Cellulose nanocrystals in nanoarchitectonics – towards photonic functional materials
Michael
Giese
 * and
Matthias
Spengler
* and
Matthias
Spengler
Institute of Organic Chemistry, University Duisburg-Essen, Universitätsstr. 7, 45141 Essen, Germany. E-mail: michael.giese@uni-due.de
First published on 6th December 2018
Abstract
The physical and chemical properties of nanomaterials do not only rely on the composition of the building blocks, but also on the nano-scaled structure itself. Therefore, a deep understanding of these materials calls for a close cooperation between various research fields such as physics, chemistry, materials science, engineering and biology. Nanoarchitectonics aims to unify the efforts towards the design and development of novel functional nanomaterials by combining methods of all these disciplines. The present review summarizes the recent achievements in the development of photonic functional materials based on cellulose nanocrystals (CNCs) or CNC templating. The unique self-assembly of CNCs to chiral nematic structures allows for the development of a variety of functional materials with application potential in photonic sensing, tunable reflectors or optoelectronics.
Design, System, ApplicationNanoarchitectonics forms an interdisciplinary bridge between a variety of research fields such as physics, chemistry, engineering and nanoscience towards the design of novel functional materials. In this respect, the evaporation induced self-assembly (EISA) of cellulose nanocrystals (CNCs) into chiral nematic structures has gained considerable attention for the design and fabrication of photonic functional materials. These chiral nematic structures with dimensions of the wavelength of visible light represent one-dimensional photonic crystals, which appear colorful due to the selective refraction of left-handed circularly polarized light. Transferring this structure into materials provides application potential for the development of new photonic sensors, filters, and reflectors or optoelectronic devices. |
Introduction
Materials with nanosized structural features in the regime of 1 to 100 nm have unique properties, which make them appealing for a variety of applications in e.g. electronics,1 optics,2 magnetism,3 energy storage,4 and electrochemistry.5,6 The properties of such materials are determined not only by the nature of the individual building blocks, but also by the mesoscopic orientation thereof. Therefore, one of the most important challenges in the field of nanomaterials is the development of efficient design and fabrication methods, which calls for highly interdisciplinary cooperation between researchers from several research fields.7This challenge is picked up by the concept of nanoarchitectonics, which aims to unify the efforts in physics, chemistry, materials science, engineering and biology to gain precise control over dimensions, compositions, and defects of materials on the atomic and molecular level for the design and fabrication of high-performance functional materials.8–14 Nanoarchitectonics combines methodologies from nanotechnology, supramolecular chemistry, self-assembly and organization and DNA nanotechnology to create new functional materials (Scheme 1).15–19
 | ||
| Scheme 1 Schematic illustration of the concept of nanoarchitectonics. Starting on the molecular level, self-assembly yields functional nanostructures. | ||
A promising approach for the fabrication of new nanomaterials is to employ self-assembly of molecular templates. Transferring the intrinsic structural information of these templates allows for the control of dimensions, periodicity and structure of the final material and provides access to a plethora of functional nanostructures.20 In this context lyotropic liquid crystals (LLCs) have gained considerable attention.21,22 Soft-templating in general starts with the suspension of a suitable LLC as template and the subsequent mixing with a compatible precursor. After evaporation-induced self-assembly (EISA) a nanostructured composite is obtained. Selective removal of the template finally yields a nanostructured porous material (Scheme 2). This method provides access to a variety of new functional materials and enables further post-synthetic modification of the surface or the pores as well as hard-templating. However, to ensure successful transfer of the intrinsic structural features a number of issues have to be considered:
• Compatibility of the precursors with the LLC phase and the solvent: all components should be miscible, the polarity of precursor and LLC should be similar. Furthermore, concentration, ionic strength and pH of the solution have to be compatible with the LLC (e.g. ionic precursors or extreme pH values will cause gelation and disturb the LLC phase).
• Kinetics of the condensation/polymerization reaction that forms the new material must be compatible with the self-assembly process of the template to ensure the formation of the LLC phase prior to complete solidification of the matrix around it.
• Byproducts of the condensation/polymerization should be volatile or compatible with the LLC phase of the template (e.g. MeOH, H2O).
If these parameters are aligned, the LLC phase will form above the critical concentration and a nanostructured composite will eventually be formed (Scheme 2),23 whereby the template imprints the structural features of the LC phase as a negative copy into the resulting material. Removal of the template yields porous materials with structural features on the nano-scale.
In this context, cellulose nanocrystals (CNCs) have been identified as highly promising templates for the preparation of photonic nanomaterials. They combine biocompatibility and copious availability with the unique self-assembly behaviour yielding chiral nematic superstructures providing materials with fascinating optical properties.
The focus of the present review is a view on the recent employment of CNCs to generate photonic structures with application potential in sensing and optoelectronics. For a more comprehensive overview of cellulose nanocrystals and materials based on CNCs we refer to the recent literature.24–30
The first chapter of the review focuses on the preparation of CNCs and their properties, especially their self-assembly into chiral nematic structures. Subsequently, the direct use of CNC films as photonic sensors and reflectors is summarized and the use of CNCs as template for functional nanomaterials is discussed. Finally, a short conclusion and perspective towards future developments in the use of CNCs for photonic applications will be given.
Cellulose nanocrystals – origin and properties
Natural polymers such as cellulose are highly appealing for the development of sustainable materials since they are biocompatible and degradable as well as renewable, non-toxic and inexpensive.27 With respect to functional materials, CNCs have gained considerable attention due to their fascinating electromagnetic and photonic properties.27 CNCs may be obtained by acid hydrolysis of wood, plant, or marine animal biomass.31 The treatment of cellulose with sulfuric acid, for example, selectively hydrolyses the amorphous parts of the cellulose microfibrils, yielding nanocrystalline rods (Fig. 1A) with some surface hydroxyl groups converted to sulfate esters.32–34 The dimensions of these nano rods depend on the chosen treatment as well as on the cellulose source and varies from 50 to 1000 nm in length and about ∼5 to 50 nm in diameter.35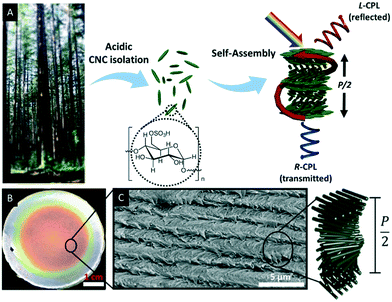 | ||
| Fig. 1 A) Schematic illustration of the CNC isolation. Acid hydrolysis of bulk cellulose material selectively removes amorphous cellulose to yield cellulose nanocrystals, which have a spindle-like morphology and can self-assemble into chiral nematic liquid crystalline phases, which can be preserved in freestanding solid films. These films selectively reflect left circularly polarized light L-CPL) of the wavelength determined by the helical pitch (P/2). Right circularly polarized light (R-CPL) penetrates the films without intensity loss; B) photograph of CNC film as obtained upon EISA; C) scanning electron micrograph of the edge of a CNC film showing the twisted layered structure. Reproduced and adapted with permission from the American Chemical Society.40 | ||
In the late 1950s, Marchessault et al. discovered the lyotropic liquid crystalline behaviour of CNCs,36 which was later identified as chiral nematic or cholesteric phase by Revol et al.37 Interestingly, the chiral nematic phase of CNC suspensions occurs at low critical concentrations, between 3 wt% and 7 wt%. A seminal work with respect to the development of photonic materials based on CNCs was reported in the late 1990s by Gray and co-workers. They were able to retain the chiral nematic organization of the CNCs suspension in solid films by evaporation-induced self-assembly (EISA) as proven by scanning electron microscopy (SEM).38,39Fig. 1B and C show a photograph and SEM image, respectively, of a typical CNC film as recently reported by Tran et al.40
The chiral nematic mesostructure of the CNC films enables interesting photonic properties since it represents a one-dimensional photonic crystal. Photonic crystals are materials with periodically changing refractive indices in one, two or three dimensions since they may selectively diffract certain wavelengths of light.41 For chiral nematic structures, the reflected wavelength depends on the pitch (P) of the helical structure, the refractive index contrast (navg) of the compound and the angle of the incident light (sin(θ)).42
| λmax = navg·P·sin(θ) |
The wavelength of the reflected colour of CNC films can be tailored across the visible spectrum by various methods such as changing the polarity and ionic strength of the suspension,43 ultra-sonication, variation of the drying conditions44 and by magnetic45 or electric fields.46 The manipulation of the structural colouration is mainly attributed to changes in the helical pitch of the chiral nematic structure. When the ionic strength of the suspension is increased by addition of salt for example, the electrostatic repulsion between the CNCs is decreased due to the surface charge of the CNCs introduced by the sulfate esters, yielding a contraction of the helical pitch and therefore a blue-shifted reflectance of the resulting films. It is important to note that the colour tuning is limited to specific ionic strength ranges and pH changes. Stronger changes in the ionic strengths will disturb the lyotropic liquid crystalline phase and may lead to gelation or precipitation.
In 2018, MacLachlan and coworkers investigated the EISA process of aqueous CNC suspensions as a function of time.40,47 The colour of the reflected light significantly red-shifted with increasing the evaporation times leading to a higher homogeneity of the resulting CNC films as proven by systematic POM and SEM studies. The authors assign this behaviour to the tactoid annealing as intermediate step between phase separation and the gel vitrification.
Recently Frka-Petesic et al. reinvestigated the self-assembly behaviour of CNCs in the presence of magnetic fields.48 By using small commercial magnets (0.5–1.2 T) they were able to tune the photonic properties of CNC films during EISA and showed unprecedented control of their angular response. As proof of concept, they were able to transfer the pattern of a polydomain magnet into a photonic pattern of the CNC film (Fig. 2A and B).
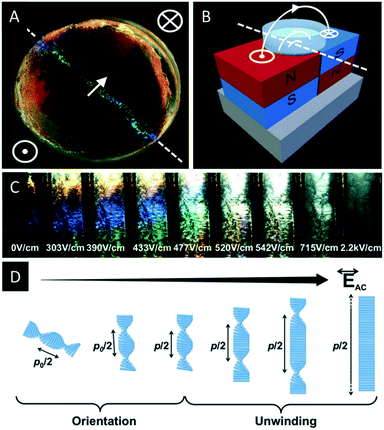 | ||
| Fig. 2 CNC films (A) obtained by evaporation in the presence of a patterned magnetic field (B) as well as evolution of the iridescence of a CNC suspension upon application of an electrical field (C). The change is attributed to the unwinding of the chiral nematic structure caused by the electrical field (D). Reproduced and adapted with permission from John Wiley and Sons.48,49 | ||
The same author also investigated the control of the iridescence of chiral nematic CNC suspensions by electric fields.49 It was found that applying an electric field to an apolar suspension of CNCs leads to an unwinding of the helical structure (Fig. 2C and D). At low electric fields a red-shift of the reflected colour is observed, finally leading to a nematic orientation of the CNCs at high electric fields (>510 V cm−1).
Nguyen et al. identified the substrate on which the CNC films were casted as a further influential factor.50 Their results indicate that the reflected colour of the dried CNC films red-shifts with decreasing polarity of the substrate surface.
Cheung et al. investigated the impact of the counter ion to the surface sulfate esters on the EISA process.51 In their systematic study they neutralized acidic CNC suspensions with different hydroxides. With increasing size of the cation, a blue shift in the reflected colour was observed while increasing the hydrophobicity of the alkylammonium cations led to a red shift of the signal. In addition, the counter ion also has an impact on the critical concentration for the formation of the chiral nematic phase as well as on the thermal stability and redispersibility of the dried films. While the acidic form of the CNCs (CNC-H) are not soluble in organic solvents such as DMF or DMSO their counter parts with sodium or potassium cations are readily dispersible in these solvents.52,53
Lizundia et al. reported a detailed study on the surface modification of CNC films and investigated the impact on the photonic properties.54 Therefore, the common CNC films, bearing sulfate groups on their surface, were obtained by EISA. Subsequently, these films were modified by TEMPO-oxidation, acetylation, desulfation and cationization and characterized with respect to their structural, thermal and optical properties. It was found that the modified CNC films showed a superior thermal stability and a significant change in the reflected colour (Fig. 3A), depending on the surface treatment. In-turn, the surface modification provides control over the swelling behaviour of the CNC films in polar solvents (Fig. 3B), which might be useful for sensing applications.
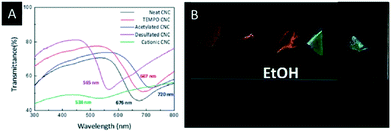 | ||
| Fig. 3 A) UV-vis spectra of the surface-modified CNC films; B) photograph of the different surface modified CNC films soaked in ethanol. Reproduced and adapted with permission from the Royal Chemical Society.54 | ||
Functional CNC materials
The use of CNCs as template for nanostructured materials with photonic properties has gained tremendous attention since the discovery of Gray and co-workers that the structure of the chiral nematic lyotropic phase of CNC suspensions can be retained in solid films.38,39 Materials chemists have been inspired by colouration due to diffraction of nanoscale structures for decades, as it offers a pathway to colours that can be tuned, will not fade and do not require toxic dyes.55,56Tuning the polarization of CNC films
As mentioned before, CNC films usually reflect left circularly polarized light, while right circularly polarized light is transmitted. In 2017, Godinho and coworkers reported an interesting approach to control the handedness of the circularly polarized light reflected by CNC films.57 They took advantage of the heterogeneity of the chiral nematic structure of CNC films attributed to the different non-equilibrium stages during the EISA process. They used gaps in the material and infiltrated these with 4-cyano-4′-pentylbiphenyl (5CB), a nematic liquid crystal. The cellulose films align the 5CB molecules parallel to the surface, thus letting it act as λ/2 retardation plate, converting the left circularly polarized light into right circularly polarized light and vice versa.58 In order to explain the photonic behaviour of their hybrid materials, the authors suggest a simplified model with two layers of cholesteric CNC films with different pitches (520 and 600 nm, Fig. 4A). The left-handed circularly polarized light is completely reflected by the green cholesteric domain, λ0 ≈ 520 nm of the top layer, while the right-handed circularly polarized light is transmitted without any loss. The 5CB layer, acting as λ/2 retardation plate, changes the right-handed circular polarization into the left-handed one. The converted left-handed circularly polarized light is then selectively reflected by the second cholesteric layer (λ0 ≈ 600 nm). On its way back, it crosses the anisotropic 5CB layer again and the left-handed circular polarization is reversed back into the right-handed one, which penetrates the top layer without losses (Fig. 4B). Consequently, both left- and right-handed circularly polarized light is successfully reflected by the hybrid material. The use of a liquid crystal as an anisotropic λ/2 retardation plate allows to tune the reflection of R-CPL or L-CPL by electric fields and by temperature (Fig. 4C and D).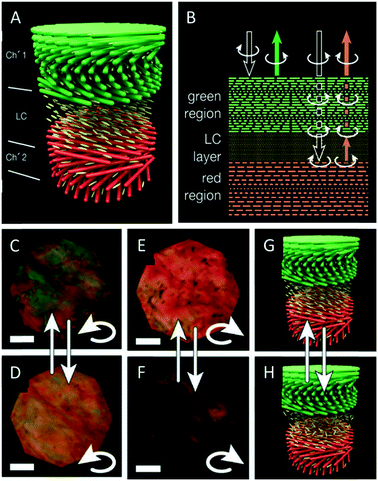 | ||
| Fig. 4 Schematic representation of the CNC films showing the gap between two chiral nematic layers filled with 5CB (A) as well as a scheme clarifying the reflection and transmittance of polarized light by the CNC films (B). The thermal induced tuning of the polarization of the reflected light is demonstrated (C–H). Reproduced and adapted with permission from John Wiley and Sons.57 | ||
Hiratani et al. manipulated the polarization of the reflected light of CNC films by employing quasinematic CNC films.59 The quasinematic CNC films were obtained by EISA from highly concentrated, aqueous CNC suspensions (∼10 wt%). Subsequently, they mounted the quasinematic CNC layer (CF5) on top of an iridescent and chiral nematic CNC film (CF1). Irradiation of this stack of CNC films led to a depolarization of R-CPL by the quasinematic layer (Fig. 5A). This effect was used to implement photonic patterns into the multilayered CNC films, which can be recognized by L- or R-circularly polarized filters (CPF) (Fig. 5B–D). Therefore, the “UBC” pattern was drop-casted as quasinematic layer (CF5) on a chiral nematic CNC film (CF1). When observed by the naked eye, the “UBC” pattern is only visible as an artefact. Upon observation under CPF, however, the pattern becomes as distinct bright letters on a black background under R-CPF and the reverse image is visible under L-CPF. Just recently, Zheng et al. investigated the ability of photonic cellulose hybrids to generate and manipulate circularly polarized light.60 They could show that incorporation of achiral luminophores into photonic cellulose films allows for selective manipulation of R-CPL emission.
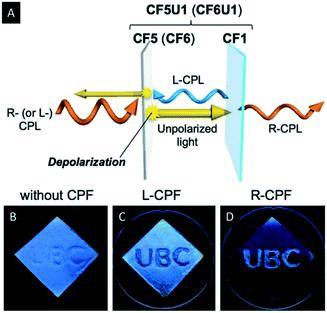 | ||
| Fig. 5 Schematic representation illustrating the CPL reflection and transmission mechanism through the double-layered samples (A) as well as photographs of the photonic pattern on CF1 as observed by the naked eye (B) under L-CPF (C) and R-CPF (D). Reproduced and adapted with permission from John Wiley and Sons.59 | ||
Functional CNC blends
In the following section a series of CNC composites or CNC films with additives will be introduced and their use as photonic sensors and reflectors will be described. These materials are obtained by co-assembling CNCs with additives during EISA (Scheme 3), which may range from small organic compounds, to nanoparticles (NPs) to polymers. In some cases, these additives were polymerized to obtain a more durable composite material.In 2017, Zhou and coworkers reported iridescent CNC/poly(ethylene glycol) (PEG) blends which showed improved mechanical strength and thermal stability compared to pristine CNC films.61 The authors were able to tune the colour of the composite films from blue to red by variation of the PEG content (Fig. 6A and B). In addition, the authors proved the reversible colour change between green and transparent upon increase of the relative humidity (Fig. 6C and D).
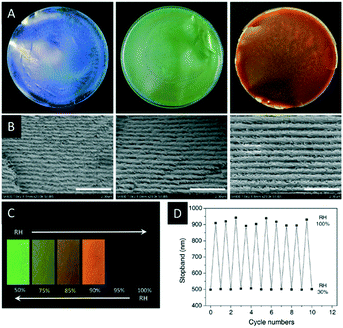 | ||
| Fig. 6 Photographs of the CNC films with varying PEG content (A, PEG wt%: 10, 20, 30; diameter of the films: 9 cm) as well as the corresponding SEM images showing the changing helical pitch (B, scale bar: 2 μm). The water uptake of CNC-2/PEG composite (80/20) films at different relative humidity (RH, C) as well as a plot demonstrating the reversible shift of the reflected colour (D). Reproduced and adapted with permission from John Wiley and Sons.61 | ||
Very similar results were found by Xu et al., who reported on CNC blends with glycerol.62 Also in this case improved mechanical properties were found and the potential for photonic sensing of the relative humidity was demonstrated.
Another example is the addition of different surfactants to CNC dispersions to yield CNC films whose structural colour can be tuned across the visible light spectrum by varying the amount and the type of surfactant.63
As already mentioned before, Cheung et al. investigated the impact of the counter cation in CNC suspensions (CNC-X, X = Li+, Na+, K+, NH4+, NMe4+, NBu4+) and the solubility of the freeze-dried CNCs.51 While the acidic CNC-H form is exclusively soluble in aqueous media, the CNC-Xs are readily dispersible in polar aprotic organic solvents such as dimethylsulfoxide (DMSO), formamide, N-methylformamide (NMF) or dimethylformamide (DMF). The authors attribute the improved dispersibility to the neutralization of the surface sulfate species, which leads to a weakening of the interparticle and solvent-particle H-bonding. The CNC-Xs suspensions in polar organic solvents formed chiral nematic phases yielding chiral photonic films upon EISA (Fig. 7A).
 | ||
| Fig. 7 POM image of 3% CNC-Na suspension in DMF showing the formation of spherulite like structures during evaporation (A). Photograph of an iridescent CNC/polymer composite film (B) as reported by Cheung et al. photographs of CNC/WPU composite films demonstrating multiple functions with the selected “ink” (water or sodium chloride solution) under different conditions (C). Reproduced and adapted with permission from the American Chemical Society.51,64 | ||
Since DMF dissolves a variety of polymers such as polystyrene (PS), poly(methyl methacrylate) (PMMA), polycarbonate (PC) and poly(9-vinylcarbazole) (PVK), the authors were able to demonstrate the formation of photonic polymer composite films by mixing DMF solutions of these polymers with CNC-X dispersions. Upon slow evaporation of DMF under dry conditions iridescent composite films (Fig. 7B) with a homogenous chiral nematic structure were obtained, as proven by SEM. The reflected colour of the composite films ranged from ∼500 nm (PC) to ∼800 nm (PS), indicating a significant impact of hydrophobicity of the polymers on the EISA of CNCs.
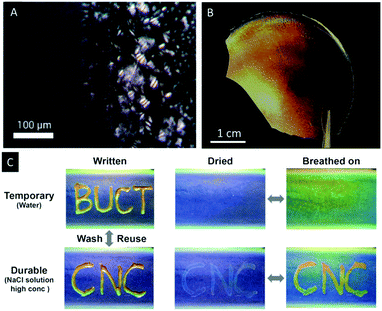
Wan et al. reported a similar approach to CNC/polymer composites by mixing waterborne polyurethane (WPU) latex particles with aqueous CNC suspensions.64 The authors were able to adjust the colour of the composites by changing the CNC/WPU ratio and found fast and reversible response to water, wet gases and NaCl solutions. In addition, the potential as photonic paper was demonstrated by treating a composite sample of blue colour (CNC/WUF = 20 wt%) with a concentrated sodium chloride solution (Fig. 7C). The wetted areas swelled and showed a red-shift of the wavelength of the UV/Vis signal from ∼350 to 385 nm.
A completely different approach was followed by Gilman and coworkers who mixed short wood-derived cellulose nanocrystals (w-CNCs) with different amounts of long tunicate-derived CNCs (t-CNCs).65 This led to an increased overlap length of the CNC nanorods in the resulting films enhancing the shear transfer and energetic barriers to deformation. The obtained materials showed improved mechanical properties and superior resilience to cracking upon straining.
Although these examples already show the great potential for CNC and CNC/polymer composite films in applications like photonic sensors and reflectors as well as for security features on documents, these applications remain significantly limited due to the fact that solvents, especially water, will redisperse the materials and thereby destroy the nanostructure causing the photonic behaviour to disappear.
In order to tackle this challenge, Kelly et al. reported a series of responsive photonic hydrogels by self-assembly of an aqueous CNC dispersion with various hydrogel monomers,66 including acrylamide (AAm), N-isopropylacrylamide (NIPAm), acrylic acid (AAc), 2-hydroxyethylmethacrylate (HEMa), polyethylene glycol dimethacrylate (DiPEGMa), and polyethylene glycol methacrylate (PEGMa). The monomers were combined with a crosslinker, a photo initiator and an aqueous suspension of CNCs. After EISA, the chiral nematic structure was locked in place by UV-initiated polymerization. After photopolymerization, the CNC/polymer composites were tested with respect to their ability to sense changes in the polarity of solvents, pH or temperature.66 In water and other polar solvents, the composite materials undergo fast and reversible swelling, which is accompanied with a significant red shift caused by stretching of the helical pitch of the chiral nematic structure. The authors were able to differ between several solvents, which varied in their polarity. In addition, it was proven that the synthetic approach to these hydrogels via photopolymerization allows to incorporate latent images into the photonic hydrogel. By masking some regions of the photonic hydrogel upon UV-irradiation, the swelling behaviour is affected. Regions which were covered during the photopolymerization process swell in water until they reflect near IR light. The reflection of the uncovered areas stays in the visible region since the swelling is limited due to stronger crosslinking (Fig. 8A).
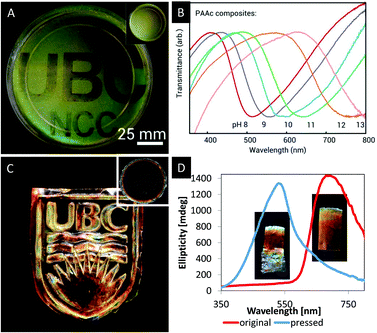 | ||
| Fig. 8 A) Photograph of a photopatterned iridescent hydrogel film revealing a latent image upon swelling of the dry film (inset) in water; B) transmission spectra showing the shifting reflection of the photonic CNC/PAAc hydrogel upon addition of base; C) photograph of a MUF-CNC composite with an imprinted photonic pattern as obtained from the film shown in the inset; D) CD spectra and inserted photographs of the MUF/CNC composite stripes before and after pressing. Reproduced and adapted with permission from John Wiley and Sons and the American Chemical Society.66,68 | ||
Furthermore, the study reports a variety of modifications of the hydrogel composition to create tailor-made photonic hydrogels that respond to different stimuli. By choosing a polyacrylic acid-CNC based hydrogel, a photonic pH sensor (Fig. 8C) was obtained and PNIPAm-CNC composites allowed temperature sensing due to a temperature-induced reversible blue shift upon reaching the lower critical solution temperature of PNIPAm at 31 °C. The colour change is causes by contraction of the swollen hydrophilic sample at the lower critical solution temperature.
Khan, Giese and coworkers reported a series of CNC/polymer composites obtained via mixing of aqueous CNC suspensions (3–5 wt% at pH = 2.4–6.9) with water soluble phenol-formaldehyde (PF), melamine-urea-formaldehyde (MUF) or urea-formaldehyde (UF) precursors.67,68,69 After EISA and simultaneous condensation reaction of the polymer precursors, freestanding composite films with iridescent appearance were obtained. Please note that only the properties of the MUF-CNC composites will be discussed in this section. The PF and UF composites with CNC are intermediates towards mesoporous photonic materials and their properties will be discussed in one of the following sections. The MUF-CNC films showed the typical iridescent appearance which is indicative for the preservation of the chiral nematic structure.68 In contrast to other CNC/polymer composites, these materials are highly flexible after EISA allowing for post-synthetic colour tuning. As a proof-of-concept, samples with a high content of MUF polymer (40 wt%) were synthesized showing a helical pitch within the near IR region. However, by pressing of the film the pitch of the helical structure is reduced, blue-shifting the reflected colour to the visible region of the electromagnetic spectrum. The change in colour as a result of the reduced helical pitch was proven by UV-vis and CD spectroscopy (Fig. 8D) as well as by SEM. Curing the film at 100 °C for 5 h preserves the structure and thereby the photonic pattern. This post-synthetic modification of the photonic properties allowed the imprinting of colourful patterns (Fig. 8C) and may find application as security feature for documents or currency.
Just recently Chen et al. reported an interesting CNC/polymer composite based on the co-assembly of boronic ester crosslinked poly(vinyl alcohol)-polyacrylamide with CNCs.70 The dynamic covalent bond of the boronic ester groups enables self-healing of the photonic material, which allows for the construction of patterns and stacked structures as well as for anchoring on surfaces (Fig. 9A and B). In addition, the use of the material as security feature was proven. Therefore, a QR code was printed on paper and covered by a thin film of the reported CNC/polymer composite. They were able to show that the QR code was exclusively encoded by a cell phone, when right-handed circularly polarized light (R-CPL) was used. The reason for this observation is that the R-CPL passes the photonic film without disturbing reflection allowing the cell phone to encode the QR pattern (Fig. 9C).
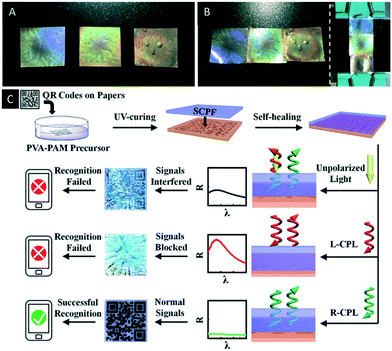 | ||
| Fig. 9 A) Photograph of three CNC/polymer composites with different structural colours (from left to right, PVA-PAM/CNC = 14%, 30% and 50%, respectively); B) photograph of the patterned composite films by self-healing process; C) schematic illustrations of the encryption and recognition of the paper-printed QR codes. The optical images and reflectance spectra of films on the QR codes were taken under natural incident light. Reproduced and adapted with permission from John Wiley and Sons and the Royal Society of Chemistry.70 | ||
Also composite materials obtained from the co-assembly of CNCs with various nanoparticles for induction of chiroptical properties have being reported.71–73 In 2017, Nguyen, Hamad and MacLachlan reported the first upconverting photonic films with chiral nematic ordering by co-assembly of well-defined NaYF4:Yb, Er nanoparticles (UCNPs). Their composite films clearly show the upconversion of near infrared light into the visible region of the electromagnetic spectrum combined with the tunable photonic properties of the CNC films, which might be promising for novel security features on documents or the development of green-emitting photonic bioplastics (Fig. 10A and B).74
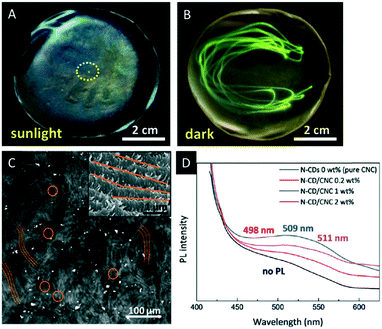 | ||
| Fig. 10 A) Photograph of the CNC film decorated with UCNPs excited with a laser beam (980 nm) revealing the green-emission under UV light irradiation (B); C) two-photon laser-scanning micrograph (485 × 485 μm) of the chiral nematic N-CD/CNC composites demonstrating the characteristic fingerprint texture (inset: corresponding high-magnification SEM image); D) photoluminescence spectroscopy of N-CD/CNC films. Reproduced and adapted with permission from John Wiley and Sons and the Royal Society of Chemistry.73,74 | ||
Also carbon dots have gained considerable attention due to their low costs, stable photoluminescence (PL) and physicochemical properties.75 Lizundia et al. recently reported composite films combining the chiral nematic nanostructure of CNCs with the photoluminescent behaviour of nitrogen-doped carbon dots (N-CDs).73 The composite materials were obtained by co-assembly of CNCs with N-CDs processed by hydrothermal reaction of melamine with D-glucose. Hybrid materials containing up to 2 wt% N-CDs were obtained and investigated with respect to their optical properties. All films showed the typical chiral nematic orientation of the CNCs as proven by SEM (Fig. 10C) and the strong positive ellipticity in the CD spectrum (Fig. 10D). Under ambient light, the materials show the intense iridescence of the chiral nematic structures with blue emission when exposed to UV light (365 nm), which is attributed to the N-CDs embedded in the chiral nanostructure of the CNC film. These composite materials are appealing for applications in luminescent chemo-sensing and bio-imaging.
CNCs as template for mesoporous functional nanomaterials
With respect to the use of CNCs as template for nanostructured and photonic materials MacLachlan and coworkers reported a seminal study in 2010.76–78 They discovered that the self-assembly process of aqueous CNC suspensions is compatible with alkoxysilane precursors such as tetramethoxysilane (TMOS) yielding iridescent composite films whose reflected colour can be varied from the UV across the visible out into the near IR spectral region by increasing the silica precursor content. The shift in the reflected wavelength is predominately due to an increase of the helical pitch. The strong positive ellipticity as observed by circular dichroism (CD) spectroscopy shows that the colour origins exclusively from the reflection of left-handed circularly polarized light, which was further confirmed by scanning electron microscopy.Upon removal of the CNC template by calcination of the composites at 540 °C chiral nematic mesoporous silica (CNMS) films were obtained (Fig. 11A and C). A net blue-shift in the reflected colour of the mesoporous films was observed due to contraction upon heating (due to further condensation of the silica walls) resulting in a decreased helical pitch.
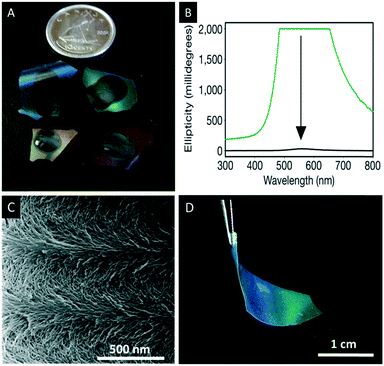 | ||
| Fig. 11 A) Photograph of the chiral nematic mesoporous silica films losing their iridescence due to infiltration by water; B) the corresponding CD spectra before (green) and after (black) immersing the films in water; C) SEM image of the chiral nematic structure showing the twisted layers on the nanoscale level; D) photographs of chiral nematic ethylene-bridged organosilica films showing their iridescence and flexibility. Reproduced and adapted with permission from Nature Publishing Group, the Royal Society of Chemistry and the American Chemical Society.76,77,80 | ||
Following the simple EISA approach MacLachlan and coworkers employed other sol–gel precursors of the type (RO)3Si–R′–Si(OR)3 (R′ = aliphatic, aryl) yielding a variety of iridescent composite materials.76 However, precursors with organic linker groups cannot withstand the calcination procedure to remove CNCs.79 For these materials an alternative removal method was developed. The ethylene-bridged organosilica composites, for instance, were treated with hot 6 M sulfuric acid followed by repeated rinsing with piranha solution to remove the CNC without damaging the organosilica host material. The major advantage of the resulting chiral nematic mesoporous organo silica (CNMO) films is their improved mechanical properties, which make them flexible and easier to handle (Fig. 11D). Related materials with bridging benzene precursors were also prepared and in this case, the CNC template was removed by treatment of the composites with hot HCl followed by a silver-activated hydrogen peroxide wash.80
With respect to the application of the reported films a major limitation is that the films tend to crack during the last stages of EISA into centimeter-sized pieces, which results from the capillary pressure gradients generated during evaporation. Kelly et al. addressed that issue and found that the cracking of mesoporous silica films can be reduced or completely prevented by the addition of polyols like glucose to the CNC suspension. The addition of polyols changes the sol–gel curing kinetics leading to crack-free iridescent silica films of up to 15 cm in diameter which are retained even after pyrolysis (Fig. 12A).81
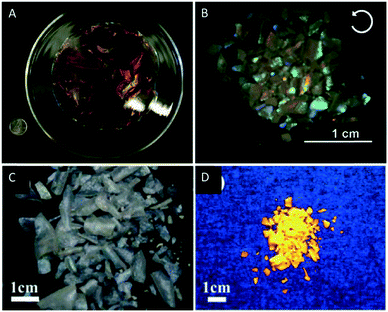 | ||
| Fig. 12 A) Photograph of the chiral nematic mesoporous silica films by MacLachlan and coworkers (25 cent for scaling); B) photograph of chiral nematic titania films viewed under a left-handed circular polarizer; photograph of the chiral nematic Eu3+-doped zirconia films under white (C) and under UV-light (D). Reproduced and adapted with permission from John Wiley and Sons and the Royal Society of Chemistry.81,89,90 | ||
As mentioned earlier, the colour of chiral nematic films can be tuned by either changing the helical pitch or the refractive index contrast. The rigidity of the silica films limits the manipulation of the reflected colour by changing the pitch. However, Shopsowitz et al. tuned the colour of CNMO films by infiltration of the pores with isotropic liquids. Water, for instance, is absorbed by the materials and leads to completely transparent films by quenching the colourful iridescence (Fig. 11A). This observation can be explained by the approximate refractive index matching between the silica matrix (SiO2: n = 1.46),82 and the water inside the pores (n = 1.33) and is in line with observations made for related systems.83–86 By eye, the iridescence is completely extinguished, however, the CD spectra reveal a red-shifted residual signal due to the small contrast in the refractive indices (Fig. 11B). By infiltration of the pores with solvents with other refractive indices matching the one of silica better (isopropanol: n = 1.38), a stronger red shift and, for DMSO (n = 1.48), a complete elimination of the CD signal was reported. The impact of the infiltration of the pores on the photonic properties of the CNMO films was used by Kelly et al. to sense changes in refractive indices of aqueous sucrose solutions.87 It could be shown that mesoporous silica and organosilica materials are suitable platforms for sensing by following the UV-vis or the CD signal, whereby the latter method has the advantage of being more sensitive which makes it useful even in the presence of intense dyes.
In 2013, MacLachlan and coworkers reported a procedure to functionalize chiral nematic materials by surface modification, which improves the ability of the silica materials to host non-polar guests.88 The functionalization of the pores was achieved by treating the chiral nematic mesoporous ethylene-bridged organosilica films with 1-(triethoxysilyl)octane. The characterization of the surface-modified CNMO by elemental analysis (EA) and thermogravimetric analysis (TGA) revealed a degree of functionalization of octyl groups to ethylene bridges of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. These modified films were further used for preparation of hybrid materials hosting hydrophobic guests inside the octyl-functionalized pores.
10. These modified films were further used for preparation of hybrid materials hosting hydrophobic guests inside the octyl-functionalized pores.
Hard templating
Hard-templating is a powerful tool to prepare new nanostructured materials with photonic properties.91–94 This method uses a mesoporous host, which transfers a negative image of its own nanostructure to a second material. Selective removal of the host template by e.g. etching yields a new nanostructured material (Scheme 4).This method is especially interesting for the synthesis of nanostructured materials whose precursors are incompatible with the lyotropic liquid crystalline phase of a soft-template (e.g. the chiral nematic phase of the CNC suspensions).95,96 In 2012, Shopsowitz et al. utilized mesoporous silica films as template for the synthesis of chiral nematic titania.89 Several attempts to combine the peptized TiCl4 solutions with CNC suspensions failed since the titania precursors were incompatible with the EISA process of aqueous CNC dispersions yielding gels instead of the desired chiral nematic phase. However, loading the pores of mesoporous chiral nematic silica films with the peptized TiCl4 solution led to silica–titania composites. The loading was repeated and drying at 80 °C followed by annealing at 200 °C was performed in each cycle. After final calcination of the composite films at 600 °C, the silica hard-template was removed by etching with 2 M NaOH to yield mesoporous titania films with the characteristic iridescence of the chiral nematic structure (Fig. 12B). The replication of the helical nanostructure of the titania films by hard templating was confirmed by SEM. Interestingly, the nanoconfinement during crystallization inside the mesoporous silica templates supported the formation of the anatase polymorph instead of the thermodynamically-favored rutile phase for bulk titania as proven by XRD analysis. These porous titania films may find application in dye-sensitized solar cells, photocatalysts or sensors and batteries.
Chu et al. adapted this hard templating approach to synthesise chiral nematic zirconia (ZrO2) and europium-doped zirconia films (ZrO2/Eu3+).90 They repeatedly loaded chiral nematic mesoporous silica films with an aqueous solution of ZrOCl2 or ZrOCl2/Eu(NO3)3 followed by drying and calcination of the composite. The silica support was finally removed under basic aqueous conditions to yield the ZrO2 and ZrO2/Eu3+ films (Fig. 12C) respectively. The iridescence, CD spectra and the SEM images prove the replication of the chiral nanostructure of the silica template. The ZrO2/Eu3+ samples combine the photonic properties of the chiral nematic structure with the characteristic luminescence of Eu3+ (Fig. 12D). The decay time constants of the composite films were found to be significantly higher than those of non-chiral reference samples, which indicates differences in the local environments of the Eu3+ ions.97 Since the XPS data for the sample and the corresponding reference did not show significant differences of the chemical environments, the authors attributed the deviation in luminescence to the chiral nematic order of the ZrO2/Eu3+ sample.
These results prove that chiral nematic mesoporous silica films are suitable for hard-templating of solid-state materials that combine photonic properties, mesoporosity and luminescence.
Functionalization of CNMS
The following section describes the attempts which have been made towards the post-synthetic modification of chiral nematic mesoporous materials. Some examples are the decoration of the mesoporous materials with metal nanoparticles (NPs), quantum dots (QDs), dyes or polymers (Scheme 5).Metal NPs have gained considerable attention due to their bioactivity and their potential in chemical sensing using surface plasmon resonance (SPR). In order to take full advantage of the properties of metal NPs, the control over their supramolecular organization is crucial.98 For this purpose, Qi et al. investigated the synthesis of silver, gold and platinum NPs in the pores of chiral nematic organosilica films.99,100 The obtained hybrid materials showed a CD signal assigned to the SPR that arises from the metal NPs in a chiral environment (Fig. 13A and B). In order to show that the chirality of the metal NPs is induced by the chiral nematic structure of the silica films two different synthetic approaches were followed. In the first method the silver NPs were synthesized inside the pores of the silica films, while in the second small quantities of the NP precursors were added to the CNC/silica gel. The accompanying CD measurements indicated that the optical activity of the SPR signal arises exclusively from the chiral nematic long-range order of the chiral host. This was the first example showing a chiral transfer to metal NPs, which was not induced by coordination of chiral ligands.101–103
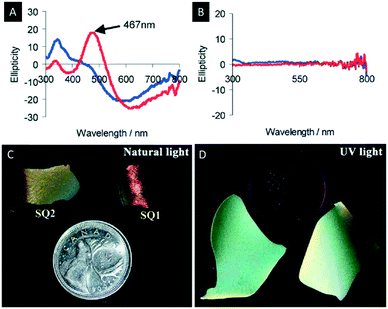 | ||
| Fig. 13 A) CD spectra of chiral nematic mesoporous silica films doped with silver NPs; B) CD spectra of achiral mesoporous silica films doped with silver NPs (blue before and red after soaking with water); C) photographs of the chiral nematic silica films doped with CdS QDs showing the characteristic iridescence of the host material under natural light; D) luminescence of the QDs evident under UV-light. Reproduced and adapted with permission from John Wiley and Sons and the Royal Society of Chemistry.72,99 | ||
Besides nanoparticle composites, the fabrication of quantum dot hybrid materials has gained considerable attention.104 In this respect Nguyen et al. prepared a hybrid material combining the iridescence of the chiral nematic silica (Fig. 13C) with the luminescence of CdS QDs (Fig. 13D).72 The luminescence lifetimes of the hybrid materials before and after calcination were found to be 1.55 and 1.75 ns, respectively, which is in line with related materials,105,106 and verifies the retention of the luminescence of the QDs after calcination. The high porosity of the hybrid films provides excellent accessibility of the QDs for analytes and makes these systems appealing for application in sensing of explosives.107–111 As a proof-of-principle experiment the luminescence quenching of the CdS-QDs by solutions and vapours of 2,4,6-trinitrotoluene (TNT) was studied. When the hybrid silica films are exposed to a 5.5 × 10−3 mM solution of TNT in toluene a complete loss of luminescence was observed, which is caused by an electron transfer from the CdS QDs to the electron-deficient π-system of the analyte.112–115 Removal of the TNT by washing recovers the luminescence of the films. Similar results were found when exposing the CdS/silica films to TNT vapours. However, in this case the emission intensity decreased gradually reaching a steady-state value after 10 min. These first experiments already indicate the potential of functional materials combining the optical properties of photonic crystals with the electronic characteristics of QDs and might find applications in sensing or optical amplifiers.116–119
In 2013, Mehr et al. polymerized poly(p-phenylenevinylene) (PPV) inside the pores of chiral nematic mesoporous organosilica.120 As a conjugated organic polymer PPV is discussed as useful material in a number of applications such as organic electronics,121–124 lasers,125,126 sensing127 and nanocomposites.128–130 In this process, the performance of such materials strongly depends on the alignment and organization of the PPV.131 The hybrids combine the photonic properties of the chiral nematic structure of the CNMO films with the luminescence of PPV (Fig. 14A). As proven by gas adsorption analysis, the PPV/CNMO samples retained their mesoporosity after polymerization of the PPV inside the channels of the CNMO films also enabling their use as sensor for explosives such as TNT. Exposing the composite films to a solution of TNT in ethanol led to an instantaneous quenching of the characteristic luminescence of PPV, which can be followed with the naked eye under UV-light irradiation (Fig. 14A and B). The films showed a slightly red iridescence due to their chiral nematic structure. Removal of the analyte by washing the films with ethanol recovered the fluorescence.
 | ||
| Fig. 14 A) Photographs of chiral nematic PPV/organosilica composite films viewed under UV light without TNT (upper image) and with TNT (lower image); B) fluorescence quenching of a sample upon exposure to a solution of TNT in ethanol as observed by UV/vis spectroscopy; C) illustration of the erasable nature of the mesoporous organosilica films through UV irradiation and exposure to white LED light; D) photographs demonstrating the colour changes of the CNMO films upon complexation of metal ions. Reproduced and adapted with permission from the American Chemical Society and John Wiley and Sons.120,132 | ||
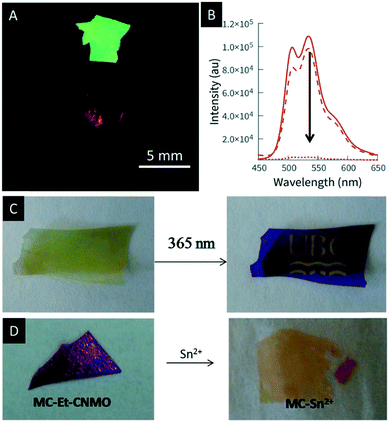
In 2017, Terpstra et al. functionalized CNMO films with a spiropyran-based dye to create photochromic films.132 Spiropyrans (SPs) are known to be efficient photo-switches which undergo reversible isomerisation between the open merocyanine (MC) and closed SP form controlled by light.133,134 The authors successfully showed the reversible photo-patterning of the hybrid material by irradiation with UV-light (Fig. 14C). In addition, they used the MC form of the hybrid material for photo-switchable metal ion sensing. The open MC form is well known to bind metal ions such as Sn(II), Ni(II) or Cu(II).135–137 The binding of the metals to the hybrid materials leads to a visible change in colour and is reversible in the presence of ethanol and white light (Fig. 14D). The authors were able to prove that the observed colour changes solely arise from the binding of the metal ion to the MC and that the structural colour of the films is not affected by the binding event.
It should be noted that the functionality of the CdS QDs and the PPV doped organosilica films mentioned so far, does not result from the chiral nematic structure. Nevertheless, it benefits from the mesoporosity and the freestanding nature of these films proving that the luminophores in these hybrid materials are accessible to analytes. Future studies have to prove their use in sensing and optoelectronic applications.
 | ||
| Scheme 6 Schematic illustration of the preparation of photonic hybrid materials by infiltration of CNMO films. | ||
With respect to the manipulation of the photonic properties of such hybrid materials thermotropic liquid crystals are interesting guests, since they show large changes in their refractive indices as a function of the temperature, which is attributed to the changes in their molecular orientations.138,139 This difference in the refractive index leads to a change in the average refractive index contrast of the composite material and thus to a change of the structural colour of the hybrid material. Although a few hybrid materials derived from mesoporous silica and LCs were reported so far, the mutual control of physical properties by the host–guest interactions remains an unclear phenomenon.10 In 2013, Giese et al. infiltrated the pores of n-octyl functionalized organosilica films with 4-cyano-4′-octylbiphenyl (8CB), a thermotropic liquid crystal.88,140 At room temperature, the composite films appeared with a green iridescence (Fig. 15A). Upon heating, however, the phase transition of 8CB from nematic to isotropic yielded an opaque sample at 50 °C (Fig. 15A). It could be shown that the observed colour change is reversible showing a small hysteresis effect observed through repeated heating and cooling cycles (Fig. 15B). To understand the temperature-dependant behaviour of the hybrid materials on a molecular level, a 15N-labeled analogue of 8CB (15N-8CB) was synthesized and inserted into the pores of the octyl-functionalized organosilica films. The samples were subsequently investigated by variable-temperature 15N-solid-state NMR spectroscopy, which is a powerful tool to investigate the molecular dynamics of liquid crystalline systems. The collected spectra showed broad signals at 21 °C corresponding to the 15N-labeled 8CB in its liquid crystalline state. However, upon heating the hybrid materials to the isotropic state the peaks collapsed to a sharp signal, which supports the assumption that colour change of the hybrid material is mainly caused by the change of the refractive index contrast due to the differences in the alignment upon phase transition from the LC phase to the isotropic state.
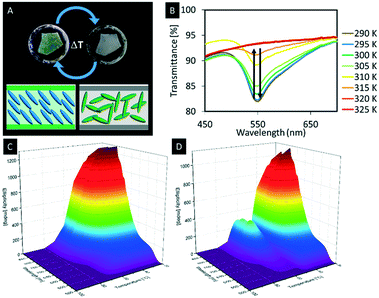 | ||
| Fig. 15 A) Photograph of the thermochromic n-octyl-functionalized organosilica films infiltrated with 8CB at room temperature (left) and at 41 °C (right); B) the UV-vis spectra showing the decreasing signal intensity with rising temperature; 3D plots of variable-temperature CD spectra demonstrating the thermochromic behavior of the hybrid materials based on hydrogen-bonded liquid crystals in the confinement of mesoporous organosilica films upon heating (C) and cooling (D). Reproduced and adapted with permission from the American Chemical Society and John Wiley and Sons.88,141 | ||
Gaining control over the self-assembly in confined spaces will open an attractive route to novel multi-functional hybrid materials.10 In 2015, Giese, MacLachlan and coworkers reported the first study on hydrogen-bonded liquid crystals (HB-LCs) in mesoporous silica films in order to tune the optical properties of the hybrid materials.141,142 These materials combine the structural colour of chiral nematic mesoporous organosilica films with the thermoresponsive behavior of HB-LCs yielding thermochromic hybrid materials. In a follow-up study the mutual effects of self-assembly in confined spaces and the manipulation of the photonic properties by temperature-dependent changes of the refractive index contrast was investigated in detail. For this study, a series of hybrid materials based on chiral nematic mesoporous organosilica films infiltrated with liquid crystalline hydrogen-bonded assemblies was prepared and the obtained photonic hybrid materials were investigated with respect to their thermo- and photo-responsive behaviour. Variable-temperature POM, UV-vis and CD spectroscopy (Fig. 15C and D) as well as DSC studies indicate a crucial impact of the confinement on the mesomorphic behaviour of the hydrogen-bonded liquid crystalline guests and the photonic properties of the systems. Hybrid materials based on CNMO films with smaller pore sizes or surface functionalized pores (by octyl groups) show a strong confinement of the HB-LCs leading to less pronounced changes in the optical properties of the hybrid films or even prevention of the formation of a liquid crystalline phase. Accompanying variable-temperature 19F solid state NMR studies revealed, that the HB-LCs can only properly orient inside the mesoporous materials with a minimum pore size of 11 nm. Furthermore, it was found that for CNMO films functionalized with octyl chains, no formation of a liquid crystalline phase was found. This observation was attributed to strong intermolecular interactions of the guests with the octyl-functionalized surface, suppressing the liquid crystalline phase. For hybrid materials showing the formation of the LC phase inside the pores photo-switching experiments with a 365 nm LED laser have been performed to demonstrate the reversible response of the hybrids upon irradiation with UV-light. The reversible thermo- and photochromic behaviour of the hybrid materials offers a new route towards tuneable optical filters or temperature sensors.
Mesoporous polymers
As mentioned earlier, CNCs are interesting templates to introduce structural colouration into polymers.51,68 However, with respect to potential applications, the removal of the CNC template and introduction of mesoporosity is highly desirable. In 2013, MacLachlan and coworkers successfully removed the CNC template from the earlier mentioned phenol formaldehyde (PF) resin to yield iridescent and mesoporous polymer films.69 The colour of the PF films can be controlled by the synthetic procedure ranging from UV to the near IR. Treating the composite film with 16 wt% aqueous NaOH solution at 70 °C for 8–12 h removed 85–90% of CNCs from the polymer matrix, which was proven by FTIR, solid-state NMR spectroscopy, and TGA. Interestingly, the CNC containing composite films appeared brittle, while the mesoporous resin films were highly flexible after removal of the CNCs with tensile strengths up to 89 MPa and 4.5% elongation at failure. In order to preserve the mesoporosity of the films, it turned out to be crucial to dry the samples from ethanol by supercritical CO2 leading to Brunauer–Emmett–Teller (BET) surface areas and pore volumes of 310–365 m2 g−1 and 0.5–0.7 cm3 g−1, and Barrett–Joyner–Halenda (BJH) pore size distribution is around 7 nm. The retention of the chiral nematic order of the mesoporous resins was proven by SEM as well as by the selective reflection of L-CPL by the films (Fig. 16A). The swelling behaviour of the mesoporous PF resins was tested by soaking the films in mixtures of polar solvents (water/ethanol). Since the swelling of the films is related to a stretching of the chiral nematic structure, the structural colour changes within seconds. In pure ethanol the films appear blue, while in pure water they rapidly swell to the IR region of the electromagnetic spectrum. The swelling behaviour was quantified by UV-vis and CD spectroscopy and enabled to sense the polarity/ethanol content in water/ethanol mixtures (Fig. 16B).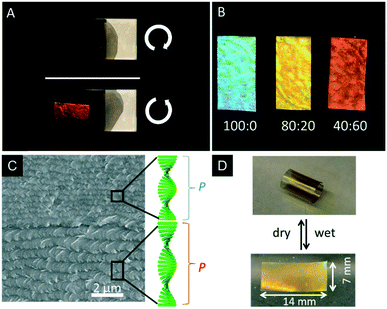 | ||
Fig. 16 A) Photograph of the photonic PF resin under left- and right-handed polarizers demonstrating the selective reflection of L-CPL; B) photograph of a mesoporous PF films after being immersed in different ratios of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water; C) SEM image of a cross-section of the mesoporous polymer bilayer showing the different helical pitches of the layers; D) photographs of the curling and uncurling of the bilayered mesoporous polymer films upon alternately soaking in water and drying. Reproduced and adapted with permission from the American Chemical Society and John Wiley and Sons.69,143 water; C) SEM image of a cross-section of the mesoporous polymer bilayer showing the different helical pitches of the layers; D) photographs of the curling and uncurling of the bilayered mesoporous polymer films upon alternately soaking in water and drying. Reproduced and adapted with permission from the American Chemical Society and John Wiley and Sons.69,143 | ||
Khan et al. used the same system to prepare bilayer mesoporous photonic resins which were obtained by layer-by-layer deposition followed by removal of the CNC template.143 The formed composite films only differed in the helical pitch of the chiral nematic structure. Interestingly, these materials showed actuator behaviour. Usually, bilayered actuators are prepared by deposition of an active layer on a substrate.144 In contrast, the bilayered mesoporous PF films consist of two active layers with different nanostructures (Fig. 16C), which results in an asymmetric swelling behaviour, providing actuator properties. The authors found that the layer of longer helical pitch and larger pore size swells stronger than the one with a shorter helical pitch leading to fast and directional curling and uncurling upon drying and swelling in polar solvents (Fig. 16D). The directional curling was attributed to different permeability of the individual layers yielding differences in expansion or shrinkage of the bilayered films.
A similar system was reported by Guo and coworkers in 2016, where they sandwiched an uniaxially oriented PA-6 film (half-wave retarder) between two CNC polyethylene glycol diacrylate (PEGDA) layers to obtain a nanocomposite with remarkable reflection and actuator properties due to different swelling of the CNC/PEGDA layers in the presence of humidity.145 The remarkable fast, dynamic mechanical and photonic response of the bilayered materials has the potential for application in optics and soft robotics.
In 2015, Khan et al. used the photonic mesoporous PF resins to create dynamic photonic patterns by post-synthetic treatment with aqueous acid or formaldehyde solution.146 Regions of the mesoporous resin films treated with HCl solutions show enhanced crosslinking of the resin. The crosslinking, in-turn, reduces the number of methylol groups on the surface making the films more hydrophobic. In contrast, the formaldehyde treatment increases the density of methylol groups on the surface, making these regions more hydrophilic. Consequently, the HCl treated areas swell to a smaller extend in polar solvents than the formaldehyde treated parts of the film (Fig. 17A). This enables the implementation of latent images onto the photonic films by inkjet printing (Fig. 17B and C). These materials might find application as security feature for documents or visual graphic signage.
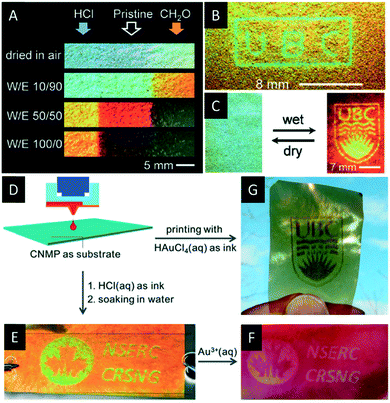 | ||
| Fig. 17 A) Photographs of the mesoporous photonic resin films showing the changes in colour upon exposure to water/ethanol mixtures. The left and right ends of the strip were treated with HCl and formaldehyde, respectively, while the middle part remained untreated; B) photograph of a polymer film patterned with “UBC”; C) photograph of the latent UBC crest (dry state, left) revealing the pattern upon swelling in water (right); D) schematic presentation of the surface patterning by a custom inkjet printer; E) photograph of the pattern printed with aqueous HCl solution revealed by soaking in water; F) photograph of the same patterned film treated with aqueous HAuCl4 solution; G) photograph of the patterned film obtained by direct printing using aqueous HAuCl4 as ink. Reproduced and adapted with permission from John Wiley and Sons and the Royal Society of Chemistry.147,149 | ||
In 2016, Zamarion et al. decorated the mesoporous PF resins with gold, silver and palladium nanoparticles by in situ reduction.147 They were able to control the size and loading of the metal nanoparticles by varying the reagent concentration and the deposition time. In addition, they developed a new method to pattern the resin films with metal nanoparticles by ink-jet printing (Fig. 17D–G). Currently, the authors investigate the chiroptical properties of these hybrid materials and its application in sensing. Just recently, Leng et al. reported on the synthesis of porous latex photonic films by removal of the CNC template from silicone-modified acrylic latex (SAL)/CNC composites by alkaline treatment.148 The authors were able to blueshift the reflectance wavelength of the SAL/CNC composite across the visible spectrum by variation of the latex content from a SAL to CNC ratio of 0.2 to 1.0. Upon removal of the CNC template the obtained two dimensional porous latex films show remarkable flexibility and reversible colour tunability by swelling in different solvents.
In 2014, Giese et al. treated urea formaldehyde (UF) CNC resins with an alkaline solution to obtain mesoporous photonic cellulose (MPC), an active form of “paper” (Scheme 7).150
The mesoporosity combined with the chiral nematic structure allows fast and reversible changes of the structural colour by swelling in polar solvents, enabling to follow the changes in the polarity by the naked eye (Fig. 18A). The MPC films appear deep blue with a peak reflection wavelength at 430 nm in pure ethanol. Increasing the water content yields a systematic red shift in the reflected colour up to 840 nm for pure water. The fast photonic response occurs within 10 s and was quantified by UV-vis and CD spectroscopy. In addition, the MPC films are highly flexible enabling reversible piezochromic behaviour of the water-swollen samples (Fig. 18B). Initially, these MPC samples appear colourless, reflecting near IR-light. However, by pressing the sample the helical pitch of the chiral nematic structure is compressed shifting the reflected colour by more than 100 nm into the visible region of the electromagnetic spectrum. The piezochromic behaviour of MPC provides a novel method to sense pressure.
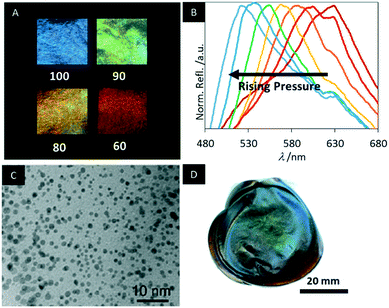 | ||
| Fig. 18 A) Photographs of MPC samples immersed in mixtures of water and ethanol (the value corresponds to vol% ethanol); B) reflectance spectra of water-swollen samples upon pressing C) TEM image of the MPC films decorated with gold NPs; D) photograph of the cobalt ferrite/MPC hybrid films. Reproduced and adapted with permission from John Wiley and Sons, the American Chemical Society and the Royal Society of Chemistry.150–152 | ||
In 2015, Schlesinger et al. decorated MPC films with gold nanoparticles and investigated the chiroptical activity of the composites.151 The hybrid materials were obtained by soaking the MPC films with auric acid (HAuCl4) solution and subsequent reduction by sodium borohydride (NaBH4). It was shown that the mesoporous cellulose films stabilize nearly monodisperse Au NPs and that size (4.5–7.1 nm) and particle amount (0.2–1.9 wt%) could easily be controlled by the concentration of the HAuCl4 solution (Fig. 18C). To demonstrate the application potential of these composite films they were exposed to an aqueous solution of 2-mercaptoethanol, which yielded a significant colour change.
Giese et al. prepared a MPC composite with cobalt ferrite nanoparticles by soaking the MPC films in an aqueous solution of CoCl2 and FeSO4 followed by oxidative treatment with a solution of KNO3 in order to oxidize ferrous to ferric ions.152 The obtained samples show the iridescent colour of the MPC combined with the brownish colour of the cobalt ferrite NPs providing magnetic and dielectric properties (Fig. 18D). Swelling of the samples in water led to a significant red shift in the reflected colour due to stretching of the helical pitch. However, even more interesting was the dielectricity of the MPC composites which was tremendously increased during swelling in water. This finding makes them appealing for electromagnetic interference shielding applications.
In 2015, Schlesinger et al. reported an alternative approach to MPC films.153 Here, the CNC silica composites reported by Shopsowitz et al.76 were treated with base to obtain chiral nematic mesoporous cellulose films. With this method they were able to obtain films with different helical pitches, but identical porosity as proven by SEM. However, the films obtained with this method appear opaque making them less appealing for photonic applications. The authors prepared composites with gold NPs and investigated their chiroptical properties and were able to attribute the chiroptical behaviour to interactions of the surface plasmon resonance of the gold particles with the chiral nematic structure of the cellulose films. These films may find application as biosensors, security features or selective membranes.
Conclusions and outlook
The ability of CNC suspensions to self-assemble into chiral-nematic structures, representing 1D photonic crystals, yielded a series of fascinating photonic functional nanomaterials with brilliant colours. The presented concepts and proof-of-principle studies have shown the use of the materials in sensing and optoelectronic devices. However, although a number of interesting materials have been reported some fundamental questions have to be addresses, such as: how do these materials form? What kind of processes occur inside the pores of the chiral materials? Clarifying these aspects will open up new directories towards the development of novel functional nano- and hybrid materials. The next challenge now is to transfer the preparation methods for these materials to an industrial scale. With all this in mind these materials will lead us into a brighter and more colourful future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. S. and M. G. are thankful for genereous support by the Professor Werdelmann-Stiftung. M. G. thanks the Boehringer Ingelheim Foundation for financial support.Notes and references
- W. Lu and C. M. Lieber, Nanoelectronics from the bottom up, Nat. Mater., 2007, 6(11), 841–850 CrossRef CAS.
- Y. Fang, B. M. Phillips, K. Askar, B. Choi, P. Jiang and B. Jiang, Scalable bottom-up fabrication of colloidal photonic crystals and periodic plasmonic nanostructures, J. Mater. Chem. C, 2013, 1(38), 6031–6047 RSC.
- E. Burstein, M. L. Cohen, D. L. Mills and P. J. Stiles, Nanomagnetism: Ultrathin Films, Multilayers and Nanostructures, Elsevier, London, UK, 2006 Search PubMed.
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Nanomaterials for energy conversion and storage, Chem. Soc. Rev., 2013, 42(7), 3127–3171 RSC.
- J. Wang, Carbon-Nanotube Based Electrochemical Biosensors: A Review, Electroanalysis, 2005, 17(1), 7–14 CrossRef CAS.
- J. J. Gooding, Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing, Electrochim. Acta, 2005, 50(15), 3049–3060 CrossRef CAS.
- A. Huczko, Template-based synthesis of nanomaterials, Appl. Phys. A: Mater. Sci. Process., 2000, 70(4), 365–376 CrossRef CAS.
- K. Ariga, T. Nakanishi and J. P. Hill, Self-assembled microstructures of functional molecules, Curr. Opin. Colloid Interface Sci., 2007, 12(3), 106–120 CrossRef CAS.
- K. Sakakibara, J. P. Hill and K. Ariga, Thin-Film-Based Nanoarchitectures for Soft Matter: Controlled Assemblies into Two-Dimensional Worlds, Small, 2011, 7(10), 1288–1308 CrossRef CAS PubMed.
- M. Ramanathan, I. I. S. M. Kilbey, Q. Ji, J. P. Hill and K. Ariga, Materials self-assembly and fabrication in confined spaces, J. Mater. Chem., 2012, 22(21), 10389–10405 RSC.
- K. Ariga, Q. Ji, J. P. Hill, Y. Bando and M. Aono, Forming nanomaterials as layered functional structures toward materials nanoarchitectonics, NPG Asia Mater., 2012, 4, e17 CrossRef.
- K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Nanoarchitectonics: a new materials horizon for nanotechnology, Mater. Horiz., 2015, 2(4), 406–413 RSC.
- K. Ariga and J. Li, Nanoarchitectonics for Advanced Materials: Strategy Beyond Nanotechnology, Adv. Mater., 2016, 28(6), 987–988 CrossRef CAS PubMed.
- K. Ariga, D. T. Leong and T. Mori, Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications, Adv. Funct. Mater., 2018, 28(27), 1702905 CrossRef.
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Amphiphile nanoarchitectonics: from basic physical chemistry to advanced applications, Phys. Chem. Chem. Phys., 2013, 15(26), 10580–10611 RSC.
- K. Ariga, Y. Yamauchi, G. Rydzek, Q. Ji, Y. Yonamine, K. C.-W. Wu and J. P. Hill, Layer-by-layer Nanoarchitectonics: Invention, Innovation, and Evolution, Chem. Lett., 2014, 43(1), 36–68 CrossRef CAS.
- L. Zhang, T. Wang, Z. Shen and M. Liu, Chiral Nanoarchitectonics: Towards the Design, Self-Assembly, and Function of Nanoscale Chiral Twists and Helices, Adv. Mater., 2016, 28(6), 1044–1059 CrossRef CAS PubMed.
- T. Govindaraju and M. B. Avinash, Two-dimensional nanoarchitectonics: organic and hybrid materials, Nanoscale, 2012, 4(20), 6102–6117 RSC.
- R. Rajendran, L. K. Shrestha, R. M. Kumar, R. Jayavel, J. P. Hill and K. Ariga, Composite Nanoarchitectonics for Ternary Systems of Reduced Graphene Oxide/Carbon Nanotubes/Nickel Oxide with Enhanced Electrochemical Capacitor Performance, J. Inorg. Organomet. Polym. Mater., 2015, 25(2), 267–274 CrossRef CAS.
- Y. S. Lee, Self-Assembly and Nanotechnology: A Force Balance Approach, John Wiley & Sons, New Jersey, 2008 Search PubMed.
- M. Antonietti, Silica nanocasting of lyotropic surfactant phases and organized organic matter: material science or an analytical tool?, Philos. Trans. R. Soc., A, 2006, 364, 2817–2840 CrossRef CAS PubMed.
- S. E. Friberg, C. C. Yang, R. Goubran and R. E. Partch, Ammonia microemulsions and ammonolysis of silicon tetrachloride, Langmuir, 1991, 7(6), 1103–1106 CrossRef CAS.
- C. G. Göltner and M. Antonietti, Mesoporous materials by templating of liquid crystalline phases, Adv. Mater., 1997, 9(5), 431–436 CrossRef.
- J. P. F. Lagerwall, C. Schutz, M. Salajkova, J. Noh, J. Hyun Park, G. Scalia and L. Bergstrom, Cellulose nanocrystal-based materials: from liquid crystal self-assembly and glass formation to multifunctional thin films, NPG Asia Mater., 2014, 6, e80 CrossRef CAS.
- E. Kontturi, P. Laaksonen, M. B. Linder, Nonappa, A. H. Gröschel, O. J. Rojas and O. Ikkala, Adv. Mater., 2018, 30(24), 1703779 CrossRef PubMed.
- S. Elazzouzi-Hafraoui, J.-L. Putaux and L. Heux, Self-assembling and Chiral Nematic Properties of Organophilic Cellulose Nanocrystals, J. Phys. Chem. B, 2009, 113(32), 11069–11075 CrossRef CAS PubMed.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications, Chem. Rev., 2010, 110(6), 3479–3500 CrossRef CAS PubMed.
- A. Dufresne, Nanocellulose: From Natur to High Performance Tailored Materials, Walter de Gruyter GmbH, Göttingen, 2012 Search PubMed.
- W. Y. Hamad, Photonic and Semiconductor Materials Based on Cellulose Nanocrystals, in Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, ed. O. J. Rojas, Springer International Publishing, Cham, 2016, pp. 287–328 Search PubMed.
- K. J. De France, T. Hoare and E. D. Cranston, Review of Hydrogels and Aerogels Containing Nanocellulose, Chem. Mater., 2017, 29(11), 4609–4631 CrossRef CAS.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Cellulose nanomaterials review: structure, properties and nanocomposites, Chem. Soc. Rev., 2011, 40(7), 3941–3994 RSC.
- B. G. Rånby, Aqueous Colloidal Solutions of Cellulose Micelles, Acta Chem. Scand., 1949, 3, 649–650 CrossRef.
- B. G. Rånby and E. Ribi, Über den Feinbau der Zellulose, Experientia, 1950, 6(1), 12–14 CrossRef.
- B. G. Rånby, Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles, Discuss. Faraday Soc., 1951, 11(0), 158–164 RSC.
- B. L. Peng, N. Dhar, H. L. Liu and K. C. Tam, Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective, Can. J. Chem. Eng., 2011, 89(5), 1191–1206 CrossRef CAS.
- R. H. Marchessault, F. F. Morehead and N. M. Walter, Liquid Crystal Systems from Fibrillar Polysaccharides, Nature, 1959, 184(4686), 632–633 CrossRef CAS.
- J. F. Revol, H. Bradford, J. Giasson, R. H. Marchessault and D. G. Gray, Helicoidal self-ordering of cellulose microfibrils in aqueous suspension, Int. J. Biol. Macromol., 1992, 14(3), 170–172 CrossRef CAS PubMed.
- J. F. Revol, D. L. Godbout and D. G. Gray, Solidified liquid crystals of cellulose with optically variable properties, 1997 Search PubMed.
- J.-F. Revol, L. Godbout and D. G. Gray, Solid self-assembled films of cellulose with chrial nematic order and optically variable properties, J. Pulp Pap. Sci., 1998, 24, 146–149 CAS.
- A. Tran, W. Y. Hamad and M. J. MacLachlan, Tactoid Annealing Improves Order in Self-Assembled Cellulose Nanocrystal Films with Chiral Nematic Structures, Langmuir, 2018, 34(2), 646–652 CrossRef CAS PubMed.
- G. von Freymann, V. Kitaev, B. V. Lotsch and G. A. Ozin, Bottom-up assembly of photonic crystals, Chem. Soc. Rev., 2013, 42(7), 2528–2554 RSC.
- H. De Vries, Rotatory power and other optical properties of certain liquid crystals, Acta Crystallogr., 1951, 4(3), 219–226 CrossRef CAS.
- V. Sharma, M. Crne, J. O. Park and M. Srinivasarao, Structural Origin of Circularly Polarized Iridescence in Jeweled Beetles, Science, 2009, 325(5939), 449 CrossRef CAS PubMed.
- B. R. Harkness and D. G. Gray, Left- and right-handed chiral nematic mesophase of (trityl)(alkyl)cellulose derivatives, Can. J. Chem., 1990, 68(7), 1135–1139 CrossRef CAS.
- J. Pan, W. Hamad and S. K. Straus, Parameters Affecting the Chiral Nematic Phase of Nanocrystalline Cellulose Films, Macromolecules, 2010, 43(8), 3851–3858 CrossRef CAS.
- Y. Habibi, T. Heim and R. Douillard, AC electric field-assisted assembly and alignment of cellulose nanocrystals, J. Polym. Sci., Part B: Polym. Phys., 2008, 46(14), 1430–1436 CrossRef CAS.
- P.-X. Wang, W. Y. Hamad and M. J. MacLachlan, Structure and transformation of tactoids in cellulose nanocrystal suspensions, Nat. Commun., 2016, 7, 11515 CrossRef.
- B. Frka-Petesic, G. Guidetti, G. Kamita and S. Vignolini, Controlling the Photonic Properties of Cholesteric Cellulose Nanocrystal Films with Magnets, Adv. Mater., 2017, 29(32), 1701469 CrossRef PubMed.
- B. Frka-Petesic, H. Radavidson, B. Jean and L. Heux, Dynamically Controlled Iridescence of Cholesteric Cellulose Nanocrystal Suspensions Using Electric Fields, Adv. Mater., 2017, 29(11), 1606208 CrossRef PubMed.
- T.-D. Nguyen, W. Y. Hamad and M. J. MacLachlan, Tuning the iridescence of chiral nematic cellulose nanocrystals and mesoporous silica films by substrate variation, Chem. Commun., 2013, 49(96), 11296–11298 RSC.
- C. C. Y. Cheung, M. Giese, J. A. Kelly, W. Y. Hamad and M. J. MacLachlan, Iridescent Chiral Nematic Cellulose Nanocrystal/Polymer Composites Assembled in Organic Solvents, ACS Macro Lett., 2013, 2(11), 1016–1020 CrossRef CAS.
- X. M. Dong, T. Kimura, J.-F. Revol and D. G. Gray, Effects of Ionic Strength on the Isotropic–Chiral Nematic Phase Transition of Suspensions of Cellulose Crystallites, Langmuir, 1996, 12(8), 2076–2082 CrossRef CAS.
- X. M. Dong and D. G. Gray, Effect of Counterions on Ordered Phase Formation in Suspensions of Charged Rodlike Cellulose Crystallites, Langmuir, 1997, 13(8), 2404–2409 CrossRef CAS.
- E. Lizundia, T.-D. Nguyen, J. L. Vilas, W. Y. Hamad and M. J. MacLachlan, Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films, J. Mater. Chem. A, 2017, 5(36), 19184–19194 RSC.
- J. Xu and Z. Guo, Biomimetic photonic materials with tunable structural colors, J. Colloid Interface Sci., 2013, 406(0), 1–17 CrossRef CAS PubMed.
- K. Yu, T. Fan, S. Lou and D. Zhang, Biomimetic optical materials: Integration of nature's design for manipulation of light, Prog. Mater. Sci., 2013, 58(6), 825–873 CrossRef.
- S. N. Fernandes, P. L. Almeida, N. Monge, L. E. Aguirre, D. Reis, C. L. P. de Oliveira, A. M. F. Neto, P. Pieranski and M. H. Godinho, Mind the Microgap in Iridescent Cellulose Nanocrystal Films, Adv. Mater., 2017, 29(2), 1603560 CrossRef PubMed.
- L. E. Aguirre, A. de Oliveira, D. Seč, S. Čopar, P. L. Almeida, M. Ravnik, M. H. Godinho and S. Žumer, Sensing surface morphology of biofibers by decorating spider silk and cellulosic filaments with nematic microdroplets, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(5), 1174–1179 CrossRef CAS PubMed.
- T. Hiratani, W. Y. Hamad and M. J. MacLachlan, Transparent Depolarizing Organic and Inorganic Films for Optics and Sensors, Adv. Mater., 2017, 29(13), 1606083 CrossRef PubMed.
- H. Zheng, W. Li, W. Li, X. Wang, Z. Tang, S. X.-A. Zhang and Y. Xu, Uncovering the Circular Polarization Potential of Chiral Photonic Cellulose Films for Photonic Applications, Adv. Mater., 2018, 30(13), 1705948 CrossRef PubMed.
- K. Yao, Q. Meng, V. Bulone and Q. Zhou, Flexible and Responsive Chiral Nematic Cellulose Nanocrystal/Poly(ethylene glycol) Composite Films with Uniform and Tunable Structural Color, Adv. Mater., 2017, 29(28), 1701323 CrossRef PubMed.
- M. Xu, W. Li, C. Ma, H. Yu, Y. Wu, Y. Wang, Z. Chen, J. Li and S. Liu, Multifunctional chiral nematic cellulose nanocrystals/glycerol structural colored nanocomposites for intelligent responsive films, photonic inks and iridescent coatings, J. Mater. Chem. C, 2018, 6(20), 5391–5400 RSC.
- G. Guidetti, S. Atifi, S. Vignolini and W. Y. Hamad, Flexible Photonic Cellulose Nanocrystal Films, Adv. Mater., 2016, 28(45), 10042–10047 CrossRef CAS PubMed.
- H. Wan, X. Li, L. Zhang, X. Li, P. Liu, Z. Jiang and Z.-Z. Yu, Rapidly Responsive and Flexible Chiral Nematic Cellulose Nanocrystal Composites as Multifunctional Rewritable Photonic Papers with Eco-Friendly Inks, ACS Appl. Mater. Interfaces, 2018, 10(6), 5918–5925 CrossRef CAS.
- B. Natarajan, A. Krishnamurthy, X. Qin, C. D. Emiroglu, A. Forster, E. J. Foster, C. Weder, D. M. Fox, S. Keten, J. Obrzut and J. W. Gilman, Binary Cellulose Nanocrystal Blends for Bioinspired Damage Tolerant Photonic Films, Adv. Funct. Mater., 2018, 28(26), 1800032 CrossRef.
- J. A. Kelly, A. M. Shukaliak, C. C. Y. Cheung, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Responsive Photonic Hydrogels Based on Nanocrystalline Cellulose, Angew. Chem., Int. Ed., 2013, 52(34), 8912–8916 CrossRef CAS PubMed.
- M. Giese, L. K. Blusch, M. K. Khan and M. J. MacLachlan, Functional Materials from Cellulose-Derived Liquid-Crystal Templates, Angew. Chem., Int. Ed., 2015, 54(10), 2888–2910 CrossRef CAS PubMed.
- M. Giese, M. K. Khan, W. Y. Hamad and M. J. MacLachlan, Imprinting of Photonic Patterns with Thermosetting Amino-Formaldehyde-Cellulose Composites, ACS Macro Lett., 2013, 2(9), 818–821 CrossRef CAS.
- M. K. Khan, M. Giese, M. Yu, J. A. Kelly, W. Y. Hamad and M. J. MacLachlan, Flexible Mesoporous Photonic Resins with Tunable Chiral Nematic Structures, Angew. Chem., Int. Ed., 2013, 52(34), 8921–8924 CrossRef CAS.
- J. Chen, L. Xu, X. Lin, R. Chen, D. Yu, W. Hong, Z. Zheng and X. Chen, Self-healing responsive chiral photonic films for sensing and encoding, J. Mater. Chem. C, 2018, 6(29), 7767–7775 RSC.
- G. Chu, X. Wang, T. Chen, J. Gao, F. Gai, Y. Wang and Y. Xu, Optically Tunable Chiral Plasmonic Guest–Host Cellulose Films Weaved with Long-range Ordered Silver Nanowires, ACS Appl. Mater. Interfaces, 2015, 7(22), 11863–11870 CrossRef CAS PubMed.
- T.-D. Nguyen, W. Y. Hamad and M. J. MacLachlan, CdS Quantum Dots Encapsulated in Chiral Nematic Mesoporous Silica: New Iridescent and Luminescent Materials, Adv. Funct. Mater., 2014, 24(6), 777–783 CrossRef CAS.
- E. Lizundia, T.-D. Nguyen, J. L. Vilas, W. Y. Hamad and M. J. MacLachlan, Chiroptical luminescent nanostructured cellulose films, Mater. Chem. Front., 2017, 1(5), 979–987 RSC.
- T.-D. Nguyen, W. Y. Hamad and M. J. MacLachlan, Near-IR-Sensitive Upconverting Nanostructured Photonic Cellulose Films, Adv. Opt. Mater., 2017, 5(1), 1600514 CrossRef.
- S. N. Baker and G. A. Baker, Luminescent Carbon Nanodots: Emergent Nanolights, Angew. Chem., Int. Ed., 2010, 49(38), 6726–6744 CrossRef CAS PubMed.
- K. E. Shopsowitz, H. Qi, W. Y. Hamad and M. J. MacLachlan, Free-standing mesoporous silica films with tunable chiral nematic structures, Nature, 2010, 468(7322), 422–425 CrossRef CAS PubMed.
- K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Flexible and Iridescent Chiral Nematic Mesoporous Organosilica Films, J. Am. Chem. Soc., 2012, 134(2), 867–870 CrossRef CAS PubMed.
- A. S. Terpstra, L. P. Arnett, A. P. Manning, C. A. Michal, W. Y. Hamad and M. J. MacLachlan, Iridescent Chiral Nematic Mesoporous Organosilicas with Alkylene Spacers, Adv. Opt. Mater., 2018, 6(13), 1800163 CrossRef.
- J. A. Kelly, M. Giese, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, The Development of Chiral Nematic Mesoporous Materials, Acc. Chem. Res., 2014, 47(4), 1088–1096 CrossRef CAS.
- A. S. Terpstra, K. E. Shopsowitz, C. F. Gregory, A. P. Manning, C. A. Michal, W. Y. Hamad, J. Yang and M. J. MacLachlan, Helium ion microscopy: a new tool for imaging novel mesoporous silica and organosilica materials, Chem. Commun., 2013, 49(16), 1645–1647 RSC.
- J. A. Kelly, M. Yu, W. Y. Hamad and M. J. MacLachlan, Large, Crack-Free Freestanding Films with Chiral Nematic Structures, Adv. Opt. Mater., 2013, 1(4), 295–299 CrossRef.
- K. Robbie, D. J. Broer and M. J. Brett, Chiral nematic order in liquid crystals imposed by an engineered inorganic nanostructure, Nature, 1999, 399(6738), 764–766 CrossRef CAS.
- R. C. Schroden, M. Al-Daous, C. F. Blanford and A. Stein, Optical Properties of Inverse Opal Photonic Crystals, Chem. Mater., 2002, 14(8), 3305–3315 CrossRef CAS.
- G. I. N. Waterhouse and M. R. Waterland, Opal and inverse opal photonic crystals: Fabrication and characterization, Polyhedron, 2007, 26(2), 356–368 CrossRef CAS.
- C. I. Aguirre, E. Reguera and A. Stein, Tunable Colors in Opals and Inverse Opal Photonic Crystals, Adv. Funct. Mater., 2010, 20(16), 2565–2578 CrossRef CAS.
- S. O. Klimonsky, V. A. Vera, S. S. Alexander and D. T. Yuri, Photonic crystals based on opals and inverse opals: synthesis and structural features, Russ. Chem. Rev., 2011, 80(12), 1191 CrossRef CAS.
- K. E. Shopsowitz, J. A. Kelly, W. Y. Hamad and M. J. MacLachlan, Biopolymer Templated Glass with a Twist: Controlling the Chirality, Porosity, and Photonic Properties of Silica with Cellulose Nanocrystals, Adv. Funct. Mater., 2014, 24(3), 327–338 CrossRef CAS.
- M. Giese, J. C. De Witt, K. E. Shopsowitz, A. P. Manning, R. Y. Dong, C. A. Michal, W. Y. Hamad and M. J. MacLachlan, Thermal Switching of the Reflection in Chiral Nematic Mesoporous Organosilica Films Infiltrated with Liquid Crystals, ACS Appl. Mater. Interfaces, 2013, 5(15), 6854–6859 CrossRef CAS PubMed.
- K. E. Shopsowitz, A. Stahl, W. Y. Hamad and M. J. MacLachlan, Hard Templating of Nanocrystalline Titanium Dioxide with Chiral Nematic Ordering, Angew. Chem., Int. Ed., 2012, 51(28), 6886–6890 CrossRef CAS PubMed.
- G. Chu, J. Feng, Y. Wang, X. Zhang, Y. Xu and H. Zhang, Chiral nematic mesoporous films of ZrO2:Eu3+: new luminescent materials, Dalton Trans., 2014, 43(41), 15321–15327 RSC.
- R. Caruso, Nanocasting and Nanocoating, in Colloid Chemistry I, ed. M. Antonietti, Springer Berlin Heidelberg, 2003, vol. 226, pp. 91–118 Search PubMed.
- H. Liu, G. Wang, J. Liu, S. Qiao and H. Ahn, Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance, J. Mater. Chem., 2011, 21(9), 3046–3052 RSC.
- G. S. Armatas, A. P. Katsoulidis, D. E. Petrakis, P. J. Pomonis and M. G. Kanatzidis, Nanocasting of Ordered Mesoporous Co3O4-Based Polyoxometalate Composite Frameworks, Chem. Mater., 2010, 22(20), 5739–5746 CrossRef CAS.
- T. Waitz, B. Becker, T. Wagner, T. Sauerwald, C. D. Kohl and M. Tiemann, Ordered nanoporous SnO2 gas sensors with high thermal stability, Sens. Actuators, B, 2010, 150(2), 788–793 CrossRef CAS.
- D. Gu and F. Schüth, Synthesis of non-siliceous mesoporous oxides, Chem. Soc. Rev., 2014, 43(1), 313–344 RSC.
- Y. Li and J. Shi, Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications, Adv. Mater., 2014, 26(20), 3176–3205 CrossRef CAS PubMed.
- I. S. Nikolaev, P. Lodahl and W. L. Vos, Fluorescence Lifetime of Emitters with Broad Homogeneous Linewidths Modified in Opal Photonic Crystals, J. Phys. Chem. C, 2008, 112(18), 7250–7254 CrossRef CAS.
- M.-C. Daniel and D. Astruc, Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology, Chem. Rev., 2003, 104(1), 293–346 CrossRef PubMed.
- H. Qi, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Chiral Nematic Assemblies of Silver Nanoparticles in Mesoporous Silica Thin Films, J. Am. Chem. Soc., 2011, 133(11), 3728–3731 CrossRef CAS PubMed.
- J. A. Kelly, K. E. Shopsowitz, J. M. Ahn, W. Y. Hamad and M. J. MacLachlan, Chiral Nematic Stained Glass: Controlling the Optical Properties of Nanocrystalline Cellulose-Templated Materials, Langmuir, 2012, 28(50), 17256–17262 CrossRef CAS PubMed.
- C. Noguez and I. L. Garzon, Optically active metal nanoparticles, Chem. Soc. Rev., 2009, 38(3), 757–771 RSC.
- C. Gautier and T. Bürgi, Chiral Gold Nanoparticles, ChemPhysChem, 2009, 10(3), 483–492 CrossRef CAS PubMed.
- V. Kitaev, Chiral nanoscale building blocks-from understanding to applications, J. Mater. Chem., 2008, 18(40), 4745–4749 RSC.
- A. P. Alivisatos, Semiconductor Clusters, Nanocrystals, and Quantum Dots, Science, 1996, 271(5251), 933–937 CrossRef CAS.
- P. P. Jha and P. Guyot-Sionnest, Trion Decay in Colloidal Quantum Dots, ACS Nano, 2009, 3(4), 1011–1015 CrossRef CAS PubMed.
- S. S. Liji Sobhana, M. Vimala Devi, T. P. Sastry and A. Mandal, CdS quantum dots for measurement of the size-dependent optical properties of thiol capping, J. Nanopart. Res., 2011, 13(4), 1747–1757 CrossRef CAS.
- A. Rose, Z. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Sensitivity gains in chemosensing by lasing action in organic polymers, Nature, 2005, 434(7035), 876–879 CrossRef CAS PubMed.
- Y. Xia, L. Song and C. Zhu, Turn-On and Near-Infrared Fluorescent Sensing for 2,4,6-Trinitrotoluene Based on Hybrid (Gold Nanorod)–(Quantum Dots) Assembly, Anal. Chem., 2011, 83(4), 1401–1407 CrossRef CAS PubMed.
- R. Freeman, T. Finder, L. Bahshi, R. Gill and I. Willner, Functionalized CdSe/ZnS QDs for the Detection of Nitroaromatic or RDX Explosives, Adv. Mater., 2012, 24(48), 6416–6421 CrossRef CAS PubMed.
- W. Wei, X. Huang, K. Chen, Y. Tao and X. Tang, Fluorescent organic-inorganic hybrid polyphosphazene microspheres for the trace detection of nitroaromatic explosives, RSC Adv., 2012, 2(9), 3765–3771 RSC.
- S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Highly Selective Detection of Nitro Explosives by a Luminescent Metal–Organic Framework, Angew. Chem., Int. Ed., 2013, 52(10), 2881–2885 CrossRef CAS PubMed.
- E. R. Goldman, I. L. Medintz, J. L. Whitley, A. Hayhurst, A. R. Clapp, H. T. Uyeda, J. R. Deschamps, M. E. Lassman and H. Mattoussi, A Hybrid Quantum Dot–Antibody Fragment Fluorescence Resonance Energy Transfer-Based TNT Sensor, J. Am. Chem. Soc., 2005, 127(18), 6744–6751 CrossRef CAS PubMed.
- R. Tu, B. Liu, Z. Wang, D. Gao, F. Wang, Q. Fang and Z. Zhang, Amine-Capped ZnS–Mn2+ Nanocrystals for Fluorescence Detection of Trace TNT Explosive, Anal. Chem., 2008, 80(9), 3458–3465 CrossRef CAS PubMed.
- K. Zhang, H. Zhou, Q. Mei, S. Wang, G. Guan, R. Liu, J. Zhang and Z. Zhang, Instant Visual Detection of Trinitrotoluene Particulates on Various Surfaces by Ratiometric Fluorescence of Dual-Emission Quantum Dots Hybrid, J. Am. Chem. Soc., 2011, 133(22), 8424–8427 CrossRef CAS PubMed.
- Y. Ma, H. Li, S. Peng and L. Wang, Highly Selective and Sensitive Fluorescent Paper Sensor for Nitroaromatic Explosive Detection, Anal. Chem., 2012, 84(19), 8415–8421 CrossRef CAS PubMed.
- K. Aoki, D. Guimard, M. Nishioka, M. Nomura, S. Iwamoto and Y. Arakawa, Coupling of quantum-dot light emission with a three-dimensional photonic-crystal nanocavity, Nat. Photonics, 2008, 2(11), 688–692 CrossRef CAS.
- A. C. Arsenault, T. J. Clark, G. von Freymann, L. Cademartiri, R. Sapienza, J. Bertolotti, E. Vekris, S. Wong, V. Kitaev, I. Manners, R. Z. Wang, S. John, D. Wiersma and G. A. Ozin, From colour fingerprinting to the control of photoluminescence in elastic photonic crystals, Nat. Mater., 2006, 5(3), 179–184 CrossRef CAS.
- O. Painter, R. K. Lee, A. Scherer, A. Yariv, J. D. O'Brien, P. D. Dapkus and I. Kim, Two-Dimensional Photonic Band-Gap Defect Mode Laser, Science, 1999, 284(5421), 1819–1821 CrossRef CAS PubMed.
- S. Ogawa, M. Imada, S. Yoshimoto, M. Okano and S. Noda, Control of Light Emission by 3D Photonic Crystals, Science, 2004, 305(5681), 227–229 CrossRef CAS PubMed.
- S. H. M. Mehr, M. Giese, H. Qi, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Novel PPV/Mesoporous Organosilica Composites: Influence of the Host Chirality on a Conjugated Polymer Guest, Langmuir, 2013, 29(40), 12579–12584 CrossRef CAS PubMed.
- P. K. H. Ho, D. S. Thomas, R. H. Friend and N. Tessler, All-Polymer Optoelectronic Devices, Science, 1999, 285(5425), 233–236 CrossRef CAS.
- G. Yu and A. J. Heeger, High efficiency photonic devices made with semiconducting polymers, Synth. Met., 1997, 85(1–3), 1183–1186 CrossRef CAS.
- A. Teichler, R. Eckardt, C. Friebe, J. Perelaer and U. S. Schubert, Film formation properties of inkjet printed poly(phenylene-ethynylene)-poly(phenylene-vinylene)s, Thin Solid Films, 2011, 519(11), 3695–3702 CrossRef CAS.
- A. J. Heeger, Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture), Angew. Chem., Int. Ed., 2001, 40(14), 2591–2611 CrossRef CAS PubMed.
- D. P. Puzzo, F. Scotognella, M. Zavelani-Rossi, M. Sebastian, A. J. Lough, I. Manners, G. Lanzani, R. Tubino and G. A. Ozin, Distributed Feedback Lasing from a Composite Poly(phenylene vinylene)–Nanoparticle One-Dimensional Photonic Crystal, Nano Lett., 2009, 9(12), 4273–4278 CrossRef CAS PubMed.
- A. O. Furmanchuk, J. Leszczynski, S. Tretiak and S. V. Kilina, Morphology and Optical Response of Carbon Nanotubes Functionalized by Conjugated Polymers, J. Phys. Chem. C, 2012, 116(12), 6831–6840 CrossRef CAS.
- L. Feng, H. Li, Y. Qu and C. Lu, Detection of TNT based on conjugated polymer encapsulated in mesoporous silica nanoparticles through FRET, Chem. Commun., 2012, 48(38), 4633–4635 RSC.
- R. C. Smith, W. M. Fischer and D. L. Gin, Ordered Poly(p-phenylenevinylene) Matrix Nanocomposites via Lyotropic Liquid-Crystalline Monomers, J. Am. Chem. Soc., 1997, 119(17), 4092–4093 CrossRef CAS.
- H. Skaff, K. Sill and T. Emrick, Quantum Dots Tailored with Poly(para-phenylene vinylene), J. Am. Chem. Soc., 2004, 126(36), 11322–11325 CrossRef CAS.
- M. Kyotani, S. Matsushita, M. Goh, T. Nagai, Y. Matsui and K. Akagi, Entanglement-free fibrils of aligned polyacetylene films that produce single nanofibers, Nanoscale, 2010, 2(4), 509–514 RSC.
- S. Matsushita and K. Akagi, Synthesis of Conjugated Polymers in Chiral Nematic Liquid Crystal Fields, Isr. J. Chem., 2011, 51(10), 1075–1095 CrossRef CAS.
- A. S. Terpstra, W. Y. Hamad and M. J. MacLachlan, Photopatterning Freestanding Chiral Nematic Mesoporous Organosilica Films, Adv. Funct. Mater., 2017, 27(45), 1703346 CrossRef.
- S. Bénard and P. Yu, New Spiropyrans Showing Crystalline-State Photochromism, Adv. Mater., 2000, 12(1), 48–50 CrossRef.
- Y. Hirshberg and E. Fischer, Photochromism and reversible multiple internal transitions in some spiropyrans at low temperatures. Part I, J. Chem. Soc., 1954,(0), 297–303 RSC.
- C. T. Burns, S. Y. Choi, M. L. Dietz and M. A. Firestone, Acidichromic Spiropyran-Functionalized Mesoporous Silica: Towards Stimuli-Responsive Metal Ion Separations Media, Sep. Sci. Technol., 2008, 43(9–10), 2503–2519 CrossRef CAS.
- A. K. Chibisov and H. Görner, Complexes of spiropyran-derived merocyanines with metal ions: relaxation kinetics, photochemistry and solvent effects, Chem. Phys., 1998, 237(3), 425–442 CrossRef CAS.
- K. H. Fries, J. D. Driskell, G. R. Sheppard and J. Locklin, Fabrication of Spiropyran-Containing Thin Film Sensors Used for the Simultaneous Identification of Multiple Metal Ions, Langmuir, 2011, 27(19), 12253–12260 CrossRef CAS PubMed.
- H. S. Kitzerow, A. Lorenz and H. Matthias, Tuneable Photonic Crystals obtained by Liquid Crystal Infiltration, in Nanophotonic Materials, Wiley-VCH Verlag GmbH & Co. KGaA, 2008, pp. 221–237 Search PubMed.
- H. S. Kitzerow, A. Lorenz and H. Matthias, Tuneable photonic crystals obtained by liquid crystal infiltration, Phys. Status Solidi A, 2007, 204(11), 3754–3767 CrossRef CAS.
- A. P. Manning, M. Giese, A. S. Terpstra, M. J. MacLachlan, W. Y. Hamad, R. Y. Dong and C. A. Michal, NMR of guest-host systems: 8CB in chiral nematic porous glasses, Magn. Reson. Chem., 2014, 52(10), 532–539 CrossRef CAS PubMed.
- M. Spengler, R. Y. Dong, C. A. Michal, W. Y. Hamad, M. J. MacLachlan and M. Giese, Hydrogen-Bonded Liquid Crystals in Confined Spaces—Toward Photonic Hybrid Materials, Adv. Funct. Mater., 2018, 28(26), 1800207 CrossRef.
- M. Giese, T. Krappitz, R. Y. Dong, C. A. Michal, W. Y. Hamad, B. O. Patrick and M. J. MacLachlan, Tuning the photonic properties of chiral nematic mesoporous organosilica with hydrogen-bonded liquid-crystalline assemblies, J. Mater. Chem. C, 2015, 3(7), 1537–1545 RSC.
- M. K. Khan, W. Y. Hamad and M. J. MacLachlan, Tunable Mesoporous Bilayer Photonic Resins with Chiral Nematic Structures and Actuator Properties, Adv. Mater., 2014, 26, 2323–2328 CrossRef CAS PubMed.
- W.-E. Lee, Y.-J. Jin, L.-S. Park and G. Kwak, Fluorescent Actuator Based on Microporous Conjugated Polymer with Intramolecular Stack Structure, Adv. Mater., 2012, 24(41), 5604–5609 CrossRef CAS PubMed.
- T. Wu, J. Li, J. Li, S. Ye, J. Wei and J. Guo, A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties, J. Mater. Chem. C, 2016, 4(41), 9687–9696 RSC.
- M. K. Khan, A. Bsoul, K. Walus, W. Y. Hamad and M. J. MacLachlan, Photonic Patterns Printed in Chiral Nematic Mesoporous Resins, Angew. Chem., Int. Ed., 2015, 54(14), 4304–4308 CrossRef CAS PubMed.
- V. M. Zamarion, M. K. Khan, M. Schlesinger, A. Bsoul, K. Walus, W. Y. Hamad and M. J. MacLachlan, Photonic metal–polymer resin nanocomposites with chiral nematic order, Chem. Commun., 2016, 52(50), 7810–7813 RSC.
- J. Leng, G. Li, X. Ji, Z. Yuan, Y. Fu, H. Li, M. Qin and H. Moehwald, Flexible latex photonic films with tunable structural colors templated by cellulose nanocrystals, J. Mater. Chem. C, 2018, 6(9), 2396–2406 RSC.
- M. K. Khan, W. Y. Hamad and M. J. MacLachlan, Tunable Mesoporous Bilayer Photonic Resins with Chiral Nematic Structures and Actuator Properties, Adv. Mater., 2014, 26(15), 2323–2328 CrossRef CAS PubMed.
- M. Giese, L. K. Blusch, M. K. Khan, W. Y. Hamad and M. J. MacLachlan, Responsive Mesoporous Photonic Cellulose Films by Supramolecular Cotemplating, Angew. Chem., Int. Ed., 2014, 53(34), 8880–8884 CrossRef CAS PubMed.
- M. Schlesinger, M. Giese, L. K. Blusch, W. Y. Hamad and M. J. MacLachlan, Chiral nematic cellulose–gold nanoparticle composites from mesoporous photonic cellulose, Chem. Commun., 2015, 51(3), 530–533 RSC.
- M. Giese, L. K. Blusch, M. Schlesinger, G. R. Meseck, W. Y. Hamad, M. Arjmand, U. Sundararaj and M. J. MacLachlan, Magnetic Mesoporous Photonic Cellulose Films, Langmuir, 2016, 32(36), 9329–9334 CrossRef CAS PubMed.
- M. Schlesinger, W. Y. Hamad and M. J. MacLachlan, Optically tunable chiral nematic mesoporous cellulose films, Soft Matter, 2015, 11(23), 4686–4694 RSC.
| This journal is © The Royal Society of Chemistry 2019 |