Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Siloxane-based linkers in the construction of hydrogen bonded assemblies and porous 3D MOFs†
Luke C.
Delmas
 a,
Peter N.
Horton
b,
Andrew J. P.
White
a,
Peter N.
Horton
b,
Andrew J. P.
White
 a,
Simon J.
Coles
a,
Simon J.
Coles
 b,
Paul D.
Lickiss
b,
Paul D.
Lickiss
 *a and
Robert P.
Davies
*a and
Robert P.
Davies
 *a
*a
aDepartment of Chemistry, Imperial College London, South Kensington, London SW7 2AZ, UK. E-mail: r.davies@imperial.ac.uk; p.lickiss@imperial.ac.uk
bEPSRC Crystallographic Service, Department of Chemistry, University of Southampton, Highfield, Southampton SO17 1BJ, UK
First published on 6th November 2017
Abstract
A siloxane-based hexacarboxylic acid (L1-H6) has been prepared and applied in MOF construction. L1-H6 itself crystallizes as an unusual interpenetrated 3D hydrogen-bonded framework. Reaction of L1-H6 with Zn(II) gave IMP-18 – a 3D MOF incorporating Si–O–Si functionality. Cleavage of L1-H6 gives a silanol-based triacid which is shown to give a coordination polymer (IMP-19) with Zn(II).
The rapid growth in research on metal–organic frameworks (MOFs) has largely relied upon the use of commercially available organic linkers.1,2 A further step towards the rational design of functional MOFs (through fine-tuning of these materials’ topological and chemical properties) requires the synthesis of novel ligands with unique structural and chemical features.3,4 We have been interested for some time in the synthesis of highly branched organosilicon linkers and their use in the assembly of MOFs, largely due to their synthetic accessibility compared to their carbon-centred analogues.5–10 However, despite the siloxane linkage (Si–O–Si) being ubiquitous in many classes of materials including zeolites,11,12 periodic mesoporous organosilicas,13,14 POSS hybrids and other porous materials,15,16 we were surprised to find that there has been little interest in siloxane-based linkers17–21 for MOFs, and that their incorporation into a porous 3D MOF has not been reported to date. We have thus recently focussed upon the development of siloxane-based polycarboxylates, and report herein the synthesis of the novel pseudo-octahedral hexa(4-carboxyphenyl)disiloxane molecule (L1-H6), its unusual hydrogen bonded network assembly and its application in the preparation two new MOF materials.
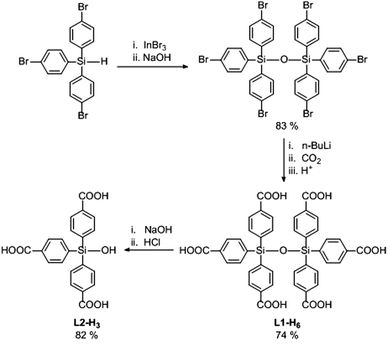
The synthesis of L1-H6 is outlined in Scheme 1. Firstly, tris(4-bromophenyl)silane22 was converted to the corresponding hexa-substituted disiloxane via modification of an In(III)-catalysed protocol for hydrosilane oxidation.23 The sterically protected siloxane linkage withstood subsequent treatment with n-BuLi, allowing reaction of the polylithiated intermediate with CO2 to afford L1-H6.8 Treatment of L1-H6 with base, followed by acid work-up, gave the tris-carboxylic acid silanol derivate L2-H3. The presence of strongly polar Si–OH groups in the pores of MOFs could potentially lead to enhanced non-covalent interactions with gas molecules giving rise to attractive adsorption properties.24,25
In some situations, polycarboxylic acids have been shown to form porous frameworks on crystallisation through hydrogen bonding.26 Moreover, some of these materials have demonstrated potential for gas storage and separation applications.27,28 Given L1-H6 is a rare example of a hexatopic ligand exhibiting an octahedral arrangement of carboxylic acid groups,8,29 we sought to explore the solid-state structure of this compound. Crystallization of L1-H6 from a mixture of acetic acid and methanol afforded crystals suitable for X-Ray analysis (see ESI†). L1-H6 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2) and the unit cell contains three crystallographically distinct molecules of L1-H6. Two of these are linear (with the central oxygen atoms sitting on centres of symmetry) while the third molecule is slightly bent with a Si–O–Si bond angle of 170.76(18)°. In all three cases, the tris(4-carboxyphenyl)silyl groups are staggered with respect to one another (looking down the respective Si–O–Si axes) disposing the carboxylic acid groups in a pseudo-octahedral geometry. The acid groups in L1-H6 aggregate through dimeric hydrogen-bonding interactions into a cubic crystalline network with H-bonding O⋯O distances in the range 2.57–2.69 Å, typical of strong hydrogen bonds.30–32
(No. 2) and the unit cell contains three crystallographically distinct molecules of L1-H6. Two of these are linear (with the central oxygen atoms sitting on centres of symmetry) while the third molecule is slightly bent with a Si–O–Si bond angle of 170.76(18)°. In all three cases, the tris(4-carboxyphenyl)silyl groups are staggered with respect to one another (looking down the respective Si–O–Si axes) disposing the carboxylic acid groups in a pseudo-octahedral geometry. The acid groups in L1-H6 aggregate through dimeric hydrogen-bonding interactions into a cubic crystalline network with H-bonding O⋯O distances in the range 2.57–2.69 Å, typical of strong hydrogen bonds.30–32
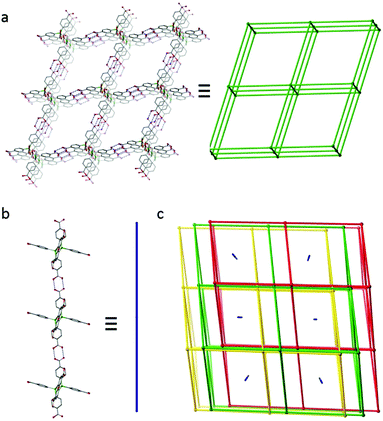
The molecules of L1-H6 assemble via dimeric hydrogen bonding interactions to give a unique structure comprising of triply interpenetrating 3D nets with 1D polymeric chains sited within the remaining pores (Fig. 1). The 3D assemblies can be considered as 6-c pcu nets with the 6-c vertices centred on the siloxane oxygen of the L1-H6 molecules (Fig. 1a). All six octahedrally disposed carboxylic acids on the L1-H6 molecules are involved in hydrogen bonding to neighbouring molecules within these nets. An alternative topological interpretation in which each 6-c vertex is replaced by two 4-c vertices, each centred on a tetrahedral silicon atom, gives the derived 4-c dia net. The calculated accessible void space33 for one of these 3D-structures is approximately 85% of its overall volume, and satisfactory packing is achieved by mutual interpenetration of two additional nets to afford a triply interlocked system (Fig. 1c). In addition, one dimensional channels still remain along the [1 0 1] crystal direction and these are filled by infinite 1D-chains of L1-H6 molecules that hydrogen bond through only two carboxyl groups as shown in Fig. 1b. The remaining four carboxylic acid groups on these molecules do not engage in any inter-molecular interactions. The overall porosity for this structure is consequently low, with a calculated solvent-accessible void volume of 4709 Å3 per unit cell or just 22% of the unit cell volume.33 Efforts to exploit L1-H6 in the preparation of a non-interpenetrated hydrogen-bonded array are ongoing.
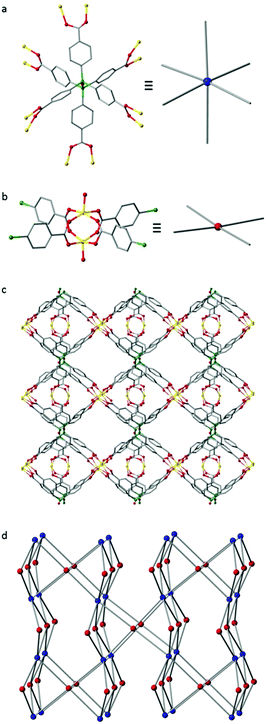
We then turned our attention to the complexation of L1-H6 with transition metals for the generation of MOF materials. Reaction of L1-H6 with Zn(NO3)2·6H2O in DMF at 85 °C in a sealed vial for 2 days afforded colourless needle-like crystals of IMP-18, where IMP is short for Imperial College London. These crystals were characterised by single crystal X-ray analysis to be [Zn3(L1)(H2O)3]·7DMF and the bulk purity of the sample was confirmed by powder X-ray diffraction (Fig. S3, ESI†).34 The crystals were found to comprise of a porous 3D-connected MOF built from discrete bimetallic zinc paddle-wheel nodes linked together by the fully deprotonated ligand L1 (Fig. 2). The siloxane linkages in the L1 ligands are close to linear [∠Si–O–Si 176.0(12) and 167.9(17)°] with staggered aryl groups, resulting in the six carboxylate branches being arranged in a pseudo-octahedral disposition. In order to better understand the framework topology the Zn2-dimer can be reduced to a 4-c node and the L1 ligand reduced to a 6-c node to give a (4,6)-c fsy basic net, rarely encountered in MOFs.35,36 After theoretical removal of both the coordinated and non-coordinated solvent, PLATON33 estimates the solvent-accessible void volume for IMP-18 to be 9006 Å3 or 59% of the unit cell volume. MOFs with hexatopic linkers remain relatively few in number and the majority of those reported feature ligands of hexagonal shape.29,37 An alternative topological description in which the 6-c ligand based vertex is replaced by two 4-c silicon centred vertices gives a new (4,4,4)-connected topology whose point symbol is {4·63·82}4{42·62·82}2{62·84} (see also Fig. S2, ESI†). PXRD measurements on a desolvated IMP-18 sample showed retention of the structure, albeit with slight broadening of the diffraction peaks. Initial sorption analysis studies on IMP-18 show a reversible type-I N2 adsorption isotherm at 77 K (Fig. S5, ESI†) with a calculated BET surface area of 358 m2 g−1.
The siloxane linkage in L1-H6 is susceptible to base cleavage. Indeed treatment of L1-H6 with an excess of 1 M NaOH affords, after protonation, tris(4-carboxyphenyl)silanol (L2-H3). L2-H3 is a novel and interesting linker since, in addition to the coordinating abilities of the carboxylate groups, the Si–OH group is a good hydrogen bond donor38 and a potential site for further functionalization of the ligand (or post-synthetic modification of derived MOFs). Silanol-based linkers are not common in the MOF literature but Sun and co-workers have reported two 3D frameworks based on biphenyl-substituted silanols.39,40
The reaction of tricarboxylic acid L2-H3 with Zn(NO3)2·6H2O in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
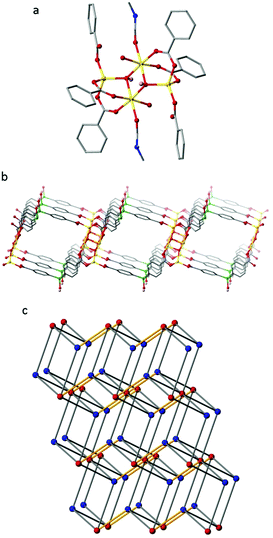
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DMF and H2O at 80 °C for 2 days afforded colourless blade-like crystals. These crystals were characterised by single crystal X-ray analysis to be [Zn2(L2)(OH)(H2O)(DMF)]·2H2O·3DMF (IMP-19) and the bulk purity of the sample was confirmed by powder X-ray diffraction (Fig. S7, ESI†). The crystals were found to comprise 2D polymeric sheets built from tetrametallic nodes (comprising four Zn atoms held together by two μ3-bridging hydroxides) linked together by the triply deprotonated L2 (Fig. 3) to give a (3,6)-connected net of kgd topology.
1 mixture of DMF and H2O at 80 °C for 2 days afforded colourless blade-like crystals. These crystals were characterised by single crystal X-ray analysis to be [Zn2(L2)(OH)(H2O)(DMF)]·2H2O·3DMF (IMP-19) and the bulk purity of the sample was confirmed by powder X-ray diffraction (Fig. S7, ESI†). The crystals were found to comprise 2D polymeric sheets built from tetrametallic nodes (comprising four Zn atoms held together by two μ3-bridging hydroxides) linked together by the triply deprotonated L2 (Fig. 3) to give a (3,6)-connected net of kgd topology.
These 2D sheets can be considered to further assemble into a 3D networked structure through hydrogen bonding between the peripheral silanol group of an adjacent layer (H-bond donor) and the non-coordinated oxygen atom of the L2 monodentate carboxylate group with a typical D–H⋯A distance of 1.88 Å.41 Considering these hydrogens bonds as topological connections, the overall supramolecular structure of IMP-19 is determined by TOPOS42 to be a 4,8-connected net of flu topology. The IMP-19 framework has solvent-filled channels with the largest window size being ca. 12 × 12 Å2. After theoretical removal of both the coordinated and non-coordinated solvent, the PLATON33 estimated solvent-accessible void volume for IMP-19 is 2070 Å3 or 58% of the unit cell volume. However, in practice evacuation of the material led to its decomposition and a complete loss of porosity.
In summary, the novel siloxane-based hexacarboxylic acid L1-H6 has been prepared and shown to self-assemble into a 3D triply interpenetrated hydrogen-bonded organic framework with guest 1D H-bonded chain polymers in its pores. Treatment of L1-H6 with Zn(II) gives IMP-18, which in our knowledge is the first 3D-connected MOF structure which incorporates a disiloxane-based linker. We believe these initial results will lead to the use of other siloxane scaffolds in the assembly of porous MOFs, including the use of cyclic and cage-like polysiloxane linkers. We are also interested in further investigating the use of silanol-based linkers in the preparation of porous MOFs.
The Imperial College President's PhD Scholarship Scheme (LD) and the EPSRC (EP/M507878/1) are acknowledged for funding this work. We are grateful to A. R. Kucernak for assistance with BET measurements. Special thanks to Amanda Zammit and Yu Hin Lai for assistance with some of the experimental work.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- F. A. Almeida Paz, J. Klinowski, S. M. F. Vilela, J. P. C. Tome, J. A. S. Cavaleiro and J. Rocha, Chem. Soc. Rev., 2012, 41, 1088–1110 RSC.
- R. P. Davies, R. J. Less, P. D. Lickiss, K. Robertson and A. J. P. White, Inorg. Chem., 2008, 47, 9958–9964 CrossRef CAS PubMed.
- R. P. Davies, R. Less, P. D. Lickiss, K. Robertson and A. J. P. White, Cryst. Growth Des., 2010, 10, 4571–4581 CAS.
- R. P. Davies, P. D. Lickiss, K. Robertson and A. J. P. White, Aust. J. Chem., 2011, 64, 1239–1246 CrossRef.
- R. P. Davies, P. D. Lickiss, K. Robertson and A. J. P. White, CrystEngComm, 2012, 14, 758–760 RSC.
- I. Timokhin, J. Baguna Torres, A. J. P. White, P. D. Lickiss, C. Pettinari and R. P. Davies, Dalton Trans., 2013, 42, 13806–13808 RSC.
- I. Timokhin, A. J. P. White, P. D. Lickiss, C. Pettinari and R. P. Davies, CrystEngComm, 2014, 16, 8094–8097 RSC.
- B. Sels and L. Kustov, Zeolites and Zeolite-like Materials, Elsevier, 2016 Search PubMed.
- P. Eliasova, M. Opanasenko, P. S. Wheatley, M. Shamzhy, M. Mazur, P. Nachtigall, W. J. Roth, R. E. Morris and J. Cejka, Chem. Soc. Rev., 2015, 44, 7177–7206 RSC.
- P. Van Der Voort, D. Esquivel, E. De Canck, F. Goethals, I. Van Driessche and F. J. Romero-Salguero, Chem. Soc. Rev., 2013, 42, 3913–3955 RSC.
- J. G. Croissant, X. Cattoen, M. Wong Chi Man, J.-O. Durand and N. M. Khashab, Nanoscale, 2015, 7, 20318–20334 RSC.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- Q. Ye, H. Zhou and J. Xu, Chem. – Asian J., 2016, 11, 1322–1337 CrossRef CAS PubMed.
- M. S. Deshmukh, T. Vijayakanth and R. Boomishankar, Inorg. Chem., 2016, 55, 3098–3104 CrossRef CAS PubMed.
- A. Vlad, M. Cazacu, M.-F. Zaltariov, A. Bargan, S. Shova and C. Turta, J. Mol. Struct., 2014, 1060, 94–101 CrossRef CAS.
- A. Vlad, M.-F. Zaltariov, S. Shova, G. Novitchi, C.-D. Varganici, C. Train and M. Cazacu, CrystEngComm, 2013, 15, 5368–5375 RSC.
- C. Racles, S. Shova, M. Cazacu and D. Timpu, Polymer, 2013, 54, 6096–6104 CrossRef CAS.
- D. M. L. Goodgame, P. D. Lickiss, S. J. Rooke, A. J. P. White and D. J. Williams, Inorg. Chim. Acta, 2003, 343, 61–73 CrossRef CAS.
- M. Wander, P. J. C. Hausoul, L. A. J. M. Sliedregt, B. J. van Steen, G. van Koten and R. J. M. Klein Gebbink, Organometallics, 2009, 28, 4406–4415 CrossRef CAS.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, M. Kumara Swamy, B. Mahesh and M. K. Kumar Reddy, Tetrahedron Lett., 2009, 50, 7166–7168 CrossRef CAS.
- S. Wang, L. Tan, C. Zhang, I. Hussain and B. Tan, J. Mater. Chem. A, 2015, 3, 6542–6548 CAS.
- R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173–1177 RSC.
- Y.-F. Han, Y.-X. Yuan and H.-B. Wang, Molecules, 2017, 22, 266–300 CrossRef PubMed.
- J. Lü and R. Cao, Angew. Chem., Int. Ed., 2016, 55, 9474–9480 CrossRef PubMed.
- F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101–2104 CrossRef CAS PubMed.
- M. Li, D. Li, M. O’Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- D. J. Duchamp and R. E. Marsh, Acta Crystallogr., Sect. B: Struct. Sci., 1969, 25, 5–19 CrossRef CAS.
- M. Bailey and C. J. Brown, Acta Crystallogr., 1967, 22, 387–391 CrossRef CAS.
- R. Alcala and S. Martinez-Carrera, Acta Crystallogr., Sect. B: Struct. Sci., 1972, 28, 1671–1677 CrossRef CAS.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC.
- S. Zhang, W. Shi, L. Li, E. Duan and P. Cheng, Inorg. Chem., 2014, 53, 10340–10346 CrossRef CAS PubMed.
- Y. He, Z. Zhang, S. Xiang, H. Wu, F. R. Fronczek, W. Zhou, R. Krishna, M. O'Keeffe and B. Chen, Chem. – Eur. J., 2012, 18, 1901–1904 CrossRef CAS PubMed.
- S. Kaskel, The chemistry of metal–organic frameworks: synthesis, characterization, and applications, John Wiley & Sons, 2016 Search PubMed.
- P. D. Lickiss, Adv. Inorg. Chem., 1995, 42, 147–262 CrossRef CAS.
- X. Zhao, D. Sun, S. Yuan, S. Feng, R. Cao, D. Yuan, S. Wang, J. Dou and D. Sun, Inorg. Chem., 2012, 51, 10350–10355 CrossRef CAS PubMed.
- H. Ma, D. Sun, L. Zhang, R. Wang, V. A. Blatov, J. Guo and D. Sun, Inorg. Chem., 2013, 52, 10732–10734 CrossRef CAS PubMed.
- J. O. Bauer and C. Strohmann, J. Organomet. Chem., 2015, 797, 52–56 CrossRef CAS.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details of the synthesis, characterisation data and the crystallographic protocols employed in this study. PXRD and TGA plots for IMP-18 and IMP-19 and N2 sorption isotherm for IMP-18. Details of additional topological analyses for structures are also presented. CCDC 1566035–1566037. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc06022j |
| This journal is © The Royal Society of Chemistry 2017 |