Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid access to substituted 2-naphthyne intermediates via the benzannulation of halogenated silylalkynes†
Samuel J.
Hein
 ab,
Dan
Lehnherr
ab,
Dan
Lehnherr
 b and
William R.
Dichtel
b and
William R.
Dichtel
 *a
*a
aDepartment of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208, USA. E-mail: wdichtel@northwestern.edu
bDepartment of Chemistry and Chemical Biology, Baker Laboratory, Cornell University, Ithaca, New York 14853, USA
First published on 9th June 2017
Abstract
Aryne intermediates are versatile and important reactive intermediates for natural product and polymer synthesis. 2-Naphthynes are relatively unexplored because few methods provide precursors to these intermediates, especially for those bearing additional substituents. Here we report a general synthetic strategy to access 2-naphthyne precursors through an Asao-Yamamoto benzannulation of ortho-(phenylethynyl)benzaldehydes with halo-silylalkynes. This transformation provides 2-halo-3-silylnaphthalenes with complete regioselectivity. These naphthalene products undergo desilylation/dehalogenation in the presence of F− to generate the corresponding 2-naphthyne intermediate, as evidenced by furan trapping experiments. When these 2-naphthynes are generated in the presence of a copper catalyst, ortho-naphthalene oligomers, trinaphthalene, or binaphthalene products are formed selectively by varying the catalyst loading and reaction temperature. The efficiency, mild conditions, and versatility of the naphthalene products and naphthyne intermediates will provide efficient access to many new functional aromatic systems.
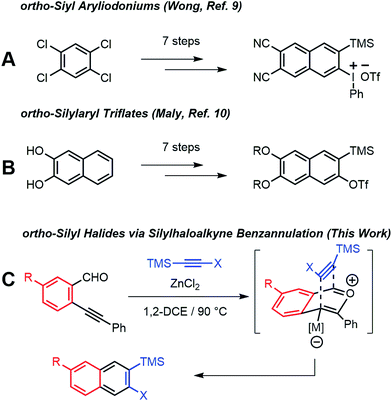
ortho-Arynes are reactive intermediates generated through the elimination of adjacent functional groups from an aromatic system.1 These species have a rich history in physical and synthetic organic chemistry since their existence was first postulated by Stoermer and Kahlert.2 Transformations involving nucleophilic additions, bond insertions, pericyclic reactions, and multicomponent coupling reactions involving arynes have been employed for the synthesis of natural products and polycyclic aromatic hydrocarbons.3 Although early arynes were first generated using strong bases with limited functional group compatibility, Kobayashi reported that ortho-silylaryl triflates generate arynes efficiently in the presence of fluoride ions.4 The mild conditions and excellent functional group tolerance of this approach has led to ortho-silylaryl triflates becoming popular aryne precursors. Yet few ortho-silylaryl triflates are commercially available, and their synthesis is often laborious, despite the recent development of cycloaddition-based strategies by Harrity5 and aryl C–H bond silylations by Daugulis.6
Arynes derived from other aromatic systems7,8 enable the synthesis of more complex structures, yet access to appropriately substituted precursors is even more limited. Wong9 and Maly10 reported substituted 2-naphthyne precursors, but the seven step syntheses of each method limit general adoption (Scheme 1a and b). We recently noted that the Asao-Yamamoto benzannulation,11 which was known to provide 2,3-diarylnaphthalenes from o-phenylethynyl-benzaldehydes and diarylalkynes, is capable of modifying conjugated polymer backbones with high efficiency12 and is regioselective for alkynes with electronically different aryl substituents.13 The scope of these benzannulation reactions has since been expanded to silyl- and haloalkynes, enabling the synthesis of sterically hindered14 and polyheterohalogenated naphthalenes.15 Here we demonstrate a new variant of the Asao-Yamamoto benzannulation that provides 2-silyl-3-halonaphthalenes as single regioisomers (Scheme 1c). These compounds generate 2-naphthyne intermediates under mild conditions, which were trapped by furan, oligomerized, and found to undergo cyclodimerization or cyclotrimerization to bi- and trinaphthalenes, respectfully. Several examples featuring substituted o-phenylethynylbenzaldehydes demonstrate the generality of this approach to access substituted 2-naphthyne intermediates.
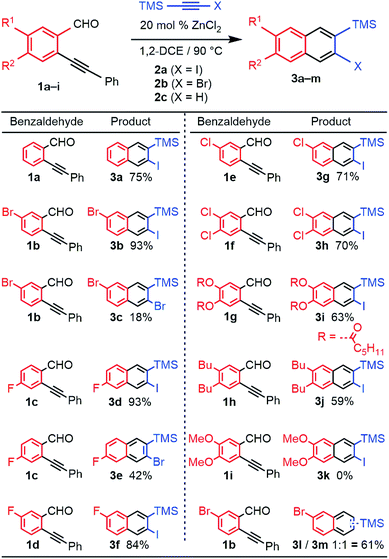
We evaluated the scope and regioselectivity of the benzannulation reaction using substituted benzaldehydes and silylhaloalkynes (Fig. 1). Previously, catalytic Cu(OTf)2 in the presence of CF3CO2H had been employed for the benzannulation of substituted acetylenes, but these conditions favour protodesilylation of the trimethylsilyl (TMS) substituent.14 ZnCl2 is a milder catalyst that does not require acidic additives.16 Naphthalene products 3a–j were obtained as single regioisomers, often in good to excellent yield. The reaction was more efficient for iodoalkynes than bromoalkynes, as shown for benzaldehyde 1b with 2a (X = I, 93% yield) and 2b (X = Br, 18% yield). Likewise, the reaction of 1c with 2a and 2b provided 3d (93%) and 3e (42%). Unsubstituted and halogenated benzaldehydes (1a–f) performed well with yields between 70–93% whereas those for benzaldehydes bearing two phenolic esters (1g) and two alkyl groups (1h) were 63% and 59%, respectively. Benzaldehydes bearing stronger electron donating groups, such as dimethoxy benzaldehyde 1i, did not undergo benzannulation, and a mixture of aldehyde decomposition products and unreacted alkyne was obtained.
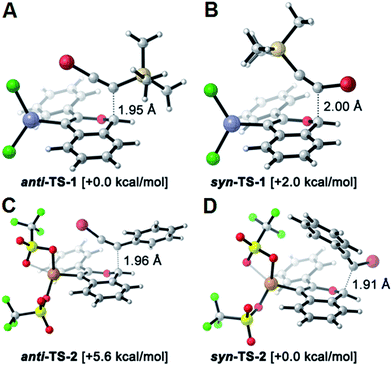
The benzannulation reactions of 2a and 2b (Fig. 1) provide substituted naphthalene products as single regioisomers when non-pseudosymmetric benzaldehydes (e.g., 1c, 1d) are employed. Our previous studies of diarylalkynes and haloarylalkynes indicated that regioselectivity arises from the ability of the alkyne substituents to preferentially stabilize developing positive charge at one of the alkyne carbons.13,15 For example, when brominated benzaldehyde 1b undergoes benzannulation with an aryl-haloalkyne, using either Cu(OTf)2 or ZnCl2 catalysts, the syn-regioisomer with respect to the iodine and bromine positions is obtained (Fig. 2a). The opposite regioisomer is obtained for silylhaloalkyne substrates; 1b reacts with 2a and 2b to provide the corresponding anti-regioisomers 3b and 3c, respectively. These outcomes were confirmed by single crystal X-ray crystallography of 3c (Fig. 2a), compared to that obtained for a typical arylhaloalkyne. The regiochemical outcome is insensitive to substitution patterns on the benzaldehyde, as demonstrated for the benzannulations of monofluorinated benzaldehyde regioisomers 1c and 1d with 2a. Each reaction provides a single fluoronaphthalene regioisomer, whose structures were assigned by 19F and 2D NMR spectroscopy (see ESI†). Finally, halogenation of the silylalkyne is also essential for regioselectivity, as the benzannulation reaction of trimethylsilylacetylene (2c) and 1b provides a nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the two regioisomers. The regiochemical outcome of these ZnCl2-catalyzed benzannulations with silylarylalkynes are congruent with those using silylhaloalkynes. This observation supports our hypothesis that the preferred regiochemical outcome is based on going through the more stabilized regioisomeric cation, in this case the cation stabilized by both the beta-silyl effect and formation of a benzylic cation (see ESI†).
1 ratio of the two regioisomers. The regiochemical outcome of these ZnCl2-catalyzed benzannulations with silylarylalkynes are congruent with those using silylhaloalkynes. This observation supports our hypothesis that the preferred regiochemical outcome is based on going through the more stabilized regioisomeric cation, in this case the cation stabilized by both the beta-silyl effect and formation of a benzylic cation (see ESI†).
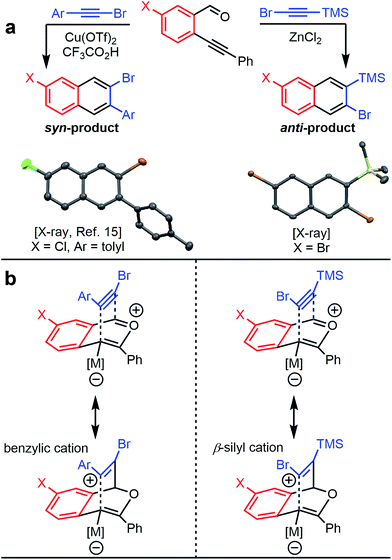 | ||
| Fig. 2 (a) Haloarylalkynes and halosilylalkynes provide opposite regioselectivity in benzannulation reactions, as demonstrated by X-ray crystallography.17 Ellipsoids set to 50% probability level for 3c. (b) Rationale for the regioselectivity of each reaction. The silicon substituent stabilizes developing positive charge on the carbon adjacent to the halogen, which makes the observed regioselectivity consistent with other Asao-Yamamoto benzannulation reactions. | ||
The reversed regioselectivity of halosilylalkyne benzannulations likely originates from the combined ability of the silicon and halogen substituents to stabilize developing positive charge on the alkyne carbon beta to the silicon atom.18 In contrast, when arylhaloalkynes are benzannulated, the aromatic ring stabilizes a developing positive charge on the alkyne carbon alpha to the ring (Fig. 2b). A DFT model of the proposed transition states leading to either the syn- or anti-regioisomer correctly predicts the reversal in regioselectivity observed experimentally between silylhaloalkynes and arylhaloalkynes (Fig. 3). The B3LYP/6-31G(d) calculated ZPE-corrected electronic energies of regioisomeric transition states predict a 2.0 kcal mol−1 preference for the anti-regioisomer in the case of silylhaloalkyne 2b compared to 5.6 kcal mol−1 in favor of the syn-regioisomer for phenylbromoalkyne. An alternate mechanism in which the metal is not bound to the benzopyrylium intermediate is also plausible, and transition state energies of those structures also support the observed regioselectivity (see ESI†).
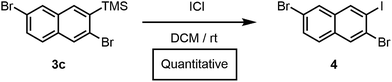
Furthermore, we had previously accessed a library of polyheterohalogenated naphthalenes through the benzannulation of arylhaloalkynes. Although this approach was used to prepare more than twenty polyheterohalogenated naphthalenes as single regioisomers, it was limited to 2-arylnaphthalene derivatives.15 Silylhaloalkyne benzannulations eliminate this restriction, and instead provide 2-trimethylsilylnaphthalenes with a silyl group that is easily removed or further transformed. For example, treatment of 3c with ICl afforded polyheterohalogenated naphthalene 4 in quantitative yield (Scheme 2). The TMS group incorporated into the naphthalene products serves as a versatile handle for further functionalization, as C(aryl)–Si bonds are readily transformed into I, Br, Cl, H, OH, CF3, Me, and others, highlighting the vast chemical space available through this method.19
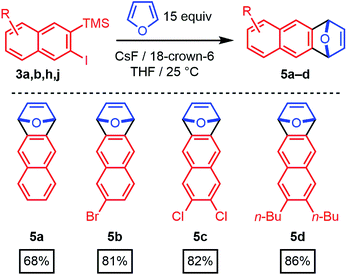
The 2-silyl-3-iodonaphthalenes serve as efficient precursors of 2-naphthyne intermediates in the presence of F− at room temperature, as demonstrated by furan trapping experiments.20–22 Unsubstituted (3a), 6-bromo- (3b), 6,7-dichloro- (3h), and 6,7-dibutyl-substituted (3j) 2-naphthynes provided [2.2.1]oxabicyclic alkenes (5a–d) in good to excellent isolated yields (68–86%, Scheme 3). These structures are of interest as precursors of poly(ortho-phenylene)s, iptycenes, acenes, and other aromatic structures.23–31 Given the availability of many substituted benzaldehyde cycloaddition partners, these findings demonstrate that benzannulation chemistry provides rapid entry to many substituted 2-naphthyne intermediates.
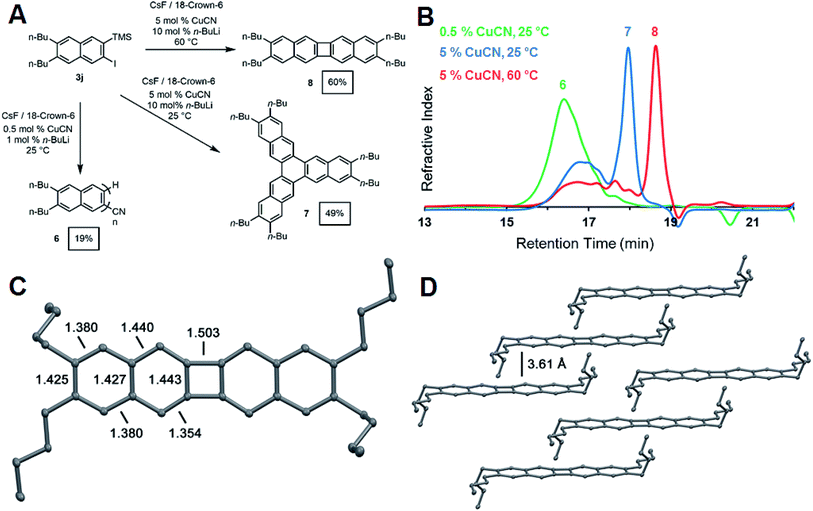
The synthesis of oligo- and poly(o-arylene)s represents a long-standing synthetic challenge. Oligomers have been prepared through stepwise cross-coupling strategies32,33 or cycloaddition approaches34 and adopt preferred or exclusive helical conformations. Formal or direct aryne polymerizations have provided the first polymers with moderate to high degrees of polymerization (DP: 20–100).23,35 We explored the CuCN-mediated polymerization of 3j (Fig. 4a) under conditions adapted from Uchiyama's pioneering study.35 Naphthalene derivative 3j (0.235 mM) was polymerized in THF at 25 °C in the presence of CsF (2 equiv.), CuCN (0.05 equiv.). n-BuLi (0.10 equiv.) and 18-crown-6 (4 equiv.). The addition of n-BuLi to CuCN generates a Lipshultz-type cuprate that is thought to be the active catalytic species in this reaction.35,36 These conditions provided 6 as an oligomeric mixture in modest isolated yield (19%). Size-exclusion chromatography (SEC) of 6 (Fig. 3) indicated a monomodal molecular weight distribution with Mn = 1900 g mol−1 compared to polystyrene standards, with tailing to low molecular weight, corresponding to desilylated and/or dehalogenated naphthalene side products. MALDI-TOF mass spectrometry (see ESI†) showed peaks with spacings consistent with the expected dibutylnaphthalene repeat unit and suggested that these species had well-defined nitrile end group on one end of the oligomer chain. These results confirm the expected reactivity of 3j under reported aryne polymerization conditions, despite the formation of low molecular weight species. It may ultimately prove possible to achieve higher yields and DP by optimizing the rate of 2-naphthyne generation. However, our efforts to explore the role of reaction conditions, such as increased catalyst loading and temperatures, provided unexpected and previously undescribed cyclooligomerization products in synthetically useful yields (Fig. 4a).
During our attempts to obtain higher molecular weight polymers, we found the product distribution drastically changed when the CuCN loading was increased to 5 mol% and the reaction run at room temperature. Analysis of the GPC trace of the precipitated product revealed a significant shift in the retention time of the oligomeric peak reflective of shorter oligomers (Fig. 4b). A sharp peak emerged at significantly longer retention times, suggesting a well-defined, low molecular weight compound. The 1H and 13C NMR spectra indicated the formation of a single naphthyl species with only two aromatic 1H resonances and five aromatic carbon shifts, suggesting the formation of a cyclic compound with a high degree of symmetry (see ESI Fig. S40 and S41†). Finally, high-resolution mass spectrometry of the isolated product after purification by chromatography identified it as the cyclic hexabutyltrinaphthylene 7, which was isolated in 49% yield (Fig. 4a). This extended form of triphenylene is of interest for discotic liquid crystals and as a potential monomer for covalent organic frameworks (COFs).37
When the above reaction is run at elevated temperature, a different molecular product dominates, as indicated by a shift to longer retention time in the GPC trace of the crude reaction mixture (Fig. 4b). We identified this species as the tetrabutylbinaphthalene 8 through 1H and 13C NMR (ESI Fig. S42 and S43†) spectroscopy and high-resolution mass spectrometry. Single-crystal X-ray crystallography unambiguously identified the structure as tetrabutylbinaphthalene product 8 (Fig. 4c). The solid-state arrangement of 8 features cofacial π-stacking with an unusually long interplanar distance of 3.61 Å, compared to the typical 3.4 Å distance for acenes (Fig. 4d).38 This arrangement arises from the butyl side chains, whose conformation places their termini perpendicular to the arene plane, preventing closer cofacial packing. [N]Phenylenes are traditionally challenging synthetic targets. Vollhardt and coworkers have prepared many of these compounds through a cobalt-catalyzed [2 + 2 + 2] cycloaddition of phenylene ethynylenes,39 and new synthetic strategies have been reported by Swager,40 Xia,41 and Bunz.42 These compounds consist of alternating aromatic rings fused cyclobutadienes with strong anti-aromatic character. The X-ray crystal structure showed a nearly planar geometry with torsion angles of 1.56° and 0.55° around the phenylene link. Analysis of the bond lengths confirms localization of the π-bonds around the 4-membered ring to offset the antiaromatic character of the cyclobutadienoid with alternating 1.44 Å endocyclic and 1.35 Å exocyclic bond distances. Bonds linking the acenoid segments have a larger single bond character with a bond length of 1.50 Å (Fig. 4c).43 UV-vis absorption and photoemission spectra of phenylenes 7 and 8 also show distinct differences to naphthyne precursor 3j and oligo(ortho-naphthalene) 6 (see ESI†).
Conclusions
The benzannulation of halosilylalkynes provides a general and rapid method to produce substituted 2-halo-3-silylnaphthalenes with high regioselectivity. The addition of a fluoride anion generates 2-naphthyne reactive intermediates, which were trapped as the [2.2.1]oxabicyclic alkene. These trapped arynes are themselves synthetically useful building blocks.23–31 A copper mediated aryne polymerization afforded low molecular weight oligomers, however when higher catalyst loadings are used the [2 + 2 + 2] and [2 + 2] cycloaddition products were observed in synthetically useful yields. These results show the utility of sequential zinc-catalyzed benzannulation reaction and naphthyne generation to access substituted tri- and binaphthalenes that are otherwise not easily prepared. We anticipate that the control of the halogenation pattern in conjunction with the diversity of available aryne reactions will enable rapid access to diverse and unique polycyclic conjugated aromatic architectures, including functionalized acenes and poly(arylene)s, among others.Acknowledgements
This work was supported by the Beckman Young Investigator Program of the Arnold and Mabel Beckman Foundation. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC program (DMR-1120296) and the IMSERC at Northwestern University, which has received support from the NSF (CHE-0923236, CHE-1048773); Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF NNCI-1542205); the State of Illinois and International Institute for Nanotechnology (IIN). We thank Christos D. Malliakas (X-ray Diffraction Facility, Northwestern University) for X-crystallographic analysis.Notes and references
- H. H. Wenk, M. Winkler and W. Sander, Angew. Chem., Int. Ed., 2003, 42, 502–528 CrossRef CAS PubMed
.
- R. Stoermer and B. Kahlert, Berichte Dtsch. Chem. Ges., 1902, 35, 1633–1640 CrossRef CAS
.
- For comprehensive reviews of the history of arynes in synthetic chemistry, see:
(a) S. S. Bhojgude, A. Bhunia and A. T. Biju, Acc. Chem. Res., 2016, 49, 1658–1670 CrossRef CAS PubMed
; (b) H. Yoshida, in Comprehensive Organic Synthesis II, ed. P. Knochel, Elsevier, Amsterdam, 2nd edn, 2014, pp. 517–579 Search PubMed
; (c) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550–3577 CrossRef CAS PubMed
; (d) D. Wu, H. Ge, S. Hua Liu and J. Yin, RSC Adv., 2013, 3, 22727–22738 RSC
; (e) E. Guitián, D. Pérez and D. Peña, in Palladium in Organic Synthesis, ed. J. Tsuji, Springer, Berlin, Heidelberg, 2005, pp. 109–146, Topics in Organometallic Chemistry Search PubMed
; (f) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2012, 11, 191–218 RSC
; (g) A. M. Dyke, A. J. Hester and G. C. Lloyd-Jones, Synthesis, 2006, 2006, 4093–4112 CrossRef
.
- Y. Himeshima, T. Sonoda and H. Kobayashi, Chem. Lett., 1983, 12, 1211–1214 CrossRef
.
- J. A. Crossley, J. D. Kirkham, D. L. Browne and J. P. A. Harrity, Tetrahedron Lett., 2010, 51, 6608–6610 CrossRef CAS
.
- M. Mesgar and O. Daugulis, Org. Lett., 2016, 18, 3910–3913 CrossRef CAS PubMed
.
-
(a) T. Kitamura, N. Fukatsu and Y. Fujiwara, J. Org. Chem., 1998, 63, 8579–8581 CrossRef CAS
; (b) K. Gondo and T. Kitamura, Adv. Synth. Catal., 2014, 356, 2107–2112 CrossRef CAS
; (c) C. S. LeHoullier and G. W. Gribble, J. Org. Chem., 1983, 48, 2364–2366 CrossRef CAS
; (d) J. A. Dodge, J. D. Bain and A. R. Chamberlin, J. Org. Chem., 1990, 55, 4190–4198 CrossRef CAS
; (e) Y. L. Chen, J. Q. Sun, X. Wei, W. Y. Wong and A. W. M. Lee, J. Org. Chem., 2004, 69, 7190–7197 CrossRef CAS PubMed
.
- For examples of heterocyclic arynes see:
(a) A. E. Goetz and N. K. Garg, J. Org. Chem., 2014, 79, 846–851 CrossRef CAS PubMed
; (b) J. M. Medina, M. K. Jackl, R. B. Susick and N. K. Garg, Tetrahedron, 2016, 72, 3629–3634 CrossRef CAS
; (c) G. Y. J. Im, S. M. Bronner, A. E. Goetz, R. S. Paton, P. H. Y. Cheong, K. N. Houk and N. K. Garg, J. Am. Chem. Soc., 2010, 132, 17933–17944 CrossRef CAS PubMed
; (d) T. Ikawa, H. Urata, Y. Fukumoto, Y. Sumii, T. Nishiyama and S. Akai, Chem.–Eur. J., 2014, 20, 16228–16232 CrossRef CAS PubMed
; (e) N. Saito, K. Nakamura, S. Shibano, S. Ide, M. Minami and Y. Sato, Org. Lett., 2013, 15, 386–389 CrossRef CAS PubMed
.
- C. Y. Yick, S. H. Chan and H. N. C. Wong, Tetrahedron Lett., 2000, 41, 5957–5961 CrossRef CAS
.
- P. T. Lynett and K. E. Maly, Org. Lett., 2009, 11, 3726–3729 CrossRef CAS PubMed
.
-
(a) N. Asao, T. Nogami, S. Lee and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 10921–10925 CrossRef CAS PubMed
; (b) N. Asao and Y. Yamamoto, Gold-Catalyzed Benzannulations: Asao-Yamamoto Benzopyrylium Pathway, in Modern Gold Catalyzed Synthesis, ed. S. K. Hashimi and F. D. Toste, Wiley-VCH, Weinheim, 1st edn, 2012, pp. 35–75 Search PubMed
.
- H. Arslan, J. D. Saathoff, D. N. Bunck, P. Clancy and W. R. Dichtel, Angew. Chem., Int. Ed., 2012, 51, 12051–12054 CrossRef CAS PubMed
.
-
(a) H. Arslan, K. L. Walker and W. R. Dichtel, Org. Lett., 2014, 16, 5926–5929 CrossRef CAS PubMed
; (b) H. Arslan, F. J. Uribe-Romo, B. J. Smith and W. R. Dichtel, Chem. Sci., 2013, 4, 3973–3978 RSC
.
- S. J. Hein, H. Arslan, I. Keresztes and W. R. Dichtel, Org. Lett., 2014, 16, 4416–4419 CrossRef CAS PubMed
.
- D. Lehnherr, J. M. Alzola, E. B. Lobkovsky and W. R. Dichtel, Chem.–Eur. J., 2015, 21, 18122–18127 CrossRef CAS PubMed
.
- X. L. Fang, R. Y. Tang, X. G. Zhang, P. Zhong, C. L. Deng and J. H. Li, J. Organomet. Chem., 2011, 696, 352–356 CrossRef CAS
.
- Crystallographic data for 2-bromo-3-tosyl-7-chloronaphthalene was reported by Dichtel and coworkers, see ref. 15.
-
(a) S. G. Wierschke, J. Chandrasekhar and W. L. Jorgensen, J. Am. Chem. Soc., 1985, 107, 1496–1500 CrossRef CAS
; (b) J. B. Lambert, G. T. Wang, R. B. Finzel and D. H. Teramura, J. Am. Chem. Soc., 1987, 109, 7838–7845 CrossRef CAS
; (c) H. U. Siehl, Pure Appl. Chem., 2009, 67, 769–775 Search PubMed
.
-
(a) C. Zarate, M. Nakajima and R. Martin, J. Am. Chem. Soc., 2017, 139, 1191–1197 CrossRef CAS PubMed
; (b) R. L. Funk and K. P. C. Vollhardt, J. Am. Chem. Soc., 1980, 102, 5245–5253 CrossRef CAS
; (c) J. Morstein, H. Hou, C. Cheng and J. F. Hartwig, Angew. Chem., Int. Ed., 2016, 55, 8054–8057 CrossRef CAS PubMed
; (d) B. Shao, A. L. Bagdasarian, S. Popov and H. M. Nelson, Science, 2017, 355, 1403–1407 CrossRef CAS PubMed
; (e) M. Tredwell and V. Gouverneur, Org. Biomol. Chem., 2006, 4, 26–32 RSC
.
- Q. Chen, H. Yu, Z. Xu, L. Lin, X. Jiang and R. Wang, J. Org. Chem., 2015, 80, 6890–6896 CrossRef CAS PubMed
.
- R. Harrison, H. Heaney and P. Lees, Tetrahedron, 1968, 24, 4589–4594 CrossRef CAS
.
- E. Masson and M. Schlosser, Eur. J. Org. Chem., 2005, 2005, 4401–4405 CrossRef
.
- S. Ito, K. Takahashi and K. Nozaki, J. Am. Chem. Soc., 2014, 136, 7547–7550 CrossRef CAS PubMed
.
- C. Romero, D. Peña, D. Pérez and E. Guitián, J. Org. Chem., 2008, 73, 7996–8000 CrossRef CAS PubMed
.
- S. Ito, W. Wang, K. Nishimura and K. Nozaki, Macromolecules, 2015, 48, 1959–1962 CrossRef CAS
.
- J. M. Medina, J. H. Ko, H. D. Maynard and N. K. Garg, Macromolecules, 2017, 50, 580–586 CrossRef CAS
.
- S. W. Thomas, T. M. Long, B. D. Pate, S. R. Kline, E. L. Thomas and T. M. Swager, J. Am. Chem. Soc., 2005, 127, 17976–17977 CrossRef CAS PubMed
.
- Z. Chen, J. P. Amara, S. W. Thomas and T. M. Swager, Macromolecules, 2006, 39, 3202–3209 CrossRef CAS
.
- B. J. Pei, W. H. Chan and A. W. M. Lee, Org. Lett., 2011, 13, 1774–1777 CrossRef CAS PubMed
.
- G. E. Morton and A. G. M. Barrett, J. Org. Chem., 2005, 70, 3525–3529 CrossRef CAS PubMed
.
- C. Kitamura, Y. Abe, T. Ohara, A. Yoneda, T. Kawase, T. Kobayashi, H. Naito and T. Komatsu, Chem.–Eur. J., 2010, 16, 890–898 CrossRef CAS PubMed
.
-
(a) S. M. Mathew and C. S. Hartley, Macromolecules, 2011, 44, 8425–8432 CrossRef CAS
; (b) S. M. Mathew, J. T. Engle, C. J. Ziegler and C. S. Hartley, J. Am. Chem. Soc., 2013, 135, 6714–6722 CrossRef CAS PubMed
; (c) C. S. Hartley, Acc. Chem. Res., 2016, 49, 646–654 CrossRef CAS PubMed
.
- S. Ando, E. Ohta, A. Kosaka, D. Hashizume, H. Koshino, T. Fukushima and T. Aida, J. Am. Chem. Soc., 2012, 134, 11084–11087 CrossRef CAS PubMed
.
- D. Lehnherr, C. Chen, Z. Pedramrazi, C. R. DeBlase, J. M. Alzola, I. Keresztes, E. B. Lobkovsky, M. F. Crommie and W. R. Dichtel, Chem. Sci., 2016, 7, 6357–6364 RSC
.
- Y. Mizukoshi, K. Mikami and M. Uchiyama, J. Am. Chem. Soc., 2015, 137, 74–77 CrossRef CAS PubMed
.
-
(a) B. H. Lipshutz, S. Sharma and E. L. Ellsworth, J. Am. Chem. Soc., 1990, 112, 4032–4034 CrossRef CAS
; (b) R. M. Gschwind, Chem. Rev., 2008, 108, 3029–3053 CrossRef CAS PubMed
.
-
(a) C. R. DeBlase and W. R. Dichtel, Macromolecules, 2016, 49, 5297–5305 CrossRef CAS
; (b) J. W. Colson and W. R. Dichtel, Nat. Chem., 2013, 5, 453–465 CrossRef CAS PubMed
; (c) Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC
.
-
(a) M. Bendikov, F. Wudl and D. F. Perepichka, Chem. Rev., 2004, 104, 4891–4946 CrossRef CAS PubMed
; (b) J. E. Anthony, Chem. Rev., 2006, 106, 5028–5048 CrossRef CAS PubMed
.
- Representative examples of Vollhardt's syntheses of [N]phenylenes:
(a) O. Š. Miljanić, D. Holmes and K. P. C. Vollhardt, Org. Lett., 2005, 7, 4001–4004 CrossRef PubMed
; (b) D. Holmes, S. Kumaraswamy, A. J. Matzger and K. P. C. Vollhardt, Chem.–Eur. J., 1999, 5, 3399–3412 CrossRef CAS
; (c) A. Fonari, J. C. Röder, H. Shen, T. V. Timofeeva and K. P. C. Vollhardt, Synlett, 2014, 25, 2429–2433 CrossRef CAS
; (d) O. Š. Miljanić and K. P. C. Vollhardt, in Carbon-Rich Compounds, ed. M. M. Haley and R. R. V. Tykwinski, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 140–197 Search PubMed
; (e) S. Han, D. R. Anderson, A. D. Bond, H. V. Chu, R. L. Disch, D. Holmes, J. M. Schulman, S. J. Teat, K. P. C. Vollhardt and G. D. Whitener, Angew. Chem., Int. Ed., 2002, 41, 3227–3230 CrossRef CAS
; (f) D. T. Y. Bong, E. W. L. Chan, R. Diercks, P. I. Dosa, M. M. Haley, A. J. Matzger, O. Š. Miljanić, K. P. C. Vollhardt, A. D. Bond, S. J. Teat and A. Stanger, Org. Lett., 2004, 6, 2249–2252 CrossRef CAS PubMed
; (g) B. C. Berris, G. H. Hovakeemian, Y. H. Lai, H. Mestdagh and K. P. C. Vollhardt, J. Am. Chem. Soc., 1985, 107, 5670–5687 CrossRef CAS
.
- R. R. Parkhurst and T. M. Swager, J. Am. Chem. Soc., 2012, 134, 15351–15356 CrossRef CAS PubMed
.
- Z. Jin, Y. C. Teo, N. G. Zulaybar, M. D. Smith and Y. Xia, J. Am. Chem. Soc., 2017, 139, 1806–1809 CrossRef CAS PubMed
.
- P. Biegger, M. Schaffroth, O. Tverskoy, F. Rominger and U. H. F. Bunz, Chem.–Eur. J., 2016, 22, 15896–15901 CrossRef CAS PubMed
.
- Bond distances and torsional angles are comparable to similar [N]phenylenes reported by Swager, Xia, Vollhardt, and Bunz. See ref. 39–42.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterization data, and DFT calculations. CCDC 1539450 and 1539451. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc01625e |
| This journal is © The Royal Society of Chemistry 2017 |