPO-assisted CuO electrodes are compared with those of bare CuO-3 electrodes [see the ESI†].
Fig. 2A–E show the specific surface area and pore-size distribution study of the TOPO-assisted CuO samples performed by N2 adsorption and desorption. The isotherms are shown in Fig. 2A–E with the inset figures showing the corresponding BJH pore size distribution plot of samples. Nitrogen isotherms of all CuO samples are type IV and present H3 type hysteresis.53 The BET specific surface area and the pore radius of all samples are given in Table 1. The capping agent TOPO modifies the surface area of CuO samples with a change in the concentration of the Cu precursor. It was observed that from sample CuOT1 to CuOT3, the surface area increases with increasing pore size. The sample CuOT1 shows a yarn ball-like microstructure with a less surface area and a small pore size. For the CuOT2 sample nano-petals were observed on the yarn ball-like microstructure, which increased the surface area and pore size. The sample CuOT3 shows a high surface area (24.12 m2 g−1) and (4.8 nm) pore size distribution. The ratio of the concentration of TOPO and the concentration of the Cu precursor, which implies the variation of the pore size distribution, correlates with the structure and packing i.e. after increasing the concentration of the Cu precursor, the (CuOT4 and CuOT5) surface area and pore size decrease, because of agglomeration of nano-petals on the yarn ball-like microstructure. The nano-petals on the yarn ball-like microstructure have a good surface area and pore radius, which provide more active sites for the redox reaction, useful for the energy storage mechanism. The less specific surface area and small pore radius due to the agglomeration of nano-petals on the yarn ball-like microstructure reduce the charge storage capacity of the CuO electrodes in supercapacitor applications.
 | ||
| Fig. 2 N2 adsorption–desorption isotherms (A) CuOT1, (B) CuOT2, (C) CuOT3, (D) CuOT4 and (E) CuOT5. Inset: pore size distribution curves. | ||
| Sample code | Surface area (m2 g−1) | Average pore radius (nm) | Deposited wt (g) | Specific capacitance (F g−1) | Energy density (W h kg−1) | Series resistance Rs (Ω) | Charge transfer resistance Rct (Ω) |
|---|---|---|---|---|---|---|---|
| CuOT1 | 17.92 | 3.8 | 0.0013 | 53 | 5.7 | 0.47 | 1.08 |
| CuOT2 | 23.26 | 4.3 | 0.0015 | 127 | 15.5 | 0.57 | 0.79 |
| CuOT3 | 24.12 | 4.8 | 0.0016 | 143 | 18.1 | 0.53 | 0.76 |
| CuOT4 | 20.38 | 4.7 | 0.0023 | 113 | 11.3 | 0.38 | 0.67 |
| CuOT5 | 21.15 | 4.4 | 0.0033 | 97 | 10.9 | 0.54 | 0.92 |
To measure redox potentials and the effects of the capping agent on the electrochemical performance at different concentrations of the Cu precursor, cyclic voltammograms of all TOPO-assisted CuO samples were recorded within the potential range of 0 to +0.55 V vs. SCE at 10 mV s−1 scan rate in 1 M aq. KOH. All CuO samples exhibit the flow of cathodic current associated with the Cu2+ ↔ Cu+ reduction process. Similarly, anodic current flows are associated with the Cu+ ↔ Cu2+ oxidation process. The redox process in the cell has following steps.32,54
| ½Cu2O + OH− → CuO + ½H2O + e− | (5) |
| ½Cu2O + ½H2O + OH− → Cu(OH) + e− | (6) |
| CuOH + OH− → CuO + H2O + e− | (7) |
| CuOH + OH− → Cu(OH)2 + e− | (8) |
Fig. 3A shows the recorded CV curves, for the TOPO-assisted CuO sample with different concentrations of the Cu precursor at 10 mV s−1 exhibiting pseudocapacitance behavior. The broad anodic peak can be attributed to the oxidation of Cu2O and/or Cu(OH) to both CuO and/or Cu(OH)2. The broad cathodic peak can be ascribed to the reduction of CuO and/or Cu(OH)2 to Cu2O and/or Cu(OH). The peak broadness is due to the overlap of the redox peaks given in eqn (5)–(8). The CV did not show any distinct peaks, which is ascribed to the complex redox reactions of CuO in alkaline solution.55,56 The electrochemical capacitance is mostly due to redox reactions rather than the electric double layer capacitance having a regular rectangular CV shape.
As the concentration of the Cu precursor increases from 0.1 M to 0.3 M, the specific capacitance increases from 53 to 143 F g−1, which is because of the large active surface area along with a large pore size in contact with the electrolyte. Further increment in the concentration of the Cu precursor (CuOT4 and CuOT5) causes a decrease in the specific capacitance which is related to the agglomeration of nanostructures observed from SEM, reduction in the surface area and pore size distribution, and consequently a less active surface in contact with the electrolyte. The specific capacitance of each film was calculated from CV curves using the following equation57
 | (9) |
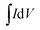 is the integrated area of the CV curve, M is the mass of the active material, dV is the potential range, and S is the scan rate.
is the integrated area of the CV curve, M is the mass of the active material, dV is the potential range, and S is the scan rate.
The inset of Fig. 3A shows the specific capacitance of all TOPO-assisted CuO samples. Table 1 lists the sample codes along with the deposited mass, specific capacitance and energy density of CuO electrodes. The maximum specific capacitance of about 143 F g−1 is observed for sample CuOT3 at the 10 mV s−1 scan rate. The charge storage mechanism is generally based on the adsorption and intercalation including surface and bulk phenomena in the CuO electrode.33,58 The first possible mechanism is the intercalation and de-intercalation of smaller or bigger alkali metal cations in the matrix of the material during the reduction and oxidation processes, respectively.33 The other possible mechanism is based on the adsorption of the ions on the surface, rather than in the bulk of the sample.35,36 It is realized from the CV curve (Fig. 3B) that current density increases as the scan rate increases, and the potential of the anodic peak moves in a positive direction and those of the cathodic peaks towards negative directions. This is due to the fact that at lower scan rates both outer and inner pore surfaces of the porous electrode materials are effectively utilized for intercalation, while the outer region of pores is mainly utilized by ions at high scan rates.35,57
Cycling stability is an important requirement for practical application of supercapacitor devices. The cycling stability of the CuOT3 sample, which has higher specific capacitance, was tested over 1000 CV cycles at a 100 mV s−1 scan rate. Fig. 6 shows the plot of variation of specific capacitance with the number of CV cycles and the percentage stability of the CuOT3 electrode. It was observed that 82% capacitance was retained after 1000 cycles for the CuOT3 sample. TOPO plays an important role in capping Cu2+ ions due to which stable and controlled growth of nanostructured CuO occurs. The CuOT3 sample did not peel off during CV cycles. The reason for decreased capacitance with the number of cycles may involve the change in the microstructure, the active surface area loss and an increase in resistance.
Fig. 4 shows the charge–discharge curves of all TOPO-assisted CuO electrodes at 1 mA cm−2 current density in the potential range between 0 and +0.53 V vs. SCE using 1 M aq. KOH electrolyte. It was observed that the shape of the charge–discharge curve is triangular in nature, which leads to ideal capacitive behavior; the charge–discharge curve is almost symmetrical. Usually, three steps are involved during the discharging curve such as (1) internal resistance of the material due to an initial drop in the voltage. (2) The linear variation of potential with respect to time, which corresponds to double layer capacitance formation at the electrode and electrolyte interface. (3) The slope variation of potential with time is attributed to the redox reaction between the electrolyte and electrode material.59 The specific energy of all TOPO-assisted CuO electrodes was calculated from the discharge cycle using the following equation:
 | (10) |
 | ||
| Fig. 4 Galvanostatic charge discharge curves of (a) CuOT1, (b) CuOT2, (c) CuOT3, (d) CuOT4 and (e) CuOT5 at 1 mA cm−2 current density. | ||
Electrochemical impedance spectroscopy (EIS) gives useful information about series resistance and charge transfer resistance (Rct) of a material. Fig. 5 shows the Nyquist plot of TOPO-assisted CuO for different concentrations of CuSO4 at open circuit potential in 1 M aq. KOH. The frequency varies from 1 Hz to 10 kHz. The Nyquist plot intercept at the X-axis corresponds to series resistance, which is due to electronic and ionic resistances. A semicircle which appears in the high-frequency region in the Nyquist plot describes the charge transfer resistance which represents the faradaic reaction and double-layer capacitance at the electrode–electrolyte interface.60 Series resistance and charge transfer resistance play an important role in electrochemistry. Among all the samples, the CuOT4 sample shows lower series resistance (≈Rs = 0.38 Ω) and charge transfer resistance (≈Rct = 0.67 Ω). Table 1 summarizes the sample codes along with series resistance (Rs) and charge transfer resistance (Rct) of TOPO-assisted CuO samples. However, the CuOT3 sample shows higher specific capacitance which is due to the high active surface area (24.12 m2 g−1) and not due to the series resistance (0.53 Ω) and charge transfer resistance (0.76 Ω) and these are not much less than those of the CuOT4 sample. Here we studied electrochemical measurements such as cyclic voltammograms, charge–discharge curves and electrochemical impedance spectroscopy for comparison of bare CuO3 and CuOT3 [see the ESI†].
 | ||
| Fig. 5 Nyquist plot of samples (a) CuOT1, (b) CuOT2, (c) CuOT3, (d) CuOT4 and (e) CuOT5 in 1 M aq. KOH electrolyte. | ||
Statistical model of the supercapacitor
In some real life situations, statistical methods are used to model the sample data, which are important for the prediction of future events. In general, statistical modeling deals with the mathematical equation(s) which consist of one or more random variables. In the present study, we have used the exponential decay model to describe the degradation process of the CuO supercapacitor. In general, the exponential decay model is defined as,| f(t) = ae−b×t, t > 0 | (11) |
From the capacitance retention and specific capacitance dataset, it is clear that the capacitance retention of the supercapacitor decreases uniformly. We fit the exponential decay model to describe the degradation process. The exponential decay model for capacitance retention is given as,
| f1(t) = a1e−b1×t | (12) |
The exponential decay model for specific capacitance is given as,
| f2(t) = a2e−b2×t | (13) |
| f1(t) = 101.00272e−0.00022154×t | (14) |
Similarly, the least square estimate parameters for the specific capacitance are found to be a2 = 74.53607892 and b2 = 0.0002216. Thus, the fitted exponential decay model for specific capacitance can be written as,
| f2(t) = 74.53607892e−0.0002216×t | (15) |
The predicted values of capacitance retention and specific capacitance for various cycle numbers are shown in Table 2. The predicted results have less error, which suggested the suitability of the model. Such models can be useful for the integrated development environments (IDEs) for large circuit simulations and predictive modeling of electronic components.62
| Cycle number | Actual capacitance retention | Predicted capacitance retention | Actual specific capacitance | Predicted specific capacitance |
|---|---|---|---|---|
| 100 | 100 | 98.78970585 | 73.8 | 72.90252603 |
| 200 | 97.0123 | 96.62518365 | 71.6 | 71.30477452 |
| 300 | 94.3761 | 94.508087 | 69.7 | 69.74203977 |
| 400 | 91.82777 | 92.43737679 | 67.7 | 68.21355433 |
| 500 | 89.68366 | 90.41203667 | 66.2 | 66.7185676 |
| 600 | 87.75044 | 88.43107257 | 64.8 | 65.2563454 |
| 700 | 85.92267 | 86.49351219 | 63.3 | 63.82616966 |
| 800 | 84.32337 | 84.59840454 | 62.2 | 62.42733803 |
| 900 | 83.21617 | 82.74481946 | 61.4 | 61.05916358 |
| 1000 | 81.89807 | 80.93184717 | 60.5 | 59.72097442 |
| 1100 | — | 79.15859784 | — | 58.41211337 |
| 1200 | — | 77.42420111 | — | 57.13193767 |
| 1300 | — | 75.72780571 | — | 55.87981865 |
| 1400 | — | 74.06857902 | — | 54.6551414 |
| 1500 | — | 72.44570665 | — | 53.45730452 |
| 1600 | — | 70.85839207 | — | 52.28571976 |
| 1700 | — | 69.30585619 | — | 51.13981176 |
| 1800 | — | 67.787337 | — | 50.01901779 |
| 1900 | — | 66.30208917 | — | 48.92278745 |
| 2000 | — | 64.84938372 | — | 47.85058239 |
| 2500 | — | 58.0495762 | — | 42.83190586 |
| 3000 | — | 51.96276516 | — | 38.3395994 |
| 3500 | — | 46.51418908 | — | 34.31845613 |
| 4000 | — | 41.63692558 | — | 30.71905939 |
| 4500 | — | 37.27106944 | — | 27.49717546 |
| 5000 | — | 33.36299687 | — | 24.61320996 |
Conclusions
A novel and rapid synthesis of binder- and additive-free CuO thin films deposited on stainless steel by a hydrothermal method which is eco-friendly, simple and low cost has been reported. Effects on surface morphology and electrochemical performance of TOPO-assisted CuO by simultaneously varying the concentrations of the Cu precursor, have been studied. The highest specific capacitance of about 143 F g−1 at 10 mV s−1 in 1 M aq. KOH was achieved for sample CuOT3. The CuOT3 sample has a porous yarn-ball-like microstructure with nano-petals responsible for low charge transfer resistance, high surface area, and consequently high capacitance. The cycling stability of this electrode was measured and 82% capacitance retention after 1000 CV cycles at 100 mV s−1 was observed. The TOPO is a good capping agent for metal oxides, which modifies the morphology of the material, leading to an increased electrochemical performance. Therefore, it is an excellent candidate as a capping agent for other metal oxides to improve their performance.Acknowledgements
Authors PSP and PRJ wish to acknowledge the CSIR, New Delhi for financial assistance through major research project (sanction no. 03/(1240)/12/EMR-II). All authors are thankful to PIFC Department of Physics, Shivaji University, Kolhapur for providing the instrumental facility.References
- B. E. Conway, Electrochemical Supercapacitors Scientific Fundamentals and Technological Applications, Kluwer Academic/Plenum Publisher, 1999 Search PubMed.
- K. Leitner, A. Lerf, M. Winter, J. Besenhard, S. Villar-Rodil, F. Suarez-Garcia, A. Martinez-Alonso and J. Tascon, Nomex-derived activated carbon fibers as electrode materials in carbon based supercapacitors, J. Power Sources, 2006, 153, 419 CrossRef CAS.
- A. Pandolfo and A. Hollenkamp, Carbon properties and their role in supercapacitors, J. Power Sources, 2006, 157, 11 CrossRef CAS.
- M. Shao, F. Ning, Y. Zhao, J. Zhao, M. Wei, D. G. Evans and X. Duan, Core–shell layered double hydroxide microspheres with tunable interior architecture for supercapacitors, Chem. Mater., 2012, 24, 1192 CrossRef CAS.
- R. Zou, K. Xu, T. Wang, G. He, Q. Liu, X. Liu, Z. Zhang and J. Hu, Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors, J. Mater. Chem. A, 2013, 1, 8560 CAS.
- T. Hsieh, C. Chuang, W. Chen, J. Huang, W. Chen and C. Shu, Hydrous ruthenium dioxide/multi-walled carbon-nanotube/titanium electrodes for supercapacitors, Carbon, 2012, 50, 1740 CrossRef CAS.
- H. Zhang, G. Cao, Z. Wang, Y. Yang and Z. Gu, Electrochemical capacitive properties of carbon nanotube arrays directly grown on glassy carbon and tantalum foils, Carbon, 2008, 46, 822–824 CrossRef CAS.
- J. Chang, C. Lin and W. Tsai, Manganese oxide/carbon composite electrodes for electrochemical capacitors, Electrochem. Commun., 2004, 666–671 CrossRef CAS.
- X. Li, W. Gan and H. Ma, Preparation of active carbon/RuO2 composite electrode and its properties, Rare Met. Mater. Eng., 2012, 41(4), 733 CAS.
- G. Snook, P. Kao and A. Best, Conducting-polymer-based supercapacitor devices and electrodes, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- R. Liu, J. Duay and T. Lane, Synthesis and characterization of RuO2/poly(3,4-ethylenedioxythiophene) composite nanotubes for supercapacitors, Phys. Chem. Chem. Phys., 2010, 12(17), 4309 RSC.
- J. Chang, M. Lee and W. Tsai, In situ Mn K-edge X-ray absorption spectroscopic studies of anodically deposited manganese oxide with relevance to supercapacitor applications, J. Power Sources, 2007, 166, 590–594 CrossRef CAS.
- Y. Guo, Y. Hu, W. Sigle and J. Maier, Superior electrode performance of nanostructured mesoporous TiO2 (Anatase) through efficient hierarchical mixed conducting networks, Adv. Mater., 2007, 19, 2087–2091 CrossRef CAS.
- C. Hu, H. Guo, K. Chang and C. Huang, Anodic composite deposition of RuO2·XH2O–TiO2 for electrochemical supercapacitors, Electrochem. Commun., 2009, 11, 1631–1634 CrossRef CAS.
- L. Mao, K. Zhang, H. S. On Chan and J. Wu, J. Mater. Chem., 2012, 22(no. 5), 1845 RSC.
- T. Zheng and J. Huang, The Limitations of Energy Density for Electrochemical Capacitors, J. Electrochem. Soc., 1997, 144, 2026 CrossRef.
- W. Deng, X. Ji, Q. Chen and C. E. Banks, Electrochemical capacitors utilising transition metal oxides: an update of recent developments, RSC Adv., 2011, 1, 1171 RSC.
- H. Lee, M. Cho, I. Kim, J. Do Nam and Y. Lee, RuOx/polypyrrole nanocomposite electrode for electrochemical capacitors, Synth. Met., 2010, 160, 1055–1059 CrossRef CAS.
- Z. Wu, D. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H. Cheng, Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- C. C. Hu, W.-C. Chen and K.-H. Chang, How to Achieve Maximum Utilization of Hydrous Ruthenium Oxide for Supercapacitors, J. Electrochem. Soc., 2004, 151, A281–A290 CrossRef CAS.
- G. Wang, J. Huang, S. Chen, Y. Gao and D. Cao, Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam, J. Power Sources, 2011, 196, 5756–5760 CrossRef CAS.
- D. Jung, J. Kim, C. Nahm, H. Choi, S. Nam and B. Park, Review paper: semiconductor nanoparticles with surface passivation and surface plasmon, Electron. Mater. Lett., 2011, 7, 185–194 CrossRef CAS.
- D. Onwudiwe, M. Hrubaru and E. Ebenso, Synthesis, Structural and Optical Properties of TOPO and HDA Capped Cadmium Sulphide Nanocrystals, and the Effect of Capping Ligand Concentration, J. Nanomater., 2015, 9, 1–9 CrossRef.
- M. Hassan and A. Ali, Synthesis of nanostructured cadmium and zinc sulfides in aqueous solutions of hyperbranched polyethyleneimine, J. Cryst. Growth, 2008, 310, 5252–5258 CrossRef CAS.
- L. Manna, E. Scher and A. Alivisatos, Shape control of colloidal semiconductor nanocrystals, J. Cluster Sci., 2002, 13, 521–532 CrossRef CAS.
- M. Ghosh and C. N. R. Rao, Solvothermal synthesis of CdO and CuO nanocrystals, Chem. Phys. Lett., 2004, 393(4–6), 493–497 CrossRef CAS.
- X. Wang, W. Tian, T. Zhai, C. Zhi, Y. Bando and D. Golberg, Cobalt(II,III) oxide hollow structures: fabrication, properties and applications, J. Mater. Chem., 2012, 22, 23310 RSC.
- G. Gund, D. Dubal, D. Dhawale, S. Shinde and C. Lokhande, Porous CuO nanosheet clusters prepared by a surfactant assisted hydrothermal method for high performance supercapacitors, RSC Adv., 2013, 3, 24099 RSC.
- L. Vayssieres, A. Hagfeldt and S. E. Lindquist, Purpose-built metal oxide nanomaterials. The emergence of a new generation of smart materials, Pure Appl. Chem., 2000, 72, 47–52 CrossRef CAS.
- S. Jung, E. Oh, K. Lee, Y. Yang, C. Park, W. Park and S. Jeong, Sonochemical preparation of shape-selective ZnO nanostructures, Cryst. Growth Des., 2008, 8, 265–269 CAS.
- G. Navathe, D. Patil, P. Jadhav, D. Awale, A. Teli, S. Bhise, S. Kolekar, M. Karanjkar, J. H. Kim and P. Patil, Rapid synthesis of nanostructured copper oxide for electrochemical supercapacitor based on novel [HPMIM][Cl] ionic liquid, J. Electroanal. Chem., 2015, 738, 170–175 CrossRef CAS.
- J. Shaikh, R. Pawar, R. Devan, Y. Ma, P. Salvi, S. Kolekar and P. Patil, Synthesis and characterization of Ru doped CuO thin films for supercapacitor based on Bronsted acidic ionic liquid, Electrochim. Acta, 2011, 56, 2127–2134 CrossRef CAS.
- H. Zhang and M. Zhang, Synthesis of CuO nanocrystalline and their application as electrode materials for capacitors, Mater. Chem. Phys., 2008, 108, 184–187 CrossRef CAS.
- H. Wang, J. Xu, J. Zhu and H. Chen, Preparation of CuO nanoparticles by microwave irradiation, J. Cryst. Growth, 2002, 244, 88–94 CrossRef CAS.
- D. Dubal, D. Dhawale, R. Salunkhe, V. Jamdade and C. Lokhande, Fabrication of copper oxide multilayer nanosheets for supercapacitor application, J. Alloys Compd., 2010, 492, 26–30 CrossRef CAS.
- D. Dubal, G. Gund, C. Lokhande and R. Holze, CuO cauliflowers for supercapacitor application: Novel potentiodynamic deposition, Mater. Res. Bull., 2013, 48, 923 CrossRef CAS.
- Z. Zhang, H. Che, Y. Wang, J. Gao, X. She, J. Sun, Z. Zhong and F. Su, Flower-like CuO microspheres with enhanced catalytic performance for dimethyldichlorosilane synthesis, Catal. Sci. Technol., 2012, 2, 2254 CAS.
- A. Moura, L. Cavalcante, J. Sczancoski, D. Stroppa, E. Paris, A. Ramirez, J. Varela and E. Longo, Structure and growth mechanism of CuO plates obtained by microwave-hydrothermal without surfactants, Adv. Powder Technol., 2010, 21, 197–202 CrossRef CAS.
- S. Jung, E. Oh, K. Lee, Y. Yang, C. Park, W. Park and S. Jeong, Sonochemical preparation of shape-selective ZnO nanostructures, Cryst. Growth Des., 2008, 8, 265–269 CAS.
- P. Chand, A. Gaur, A. Kumar and U. Gau, Effect of NaOH molar concentration on morphology, optical and ferroelectric properties of hydrothermally grown CuO nanoplates, Mater. Sci. Semicond. Process., 2015, 38, 72–80 CrossRef CAS.
- H. Chen, M. Zhou, T. Wang, F. Lia and Y. Zhang, Construction of unique cupric oxide–manganese dioxide core–shell arrays on a copper grid for high performance supercapacitors, J. Mater. Chem. A, 2016, 4, 10786–10793 CAS.
- M. Huang, F. Li, F. Dong, Y. Zhang and L. Zhang, MnO2-based nanostructures for high-performance supercapacitors, J. Mater. Chem. A, 2015, 3, 21380–21423 CAS.
- Y. Zou, Y. Li, Y. Guo, Q. Zhou and D. An, Ultrasound-assisted synthesis of CuO nanostructures templated by cotton fibers, Mater. Res. Bull., 2012, 47, 3135–3140 CrossRef CAS.
- S. Seo, D. Lee, J. Kim, G. Lee and D. Kim, Room-temperature synthesis of CuO/graphene nanocomposite electrodes for high lithium storage capacity, Ceram. Int., 2013, 39, 1749–1755 CrossRef CAS.
- D. Lia, Y. Leung, A. Djuris, Z. Liua, M. Xie, J. Gao and W. Chan, CuO nanostructures prepared by a chemical method, J. Cryst. Growth, 2005, 282, 105–111 CrossRef.
- D. Volanti, D. Keyson, L. Cavalcante, A. Simões, M. Joya, E. Longo, J. Varela, P. Pizani and A. Souza, Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave, J. Alloys Compd., 2008, 459, 537–542 CrossRef CAS.
- D. Keyson, D. Volanti, L. Cavalcante, A. Simões, J. Varela and E. Longo, CuO urchin-nanostructures synthesized from a domestic hydrothermal microwave method, Mater. Res. Bull., 2008, 43, 771–775 CrossRef CAS.
- Z. Hong, Y. Cao and J. Deng, A convenient alcohothermal approach for low temperature synthesis of CuO nanoparticles, Mater. Lett., 2002, 52, 34–38 CrossRef CAS.
- R. Srivastava, M. Prathap and R. Kore, Morphologically controlled synthesis of copper oxides and their catalytic applications in the synthesis of propargylamine and oxidative degradation of methylene blue, Colloids Surf., A, 2011, 392, 271–282 CrossRef CAS.
- X. Zhang, G. Wang, X. Liu, J. Wu, M. Li, J. Gu, H. Liu and B. Fang, Different CuO Nanostructures: Synthesis, Characterization, and Applications for Glucose Sensors, J. Phys. Chem. C, 2008, 112, 16845–16849 CAS.
- J. Wang, S. He, Z. Li, X. Jing, M. Zhang and Z. Jiang, Synthesis of chrysalis-like CuO nanocrystals and their catalytic activity in the thermal decomposition of ammonium perchlorate, J. Chem. Sci., 2009, 121, 1077–1081 CrossRef CAS.
- C. Lu, L. Qi, J. Yang, D. Zhang, N. Wu and J. Ma, Simple Template-Free Solution Route for the Controlled Synthesis of Cu(OH)2 and CuO Nanostructures, J. Phys. Chem. B, 2004, 108, 17825–17831 CrossRef CAS.
- IUPAC Recommendations, Pure Appl. Chem., 1994, 66, 1739 Search PubMed.
- J. Shaikh, R. Pawar, A. Moholkar, J. Kim and P. Patil, CuO–PAA hybrid films: Chemical synthesis and supercapacitor behavior, Appl. Surf. Sci., 2011, 257, 4389–4397 CrossRef CAS.
- G. Brisard, J. Rudnicki, F. Mclarnon and E. Cairns, Application of probe beam deflection to study the electrooxidation of copper in alkaline media, Electrochim. Acta, 1995, 40, 859–865 CrossRef CAS.
- M. Jayalakshmi and K. Balasubramanian, Cyclic voltammetric behavior of copper powder immobilized on paraffin impregnated graphite electrode in dilute alkali solution, Int. J. Electrochem. Sci., 2008, 3, 1277–1287 CAS.
- D. Patil, J. Shaikh, D. Dalavi, M. Karanjkar, R. Devan, Y. Ma and P. Patil, An Mn Doped Polyaniline Electrode for Electrochemical Supercapacitor, J. Electrochem. Soc., 2011, 158, A653–A657 CrossRef CAS.
- V. Patake, S. Joshi, C. Lokhande and O. Joo, Electrodeposited porous and amorphous copper oxide film for application in supercapacitor, Mater. Chem. Phys., 2009, 114, 6–9 CrossRef CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Preparation of ruthenic acid nanosheets and utilization of its interlayer surface for electrochemical energy storage, Angew. Chem., Int. Ed., 2003, 42, 4092–4096 CrossRef CAS PubMed.
- Z. Wen, Q. Qu, Q. Gao, X. Zheng, Z. Hu, Y. Wu, Y. Liu and X. Wang, An activated carbon with high capacitance from carbonization of a resorcinol-formaldehyde resin, Electrochem. Commun., 2009, 11, 715–718 CrossRef CAS.
- G. Casella and R. Berger, Statistical Inference, Duxbury Advanced Series, Pacific Grove, CA, 2nd edn, 2002 Search PubMed.
- T. Dongale, P. Jadhav, G. Navathe, J. Kim, M. Karanjkar and P. Patil, Development of nano fiber MnO2 thin film electrode and cyclic voltammetry behavior modeling using artificial neural network for supercapacitor application, Mater. Sci. Semicond. Process., 2015, 36, 43–48 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6se00016a |
| This journal is © The Royal Society of Chemistry 2017 |



