Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible-light-induced oxidant and metal-free dehydrogenative cascade trifluoromethylation and oxidation of 1,6-enynes with water†
Sadhan
Jana
 ,
Ajay
Verma
,
Ajay
Verma
 ,
Rahul
Kadu
,
Rahul
Kadu
 and
Sangit
Kumar
and
Sangit
Kumar
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Bhopal, Bhopal By-pass Road, Bhauri, Bhopal-462066, India. E-mail: sangitkumar@iiserb.ac.in; Web: http://home.iiserbhopal.ac.in/˜sangitkumar/
First published on 10th July 2017
Abstract
Generally, oxy-trifluoromethylation in olefins is achieved using oxidants and transition metal catalysts. However, labile olefins remain unexplored due to their incompatibility with harsh reaction conditions. Here, unprecedented light-induced oxidant and metal-free tandem radical cyclization–trifluoromethylation and dehydrogenative oxygenation of 1,6-enynes have been achieved using a photoredox catalyst, CF3SO2Na, and phenanthrene-9,10-dione (PQ), Langlois’ reagent (CF3SO2Na) and water as the oxygen source. This benign protocol allows for access to various CF3-containing C3-aryloyl/acylated benzofurans, benzothiophenes, and indoles. Moreover, the oxidized undesired products, which are inherently formed by the cleavage of the vinylic carbon and heteroatom bond, have been circumvented under oxidant free conditions. The mechanistic investigations by UV-visible and ESR spectroscopy, electrochemical studies, isotope labelling and density functional theory (DFT) suggest that light induced PQ produced a CF3 radical from CF3SO2Na. The generated CF3 radical adds to the alkene, followed by cyclization, to provide a vinylic radical that transfers an electron to PQ and generates a vinylic cation. Alternatively, electron transfer may occur from the CF3-added alkene moiety, forming a carbocation, which would undergo cationic cyclization to generate a vinylic carbocation. The subsequent addition of water to the vinylic cation, followed by the elimination of hydrogen gas, led to the formation of trifluoromethylated C3-aryloyl/acylated heterocycles.
Introduction
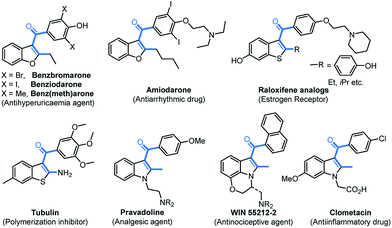
C3-Aryloylated benzofurans, benzothiophenes, indoles, and related fused polyheterocycles are privileged structures with numerous applications in materials, drugs, and biology (Fig. 1).1–5 Benzbromarone2 is being used for the treatment of gout as a urate lowering drug (ULD) and amiodarone3 is a potent inhibitor of the human cytochrome P-450 (CYP)2C19 responsible for metabolizing commonly prescribed drugs. The C3-aryloylated benzothiophenes,4 raloxifene and tubulin, which are antimitotic agents, act as an estrogen receptor and inhibitor for the polymerization of tubulin and growth of tumour cells, respectively. Similarly, 2-methyl-C3-aryloylated indoles,5 namely pravadoline, WIN55212-2, and clometacin, are nonsteroidal anti-inflammatory drugs, which are used against pain and inflammation. However, the construction of C3-aryloylated benzofuran, indole, and thiophene scaffolds is still challenging (vide infra) despite the availability of advanced synthetic strategies.15–17The incorporation of a trifluoromethyl group (–CF3) into drug molecules can dramatically change their properties, such as their solubility, lipophilicity, metabolic stability, etc.6 As a result, several trifluoromethylated heterocycles, such as efavirenz and celecoxib, are used as potential drugs for the treatment of various diseases.7 It is worth noting that the synthesis of trifluoromethylated C3-aryloyl heterocycles (shown in Fig. 1) has not been reported to date.
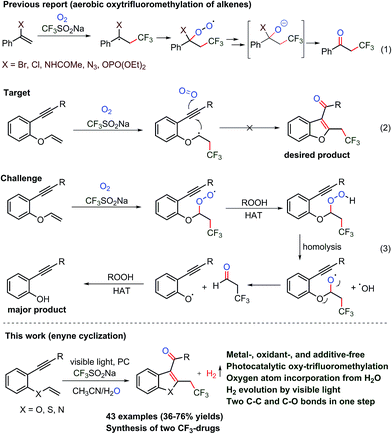
The trifluoromethylation and oxygenation of alkene and alkyne substrates have been achieved using various trifluoromethylating reagents and peroxides, persulfates, hypervalent iodine salts and oxygen as the oxidant and/or transition metal catalytic system (Scheme 1, eqn (1)).8–14 Although different valuable approaches appear to have been incorporated into the –CF3 group, still there is demand for mild and sustainable synthetic methods that avoid oxidants and heavy metals and are applicable to sensitive substrates for the construction of functionalized advanced heterocycles.
The cyclization of 1,6-enynes can be a powerful transformation that allows for the construction of C3-aryloyl/acylated heterocycles (Scheme 1, eqn (2)).15–17 1,6-Enyne substrates under transition metal (TM)-catalyzed reaction conditions are rearranged to benzofuran,15 benzothiophene16 and indole17 heterocycles, and thus restrict the use of TM to access the desired C3-aryloylated heterocycles. On the other hand, the use of oxidants such as hypervalent iodine reagents, peroxides, and oxygen, which are required for the generation of the CF3 radical and as a source of oxygen under TM-free conditions, led to the cleavage of the vinylic carbon–heteroatom (oxygen, sulfur, nitrogen, etc.)8c bond and thus provided undesired phenol, thiophenol and aniline as the main side products, respectively (eqn (3)). It is a natural inherent property of oxygen (bi-radical) to form a peroxide that leads to the cleavage of the labile carbon–heteroatom bond and thus imposes a major challenge to functionalize such a vinylic carbon–carbon double bond.8f Consequently, keeping the ether linkage intact throughout the trifluoromethylation of the vinylic C–C double bond adjacent to the heteroatom has not been reported to date, despite the presence of this skeleton in various molecules of pharmaceuticals, agrochemicals, materials, and fine chemicals.
Photocatalyzed cascade radical reactions have gained much attention in recent times as these reactions provide access to complex molecules in one pot with step and atom economy.18,19 Although several photocatalyzed trifluoromethylation reactions viz. aromatic trifluoromethylation,20a conversion of alkynes into tetra-substituted trifluoromethylated alkenes,20b and radical trifluoromethylation/cyclization cascade for CF3-containing pyrazolines and isoxazolines20c have been described, cascade oxy-trifluoromethylation has not been explored. Water is the most abundant reactant that can be used as an oxygen source in photocatalytic reactions. The incorporation of water for the oxygenation of substrates requires a strong one-electron oxidant. Alternatively, water can be added as a nucleophile to an organic substrate followed by its oxidation by weaker oxidants under light driven conditions, which could lead to the oxygenation of organic molecules.21
In the continuation of our research interest in TM-free C–C and C–heteroatom coupling reactions,22 here, we disclose a photocatalyzed reaction that not only activates CF3SO2Na for the generation of the CF3 radical but also facilitates water towards the oxygenation of organic molecules. This approach enables the oxygenation of labile 1,6-enyne substrates along with the generation of hydrogen gas under oxidant-free conditions, which circumvents an undesired, over-oxidized product. The mechanistic investigations by labelling experiments, UV-visible and ESR spectroscopy and cyclic voltammetry studies, corroborated by DFT calculations, have been carried out to understand the role of the photoredox catalyst (PC) in the oxy-trifluoromethylation reaction of 1,6-enynes under oxidant and metal-free conditions.
Results and discussion
Initial screening and photo-initiated conditions with and without oxidants
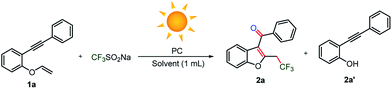
Initially, 1-(phenylethynyl)-2-(vinyloxy)benzene substrate 1a was reacted with an economical amount of trifluoromethylating Langlois’ reagent (CF3SO2Na) under an oxygen atmosphere and heated conditions (Table 1). Disappointingly, cleavage of the vinylic carbon–oxygen bond occurred, which yielded undesired 2-(phenylethynyl)phenol 2a′ instead of C3-aryloylated-2-trifluoromethylated benzofuran 2a (Table 1, entry 1). Similarly, various oxidants, such as hydrogen peroxide, benzyl peroxide, tert-butyl hydroperoxide, di-tert butyl peroxide, iodine, (diacetoxyiodo)benzene, persulfate salts, oxone, ceric ammonium nitrate, 2,3-dichloro-5,6-dicyano-p-benzoquinone and the radical initiators azobisisobutyronitrile and azobis(2,4-dimethylvaleronitrile), provided the undesired phenol 2a′ as a major product and 2a was noticed as a minor product (<20% yields, see ESI, page S3 and S4†). Next, we performed the reaction at room temperature using oxygen, and the reaction provided 2a and 2a′ in <10 and 15% yields, respectively (Table 1, entry 2). However, poor conversion was observed despite the continuation of the reaction for a longer time (24 h) and 70% of substrate 1a was recovered from the reaction.| Entry | PC (mol%) | Gas | Solvent | Yieldb2a/2a′ |
|---|---|---|---|---|
| a The reactions were carried out using 0.1 mmol of 1a, 0.3 mmol of CF3SO2Na and 0.01 mmol of photocatalyst (PC) in 1 mL of solvent at 30 °C in a 5 mL round bottom flask under O2 or Ar atmosphere, unless otherwise stated; the progress of the reaction was monitored by TLC. b Percentage isolated yield. c Under a household CFL bulb (23 W). nd = not detected. | ||||
| 1 | −(90 °C) | O2 | DMSO | Trace/74 |
| 2 | −(25 °C) | O2 | DMSO | <10/15 |
| 3 | — | O2 | Acetone | Trace/20 |
| 4 | A | O2 | Acetone | Trace/15 |
| 5 | B | O2 | Acetone | nd/10 |
| 6 | C | O2 | Acetone | 5/15 |
| 7 | D | O2 | Acetone | 5/15 |
| 8 | PQD | O2 | Acetone | 3/25 |
| 9 | PQ | O2 | Acetone | 9/30 |
| 10 | PQ | Ar | Acetone | Trace/— |
| 11 | PQ | Ar | Acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20/8 |
| 12 | PQ | Ar | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
13/5 |
| 13 | PQ | Ar | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
35/10 |
| 14 | PQ | Ar | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48/12 |
| 15 | PQ | Ar |
CH
3
CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O (9 H
2
O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
76/trace |
| 16 | PQ | Ar | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
32/5 |
| 17c | PQ | Ar | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
69/trace |
| 18 | PQ | O2 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24/40 |
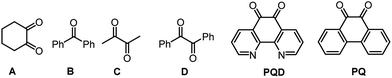
Next, we envisaged that the UV-visible irradiation of the reaction system in the presence of diketones would bring about oxy-trifluoromethylation in 1,6-enyne as n → π* triplet-excited ketones have been successfully exploited in several photoinduced chemical transformations.23 Consequently, α-diketones (A–D) and ortho-quinones 1,10-phenanthroline-5,6-dione (PQD) and phenanthrene-9,10-dione (PQ) were investigated for their UV-visible absorption properties (Fig. 2). A–D show strong absorption in the ultraviolet range below 380 nm, whereas ortho-quinones PQD and PQ exhibit absorption bands in the visible range (380–420 nm). The absorption bands for PQ appeared in the visible light region (420 and 505 nm)24 in acetone solvent, which shows a slight red shift in acetonitrile (ESI, page S12 and S13†). The electronic excitation would lead to a first triplet excited state (T1) of the carbonyl group having a diradicaloid nature. The triplet states of aromatic ketones are long-lived, and also the electronic character and reactivity of the lowest triplet state can be tuned by the solvent polarity.25 9,10-Phenanthrenequinone PQ shows an n → π* transition of an aromatic ketone that could reverse the charges on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, thus making the oxygen atom electron deficient. Due to the electron-deficient oxygen atom and “radical-like” characteristic of carbonyl in PQ, its reduction is enabled26 by the abstraction of an electron from CF3SO2Na and the generation of a CF3 radical, which can indeed initiate a cascade reaction.
O group, thus making the oxygen atom electron deficient. Due to the electron-deficient oxygen atom and “radical-like” characteristic of carbonyl in PQ, its reduction is enabled26 by the abstraction of an electron from CF3SO2Na and the generation of a CF3 radical, which can indeed initiate a cascade reaction.
The reaction system under UV-irradiation in acetone, which acts as a solvent and radical initiator,20n and under oxygen atmosphere provided only traces of 2a (Table 1, entry 3). Similar results were obtained with A–C under UV-irradiation and also with D, PQD and PQ under visible light and oxygen atmosphere (Table 1, entries 4–9). In the absence of oxygen, no product formation was realized, as expected (Table 1, entry 10). Surprisingly, when the reaction was performed in the absence of oxygen in a mixture of an organic and water solvent system, a noticeable increase in the yield of the desired product 2a was observed, moreover, the formation of undesired side product was minimized to <10% yield (Table 1, entries 11–16). The reactions presented here were optimized under sun-light irradiation. In a separate experiment, a household CFL bulb (23 W) was used, which provided nearly the same yield of 2a (69% vs. 76% in sunlight) although a longer time (6 h for sunlight vs. 16 h for one 23 W CFL bulb) is required (Table 1, entry 17). A trifluoromethylation reaction under oxygen atmosphere, instead of argon, under optimized conditions, afforded 24% yield of the desired product 2a (Table 1, entry 18). Among the various diketones screened (A–D, PQD, PQ) (Table 1, entries 3–17), PQ, which has been explored for the first time,27 provided optimum yield of the desired oxy-trifluoromethylated benzofuran 2a under sunlight irradiation.
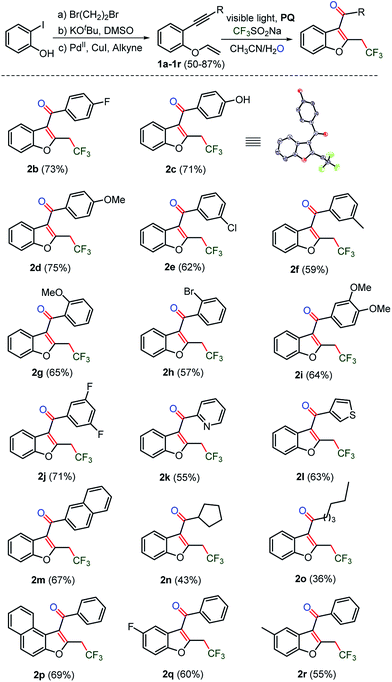
The substrate scope of the light-induced oxy-trifluoromethylation reaction to phenolic 1,6-enynes
Next, the substrate scope was studied on phenolic 1,6-enynes (1a–1r)28 under the optimized reaction conditions (Table 1, entry 15). Substrates 1b–1j, with electron donating as well as electron withdrawing substituents on the ethynyl ring, showed compatibility with the optimized reaction conditions and afforded respective trifluoromethylated benzofurans 2b–2j in 57–75% yields (Scheme 2). Moreover, the phenolic-1,6-enyene substrate 1c with OH functionality and an acidic proton was tolerated to provide hydroxyl benzofuran 2c in good yield. The heteroaromatics pyridyl and thiophenyl and other aromatic naphthyl, as the ethynyl ring containing substrates 1k–1m, were also amenable to the reaction and transformed into the respective benzofurans 2k–2m. Next, the n- and sec-alkyl substituted substrates 1n–1o underwent an oxy-trifluoromethylation reaction to afford the acyl-benzofurans 2n–2o, albeit in low yields. Substitution on the phenolic ring was also explored under the optimized conditions. Substrate 1p, containing a naphthyl ring, and substrates 1q–1r, having fluoro and methyl substituents on the phenolic ring, afforded respective trifluoromethylated benzofurans 2p and 2q–2r without any noticeable loss in the yields (55–69%).Substrate scope with regard to thiophenolic 1,6-enynes
In order to explore the synthesis of trifluoromethylated C3-aryloyl benzothiophenes, thio-linked 1,6-enyne substrates 3a–3m were prepared from 2-bromo-benzenethiols in moderate yields (Scheme 3, for details see the ESI, page S28 and S29†). In general, the sulfur-containing substrates showed sluggish reactivity under TM-catalyzed reaction conditions due to the poisoning of the catalyst by sulfur. Earlier synthetic methods involve inter or intramolecular coupling of alkynyl and sulfoxide in the presence of high loading of the Au, Hg and Pd-catalysts.16d,e To our delight, a TM-free cyclization reaction yielded C3-aryloyl trifluoromethylated benzothiophenes 4a–4m in nearly the same yields (73–36%) as those obtained for benzofurans. Moreover, a similar substrate scope was realized as thio-linked 1,6-enyne substrates 3b–3m with electron donating methyl, methoxy, [1,3]-dioxole, and tri- methoxy and electron withdrawing CF3 and F and also naphthyl, thiophenyl, and n-butyl substituents provided unaltered yields of 2-trifluoromethyl C3-aryloyl/acylated benzothiophenes 4b–4m. The cyclopropyl substituted substrate 3l also underwent ring opening of the cyclopropyl ring by the trifluoromethyl radical to yield an unexpected di-trifluoromethylated benzothiophene 4l as the major product and a di-trifluoromethylated product as the minor product.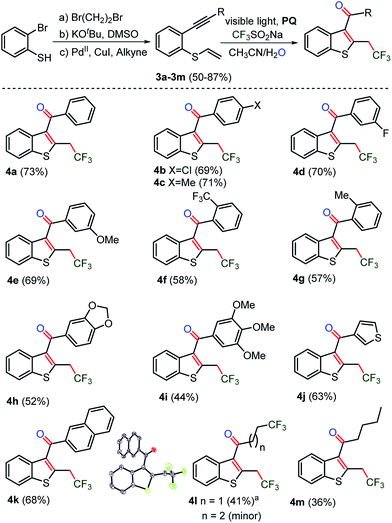 | ||
| Scheme 3 Synthesis of CF3-benzo[b]thiophenes. The reaction of 0.1 mmol of 3a, 0.3 mmol of CF3SO2Na and 0.01 mmol of photocatalyst PQ in CH3CN and H2O was irradiated under Ar and the progress of the reaction was monitored by TLC. 4l was obtained from cyclopropane substituted substrate 3l, as the major along with the minor (n = 1) product (see the ESI, page S56 and S57, and the spectra on pages S203–S207†). | ||
Substrate scope with regard to anilinic 1,6-enynes
Next, N-tosyl (Ts) and N-tert-butyloxycarbonyl (Boc) protected 1,6-enyne29 substrates were prepared to construct trifluoromethylated C3-aryloyl indoles, which are prevalent heterocycles in many drugs and materials (Scheme 4).5a,b Indeed, N-tosylated substrates 5a–5g underwent an oxy-trifluoromethylation reaction. Moreover, the removal of the tosyl (Ts) group was realized in the same pot leading to N-unprotected trifluoromethylated C3-aryloyl indoles 6a–6g in 67–56% yields. Next, we sought for the synthesis of N-protected trifluoromethylated C3-aryloyl indoles. Substrates 5h–5l, which were protected by a N-tert-butyloxycarbonyl (Boc) group, provided trifluoromethylated indoles in moderate (10–57%) yields. Both N-Ts and Boc-protected 1,6-enyne substrates, having bromo, fluoro, difluoro, methoxy, methyl and pyridyl, naphthyl and thiophenyl rings, were amenable to the oxidant and metal-free oxy-trifluoromethylation reactions. A more efficient electron delocalization occurs with the N-protected carbonyl group of carbamate rather than the sufonyl moiety of the tosyl group. The weak mesomeric effect indicates that the sulfur-centered group had increased electron density on the nitrogen, which reflected the higher aromaticity of the indole.30 As a consequence, tosyl becomes a better leaving group than carbamate. Thus the deprotection of the tosyl protecting group under the optimized reaction conditions is attributed to its labile nature. The C3-aryloyl trifluoromethylated indoles 6c and 6d, benzofuran 2c, and benzothiophene 4k are also characterized by X-ray single crystal structure analysis (for details, see ESI, pages S80–S106†).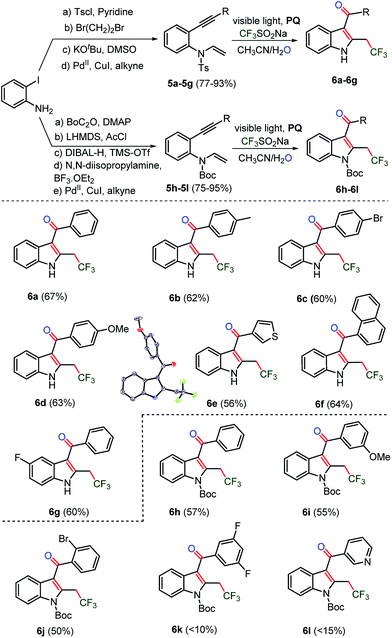 | ||
| Scheme 4 Synthesis of CF3-indoles. The reaction of 5a (0.1 mmol), 0.3 mmol of CF3SO2Na and 0.01 mmol of photocatalyst PQ in CH3CN and H2O was irradiated for 4–8 h under argon atmosphere. | ||
Synthesis of CF3-bearing drugs
The synthesis of trifluoromethylated drug molecules was explored from synthesized 2-trifluoromethyl-C3-aryloyl benzofuran 2c and indole 6f by late-stage functionalization (Scheme 5). The bromination of benzofuran 2c using N-bromosuccinimide afforded novel trifluoromethylated benzbromarone 7a in 40% yield. The N-alkylation of the synthesized oxy-trifluoromethylated indole 6f was observed to be difficult by known methods using KOH in DMSO, Cs2CO3 in DMSO or K2CO3 in DMF and failed to yield N-alkylated indole 7b and instead a decomposed product was realized.31 The addition of NaH in DMF along with n-propyl iodide at 0 °C provided the trifluoromethylated JWH-105 7b drug in moderate yield (54%).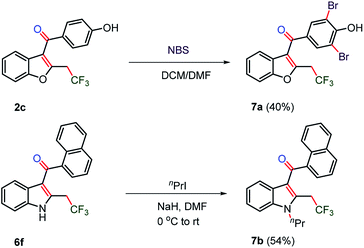 | ||
| Scheme 5 CF3-bearing drugs by post-modification. NBS (0.40 mmol) and 2c (0.20 mmol) were used for 7a. nPrI (0.12 mmol) and 6f (0.16 mmol) in DMF were used for 7b. | ||
Mechanistic study
Labelling and 1H NMR experiments
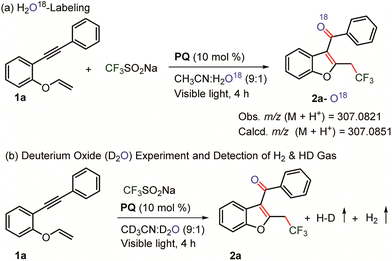
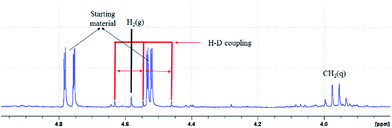
A labelling experiment was performed using H2O18 under the optimized reaction conditions (Scheme 6). Mass analysis revealed the formation of O18-labelled trifluoromethylated C3-aryloyl benzofuran 2a (M + H+ = 307.0821; calcd 307.0851).1H NMR experiments were performed on the reaction mixture to gain further insights. 1H NMR spectroscopy of the reaction mixture shows a peak (δ = 4.57 ppm) indicative of hydrogen evolution in the reaction mixture (see the ESI, page S7–S8 for details†).32 Next, a reaction was carried out in CH3CN-d3 to study the role of the solvent. Expectedly, 1H NMR spectroscopy shows the formation of H2, which suggests that acetonitrile does not participate in the reaction. When D2O was used in the reaction, the formation of H–D (δ = 4.55 ppm, J = 42.8 Hz) and H2 was realized in the 1H NMR spectrum, revealing the involvement of water in the hydrogen gas evolution (Fig. 3 and Scheme 6). The formation of H2 gas is attributed to H2O in deuterated solvents and it is the result of hydrogen and deuterium exchange (page S8†).
ESR investigation
To investigate the reaction pathways, EPR experiments were conducted on the reaction mixture. For this purpose, the generated reactive CF3 radical in the reaction was trapped by the 2-methyl-2-nitrosopropane (MNP) dimer, and its EPR spectrum was monitored (Scheme 7).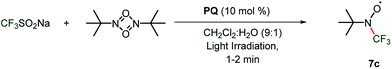 | ||
| Scheme 7 The reaction with a radical trapping reagent. The reaction was carried out using CF3SO2Na (0.3 mmol), 1a (0.1 mmol) and MNP (0.2 mmol) in an EPR tube in CH2Cl2/H2O. | ||
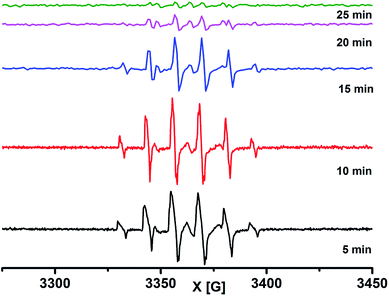
CF3SO2Na and MNP in the presence of CH3CN/H2O under light irradiation provided a triplet centred at 3364.5 G with a coupling constant 14.7 G, which is attributed to the dissociation of MNP to a tert-butyl nitroxide radical (see the ESI, Fig. S6†). The formation of the tert-butyl nitroxide radical is largely suppressed in CH2Cl2/H2O and the formation of the tert-butyl-trifluoromethyl nitroxide radical 7c (Scheme 7) is observed predominantly as the EPR spectrum shows a sextet centered at g = 2.0054 with a coupling constant 12.27 G.33 A reaction mixture of MNP, CF3SO2Na and 1a under dark conditions was realized to be EPR silent. Upon light irradiation, the reaction mixture shows a similar well-resolved sextet centered at g = 2.0089 with a coupling constant 12.38 G (Scheme 7 and Fig. 4). The intensity of the EPR signals gradually decreased with time and completely diminished after 25 minutes. The second time irradiation of the same reaction mixture again showed a sextet signal in the EPR spectrum. This suggests that continuous irradiation of the reaction mixture is necessary for the generation of the CF3 radical to achieve maximum conversion.
Absorption spectra
UV-visible spectroscopic studies were performed on the reaction mixture to understand the role of PQ. The spectra of PQ shows well-resolved absorption maxima at 420 and 510 nm in CH3CN and H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures (Fig. 5). An equimolar mixture of PQ and CF3SO2Na did not lead to any change in the absorption spectrum under dark conditions.
1) mixtures (Fig. 5). An equimolar mixture of PQ and CF3SO2Na did not lead to any change in the absorption spectrum under dark conditions.
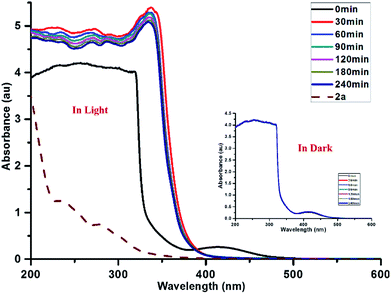 | ||
Fig. 5 Absorption spectra of PQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CF3SO2Na CF3SO2Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a = 0.1 1a = 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under visible light and dark conditions at different time intervals. 1 under visible light and dark conditions at different time intervals. | ||
The reaction mixture of PQ, CF3SO2Na and substrate 1a provided a similar absorption spectrum under dark conditions. Further, the impact of light on the reaction progress was studied for 3 h at 30 min time intervals. Upon sunlight irradiation (30 min) of the reaction mixture, the characteristic peaks of PQ completely disappeared (Fig. 5) suggesting the involvement of PQ in the oxy-trifluoromethylation reaction.
The absorption spectra of the standard reaction mixture remained nearly unchanged at various time intervals. It seems that photocatalyst PQ is transformed into another species, presumably phenanthrene-9,10-diol (PQH2), which might be the dominant species observable by UV-visible spectroscopy under the reaction conditions (Fig. S13, see the ESI page S14†). A slight increase in the absorption at 420 nm was observed with an increase in time, which could be attributed to the partial regeneration of PQ after the completion of the reaction. Further, the regeneration of PQ is confirmed by 13C NMR spectroscopy and mass spectrometry (ESI, page S16 and S17†).
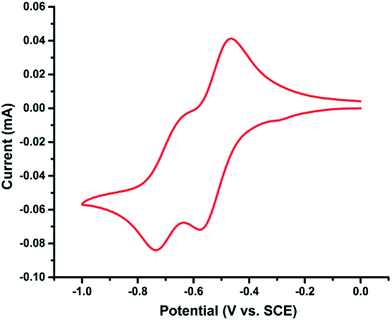
Cyclic voltammetric study
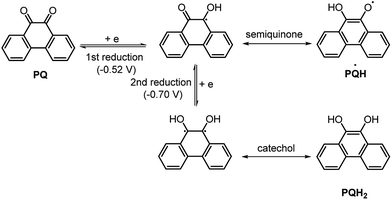
To gain insights into the redox behaviour of photocatalyst PQ, a cyclic voltammetry study was performed (Fig. 6). The cyclic voltammogram (CV) of PQ shows reversible two-electron reduction processes at −0.52 and −0.70 V (Ered1/2) attributed to the quinone → semiquinone and semiquinone → catechol redox couples, respectively (Scheme 8).35The considerably lower reduction potentials of PQ, presumably due to the presence of conjugation adjacent to the 9 and 10-positions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, provide stability to the radical PQ˙H and diol PQH2. Also, photocatalyst PQ exhibited high stability under the electrochemical redox process as it underwent 12-cycles without any loss in the redox activity.
O group, provide stability to the radical PQ˙H and diol PQH2. Also, photocatalyst PQ exhibited high stability under the electrochemical redox process as it underwent 12-cycles without any loss in the redox activity.
Photocatalyst PQ has a triplet excited state energy of 2.116 V.36a Thus, the excited state reduction potential Ered1/2* (3PQ*/PQ˙−) of PQ is 1.6 V (ref. 27f and 36b) and Langlois’ reagent exhibits an oxidation potential of 1.05 V (vs. SCE),37 which suggests that PQ is strong enough for the oxidation of CF3SO2Na by single electron transfer (ΔGPET = −12.7 kcal mol−1).
Control experiments
To study whether water alone is enough to initiate the reaction, substrate 1a was treated with water in the absence of CF3SO2Na under optimized reaction conditions (Scheme 9). The hydroxylation of the alkene or alkyne was not observed and 1,6-enyne 1a was recovered quantitatively. It seems that the substrate does not undergo photohydration of the alkynes34 to provide 1-phenyl-2-(2-(vinyloxy)phenyl)ethan-1-one, which is suggestive of the reaction procession being less likely via oxygenation followed by trifluoromethylation of substrate 1a.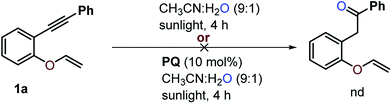 | ||
| Scheme 9 The attempted hydroxylation of 1,6-enyne 1a. The reaction was carried out using 0.1 mmol of 1a, 0.3 mmol of CF3SO2Na and 0.01 mmol of PQ in CH3CN and H2O in a 5 mL round bottom flask. | ||
When the reaction was performed in the presence of a radical scavenger, TEMPO, the formation of 2a was not observed. Instead, the coupling between CF3 and TEMPO was realized (see ESI, S11†).
Mechanism, quantum yield and DFT calculations
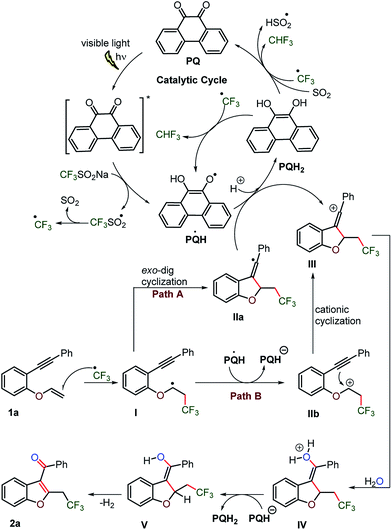
Based on the control and labelling experiments, it is reasonable to assume that the photoredox catalyst PQ, excited by visible light, activates CF3SO2Na by single electron transfer to produce a CF3 radical and SO2 (Scheme 10). The trifluoromethylated radical would add to the vinylic carbon–carbon double bond of the substrate 1a, forming a radical species I, which intramolecularly translocated to the alkyne bond via 5-exo-dig cyclization and thus rearranged to the vinylic radical IIa. The electron transfer from vinylic radical IIa to PQ would lead to vinylic carbocation III and PQH2. Although, vinylic carbocations have low thermodynamic stability due to the sp-hybridization of the carbenium centre and as a consequence show poor S1N-reactivity. However, the sp–sp2 rehybridization in the high energy state could account for its electrophilic nature.38 The second electron accepting ability of the photoredox catalyst PQ would facilitate the formation of vinylic carbocation III.Alternatively, the alkyl ether radical I may transfer an electron to PQ˙H intermolecularly and convert into carbocation IIb, which may proceed by an intramolecular cationic cyclization to provide vinylic carbocation III.39
The slow addition of water to vinylic carbocation III shall provide aquated intermediate IV, which upon release of the proton converts into enol intermediate V. Further, the photoaromatization of enol V would furnish 2-trifluromethylation C3-aryloyl benzofuran 2a along with the concomitant release of hydrogen gas.40 As inferred from the UV-visible study (vide supra), PQH2 would be the predominant species in the catalytic cycle. PQH2 could regenerate to PQ by the transfer of its electrons to sulfur dioxide37 and/or the trifluoromethyl radical to form a HSO2 radical and/or fluoroform, respectively.
The quantum yield (QY) can in particular provide valuable insight into the mechanistic understanding of the photocatalytic reaction, which involves radical chain propagation. The QY of the developed reaction, substrate 1a to product 2a, was studied using the photodecomposition of potassium ferrioxalate, which is a well-explored chemical actinometer.41 The determined QY is φ = 27 (for details, see the ESI page S17†) which suggests that 27 equivalents of product 2a are formed for every photon absorbed by the photocatalyst PQ. Therefore, the reaction may proceed via a chain mechanism. The generated HSO2 radical propagates the radical chain by reacting with Langlois’ reagent, which again provides a CF3 radical, thus continuing the radical chain reaction.
DFT calculations were explored to examine the mechanism of the reaction (Fig. 7 and Scheme 10). The thermodynamic feasibility of the intermediates and oxygenation by water were computed using DFT-B3LYP/6-31+G(d) in a Gaussian 09 suite in CH3CN (see the ESI, page S65–S80†). The Gibbs free energy of the reaction suggests that the proposed intermediates I–V are stable under the reaction conditions.38 The vinylic cation III could be obtained from radical I by two paths A and B (Fig. 7) as the energy difference between them is 2.03 kcal mol−1. The attack of a water molecule on vinylic cation III could be the key step in the transformation and may occur through a transition state TS. The energy barrier (ΔG#) for the step is +16.47 kcal mol−1, which could be feasible under the reaction conditions. Further, the abstraction of the proton from the hydronium ion IV by PQH− provides a stabilization to intermediate V by the energy difference of 63.52 kcal mol−1 and the subsequent removal of hydrogen gas from the intermediate V lowers the energy by 6.78 kcal mol−1.
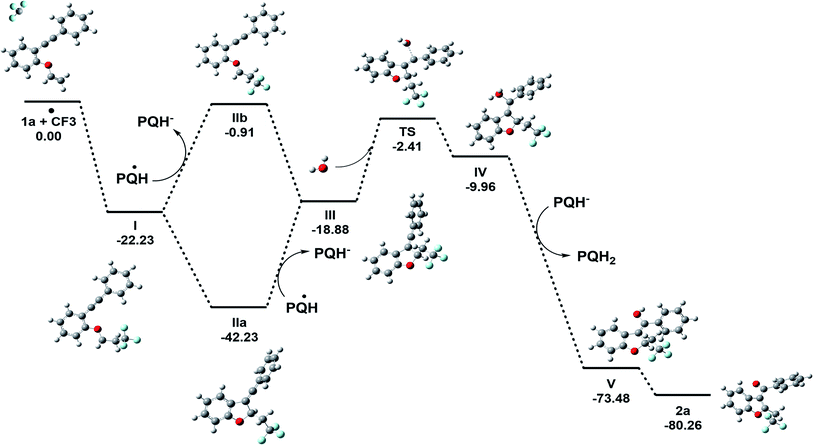 | ||
| Fig. 7 The calculated relative Gibbs’ free energy of the reaction (ΔG° in kcal mol−1) obtained at a DFT-B3LYP/6-31+G(d)/CPCM(acetonitrile) level of theory. | ||
Conclusions
In summary, we have unveiled an oxidant and TM-free visible light induced oxy-trifluoromethylation of enynes that enables access to biologically important carboxy-trifluoromethylated benzofurans, thiophenes, and indoles. The mild reaction conditions tolerate electronically diverse substrates, regardless of the substitution pattern on either ethynylic or vinylic arene, and as a consequence the methyl group in benzbromarone and JWH015 drugs has been substituted by a trifluoromethyl group. This protocol relies on the universal solvent as a source of oxygen for the oxygenation of enynes. The use of a highly practical 9,10-phenanthrequinone photoredox catalyst, which has a two electron redox property, seems crucial for the transformation as it not only generates trifluoromethyl radicals from the Langlois’ reagent by an electron transfer, but it also brings about one electron oxidation of enynes by a second electron transfer, which in turn facilitates oxygenation utilizing water followed by hydrogen gas evolution under oxidant and TM-free mild conditions. Moreover, we have shown that the di-functionalization of the vinylic double bond adjacent to the heteroatom, which is a formidable task due to the cleavage of the labile carbon–heteroatom bond, can be achieved under the developed conditions. The finding of oxy-trifluoromethylation of enyne substrates under metal and oxidant-free conditions opens a new avenue for the synthesis of trifluoromethylated advance heterocyclic molecules under atom and step economical pathways.Acknowledgements
We are grateful for the financial support from DST-SERB New Delhi (EMR/2015/000061). SJ, RK and AV acknowledge IISER Bhopal and UGC, New Delhi for fellowships. Miss Anjaly N. Vijayan for help with the preparation of precursors, Miss Debarati Roy Chowdhury and Dr Suraj Kumar Gupta for help with the electrochemical study, Dr Apurba Lal Koner for determination of the quantum yield, and the reviewers for their valuable comments are greatly acknowledged.Notes and references
- T. Liu, et al. , Org. Lett., 2016, 18, 4044 CrossRef CAS PubMed.
- (a) C. W. Locuson, H. Suzuki, A. E. Rettie and J. P. Jones, J. Med. Chem., 2004, 47, 6768 CrossRef CAS PubMed; (b) M. F. Wempe, et al. , J. Med. Chem., 2011, 54, 2701 CrossRef CAS PubMed.
- (a) T. A. Theodossiou, M. C. Galanou and C. M. Paleos, J. Med. Chem., 2008, 51, 6067 CrossRef CAS PubMed; (b) A. N. Snead, M. Miyakawa, E. S. Tan and T. S. Scanlan, Bioorg. Med. Chem. Lett., 2008, 18, 5920 CrossRef CAS PubMed.
- (a) T. A. Grese, et al. , J. Med. Chem., 1998, 41, 1272 CrossRef CAS PubMed; (b) R. Romagnoli, et al. , Bioorg. Med. Chem. Lett., 2008, 18, 5041 CrossRef CAS PubMed.
- (a) T. E. D’Ambra, et al. , J. Med. Chem., 1992, 35, 124 CrossRef; (b) O. Mazzoni, et al. , Chem. Biol. Drug Des., 2010, 75, 106 CrossRef CAS PubMed.
- (a) K. Muller, C. Faeh and F. Deiderich, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (c) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed; (d) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- (a) S. D. Young, et al. , Antimicrob. Agents Chemother., 1995, 39, 2602 CrossRef CAS PubMed; (b) T. D. Penning, et al. , J. Med. Chem., 1997, 40, 1347 CrossRef CAS PubMed.
- Oxy-trifluoromethylation of alkenes by Langlois’ reagent: (a) A. Deb, S. Manna, A. Modak, T. Patra, S. Maity and D. Maiti, Angew. Chem., Int. Ed., 2013, 52, 9747 CrossRef CAS PubMed; (b) X. Y. Jiang and F. L. Qing, Angew. Chem., Int. Ed., 2013, 52, 14177 CrossRef CAS PubMed; (c) Q. Lu, C. Liu, Z. Huang, Y. Ma, J. Zhang and A. Lei, Chem. Commun., 2014, 50, 14101 RSC; (d) X.-Z. Luo, H.-Q. Luo, Z.-P. Zhang and W. Dong, Synlett, 2014, 25, 1307 CrossRef; (e) Y. Yang, Y. Liu, Y. Jiang, Y. Zhang and D. A. Vicic, J. Org. Chem., 2015, 80, 6639 CrossRef CAS PubMed; (f) C. Liu, Q. Lu, Z. Huang, J. Zhang, F. Liao, P. Peng and A. Lei, Org. Lett., 2015, 17, 6034 CrossRef CAS PubMed; (g) T. Kawamoto, R. Sasaki and A. Kamimura, Angew. Chem., Int. Ed., 2017, 56, 1342 CrossRef CAS PubMed.
- Oxy-trifluoromethylation of alkenes by different CF3-sources: (a) C.-P. Zhang, Z.-L. Wang, Q.-Y. Chen, C.-T. Zhang, Y.-C. Gu and J.-C. Xiao, Chem. Commun., 2011, 47, 6632 RSC; (b) Y. Li and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8221 CrossRef CAS PubMed; (c) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462 CrossRef CAS PubMed; (d) H. Egami, R. Shimizu and M. Sodeoka, Tetrahedron Lett., 2012, 53, 5503 CrossRef CAS; (e) C. Feng and T.-P. Loh, Chem. Sci., 2012, 3, 3458 RSC; (f) Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef CAS PubMed; (g) P. G. Janson, I. Ghoneim, N. O. Ilchenko and K. J. Szado, Org. Lett., 2012, 14, 2882 CrossRef CAS PubMed; (h) D.-F. Lu, C.-L. Zhu and H. Xu, Chem. Sci., 2013, 4, 2478 RSC; (i) E. Kim, S. Choi, H. Kim and E. J. Cho, Chem.–Eur. J., 2013, 19, 6209 CrossRef CAS PubMed; (j) R. Zhu and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 12655 CrossRef CAS PubMed; (k) Y.-T. He, H.-L. Li, Y.-F. Yang, Y.-Q. Wang, J.-Y. Luo, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2013, 49, 5687 RSC; (l) Q. Yu and S. Ma, Chem.–Eur. J., 2013, 19, 13304 CrossRef CAS PubMed; (m) Photoredox-catalyzed: Y. Yasu, Y. Arai, R. Tomita, T. Koike and M. Akita, Org. Lett., 2014, 16, 780 CrossRef CAS PubMed; (n) H. Egami, R. Shimizu, Y. Usui and M. Sodeoka, J. Fluorine Chem., 2014, 167, 172 CrossRef CAS; (o) S. Jana, A. Ashokan, S. Kumar, A. Verma and S. Kumar, Org. Biomol. Chem., 2015, 13, 8411 RSC; (p) N. Yi, H. Zhang, C. Xu, W. Deng, R. Wang, D. Peng, Z. Zeng and J. Xiang, Org. Lett., 2016, 18, 1780 CrossRef CAS PubMed; (q) J. Ye, et al. , Angew. Chem., Int. Ed., 2016, 55, 10022 CrossRef CAS PubMed; (r) Y.-B. Wu, G.-P. Lu, T. Yuan, Z.-B. Xu, L. Wan and C. Cai, Chem. Commun., 2016, 52, 13668 RSC.
- Oxy-trifluoromethylation of alkynes by CF3SO2Na: (a) Z. He, R. Zhang, M. Hu, L. Li, C. Ni and J. Hu, Chem. Sci., 2013, 4, 3478 RSC; (b) A. Maji, A. Hazra and D. Maiti, Org. Lett., 2014, 16, 4524 CrossRef CAS PubMed.
- Various initiators for trifluoromethylation: (a) Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411 CrossRef CAS PubMed; (b) Y. Fujiwara, J. A. Dixon, F. O’Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herle, N. Sach, M. R. Collins, Y. Ishihara and P. S. Baran, Nature, 2012, 492, 95 CrossRef CAS PubMed; (c) R. Gianatassio, S. Kawamura, C. L. Eprile, K. Foo, J. Ge, A. C. Burns, M. R. Collins and P. S. Baran, Angew. Chem., Int. Ed., 2014, 53, 9851 CrossRef CAS PubMed; (d) Y. Ye, S. A. Künzi and M. S. Sanford, Org. Lett., 2012, 14, 4979 CrossRef CAS PubMed; (e) Y. Ye, S. H. Lee and M. S. Sanford, Org. Lett., 2011, 13, 5464 CrossRef CAS PubMed; (f) A. T. Parsons and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 9120 CrossRef CAS PubMed; (g) Z. Li, Z. Cui and Z.-Q. Liu, Org. Lett., 2013, 15, 406 CrossRef CAS PubMed.
- Cascade trifluoromethylation: (a) L. Zhang, Z. Li and Z. Q. Liu, Org. Lett., 2014, 16, 3688 CrossRef CAS PubMed; (b) Y. Q. Wang, Y. T. He, L. L. Zhang, X. X. Wu, X. Y. Liu and Y. M. Liang, Org. Lett., 2015, 17, 4280 CrossRef CAS PubMed; (c) Y.-T. He, et al. , Adv. Synth. Catal., 2015, 357, 3069 CrossRef CAS; (d) Q. Wang, H. Song, Y. Liu, H. Song and Q. Wang, Adv. Synth. Catal., 2016, 358, 3435 CrossRef CAS; (e) L.-Z. Yu, Q. Xu, X.-Y. Tang and M. Shi, ACS Catal., 2016, 6, 526 CrossRef CAS.
- Reviews on trifluoromethylation: (a) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed; (b) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (c) C. Zhang, Adv. Synth. Catal., 2014, 356, 2895 CrossRef CAS; (d) C. Alonso, E. Martinez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed; (e) C. Ni and J. Hu, Chem. Soc. Rev., 2016, 45, 5441 RSC.
- Cascade oxy-trifluoromethylation: H. Fu, S.-S. Wang and Y.-M. Li, Adv. Synth. Catal., 2016, 358, 3616 CrossRef CAS.
- C3-aryloyl benzofurans: (a) Y. Liao, M. Reitman, Y. Zhang, R. Fathi and Z. Yang, Org. Lett., 2002, 4, 2607 CrossRef CAS PubMed; (b) Y. Hu, Y. Zhang, Z. Yang and R. Fathi, J. Org. Chem., 2002, 67, 2365 CrossRef CAS PubMed; (c) A. Furstner, E. K. Heilmann and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 4760 CrossRef PubMed; (d) Y. Liu, J. Qian, S. Lou and Z. Xu, J. Org. Chem., 2010, 75, 6300 CrossRef CAS PubMed; (e) J. U., L.-Y. Wu, X.-C. Wang, Y.-Y. Hu, Y.-N. Niu, X.-Y. Liu, S. Yang and Y.-M. Liang, Adv. Synth. Catal., 2010, 352, 351 CrossRef; (f) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (g) N. Aljaar, C. C. Malakar, J. Conrad, S. Strobel, T. Schleid and U. Beifuss, J. Org. Chem., 2012, 77, 7793 CrossRef CAS PubMed; (h) S. Ngwerume, W. Lewis and J. E. Camp, J. Org. Chem., 2013, 78, 920 CrossRef CAS PubMed; (i) S. Bensulong, J. Boonsombat and S. Ruchirawat, Tetrahedron, 2013, 69, 9335 CrossRef CAS; (j) M. Hu, R. J. Song and J. H. Li, Angew. Chem., Int. Ed., 2015, 54, 608 CAS.
- C3-aryloyl benzothiophenes: (a) D. Fréhel, R. Boigegrain and J. Maffrand, Heterocycles, 1984, 22, 1235 CrossRef; (b) S. Dadiboyena, Eur. J. Med. Chem., 2012, 51, 17 CrossRef CAS PubMed; (c) S. Kim, N. Dahal and T. Kesharwani, Tetrahedron Lett., 2013, 54, 4373 CrossRef CAS; (d) C. H. Lin, C. C. Chen and M. J. Wu, Chem.–Eur. J., 2013, 19, 2578 CrossRef CAS PubMed; (e) A. Acharya, S. V. Kumar and H. Ila, Chem.–Eur. J., 2015, 21, 17116 CrossRef CAS PubMed.
- C3-aryloyl indole synthesis: (a) M. A. Eissenstat and J. D. Weaver III, Tetrahedron Lett., 1995, 36, 2029 CrossRef CAS; (b) M. J. Earle, K. R. Seddon and P. B. McCormac, Green Chem., 2000, 2, 261 RSC; (c) I. Nakamura, Y. Sato, S. Konta and M. Terada, Tetrahedron Lett., 2009, 50, 2075 CrossRef CAS; (d) T. Back and D. Gao, Synlett, 2013, 24, 389 CrossRef; (e) F. Huang, P. Wu, L. Wang, J. Chen, C. Sun and Z. Yu, J. Org. Chem., 2014, 79, 10553 CrossRef CAS PubMed; (f) T.-J. Gong, W.-M. Cheng, W. Su, B. Xiao and Y. Fu, Tetrahedron Lett., 2014, 55, 1859 CrossRef CAS; (g) S. Prathapan, S. Radhamani, R. Natarajan, J. Rappai and P. Unnikrishnan, Synlett, 2015, 26, 2467 CrossRef; (h) H. Johansson, A. Urruticoechea, I. Larsen and D. Sejer Pedersen, J. Org. Chem., 2015, 80, 471 CrossRef CAS PubMed; (i) A. Arcadi, M. Chiarini, L. Del Vecchio, F. Marinelli and V. Michelet, Chem. Commun., 2016, 52, 1458 RSC; (j) P. Sharma and R. S. Liu, Org. Lett., 2016, 18, 412 CrossRef CAS PubMed.
- Recent photocatalytic reactions: (a) D. A. Nagib, M. E. Scott and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875 CrossRef CAS PubMed; (b) A. E. Allen and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 4986 CrossRef CAS PubMed; (c) P. V. Pham, K. Ashton and D. W. C. MacMillan, Chem. Sci., 2011, 2, 1470 RSC; (d) P. V. Pham, D. A. Nagib and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2011, 50, 6119 CrossRef CAS PubMed; (e) S. B. Jones, B. Simmons, A. Mastracchio and D. W. C. MacMillan, Nature, 2011, 475, 183 CrossRef CAS PubMed; (f) S. Maity and N. Zheng, Angew. Chem., Int. Ed., 2012, 51, 9562 CrossRef CAS PubMed; (g) D. P. Hari, T. Hering and B. König, Org. Lett., 2012, 14, 5334 CrossRef CAS PubMed; (h) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (i) U. Wille, Chem. Rev., 2013, 113, 813 CrossRef CAS PubMed; (j) B. N. Laforteza, M. Pickworth and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2013, 52, 11269 CrossRef CAS PubMed; (k) I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Science, 2014, 346, 725 CrossRef CAS PubMed; (l) L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886 CrossRef CAS PubMed; (m) N. A. Romero and D. A. Nicewicz, J. Am. Chem. Soc., 2014, 136, 17024 CrossRef CAS PubMed; (n) J. D. Griffin, M. A. Zeller and D. A. Nicewicz, J. Am. Chem. Soc., 2015, 137, 11340 CrossRef CAS PubMed; (o) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS PubMed; (p) C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. C. MacMillan and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 11270 CrossRef CAS PubMed; (q) P. D. Morse and D. A. Nicewicz, Chem. Sci., 2015, 6, 270 RSC; (r) C. L. Cavanaugh and D. A. Nicewicz, Org. Lett., 2015, 17, 6082 CrossRef CAS PubMed; (s) A. Joshi-Pangu, F. Lévesque, H. G. Roth, S. F. Oliver, L.-C. Campeau, D. Nicewicz and D. A. DiRocco, J. Org. Chem., 2016, 81, 7244 CrossRef CAS PubMed; (t) E. Brachet, L. Marzo, M. Selkti, B. König and P. Belmont, Chem. Sci., 2016, 7, 5002 RSC; (u) X. Q. Hu, X. Qi, J. R. Chen, Q. Q. Zhao, Q. Wei, Y. Lan and W. J. Xiao, Nat. Commun., 2016, 7, 11188 CrossRef PubMed; (v) L. Li, W. Liu, X. Mu, Z. Mi and C.-J. Li, Nat. Protoc., 2016, 11, 1948 CrossRef CAS PubMed; (w) I. Ghosh, L. Marzo, A. Das, R. Shaikh and B. König, Acc. Chem. Res., 2016, 49, 1566 CrossRef CAS PubMed; (x) T. Hering, A. U. Meyer and B. König, J. Org. Chem., 2016, 81, 6927 CrossRef CAS PubMed; (y) H. Bartling, A. Eisenhofer, B. König and R. M. Gschwind, J. Am. Chem. Soc., 2016, 138, 11860 CrossRef CAS PubMed; (z) A. U. Meyer, A. Wimmer and B. König, Angew. Chem., Int. Ed., 2017, 56, 409 CrossRef CAS PubMed; (a a) D. Kalyani, K. B. McMurtrey, S. R. Neufeldt and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 18566 CrossRef CAS PubMed; (a b) L. J. Allen, P. J. Cabrera, M. Lee and M. S. Sanford, J. Am. Chem. Soc., 2014, 136, 5607 CrossRef CAS PubMed.
- Photoredox catalysis reviews: (a) J. M. Narayanam and C. R. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (b) S. Barata-Vallejo, S. M. Bonesi and A. Postigo, Org. Biomol. Chem., 2015, 13, 11153 RSC; (c) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898 CrossRef CAS PubMed; (d) N. Corrigan, S. Shanmugam, J. Xu and C. Boyer, Chem. Soc. Rev., 2016, 45, 6165 RSC; (e) E. Brachet, L. Marzo, M. Selkti, B. König and P. Belmont, Chem. Sci., 2016, 7, 5002 RSC; (f) N. Corrigan, S. Shanmugam, J. Xu and C. Boyer, Chem. Soc. Rev., 2016, 45, 6165 RSC; (g) S. P. Pitre, C. D. McTiernan and J. C. Scaiano, Acc. Chem. Res., 2016, 49, 1320 CrossRef CAS PubMed; (h) J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (i) D. Staveness, I. Bosque and C. R. Stephenson, Acc. Chem. Res., 2016, 49, 2295 CrossRef CAS PubMed; (j) T. Chatterjee, N. Iqbal, Y. You and E. J. Cho, Acc. Chem. Res., 2016, 49, 2284 CrossRef CAS PubMed; (k) T. Koike and M. Akita, Acc. Chem. Res., 2016, 49, 1937 CrossRef CAS PubMed; (l) J. Goddard, C. Ollivier and L. Fensterbank, Acc. Chem. Res., 2016, 49, 1924 CrossRef CAS PubMed; (m) M. Majek and A. Wangelin, Acc. Chem. Res., 2016, 49, 2316 CrossRef CAS PubMed; (n) K. A. Margrey and D. A. Nicewicz, Acc. Chem. Res., 2016, 49, 1997 CrossRef CAS PubMed; (o) J. J. Douglas, M. J. Sevrin and C. R. Stephenson, Org. Process Res. Dev., 2016, 20, 1134 CrossRef CAS; (p) X. Pan, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 1163 RSC.
- Photo-induced trifluoromethylation: (a) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS PubMed; (b) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed; (c) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC; (d) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, ACS Catal., 2014, 4, 2530 CrossRef CAS; (e) R. Tomita, Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2014, 53, 7144 CrossRef CAS PubMed; (f) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Org. Lett., 2014, 16, 1240 CrossRef CAS PubMed; (g) N. Noto, K. Miyazawa, T. Koike and M. Akita, Org. Lett., 2015, 17, 3710 CrossRef CAS PubMed; (h) Q. Wei, J.-R. Chen, X.-Q. Hu, X.-C. Yang, B. Lu and W.-J. Xiao, Org. Lett., 2015, 17, 4464 CrossRef CAS PubMed; (i) J. W. Beatty, J. J. Douglas, K. P. Cole and C. R. J. Stephenson, Nat. Commun., 2015, 6, 7919 CrossRef PubMed; (j) R. Tomita, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2015, 54, 12923 CrossRef CAS PubMed; (k) N. Noto, T. Koike and M. Akita, J. Org. Chem., 2016, 81, 7064 CrossRef CAS PubMed; (l) Y. Arai, R. Tomita, G. Ando, T. Koike and M. Akita, Chem.–Eur. J., 2016, 22, 1262 CrossRef CAS PubMed; (m) Q. Lefebvre, N. Hoffmann and M. Rueping, Chem. Commun., 2016, 52, 249 RSC; (n) L. Li, X. Mu, W. Liu, Y. Wang, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2016, 138, 5809 CrossRef CAS PubMed; (o) X. L. Yu, J. R. Chen, D. Z. Chen and W. J. Xiao, Chem. Commun., 2016, 52, 8275 RSC; (p) L. Jarrige, A. Carboni, G. Dagousset, G. Levitre, E. Magnier and G. Masson, Org. Lett., 2016, 18, 2906 CrossRef CAS PubMed; (q) W. Fu, X. Han, M. Zhu, C. Xu, Z. Wang, B. Ji, X.-Q. Hao and M.-P. Song, Chem. Commun., 2016, 52, 13413 RSC; (r) T. Koike and M. Akita, Org. Chem. Front., 2016, 3, 1345 RSC; (s) H. Xiang, Q. Zhao, Z. Tang, J. Xiao, P. Xia, C. Wang, C. Yang, X. Chen and H. Yang, Org. Lett., 2017, 19, 146 CrossRef CAS PubMed; (t) H.-T. Qin, S.-W. Wu, J.-L. Liu and F. Liu, Chem. Commun., 2017, 53, 1696 RSC.
- Photocatalytic oxidative activation of H2O for oxygenation: (a) S. Fukuzumi, T. Kishi, H. Kotani, Y. M. Lee and W. Nam, Nat. Chem., 2011, 3, 38 CrossRef CAS PubMed; (b) G. Zhang, X. Hu, C. W. Chiang, H. Yi, P. Pei, A. K. Singh and A. Lei, J. Am. Chem. Soc., 2016, 138, 12037 CrossRef CAS PubMed; (c) X. Hu, G. Zhang, F. Bu and A. Lei, ACS Catal., 2017, 7, 1432 CrossRef CAS; (d) K. Ohkubo, A. Fujitomo and S. FuKuzumi, J. Am. Chem. Soc., 2013, 135, 5368 CrossRef CAS PubMed.
- (a) B. S. Bhakuni, A. Yadav, S. Kumar, S. Patel, S. Sharma and S. Kumar, J. Org. Chem., 2014, 79, 2944 CrossRef CAS PubMed; (b) S. Kumar, et al. , Org. Lett., 2015, 17, 82 CrossRef CAS PubMed; (c) A. Verma, et al. , Chem. Commun., 2015, 51, 1371 RSC; (d) A. Verma and S. Kumar, Org. Lett., 2016, 18, 4388 CrossRef CAS PubMed; (e) A. Verma, S. Jana, C. D. Prasad, A. Yadav and S. Kumar, Chem. Commun., 2016, 52, 4179 RSC; (f) V. Rathore, M. Sattar, R. Kumar and S. Kumar, J. Org. Chem., 2016, 81, 9206 CrossRef CAS PubMed; (g) C. D. Prasad, M. Sattar and S. Kumar, Org. Lett., 2017, 19, 774 CrossRef CAS PubMed; (h) S. Kumar, et al. , Angew. Chem., Int. Ed., 2016, 55, 3729 CrossRef CAS PubMed.
- (a) N. C. Yang and D.-D. H. Yang, J. Am. Chem. Soc., 1958, 80, 2913 CrossRef CAS; (b) N. Hoffmann, Chem. Rev., 2008, 108, 1052 CrossRef CAS PubMed; (c) J. D. Coyle and H. A. Carless, Chem. Soc. Rev., 1972, 1, 465 RSC.
- 9,10-Phenanthroline UV-visible study: (a) D. M. Togashi, S. M. B. Costa and D. E. Nicodem, J. Mol. Struct., 2001, 565, 93 CrossRef; (b) D. M. Togashi and D. E. Nicodem, Spectrochim. Acta, Part A, 2004, 60, 3205 CrossRef PubMed; (c) R. S. Becker and L. V. Natarajan, J. Phys. Chem., 1993, 97, 344 CrossRef CAS.
- Umpolung of aromatic ketones: (a) P. Wagner and B. S. Park, Photoinduced Hydrogen Atom Abstraction Reaction by Carbonyl Compounds, in Organic Photochemistry, ed. A. Padwa, Marcel Dekker, New York, 1991, pp. 227–366 Search PubMed; (b) V. R. Kumar, N. Rajkumar, F. Ariese and S. Umapathy, J. Phys. Chem. A, 2015, 119, 10147 CrossRef CAS PubMed.
- P. A. Carapellucci, H. P. Wolf and K. Weiss, Photoreduction of, J. Am. Chem. Soc., 1969, 91, 4635 CrossRef CAS.
- PQ is readily available, air stable, and has low molecular weight and an arbitrary absorption maximum at 420 nm. Nonetheless, however, this has not been explored in synthetic transformations other than its applications in H2 activation with B(C6H5)3,27a in organic materials.27b,c: (a) L. E. Longobardi, L. Liu, S. Grimme and D. W. Stephan, J. Am. Chem. Soc., 2016, 138, 2500 CrossRef CAS PubMed; (b) Y. X. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 16267 CrossRef CAS PubMed; (c) K. Urakawa, M. Sumimoto, M. Arisawa, M. Matsuda and H. Ishikawa, Angew. Chem., Int. Ed., 2016, 55, 7432 CrossRef CAS PubMed; Organic dyes as photoredox catalysts: (d) E. Yoshioka, S. Kohtani, T. Jichu, T. Fukazawa, T. Nagai, A. Kawashima, Y. Takemoto and H. Miyabe, J. Org. Chem., 2016, 81, 7217 CrossRef CAS PubMed; (e) S. P. Pitre, C. D. McTiernan and J. C. Scaiano, ACS Omega, 2016, 1, 66 CrossRef CAS; (f) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed.
- 1-(Alkyl/arylethynyl)-2-(vinyloxy)benzene substrates 1a–1r were prepared by following the reported procedure: N. Sakiyama, K. Noguchi and K. Tanaka, Angew. Chem., Int. Ed., 2012, 51, 5976 CrossRef CAS PubMed.
- (a) J. Zhu, Y.-J. Cheng, X.-K. Kuang, L. Wang, Z.-B. Zheng and Y. Tang, Angew. Chem., Int. Ed., 2016, 55, 9224 CrossRef CAS PubMed; (b) W.-I. Lee, J.-W. Jung, J. Sim, H. An and Y.-G. Suh, Tetrahedron, 2013, 69, 7211 CrossRef CAS.
- I. Chataigner, C. Panel, H. Gerard and S. R. Piettre, Chem. Commun., 2007, 3288 RSC.
- N-alkylated indole: (a) T. Back and D. Gao, Synlett, 2013, 24, 389 CrossRef; (b) T.-J. Gong, W.-M. Cheng, W. Su, B. Xiao and Y. Fu, Tetrahedron Lett., 2014, 55, 1859 CrossRef CAS; (c) P. Sharma and R. S. Liu, Org. Lett., 2016, 18, 412 CrossRef CAS PubMed.
- For the detection of hydrogen gas in the reaction by 1H NMR spectroscopy: (a) D. P. Ojha, K. Gadde and K. R. Prabhu, Org. Lett., 2016, 18, 5062 CrossRef CAS PubMed; Synthetic photocatalytic hydrogen evolution: (b) G. Zhang, C. Liu, H. Yi, Q. Meng, C. Bian, H. Chen, J. X. Jian, L. Z. Wu and A. Lei, J. Am. Chem. Soc., 2015, 137, 9273 CrossRef CAS PubMed; (c) C.-J. Wu, Q.-Y. Meng, T. Lei, J.-J. Zhong, W.-Q. Liu, L.-M. Zhao, Z.-J. Li, B. Chen, C.-H. Tung and L.-Z. Wu, ACS Catal., 2016, 6, 4635 CrossRef CAS; (d) K. Luo, Y. Z. Chen, W. C. Yang, J. Zhu and L. Wu, Org. Lett., 2016, 18, 452 CrossRef CAS PubMed; (e) G. Zhang, L. Zhang, H. Yi, Y. Luo, X. Qi, C. H. Tung, L. Z. Wu and A. Lei, Chem. Commun., 2016, 52, 10407 RSC; (f) H. Wang, Q. Lu, C.-W. Chiang, Y. Luo, J. Zhou, G. Wang and A. Lei, Angew. Chem., Int. Ed., 2017, 56, 595 CrossRef CAS PubMed; (g) H. Yi, L. Niu, C. Song, Y. Li, B. Dou, A. K. Singh and A. Lei, Angew. Chem., Int. Ed., 2017, 56, 1120 CrossRef CAS PubMed.
- Because the reaction mixture provides an EPR signal only for dissociated MNP; tert-buyl nitroxide radical in CH3CN: H2O solvent. Therefore, the reaction mixture along with the MNP dimer was conducted in a CH2Cl2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent system for EPR study.
(a) Z. Li, Y. Zhang, L. Zhang and Z.-Q. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed;
(b) Z. Hang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3648 CrossRef CAS PubMed.
water solvent system for EPR study.
(a) Z. Li, Y. Zhang, L. Zhang and Z.-Q. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed;
(b) Z. Hang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3648 CrossRef CAS PubMed. - Hydration of alkynes: (a) P. Wan, S. Culshaw and K. Yates, J. Am. Chem. Soc., 1982, 104, 2509 CrossRef CAS; (b) C.-J. Li, Acc.Chem. Res., 2010, 43, 581 CrossRef CAS PubMed; (c) E. H. Hill, S. Goswami, D. G. Evans, K. S. Schanze and D. G. Whitten, J. Phys. Chem. Lett., 2012, 3, 1363 CrossRef CAS PubMed.
- M. W. Lehmann and D. H. Evans, J. Electroanal. Chem., 2001, 500, 12 CrossRef CAS.
- (a) Triplet exited state energy (ETo,o) of PQ is 48.8 kcal mol−1 or 2.116 V, please see: J. J. Bohning and K. Weiss, J. Am. Chem. Soc., 1966, 88, 2893 CrossRef CAS; (b) Excited state reduction potential Ered1/2* (3PQ*/PQ˙−) of PQ was calculated by following ref. 27f: ground state Ered1/2 (PQ/PQ˙−) + triplet excited state (ETo,o); −0.52 + 2.1161 = +1.60 V. Ered1/2 (PQ/PQ˙−) is determined experimentally.
- J.-B. Tommasino, A. Brondex, M. Médebielle, M. Thomalla, B. R. Langlois and T. Billard, Synlett, 2002, 10, 1697 CrossRef.
- P. A. Byrne, S. Kobayashi, E.-U. Würthwein, J. Ammer and H. Mayr, J. Am. Chem. Soc., 2017, 139, 1499 CrossRef CAS PubMed.
- W. S. Johnson, G. W. Daub, T. A. Lyle and M. Niwa, J. Am. Chem. Soc., 1980, 102, 7800 CrossRef CAS.
- (a) M. Bettoni, T. D. Giacco, C. Rol and G. V. Sebastiani, J. Phys. Org. Chem., 2006, 19, 359 CrossRef CAS; (b) R. K. Saunthwal, M. Patel and A. K. Verma, Org. Lett., 2016, 18, 2200 CrossRef CAS PubMed.
- M. A. Cismesia and T. P. Yoon, Chem. Sci., 2015, 6, 5426 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectroscopic data, 1H, 13C, 19F, mass spectra, DFT calculations and X-ray crystallographic data. CCDC 152606 0 (2c), 1526059 (4k), 1526057 (6c), and 1526058 (6d). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc02556d |
| This journal is © The Royal Society of Chemistry 2017 |