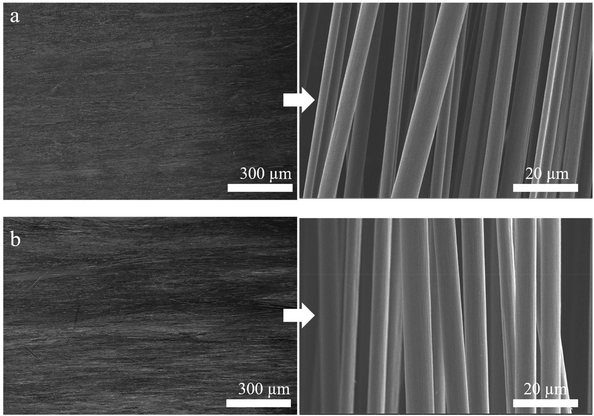
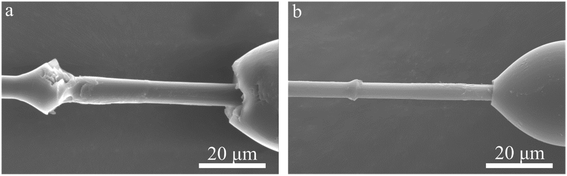
Open Access Article
Open Access ArticleRecycling of carbon fibre reinforced plastics by electrically driven heterogeneous catalytic degradation of epoxy resin†
Ji-Hua
Zhu
 a,
Pi-yu
Chen
a,
Mei-ni
Su
a,
Pi-yu
Chen
a,
Mei-ni
Su
 ab,
Chun
Pei
a and
Feng
Xing
*a
ab,
Chun
Pei
a and
Feng
Xing
*a
aGuangdong Province Key Laboratory of Durability for Marine Civil Engineering, School of Civil Engineering, Shenzhen University, Shenzhen, Guangdong 518060, PR China. E-mail: xingf@szu.edu.cn
bSchool of Mechanical, Aerospace and Civil Engineering, University of Manchester, Manchester, M1 7JR, UK
First published on 21st February 2019
Abstract
The abundant end-of-service-life carbon fibre reinforced plastic (CFRP) composites have become an increasingly significant environmental issue, making the key challenge to be how to increase the resource efficiency by turning waste into reusable materials. Existing recycling technologies generally require complicated processes, expensive facilities or toxic chemicals. Moreover, these demanding conditions limit the size of CFRP waste, resulting in greatly reduced commercial value of recycled fibres and less cost-effective applications. Here, we demonstrate a new recycling technology with the benefit of the electrochemical promotion of the catalysis effect, termed the electrically driven heterocatalytic decomposition (EHD) method. Our results show that even by using simple equipment and conventional nontoxic electrolyte components, intact carbon fibres can be efficiently recycled under atmospheric pressure and at room temperature. The residual strength of the reclaimed carbon fibres (rCFs) is close to that of the virgin carbon fibres (VCFs), i.e., a 90% residual tensile strength and 121% residual interfacial shear strength compared with the VCFs. More importantly, the simplicity of the procedure and the moderate requirements of the processing facilities removal of the CFRP waste size limit, significantly improving the commercial value of the recycled fibres and enabling large-scale implementation.
Introduction
CFRPs are composite engineered materials consisting of carbon fibres (CFs) as reinforcements and an organic epoxy resin as a matrix, a combination that renders the materials extremely strong but also lightweight. These features, along with their unique electrical conductivity, thermal stability, good resistance to corrosion, and high rigidity,1 have led to CFRPs being widely used in the aerospace, vehicle, wind power generation, and construction industries2,3 since the 1970s. In 2017, the worldwide demand for CFs was 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 tons, and this demand is expected to reach 112
400 tons, and this demand is expected to reach 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in 2020. Since the service life of CFRPs is approximately 50 years, the extensive use of CFRP is now starting to produce serious waste disposal problems.
000 tons in 2020. Since the service life of CFRPs is approximately 50 years, the extensive use of CFRP is now starting to produce serious waste disposal problems.
Although the CFRP materials reach the end of their service life, the CFs themselves generally retain their properties;4 therefore, the prospect of recycling CFRP composites without damaging the mechanical properties and dimensions of CFs is of great commercial interest. The recovery value of epoxy resin is not comparable to that of CFs; thus, in recent years, a great number of studies have been conducted on the reclamation of CFs from composite wastes, including mechanical recycling such as shredding and milling,5,6 pyrolysis at high temperature,7,8 chemical decomposition using solvents such as methanol, ethanol, 1-propanol and acetone,9–12 chemical recycling under sub-/supercritical conditions,13–15 decomposition in high-temperature fluidized bed processes16–18 and an electrochemical recycling method.19 However, as shown in Table 1, the existing techniques generally suffer from various drawbacks such as requiring complicated processes and superior facilities, pollution generation and damage to the CFRP waste. Therefore, the remaining challenge is how to eliminate the epoxy resin without damaging the dimensions and properties of CFs in CFRPs through an environmentally friendly process. In order to overcome the challenge and fully utilize the high residual value of end-of-service-life CFRP composites, our research team has made great efforts in the past few years to investigate sustainable and affordable recycling technologies. In the present study, two new reaction conditions – KOH catalyst and temperatures – are considered to improve the recycling efficiency and the quality of rCFs. In addition, other key properties of the rCFs such as interfacial shear strength and surface roughness are thoroughly studied to have a better understanding of the feasibility for remanufacturing using rCFs. Furthermore, a more detailed microstructure analysis is carried out in the present study to analyse the degradation mechanism of the epoxy resin: the cyano group of the crosslinking agent dicyandiamide and the hydroxy group of the bisphenol-A type epoxy resin form amido bond during the curing process of the epoxy resin. Electrically driven heterogeneous catalytic reactions have been shown to enable the breaking of the C–N bonds of the amido bonds, thus leading to decomposition of the epoxy resin and reclamation of CFs with the benefit of the electrochemical promotion of catalysis (EPOC) effect.20 Therefore, we reasoned that using a catalyst under these conditions should enhance this decomposition reaction, and we proposed the electrically driven heterocatalytic decomposition (EHD) method. In this study, CFRP composites were immersed in a NaCl electrolyte containing the KOH catalyst in the presence of electrical currents, and the effect of temperature was also investigated. With a view to optimize the reaction conditions to maximize the quality of the rCFs while minimizing the reaction time and energy input, we examined the effects of varying the applied current density, electrolyte concentration, catalyst concentration and temperature on the recycling of CFs. Mechanical tests and microstructural analyses are used to assess the mechanical properties and microstructures of the recycled fibres and to reveal the influences of the different reaction conditions. We found that the use of the catalyst and a moderately increased temperature with otherwise mild reaction conditions were key to obtaining high-performance CFs in an effective and efficient procedure. Finally, an optimized recycling method is suggested.
| Assessment | Methods | |||||||
|---|---|---|---|---|---|---|---|---|
| Mechanical5,6 | Thermal | Chemical | EHD method | |||||
| Milling | Combustion7,8 | Pyrolysis7,8 | Fluidized bed16–18 | Solvolysis9–12 | Supercritical/subcritical13–15 | |||
| Facility | Temperature | Room temperature | 1400–1600 °C | 400–1000 °C | 450–500 °C | ∼90 °C | 400–650 °C | Room temperature |
| Pressure | Atmospheric pressure | Atmospheric pressure | Atmospheric pressure | 10–25 kPa | Atmospheric pressure | 22.1–35 MPa | Atmospheric pressure | |
| Toxicity | Nontoxic | Nontoxic | Nontoxic | Nontoxic | Toxic | Toxic | Nontoxic | |
| Performance | Waste size limit (length) | 10–50 mm | NA | 6–25 mm | 5–10 mm | 1 μm–50 mm | 10–50 mm | No limit |
| Residual tensile strength (vs. VCF) | 50–65% | NA | 50–85% | 10–75% | 85–98% | 85–98% | 89.46% | |
| Shear strength (vs. VCF) | Unreported | NA | NA | 80% | Unreported | Unreported | 120.74% | |
| Environmental issues | Dust | Pollutant gas, dust, high energy use | Pollutant gas, high energy use | Pollutant gas, organic solvent, high energy use | Organic solvent | Organic solvent, high energy use | None | |
Our EHD method is the first green and cost-effective procedure for recycling high-strength CFs from commercial CFRP with no size limit (see Fig. 1). The chemicals used in this method are conventional, accessible and nontoxic. The easily implemented machinery and mild reaction conditions require low initial investments and energy consumption, which makes the economic value of the recycled fibres far higher than that of most existing recycled fibres. On top of the achieved merits, the research presented in this paper exploited the full potential of this new recycling technology and has a great potential for large-scale implementation.
Experimental section
Materials
The dimensions of the CFRP samples were 30 mm × 245 mm × 2 mm (width × length × thickness) (see Fig. SI1†). Each CFRP sample was divided length-wise into three regions: the test region (100 mm), which contained the rCFs; the protection region (80 mm), which insulated the test region; and the electrically conductive region (65 mm), which was connected to the power supply (see Fig. SI1†).CFRP was purchased from Carbon Composites Company Limited (Hong Kong) with an epoxy resin content of 31.5%. Each layer of CF cloth is made by orthogonal weaving of longitudinal and transverse CFs. The CFs were T700-type produced by Toray Corporation, Japan, and the epoxy resin was LAM-125/226 type. The chemical composition of the epoxy resin provided by the manufacturer is shown in Table SI 2.†
Experimental setup
The recycling setup is shown in Fig. SI1(d).† The system was composed of four parts: (1) a DC power supply, providing unidirectional currents for the system; (2) the electrodes – CFRP served as the anode, which was connected to the positive electrode of the power supply, while a stainless-steel strip served as the cathode, which was connected to the negative electrode of the power supply; (3) an electrolyte solution (9L); and (4) a datalogger – HIOKI-LR8400 Datalog from HIOKI E.E. The datalogger monitored and measured the voltage of the sample, recording the data once per hour. The measured voltages can assess CF degradation and energy consumption in different recovery processes as explained in ESI.† In all tests, the CFRP sample (anode) was placed parallel to the stainless-steel strip (cathode). System voltage and energy consumption are shown in ESI (Fig. SI 2†).Recycling process
The DC power supply was turned on to start the recycling process. The process was performed under laboratory conditions, and the solution volume was maintained throughout the experiment. At the end of the recycling process, the soft CFs were gently removed from the test sample with forceps and scissors. In order to reclaim 95% CFs from the composite wastes, the recycling process lasted for 72 hours at room temperature or 36 hours at elevated temperatures. The rCFs were then cleaned with alcohol by using an ultrasonic cleaner and rinsed three times (5 min each) with deionized water. The clean rCFs (Fig. 1) were placed in a dry box for three days at 50 °C.Experimental parameters
In this study, three series of experiments were designed to investigate the effects of the reaction conditions – NaCl concentration, KOH concentration, current density, and temperature – on the rCFs. Detailed testing groups and experimental parameters are shown in Table SI 3.†The first series of experiments investigated the effects of the current density and NaCl concentration. A total of 24 reaction conditions were considered, including six different current densities and four different NaCl concentrations. The six constant currents were 20, 40, 62.5, 78.1, 104.2, and 156.3 mA with the corresponding current densities of 3.33, 6.67, 10.42, 13.02, 17.37, and 26.01 A m−2, respectively. The NaCl solution was prepared using deionized water and sodium chloride at concentrations of 0.5, 1.0, 2.0, and 3.0%. Note that when the currents were 78.1, 104.2, and 156.3 mA, no intact rCF could be obtained; therefore, characterization was not carried out for specimens subjected to these reaction conditions.
In the second series of experiments, which were based on the optimized conditions from the first series of experiments, the concentration of the catalyst (KOH) was varied. The selected currents were 20 and 40 mA, corresponding to the current densities of 3.33 and 6.67 A m−2, respectively, and the selected NaCl concentrations were 1, 2 and 3%. Three KOH concentrations were tested: 0.5, 1, and 1.5 g L−1. Thus, a total of 18 reaction conditions were considered.
Similarly, in the third series of experiments, the optimized mix proportions obtained from the second series of experiments were used, that is, two currents (20 and 40 mA), one NaCl concentration (2%) and one KOH concentration (1 g L−1). In addition, the recycling reactions were carried out at 40, 60 and 75 °C. Thus, a total of six reaction conditions were tested.
Characterization
Results and discussion
Degradation of epoxy resin
The key factors in recycling CFs from CFRP waste are the degradation of the epoxy resin and the extent of its removal, which was measured in this study by thermogravimetric analysis. A greater extent of epoxy resin removal results in cleaner reclaimed fibres. Table 2 shows that with increasing NaCl concentration, the extent of epoxy resin removal first increased and then decreased; the experiments performed with 2% sodium chloride (NaCl) provided the greatest extent of epoxy resin removal. At 40, 60 and 75 °C, CFs were obtained after 36 hours with 99.3%–99.9% of the epoxy resin removed (see Table SI 1†). The results show that increasing the reaction temperature can significantly increase the epoxy resin decomposition efficiency. To reclaim 95% CFs from the composite wastes, the reaction time needed for the high temperature condition is only half of that at room temperature.| Test unit | Specimens | Extent of epoxy resin removal (%) | Tensile strength (GPa) | Residual strength (%) | Diameter (μm) | IFSS (MPa) | Residual strength (%) | Failure modes | R a (nm) |
|---|---|---|---|---|---|---|---|---|---|
| I = current; I20 = 20 mA; S = salt; K = KOH; K0.5 = 0.5 g L−1 KOH; T = temperature; T40 = 40 °C; DB = debonding failure within the epoxy resin; CB = debonding failure at the interface of the CFs and the epoxy resin. | |||||||||
| VCF | — | 4.641 | 100.00 | 7.00 | 31.00 | 100 | DB | 201 | |
| 1 | I20S0.5 | 68.3 | 2.634 | 56.76 | 6.97 | — | — | — | — |
| I20S1 | 89.6 | 3.472 | 74.81 | 6.95 | — | — | — | — | |
| I20S2 | 95.8 | 3.768 | 81.19 | 6.96 | — | — | — | — | |
| I20S3 | 90.4 | 3.488 | 75.16 | 6.96 | — | — | — | — | |
| I40S0.5 | 67.1 | 2.583 | 55.66 | 6.96 | — | — | — | — | |
| I40S1 | 89.3 | 3.417 | 73.63 | 6.95 | — | — | — | — | |
| I40S2 | 93.7 | 3.693 | 79.57 | 6.95 | — | — | — | — | |
| I40S3 | 89.8 | 3.458 | 74.51 | 6.95 | — | — | — | — | |
| I62.5S0.5 | 63.3 | 2.386 | 51.41 | 7.12 | — | — | — | — | |
| I62.5S1 | 65.5 | 2.471 | 53.24 | 6.97 | — | — | — | — | |
| I62.5S2 | 68.5 | 2.562 | 55.20 | 6.92 | — | — | — | — | |
| I62.5S3 | 66.1 | 2.509 | 54.06 | 6.94 | — | — | — | — | |
| 2 | I20S1K0.5 | 99.7 | 3.399 | 73.24 | 6.93 | 29.69 | 95.77 | CB | 195 |
| I20S1K1 | 99.6 | 3.165 | 68.20 | 6.91 | 33.48 | 108.00 | DB | 214 | |
| I20S1K1.5 | 100.0 | 2.952 | 63.61 | 6.85 | 32.76 | 105.68 | DB | 205 | |
| I20S2K0.5 | 99.8 | 3.426 | 73.82 | 6.94 | 29.50 | 95.16 | CB | 196 | |
| I20S2K1 | 99.7 | 3.310 | 71.32 | 6.90 | 37.43 | 120.74 | DB | 219 | |
| I20S2K1.5 | 99.5 | 3.021 | 65.09 | 6.87 | 33.06 | 106.65 | DB | 213 | |
| I20S3K0.5 | 99.9 | 3.413 | 73.54 | 6.93 | 28.83 | 93.00 | CB | 193 | |
| I20S3K1 | 99.7 | 3.198 | 68.91 | 6.92 | 34.06 | 109.87 | DB | 209 | |
| I20S3K1.5 | 99.6 | 2.966 | 63.91 | 6.86 | 33.65 | 108.55 | DB | 203 | |
| I40S1K0.5 | 99.7 | 3.357 | 72.33 | 6.87 | — | — | — | — | |
| 2 | I40S1K1 | 99.6 | 2.980 | 64.21 | 6.85 | — | — | — | — |
| I40S1K1.5 | 99.6 | 2.666 | 57.44 | 6.81 | — | — | — | — | |
| I40S2K0.5 | 99.4 | 3.365 | 72.51 | 6.88 | 27.00 | 87.11 | CB | 185 | |
| I40S2K1 | 99.8 | 2.776 | 59.81 | 6.84 | 28.08 | 90.57 | DB | 199 | |
| I40S2K1.5 | 99.6 | 2.735 | 58.93 | 6.82 | 24.70 | 79.69 | CB | 175 | |
| I40S3K0.5 | 99.5 | 3.348 | 72.14 | 6.87 | 26.53 | 85.58 | CB | 189 | |
| I40S3K1 | 99.8 | 2.910 | 62.70 | 6.85 | 28.33 | 91.39 | DB | 197 | |
| I40S3K1.5 | 99.3 | 2.660 | 57.32 | 6.82 | 25.20 | 81.29 | CB | 178 | |
| 3 | I20S2K1T40 | 99.5 | 3.759 | 81.00 | 6.99 | 25.42 | 82.00 | CB | 190 |
| I20S2K1T60 | 99.9 | 4.045 | 87.16 | 7.00 | 33.59 | 108.35 | DB | 203 | |
| I20S2K1T75 | 99.8 | 4.083 | 87.98 | 6.99 | 33.72 | 108.77 | DB | 208 | |
| I40S2K1T40 | 99.3 | 3.758 | 80.76 | 7.00 | 24.61 | 79.39 | CB | 195 | |
| I40S2K1T60 | 99.4 | 4.126 | 88.90 | 6.98 | 29.84 | 96.26 | DB | 199 | |
| I40S2K1T75 | 99.7 | 4.152 | 89.46 | 6.99 | 35.79 | 115.45 | DB | 211 | |
Note that the maximum reaction temperature in this study was 75 °C, which is far lower than the temperature required for thermal decomposition of the epoxy resin (i.e., in the range of approximately 300 to 600 °C under nitrogen or air atmosphere). Therefore, the enhanced extent of epoxy resin removal is not due to thermal decomposition of the epoxy resin. Rather, it is related to the mutual synergy between the high temperature and the catalyst (KOH). Further increasing the temperature will likely lead to a continuous increase in the extent of epoxy resin removal. However, higher temperatures might increase the requirement of the machinery, resulting in a greater initial investment. Therefore, the upper limit of temperature considered in this study is 75 °C.
The diameters of the rCFs are presented in Table 2. The results show that the diameter of the rCFs only slightly decreased compared to that of the new fibres prior to incorporation into a composite material VCF with a diameter of 7 μm, which shows intact CFs without damage to dimensions could be reclaimed from the proposed recycling technology. Under the same current density, when the catalyst (KOH) concentration was 1.5 g L−1, the diameter of the rCF was the smallest. The diameters of the rCF obtained at high temperatures (40, 60 and 75 °C) were generally the same.
Mechanical properties of rCF
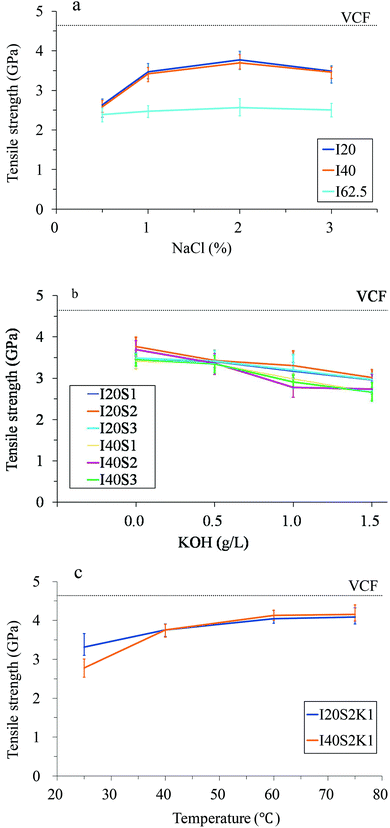 | ||
| Fig. 2 The tensile strength values of the rCFs. For an explanation of the sample numbers, see Table 2. (a) Effect of NaCl concentration; (b) effect of KOH concentration; (c) effect of temperature. | ||
The tensile strengths of the rCF decreased with the increasing current density – a slight decrease from 20 mA to 40 mA and a profound decrease from 40 mA to 62.5 mA (see Table 2). The long-term action of a high current density may damage the bulk structure of the CFs. When the current densities were 3.33 A m−2 and 6.67 A m−2 (I20 and I40 series), the tensile strengths of the rCF were much better than those of the other series (Fig. 2a). As shown in Fig. 2a, as the NaCl concentration increased from 0.5% through to 3%, the tensile strengths of the rCF first increased, reaching a maximum residual tensile strength of 81.19% at 2.0% NaCl (with a current of 20 mA) and then decreased. Fig. 2b shows that the residual tensile strength of the rCF does not improve with an increase in the KOH concentration. It is indicated that the high KOH concentration might exacerbate the oxidation of CFs and cause them mechanical damage. Conversely, KOH can significantly improve the extent of epoxy resin removal and increase the interfacial shear strength, which is discussed below. The results show that the residual tensile strength of the rCF increased with temperature (Fig. 2c). The increase was most significant from 25 to 40 °C and less significant in the range of 40 to 60 °C, whereas there was almost no change from 60 to 75 °C. The residual tensile strengths of the rCF treated at 75 °C with currents of 20 and 40 mA (specimens I20S2K1T75 and I40S2K1T75) reached 4.083 GPa and 4.152 GPa, respectively, which were 87.85% and 89.83% relative to VCF strength.
The matrix of the CF in the mechanical process of recycling has suffered severe damage, and long clean recycled fibres cannot be obtained. The residual strength of the rCF is 50 to 65% of that of VCFs.5,6 Although the mechanical process can result in cleaner, shorter lengths of CFs through thermal decomposition, due to the high temperature and surface oxidation, the mechanical properties of the CF are reduced to 50%–85%.7,8 Taking advantage of the dissolvability of super-/subcritical fluid to the polymer materials, clean CF can be obtained with the maximum retention (85%–98%) of the original mechanical properties of the CF via the solvolysis method.11–14 However, the demanding reaction conditions increase the requirements on the facilities and restrain the size of the rCF. The residual strength of the rCF obtained using the EHD method is far higher than that obtained using mechanical recycling and thermal decomposition, but slightly lower than that obtained by solvolysis.
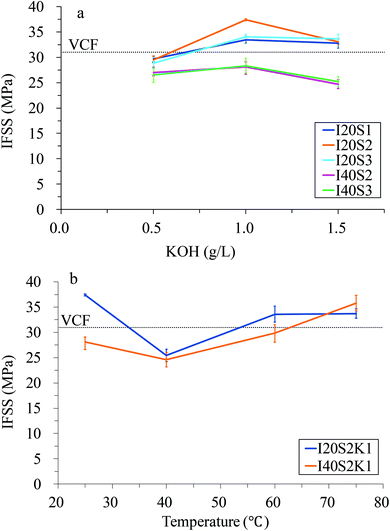 | ||
| Fig. 3 Shear strengths of the rCFs. For an explanation of the sample numbers, see Table 2. (a) Effect of KOH concentration; (b) effect of temperature. | ||
Higher current densities led to lower IFSS values of the rCF. When the NaCl concentration was increased from 1 to 3%, the IFSS of the rCF first increased, reaching a maximum value at 2% NaCl, and then decreased again. The IFSS of all samples of the rCF increased when the KOH concentration increased from 0.5 to 1.0 g L−1 but declined as the KOH concentration continued to increase from 1.0 to 1.5 g L−1 (Fig. 3a). In the I20 series, the lowest IFSS of the rCF was obtained when the KOH concentration was 0.5 g L−1, which was 4.23 to 7.0% lower than that of the VCFs. However, at KOH concentrations of 1.0 and 1.5 g L−1, the IFSS values for the rCF were higher than that of the VCFs. The IFSS of the rCF from specimen I20S2K1 was the highest (37.43 MPa), which was 20.74% higher than that of the VCFs. In the I40 series, on the other hand, the IFSS values for all rCF were lower than that of the VCFs. When the KOH concentration was ≥1.0 g L−1, the DB failure mode occurred. The relationship between the IFSS of the rCF and temperature is presented in Fig. 3b. When the temperature increased from 25 to 40 °C, the IFSS of the recycled I20 and I40 CFs dropped from 37.43 and 28.08 MPa to 25.42 and 24.61 MPa, respectively. For both samples of CF recycled at 40 °C, the failure mode was CB, the epoxy resin and CFs completely debonding, leaving barely a trace of the epoxy resin residue on the CF surface. As the temperature was increased to 60 °C, the IFSS also increased. The IFSS values of the recycled I20 and I40 CFs were 33.59 and 29.84 MPa, respectively, which are 108.35 and 96.26% of that of the VCFs. The failure mode was DB for both samples. When the temperature reached 75 °C, the IFSS values of the recycled I20 and I40 CFs were 33.72 and 35.79 MPa, respectively, which are 108.77 and 115.45% of that of the VCF; similarly, the failure mode was DB. The surface of the epoxy resin at the point of failure displayed prisms and asperities, which increased the area of the failure surface. In particular, the rCF from the I40 condition exhibited vigorous debonding and internal fracture of the droplets due to the strong force at failure. The above results show that decreasing the recycling period will reduce oxidative etching on rCF, whereas increasing the temperature will increase the surface roughness and impregnation.
Microstructural characterization of rCF
The SEM images of the rCF from the I20 and I40 series with the presence of KOH (where the current was set to 20 and 40 mA, respectively) show that all the resin was completely removed (Fig. 5), indicating that KOH can effectively decompose the epoxy resin. Very few defects were observed on the surface of the rCF obtained at a low current density and at both low and high NaCl concentrations. In the presence of high current densities or low NaCl concentrations, more oxygen is generated on the CFRP surface, and CFs are strongly oxidized and etched. In these cases, the SEM images (Fig. 5g and h) show that the surface layer of the rCF is degraded, and the decreased residual tensile strength can be attributed to the defects.
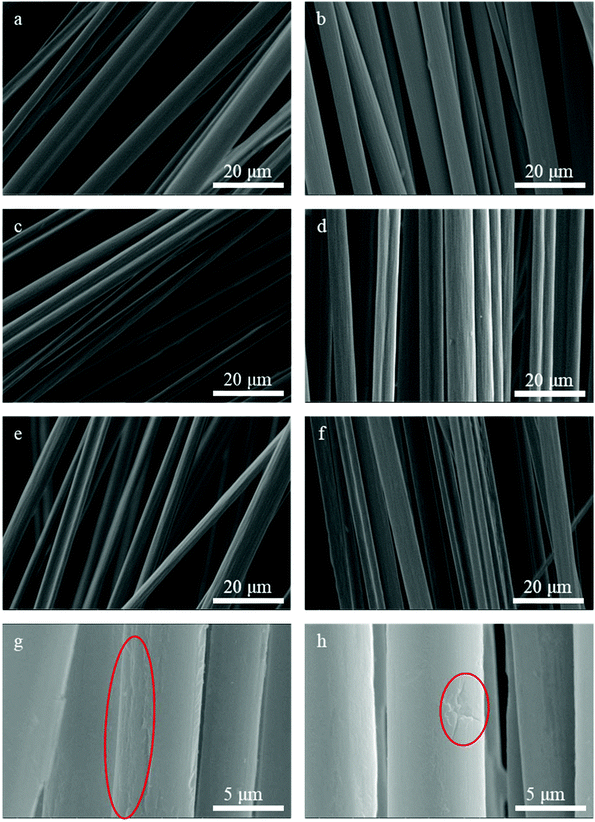 | ||
| Fig. 5 SEM images of the I20 and I40 series. For an explanation of the sample numbers, see Table 2. (a) Specimen I20S2K0.5; (b) specimen I40S2K0.5; (c) specimen I20S2K1; (d) specimen I40S2K1; (e) specimen I20S2K1.5; (f) specimen I40S2K1.5; (g) etchings and longitudinal grooves on specimen I20S2K1; (h) etchings and longitudinal grooves on specimen I20S2K1.5. | ||
The concentration of KOH catalyst is very important in this EHD process. Cl2 was generated at the anode region during electrolysis in the presence of Cl− (eqn (1)). Part of the gas reacted with water to form more oxidizing HClO (eqn (2)). ClO− would oxidize the epoxy resin by breaking down the C–N bond.19,23,24 It is critical to adjust the concentration of the KOH catalyst in order to optimize the performance of the recycling method because of the following two reasons. First, when KOH was added as a catalyst, H+ would react with OH− to form H2O, pushing the second reaction to the right side. Therefore, more ClO− would be formed, leading to the increased degradation efficiency of the epoxy resin. Due to the ionic intercalation in the electrochemical process, OH− ions would be inserted into the fibre surface and graphite layer, leading to carbon fibre cortex inflation. This phenomenon also increased the degradation of the epoxy resin and the recovery of the carbon fibres. Second, in the presence of excess OH−, the OH− would attach to the carbon atom surface. The adjacent carbon atoms and the adhered OH− would react to form oxygen, resulting in the oxidation etching effect on the carbon fibre surface. The reaction process is given in eqn (3) and (4):.
| 2Cl− → Cl2 + 2e− | (1) |
| Cl2 + H2O → HClO + H+ + Cl− | (2) |
| Cs + OH− → CsOHadsorb + e− | (3) |
| 4CsOHadsorb → 2H2O + O2 + 4Cs | (4) |
When the KOH concentration is 0.5 g L−1 (Fig. 5a and b), the surface of the CFs is relatively smooth and the curvature is unchanged, and there are no visible longitudinal grooves. This indicates that the CFs are not damaged, which explains why the tensile strength of these CFs was only slightly lower than those obtained using reaction conditions without KOH. With an increase in the KOH concentration, the oxidation-induced etching on the CFs and intercalation of OH− ions become pronounced. Therefore, longitudinal grooves can be observed on the CF surface, as shown in Fig. 5c–f. This degradation was more severe in the I40 series. Cracks can even be seen on the CF surfaces of specimens I40S2K1 (59.81% of that of the VCF) and I40S2K1.5 (58.93% of that of the VCF). For more detailed observations, these two specimens were scanned at a higher magnification of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Fig. 5g shows a small etched part of the rCFs with a reduced cross-sectional area and clear longitudinal groove structures. In Fig. 5h, the surface of the CFs has deteriorated due to oxidation and exhibits pits and crack defects.10,13,14 When the tensile test is conducted on a single fibre, this part becomes the critical section, and the stress concentration occurs near this section. Finally, fracture failure occurs in this section.
000. Fig. 5g shows a small etched part of the rCFs with a reduced cross-sectional area and clear longitudinal groove structures. In Fig. 5h, the surface of the CFs has deteriorated due to oxidation and exhibits pits and crack defects.10,13,14 When the tensile test is conducted on a single fibre, this part becomes the critical section, and the stress concentration occurs near this section. Finally, fracture failure occurs in this section.
Similarly, at elevated temperatures, the rCFs were generally found to be clean. At 40 °C, although several tiny epoxy particles could be seen on the CF surface, the CFs were otherwise clean, with no physical defects such as cracks or pits. When the temperature was increased to 60 and 75 °C, the epoxy resin was not visible at all on the CF surface; similarly, there were no visible physical defects, and furthermore, the CFs from specimen I40S2K1T75 had the highest residual tensile strength.
Note that CFs can be recycled within 36 hours at high temperature, which is only half of the time required at ambient temperature. Thus, at high temperature, CFs are exposed to the electrolyte for a shorter duration, which means that damage to the rCF due to electrochemical oxidation etching, OH− ion intercalation reactions and alkali corrosion will be less than at ambient temperature. This is the reason for the higher residual tensile strength of the CFs recycled at elevated temperatures.
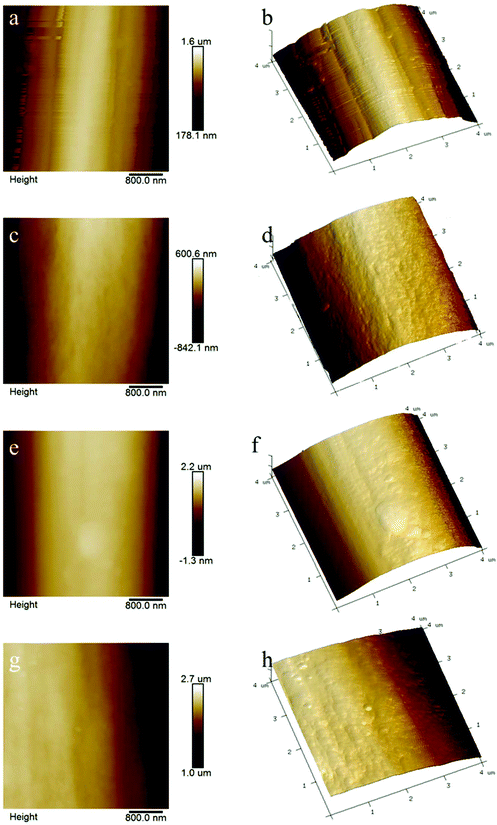 | ||
| Fig. 6 AFM images of VCF and rCFs. For an explanation of the sample numbers, see Table 2. (a) 2D image of VCF; (b) 3D image of VCF; (c) 2D image of I20S2K0.5; (d) 3D image of I20S2K0.5; (e) 2D image of I20S2K1; (f) 3D image of I20S2K1; (g) 2D image of I20S2K1.5; (h) 3D image of I20S2K1.5. | ||
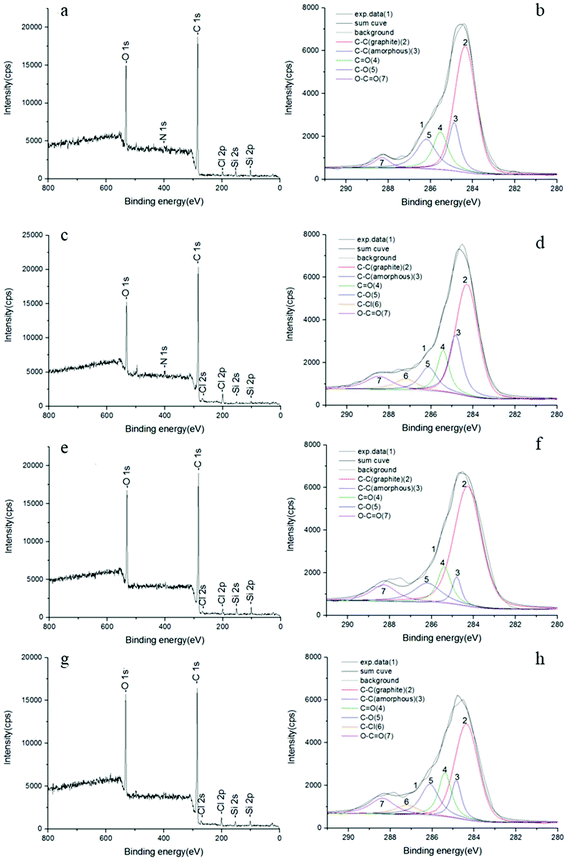 | ||
| Fig. 7 XPS spectra of VCF and rCFs. For an explanation of the sample numbers, see Table 2. (a) Full spectrum of VCF; (b) C 1s spectrum of VCF; (c) full spectrum of I20S2K0.5; (d) C 1s spectrum of I20S2K0.5; (e) full spectrum of I20S2K1; (f) C 1s spectrum of I20S2K1; (g) full spectrum of I20S2K1.5; (h) C 1s spectrum of I20S2K1.5. | ||
| Specimen no. | C | O | Cl | N | Si | O/C | C–C (graphene) | C–C (amorphous) | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
C–O | C–Cl | O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCF | 75.2 | 18.3 | 0.9 | 3.1 | 2.5 | 0.2434 | 54.9 | 14.4 | 14.6 | 15.5 | 0.0 | 4.0 |
| I20S2K0.5 | 74.2 | 19.7 | 2.2 | 3.1 | 0.8 | 0.2655 | 48.4 | 19.3 | 12.0 | 10.1 | 6.3 | 8.4 |
| I20S2K1 | 73.1 | 23.3 | 1.3 | 0.0 | 1.3 | 0.3187 | 64.8 | 5.9 | 12.0 | 12.9 | 0.0 | 10.1 |
| I20S2K1.5 | 73.0 | 23.3 | 1.5 | 0.0 | 2.2 | 0.3192 | 47.6 | 10.3 | 15.1 | 15.4 | 6.0 | 10.3 |
| I20S1K1 | 74.8 | 20.8 | 1.9 | 2.3 | 0.2 | 0.2781 | 49.7 | 11.1 | 18.2 | 12.5 | 0.0 | 13.4 |
| I20S3K1 | 72.7 | 20.4 | 4.3 | 0.8 | 1.8 | 0.2806 | 56.2 | 6.6 | 15.4 | 15.3 | 1.9 | 9.7 |
| I40S2K1 | 72.6 | 20.8 | 3.7 | 2.9 | 0.0 | 0.2865 | 36.9 | 16.4 | 19.0 | 15.6 | 6.8 | 10.1 |
| I20S2 K1T40 | 74.3 | 22.0 | 1.6 | 0.9 | 1.2 | 0.2961 | 35.6 | 20.9 | 18.0 | 18.1 | 0.0 | 12.0 |
| I20S2 K1T60 | 74.4 | 21.5 | 1.1 | 0.6 | 2.4 | 0.2890 | 49.7 | 11.8 | 17.2 | 15.0 | 0.0 | 11.1 |
| I20S2 K1T75 | 72.8 | 21.1 | 3.7 | 0.9 | 1.5 | 0.2898 | 52.8 | 10.1 | 14.6 | 16.7 | 0.0 | 10.7 |
The C 1s high-resolution narrow spectrum can be divided into six chemical bond regions by peak-fitting: graphene C–C (284.4 eV), amorphous C–C (284.8 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (285.5 eV), C–O (286.2 eV), C–Cl (287.2) and O–C
O (285.5 eV), C–O (286.2 eV), C–Cl (287.2) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.5 eV).22–26 The VCFs have relatively few oxygen-containing functional groups on their surface (the total content of C–C bonds in graphite and amorphous states is 69.3%, whereas the content of various C–O bonds is 30.7%). Consequently, they are relatively surface-inactive and water repellent. More C–O functional groups would increase their hydrophilicity and their propensity to impregnate polymer matrices such as epoxy resins.28 In addition, oxygen-containing reactive functional groups such as –COOR can increase the reaction between the CFs and epoxy resin, generating strong covalent bonds and improving interfacial bonding.29,30 A larger number of oxygen-containing functional groups might be expected to increase the chemical interactions between the CFs and epoxy resin, which might be the reason for the increased interfacial shear stress, which is consistent with the findings of other studies.31,32 Increasing the concentration of NaCl in the electrolyte solution (from 1.0% to 2.0%, i.e. specimen I20S1K1 and I20S2K1) led to an increase in the number of C–C bonds, as observed by the XPS scan, but the number of bonds decreases if the concentration of NaCl is decreased from 2 to 3.0% (i.e. specimen I20S2K1 and I20S3K1). Additionally, the O/C ratios of CFs recycled at higher temperatures (36 hours) were lower than those obtained at room temperature (72 hours) due to a shorter oxidation period. The fitting results show that the C–Cl bond content of both the higher-temperature rCF and VCF was 0, which indicates that those rCFs are not substantially corroded by chlorine. Compared to the VCFs, the total content of graphene and amorphous C–C bonds on the surface of the rCF decreased. Additionally, both the C
O (288.5 eV).22–26 The VCFs have relatively few oxygen-containing functional groups on their surface (the total content of C–C bonds in graphite and amorphous states is 69.3%, whereas the content of various C–O bonds is 30.7%). Consequently, they are relatively surface-inactive and water repellent. More C–O functional groups would increase their hydrophilicity and their propensity to impregnate polymer matrices such as epoxy resins.28 In addition, oxygen-containing reactive functional groups such as –COOR can increase the reaction between the CFs and epoxy resin, generating strong covalent bonds and improving interfacial bonding.29,30 A larger number of oxygen-containing functional groups might be expected to increase the chemical interactions between the CFs and epoxy resin, which might be the reason for the increased interfacial shear stress, which is consistent with the findings of other studies.31,32 Increasing the concentration of NaCl in the electrolyte solution (from 1.0% to 2.0%, i.e. specimen I20S1K1 and I20S2K1) led to an increase in the number of C–C bonds, as observed by the XPS scan, but the number of bonds decreases if the concentration of NaCl is decreased from 2 to 3.0% (i.e. specimen I20S2K1 and I20S3K1). Additionally, the O/C ratios of CFs recycled at higher temperatures (36 hours) were lower than those obtained at room temperature (72 hours) due to a shorter oxidation period. The fitting results show that the C–Cl bond content of both the higher-temperature rCF and VCF was 0, which indicates that those rCFs are not substantially corroded by chlorine. Compared to the VCFs, the total content of graphene and amorphous C–C bonds on the surface of the rCF decreased. Additionally, both the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and C–O bond contents increased slightly; the O–C
O bond and C–O bond contents increased slightly; the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond content was two to three times more than that of the VCFs.
O bond content was two to three times more than that of the VCFs.
Thus, the surface chemical components are an important factor influencing the mechanical properties of rCFs. The CFs recycled from specimens I40S2K1T75 and I20S2K1 had the greatest residual tensile strength and highest IFSS, respectively.
Discussion
During the recycling process, the C–N bonds of the amido bonds ruptured, followed by the cleavage of ether, benzyl and secondary and tertiary amine groups. Afterwards, the epoxy matrix on the CFRP surface was degraded gradually and subsequently the CFs were exposed to the electrolyte. Electrochemical oxidation and etching cause damage to the CFs. The rCFs suffer oxidation and OH− ion intercalation, resulting in swelling of the outermost layer and increasing the surface roughness, and thus lower residual tensile strengths but higher interfacial shear strengths.Conclusions
In this study, we investigated the recycling of a CFRP composite by using an aqueous electrolyte solution in the presence of electrical currents. The C–N bonds of the epoxy resin can be broken in an electrically driven heterogeneous catalytic reaction, leading to the decomposition of the resin component and recovery of CFs via the benefit of the EPOC effect. The properties of the rCFs are depended on the combined effect of all the variables. By optimizing the working conditions, depolymerization of the epoxy resin and reclamation of the CFs are found to be efficient even under ambient temperature and pressure using conventional and nontoxic chemicals. This method achieves a high (nearly 100%) extent of epoxy resin removal. The average residual tensile strength and IFSS are approximately 90% and 120%, respectively, of those of VCFs. Compared with existing recycling methods, this new method is simple and green (see Table 1). The commercial value of the recycled fibres based on the proposed technology is expected to dramatically increase, since (1) the simplicity of the procedure and low facility demand will remove the size restraint on the composite waste and (2) the dimensions and strength of CFs can be maintained in the recycling procedure. In addition, this technology can be implemented in an overall cost-effective manner on a large scale and will profoundly impact the management of CFRP composites and possibly other thermoset waste materials. This study is part of a realistic development process towards the longer-term goal of industrial-scale CFRP processing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to express their gratitude and sincere appreciation to the National Natural Science Foundation of China (51778370, 51861165204, 51538007), Natural Science Foundation of Guangdong Province (2017B030311004) and Shenzhen Science and Technology Funding (JCYJ20170818094820689) for financing this research work.References
- K. Yu, Q. Shi, M. L. Dunn, T. Wang and H. J. Qi, Adv. Funct. Mater., 2016, 26, 6098 CrossRef CAS.
- J. H. Zhu, L. Wei, H. Moahmoud, E. Redaelli, F. Xing and L. Bertolini, Constr. Build. Mater., 2017, 151, 127 CrossRef CAS.
- J. H. Zhu, M. N. Su, J. Y. Huang, T. Ueda and F. Xing, Constr. Build. Mater., 2018, 167, 669 CrossRef.
- Y. Yuan, Y. Sun, S. Yan, J. Zhao, S. Liu, M. Zhang, X. Zheng and L. Jia, Nat. Commun., 2017, 8, 14657 CrossRef PubMed.
- J. Palmer, O. R. Ghita, L. Savage and K. E. Evans, Composites, Part A, 2009, 40, 490 CrossRef.
- S. J. Pickering, Composites, Part A, 2006, 37, 1206 CrossRef.
- G. Marsh, Reinf. Plast., 2008, 52, 36 CrossRef.
- G. Marsh, Reinf. Plast., 2009, 53(22), 25 Search PubMed.
- J. Jiang, G. Deng, X. Chen, X. Gao, Q. Guo, C. Xu and L. Zhou, Compos. Sci. Technol., 2017, 151, 243–251 CrossRef CAS.
- Y. Wang, X. Cui, H. Ge, Y. Yang, Y. Wang, C. Zhang, J. Li, T. Deng, Z. Qin and X. Hou, ACS Sustainable Chem. Eng., 2015, 3, 3332–3337 CrossRef CAS.
- W. Nie, J. Liu, W. Liu, J. Wang and T. Tang, Polym. Degrad. Stab., 2015, 111, 247 CrossRef CAS.
- J. Li, P. L. Xu, Y. K. Zhu, J. P. Ding, L. X. Xue and Y. Z. Wang, Green Chem., 2012, 14, 3260 RSC.
- R. Piñero-Hernanz, C. Dodds, J. Hyde, J. García-Serna, M. Poliakoff, E. Lester, M. J. Cocero, S. Kingman, S. Pickering and K. H. Wong, Composites, Part A, 2008, 39, 454 CrossRef.
- R. Piñero-Hernanz, J. García-Serna, C. Dodds, J. Hyde, M. Poliakoff, M. J. Cocero, S. Kingman, S. Pickering and E. Lester, J. Supercrit. Fluids, 2008, 46, 83 CrossRef.
- A. Loppinetserani, C. Aymonier and F. Cansell, J. Chem. Technol. Biotechnol., Biotechnol., 2010, 85, 583 CrossRef CAS.
- G. Jiang, S. J. Pickering, G. S. Walker, K. H. Wong and C. D. Rudd, Appl. Surf. Sci., 2008, 254, 2588 CrossRef CAS.
- S. J. Pickering, R. M. Kelly, J. R. Kennerley, C. D. Rudd and N. J. Fenwick, Compos. Sci. Technol., 2000, 60, 509 CrossRef CAS.
- H. L. H. Yip, S. J. Pickering and C. D. Rudd, Plast., Rubber Compos., 2002, 31, 278 CrossRef CAS.
- H. Sun, G. Guo, S. A. Memon, W. Xu, Q. Zhang, J. H. Zhu and F. Xing, Composites, Part A, 2015, 78, 10 CrossRef CAS.
- A. Katsaounis, J. Appl. Electrochem., 2010, 40, 885 CrossRef CAS.
- J. S. Association , n.d.
- G. Jiang, S. J. Pickering, G. S. Walker, K. H. Wong and C. D. Rudd, Appl. Surf. Sci., 2008, 254, 2588 CrossRef CAS.
- M. Li, C. Feng, Z. Zhang, X. Lei, R. Chen and Y. Yang, J. Hazard. Mater., 2009, 171, 724–730 CrossRef CAS PubMed.
- K. Rajeshwar and J. G. Ibanez, Environmental Electrochemistry: Fundamentals and Applications in Pollution Sensors and Abatement, Academic Press, 1997 Search PubMed.
- Z. Jin, Z. Zhang and L. Meng, Mater. Chem. Phys., 2006, 97, 167 CrossRef CAS.
- N. S. Zhu, H. Y. Li, F. X. Wang and Y. S. Dai, Appl. Mech. Mater., 2014, 490–491, 298 CAS.
- K. Sawaki, S. Asai, K. Watanabe, T. Sugo and K. Saito, Membrane, 2008, 33, 32 CrossRef CAS.
- T. Takahagi and A. Ishitani, Carbon, 1984, 22, 43 CrossRef CAS.
- S. K. Ryu, B. J. Park and S. J. Park, J. Colloid Interface Sci., 1999, 215, 167 CrossRef CAS PubMed.
- S. Zhang, X. Y. Li and J. P. Chen, J. Colloid Interface Sci., 2010, 343, 232 CrossRef CAS PubMed.
- S. H. Han, H. J. Oh, H. C. Lee and S. S. Kim, Composites, Part B, 2013, 45, 172 CrossRef CAS.
- K. Giannadakis, M. Szpieg and J. Varna, Exp. Mech., 2011, 51, 767 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc03672a |
| This journal is © The Royal Society of Chemistry 2019 |