Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iron catalyzed methylation and ethylation of vinyl arenes†
Nengbo
Zhu
,
Jianguo
Zhao
and
Hongli
Bao
*
State Key Laboratory of Structural Chemistry, Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, University of Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, 350002, P. R. China. E-mail: hlbao@fjirsm.ac.cn
First published on 2nd December 2016
Abstract
Short alkyl chain Heck (type) reactions, especially methyl Heck reactions, are a difficult aspect of the alkyl Heck reaction. To provide a solution to this problem, iron-catalyzed methyl, ethyl and propyl Heck reactions were developed using readily available alkyl peroxides as alkyl sources. The reaction conditions were mild, clean, and easy to handle. No additive was needed, and no hazardous waste was generated. The products were obtained in up to 99% yield of one isomer for most situations. This reaction works for many types of olefin and tolerates a variety of functional groups. Several late-stage functionalizations of natural products and drug molecules were conducted to demonstrate the synthetic applications of this reaction.
The methyl group, which is so often considered to be chemically inert, is able to significantly alter the pharmacological properties of a molecule.1 The methyl group affects micromolecules and biomacromolecules through stereoelectronic effects to increase their potency against enzyme metabolism or changes in the physiochemical properties of bioactive compounds.2 The importance of the methyl group on arenes or heteroarenes is widely recognized, whereas the importance of the methyl group in alkyl or vinyl chains is less well understood. In fact, methyl groups in the alkyl chain or vinyl chain can profoundly affect a bioactive compound's properties.2,3
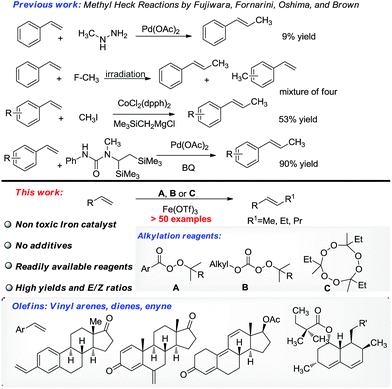
The Heck reaction is a powerful tool in chemical synthesis.4–12 Aryl Heck reactions are well established whereas alkyl Heck and alkyl Heck type reactions (for brevity, all Heck reactions including Heck-type reactions are referred to as Heck reactions), especially methyl Heck reactions are still challenging.13–23 Methyl hydrazine,24 methyl halides,13,14,25 and silanes26 have been applied to solve this problem (Scheme 1). Unfortunately, the methyl Heck reaction remains challenging and there is no practical solution to this problem using readily available methylation reagents.
Perester (A) and OO-tert-butyl (or pentyl) O-alkyl monopercarbonate (B) are inexpensive chemical feedstocks commonly used in industry as oxidants or radical initiators. These peroxides are rarely seen as alkylation reagents.27–33 Li and co-workers29 developed a procedure for the methylation of aryl C–H bonds using dicumyl peroxide as the methylation reagent, and DiRocco and co-workers developed a procedure for the late-stage methylation of heterocycles with peresters as methyl sources.31 Both examples mentioned above worked on the methylation of arenes or heterocycles,34,35 rather than double bonds.
Many peresters and OO-tert-butyl (or pentyl) O-alkyl monopercarbonates (B) are commercially available. It would be synthetically useful if a methyl Heck reaction based on these non-ordinary methylation reagents was developed. Iron is one of the most important metals in nature.36–41 Many iron compounds are observed to be essential components of biological systems and have low toxicity in living systems. The application of nontoxic iron catalysts in pharmaceutical related chemistry is highly desired as it could reduce the risk of contamination by metal catalysts. Here, we report our work on the methylation of double bonds with an iron catalyst. This iron catalyzed unprecedented short alkyl chain Heck reaction of olefins applies readily available peroxides (A, B, C) as the alkylation reagents (Scheme 1). Type B peroxides are shown to be valuable alkyl electrophiles for the first time.
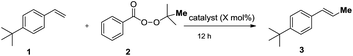
We commenced our study by screening metal catalysts for the reaction of p-tert-butyl-styrene 1 with commercially available tert-butyl peroxybenzoate (2) as the methylation reagent. Copper, nickel and palladium catalysts did not deliver the methylation product in an effective way. Styrene 1 and benzoic acid were the two major peaks observed using GC-MS (Table 1, entries 1–4). To our delight, Fe(OTf)3 (10 mol%) promoted the reaction and afforded the product in 85% yield (entry 8). Study of the reaction temperature revealed that 80 °C was optimal (entries 8–12). Solvent screening showed that THF and dioxane were both suitable for this reaction (entry 16). When reducing the usage of the catalyst from 10 mol% to 5 mol% in dioxane, the yield increased slightly (entry 17, 90% yield). The yield dropped to 36% when one equivalent of the catalyst was employed (entry 18). Fe(OTf)2 was examined under the optimal reaction conditions and 65% of the isolated product was obtained (entry 19). The product was obtained in 34% yield in the presence of HOTf (entry 20). There was no product generated without Fe(OTf)3 or under photoredox catalysis conditions (entries 21–22). The reaction conditions were mild, clean and easy to handle, used a nontoxic catalyst and required no additives. This method is potentially useful due to its low cost and environmental advantages.
| Entry | Metal (mol%) | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reactions were conducted with styrene (0.5 mmol), 2 (1.0 mmol), catalyst and solvent (2 mL) at 80 °C for 12 h. b Yield of product detected using GC. c Yield of isolated product. d 5 mol% of RuCl3(bpy)3 6H2O as photocatalyst. | ||||
| 1 | Cu(OTf)2/10 | THF | 80 | Trace |
| 2 | CuBr/10 | THF | 80 | Trace |
| 3 | Pd(TFA)2/10 | THF | 80 | Trace |
| 4 | NiCl2/10 | THF | 80 | Trace |
| 5 | FeSO4·7H2O (10) | THF | 80 | Trace |
| 6 | Fe(OAc)2 (10) | THF | 80 | Trace |
| 7 | Fe(acac)3 (10) | THF | 80 | Trace |
| 8 | Fe(OTf)3 (10) | THF | 80 | 85 |
| 9 | Fe(OTf)3 (10) | THF | rt | 46 |
| 10 | Fe(OTf)3 (10) | THF | 40 | 56 |
| 11 | Fe(OTf)3 (10) | THF | 60 | 69 |
| 12 | Fe(OTf)3 (10) | PhCH3 | 80 | 6 |
| 13 | Fe(OTf)3 (10) | CH3CN | 80 | — |
| 14 | Fe(OTf)3 (10) | DMF | 80 | 21 |
| 15 | Fe(OTf)3 (10) | DME | 80 | 70 |
| 16 | Fe(OTf)3 (10) | Dioxane | 80 | 80 |
| 17 | Fe(OTf)3 (5) | Dioxane | 80 | 90 (85)c |
| 18 | Fe(OTf)3 (100) | Dioxane | 80 | 36 |
| 19 | Fe(OTf)2(5) | Dioxane | 80 | 65c |
| 20 | HOTf (15) | Dioxane | 80 | 34 |
| 21 | — | Dioxane | 80 | Trace |
| 22d | White LED light | Dioxane | rt | Trace |
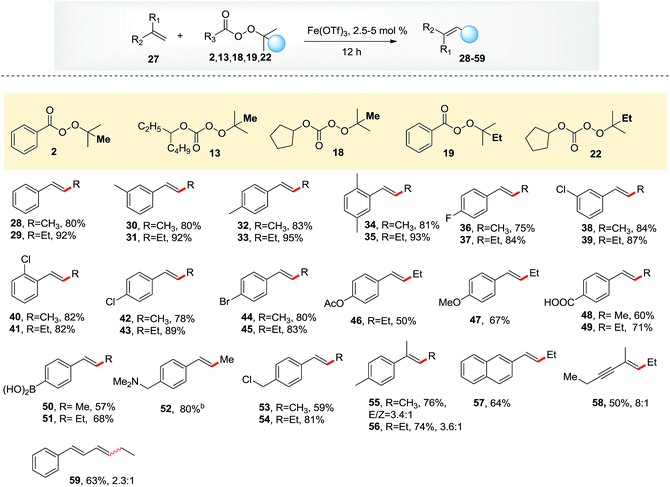
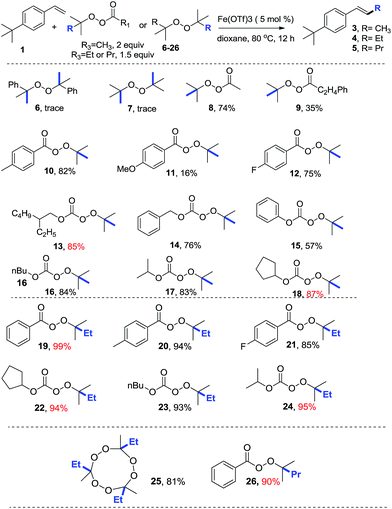
Having identified the optimal conditions, we investigated the reactivity of thirteen methylation reagents (Table 2, 6–18). The methylation product was obtained in up to an 87% yield. Effective methylation reagent 13 is commercially available, and reagents 10–12 and 14–18 were easily made from t-butyl hydroperoxide. Encouraged by these results, six ethylation reagents (19–24) and one propylation reagent (26) were synthesized and examined. Up to a 99% yield for ethylation and a 90% yield for propylation were obtained. Interestingly, an 81% yield of an ethylation product was achieved with commercially available reagent 26 (TMTETPN). It is worth noting that trans olefin was the only isomer observed. We found that peresters (A) are more reactive, while monopercarbonates (B) are milder and thus important for sensitive substrates (Table 3).
| a Yield of isolated product. |
|---|
|
Having established the reactivity of these three types of peroxides in the presence of iron, we investigated the scope of the olefins, which proved to be broad. Alkyl substituents on the phenyl ring increased the yield to 80–95%, whereas halogen substituents decreased the yield to 70–88%. Functional groups such as acetate, methoxyl, free carboxylic acid, boronic acid and amine were all tolerated. 2-Vinylnaphthalene afforded products in reasonable yields. Importantly, dienes and enynes were suitable substrates for this reaction, delivering the corresponding products in moderate yields.
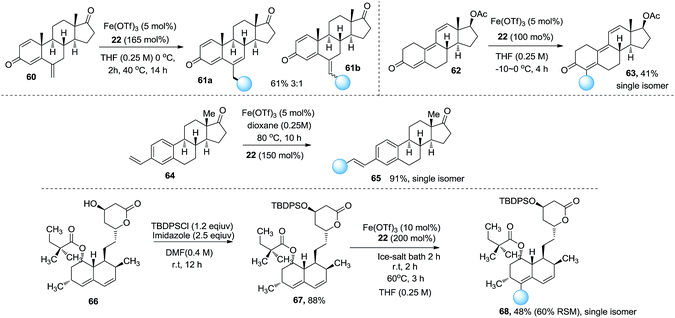
To exemplify the synthetic application of this method we examined this reaction with regard to natural products and drug molecules. The late-stage modification of exemestane 60, an aromatase inhibitor used in the treatment of breast cancer, delivered the double bond shift product 61a and the normal product 61b in 61% yield (Scheme 2). The functionalization of trenbolone acetate 62 afforded derivative 63 in a 41% yield. Remarkably, vinylestrone 64 delivered the ethyl Heck product 65 in a 91% yield. Simvastatin derivative 67 was examined in this reaction. The site selectivity was good and the ethylation product 68 was obtained in 48% yield. These examples demonstrate that this reaction is a useful method to functionalize complex molecules in the late stages.
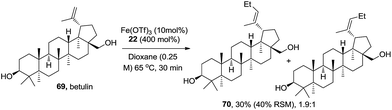
To learn more about the reactivity information of this reaction with non-conjugated olefins, natural product betulin 69 was examined (Scheme 3). The reaction did not reach full conversion and it delivered ethyl Heck product 70 as a mixture of E/Z isomers (E/Z = 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 30% yield (25% of the starting material was recovered). Other simple olefins suffer with the same conversion and E/Z selectivity problems. This reaction is less ideal for non-conjugated olefins.
1) in 30% yield (25% of the starting material was recovered). Other simple olefins suffer with the same conversion and E/Z selectivity problems. This reaction is less ideal for non-conjugated olefins.
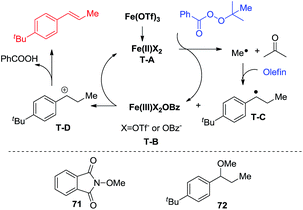
Inspired by Fenton's reaction42 and the work of Bedford, Nagashima, Nakamura, and Neidig,43–46 a single electron transfer catalytic cycle involving iron complexes, that differs only in one oxidation state, is proposed. Fe(OTf)3 is reduced to iron(II). Iron(II) complex T-A transfers an electron to the perester to form an iron(III) compound (T-B) and a methyl radical. The methyl radical reacts with styrenes to form a benzylic radical (T-C). The benzylic radical (T-C) is oxidized by the iron(III) species (T-B) to a carbocation (T-D) and reforms the iron(II) species (T-A). The carbocation (T-D) then undergoes deprotonation to deliver the desired product. To support this proposed mechanism a radical trapping experiment was conducted using NHPI (N-hydroxyphthalimide). The reaction was stopped and no desired product was observed, while radical adduct 71 was observed using GC-MS. Thereafter, MeOH was added to the reaction to see whether it would capture the carbon cation. Fortunately, methyl ether 72 was isolated from this reaction in a 30% yield (Scheme 4).
Conclusions
In summary, we have developed a practical method for the short chain alkylation of olefins using readily available peresters and monopercarbonates as alkylation reagents. Many of these reagents are commercially available and the others are easy to synthesize. The simple reaction conditions and environmental benefits of this catalytic system open up the possibility of further synthetic applications. This reaction is efficient and selective, and works for vinyl arenes, dienes, enynes, and complicated natural products or drug molecules.Acknowledgements
We thank NSFC (grant no. 21402200 and 21672213), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000) and the 100 Talents Program, “The 1000 Youth Talents Program” for financial support. We also thank Professor Weiping Su, Zhongning Chen and Daqiang Yuan from our institute for suggestions and help.Notes and references
- P. Bazzini and C. G. Wermuth, in The Practice of Medicinal Chemistry, Academic Press, San Diego, 2008, p. 431 Search PubMed.
- E. J. Barreiro, A. E. Kummerle and C. A. Fraga, Chem. Rev., 2011, 111, 5215 CrossRef CAS PubMed.
- C. S. Leung, S. S. Leung, J. Tirado-Rives and W. L. Jorgensen, J. Med. Chem., 2012, 55, 4489 CrossRef CAS PubMed.
- R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518 CrossRef CAS.
- T. Mizoroki, K. Mori and A. Ozaki, Bull. Chem. Soc. Jpn., 1971, 44, 581 CrossRef CAS.
- R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS.
- M. Oestreich, The Mizoroki-Heck reaction, Wiley-VCH, Hoboken, NJ, 2008 Search PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
- A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS PubMed.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed.
- C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef CAS.
- X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047 CrossRef CAS PubMed.
- Y. Ikeda, T. Nakamura, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2002, 124, 6514 CrossRef CAS PubMed.
- W. Affo, H. Ohmiya, T. Fujioka, Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, Y. Imamura, T. Mizuta and K. Miyoshi, J. Am. Chem. Soc., 2006, 128, 8068 CrossRef CAS PubMed.
- C. Liu, S. Tang, D. Liu, J. Yuan, L. Zheng, L. Meng and A. Lei, Angew. Chem., Int. Ed., 2012, 51, 3638 CrossRef CAS PubMed.
- T. Nishikata, Y. Noda, R. Fujimoto and T. Sakashita, J. Am. Chem. Soc., 2013, 135, 16372 CrossRef CAS PubMed.
- C. M. McMahon and E. J. Alexanian, Angew. Chem., Int. Ed., 2014, 53, 5974 CrossRef CAS PubMed.
- Y. Zou and J. S. Zhou, Chem. Commun., 2014, 50, 3725 RSC.
- S. Y. Fu, Y. Z. Li, W. Chu, C. Li and D. G. Tong, Catal. Sci. Technol., 2015, 5, 1638 CAS.
- L. Firmansjah and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 11340 CrossRef CAS PubMed.
- M. E. Weiss, L. M. Kreis, A. Lauber and E. M. Carreira, Angew. Chem., Int. Ed., 2011, 50, 11125 CrossRef CAS PubMed.
- K. S. Bloome, R. L. McMahen and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 20146 CrossRef CAS PubMed.
- A. Millan, L. Alvarez de Cienfuegos, D. Miguel, A. G. Campana and J. M. Cuerva, Org. Lett., 2012, 14, 5984 CrossRef CAS PubMed.
- F. Akiyama, H. Miyazaki, K. Kaneda, S. Teranishi, Y. Fujiwara, M. Abe and H. Taniguchi, J. Org. Chem., 1980, 45, 2359 CrossRef CAS.
- M. E. Crestoni and S. Fornarini, J. Am. Chem. Soc., 1989, 111, 6008 CrossRef CAS.
- W. Rauf and J. M. Brown, Angew. Chem., Int. Ed., 2008, 47, 4228 CrossRef CAS PubMed.
- L. F. Fieser and A. E. Oxford, J. Am. Chem. Soc., 1942, 64, 2060 CrossRef CAS.
- A. Citterio, A. Arnoldi and F. Minisci, J. Org. Chem., 1979, 44, 2674 CrossRef CAS.
- Y. Zhang, J. Feng and C.-J. Li, J. Am. Chem. Soc., 2008, 130, 2900 CrossRef CAS PubMed.
- M. Rueda-Becerril, C. Chatalova Sazepin, J. C. T. Leung, T. Okbinoglu, P. Kennepohl, J.-F. Paquin and G. M. Sammis, J. Am. Chem. Soc., 2012, 134, 4026 CrossRef CAS PubMed.
- D. A. Dirocco, K. Dykstra, S. Krska, P. Vachal, D. V. Conway and M. Tudge, Angew. Chem., Int. Ed., 2014, 53, 4802 CrossRef CAS PubMed.
- C. Pan, H. Zhang, J. Han, Y. Cheng and C. Zhu, Chem. Commun., 2015, 51, 3786 RSC.
- Y. Li, Y. Han, H. Xiong, N. Zhu, B. Qian, C. Ye, E. A. Kantchev and H. Bao, Org. Lett., 2016, 18, 392 CrossRef CAS PubMed.
- Liao and co-workers recently developed nickel catalyzed methylation of aryl halides with deuterated methyl iodide, see: L. Hu, X. Liu and X. Liao, Angew. Chem., Int. Ed., 2016, 128, 9895 CrossRef.
- Nakamura and co-workers recently disclosed their work of iron catalyzed ortho C–H methylation of carbonyl arenes with methylaluminum, see: R. Shang, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2016, 138, 10132 CrossRef CAS PubMed.
- C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed , and references there.
- I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170 CrossRef CAS PubMed , and references there.
- S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317 CrossRef CAS PubMed.
- Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed.
- C. Bolm, Nat. Chem., 2009, 1, 420 CrossRef CAS PubMed.
- C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed.
- E. Brillas, I. Sires and M. A. Oturan, Chem. Rev., 2009, 109, 6570 CrossRef CAS PubMed.
- R. B. Bedford, M. Betham, D. W. Bruce, A. A. Danopoulos, R. M. Frost and M. Hird, J. Org. Chem., 2006, 71, 1104–1110 CrossRef CAS PubMed.
- D. Noda, Y. Sunada, T. Hatakeyama, M. Nakamura and H. Nagashima, J. Am. Chem. Soc., 2009, 131, 6078–6079 CrossRef CAS PubMed.
- S. Kawamura, T. Kawabata, K. Ishizuka and M. Nakamura, Chem. Commun., 2012, 48, 9376–9378 RSC.
- S. L. Daifuku, M. H. Al-Afyouni, B. E. Snyder, J. L. Kneebone and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 9132–9143 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc04274k |
| This journal is © The Royal Society of Chemistry 2017 |