Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly selective olefin-assisted palladium-catalyzed oxidative carbocyclization via remote olefin insertion†
Youai
Qiu
,
Bin
Yang
,
Can
Zhu
and
Jan-E.
Bäckvall
*
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91, Stockholm, Sweden. E-mail: jeb@organ.su.se
First published on 2nd September 2016
Abstract
A highly selective olefin-assisted palladium-catalyzed oxidative carbocyclization via remote olefin insertion to afford cyclohexenes has been developed. It was shown that the assisting olefin moiety was indispensable for the formation of the cyclohexene product. Furthermore, preliminary studies on chiral anion-induced asymmetrical carbocyclization–borylation of enallenes have been carried out.
The development of modern methodologies for the efficient synthesis of carbocycles is of central importance in modern organic chemistry,1 given the fact that carbocycles are basic units in pharmacologically active skeletons, as well as in natural products.2 Among these methodologies, transition metal-catalyzed carbocyclizations with the involvement of π-bonds have emerged as an effective strategy for the preparation of carbocycles,3,4 considering that the π-bond moiety would not only perform as the assisting group for the formation of the carbon–metal (C–M) bond, but also as the building block for the subsequent carbocyclization. In this way, an atom-economical transformation can be achieved.
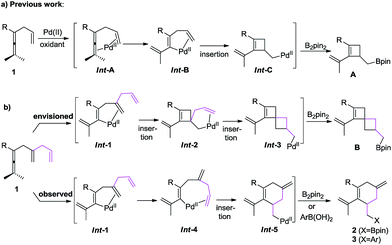
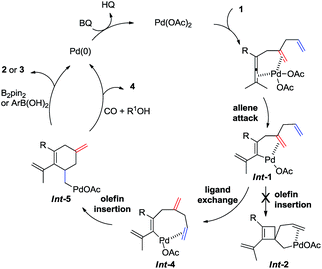
We recently reported on an olefin-directed palladium-catalyzed oxidative carbocyclization–borylation of allenes to cyclobutenes (Scheme 1a).5 In this reaction, the coordination of the olefin in Int-A triggers the allene attack on palladium,6 which results in the formation of Int-B. Subsequent olefin insertion to form a cyclobutene intermediate Int-C, followed by transmetallation and reductive elimination afforded the borylated cyclobutene derivatives A.
On the basis of these observations, we were particularly interested in the involvement of an additional double bond in a carbocyclization (Scheme 1b). We envisioned that the olefin insertion of intermediate Int-1 could lead to intermediate Int-2, and that coordination of the additional olefin to palladium would lead to a second carbocyclization to form spirocyclic intermediate Int-3, which on reaction with B2pin2 would give B. Alternatively, Int-1 may undergo ligand exchange and olefin insertion to give Int-5, which can be quenched by either B2pin2 or ArB(OH)2 to give either 2 or 3, respectively (Scheme 1b).
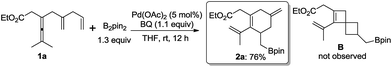
Based on this concept, we initially chose a readily accessible 3,4-dienoate 1a as the standard substrate. When 1a bearing an extra olefin was treated with Pd(OAc)2 (5 mol%), B2pin2 (1.3 equiv.), and BQ (p-benzoquinone) (1.1 equiv.) in THF at room temperature for 12 h, the envisioned spirocyclic product B was not observed (Scheme 2). Interestingly, the cyclohexene product 2a was obtained in 76% yield instead. It is obvious that intermediate Int-2 was not formed from intermediate Int-1via olefin insertion as we had envisioned, but rather the ligand exchange, from proximal olefin to remote olefin occurred in Int-1 to produce Int-4. Subsequent olefin insertion7 to give cyclic intermediate Int-5 followed by B2pin2 quenching would produce 2a (Scheme 1b). During this transformation, the proximal olefin is acting as the assisting group for the generation of palladium intermediate Int-1, while the remote olefin participates in the carbocyclization (Scheme 1c). To the best of our knowledge,8–10 the formation of six-membered rings in palladium-catalyzed oxidative carbocyclization of enallenes via olefin exchange has been rarely reported.11
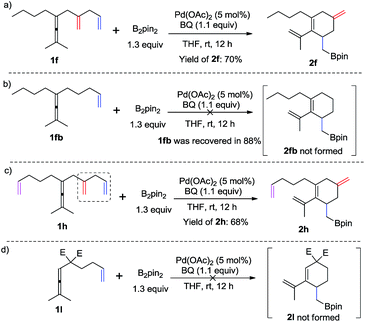
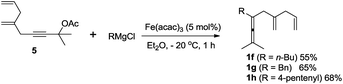
To demonstrate the necessity of the assisting olefin group, we first investigated comparative experiments with enallenes lacking the additional olefin (Scheme 3). When substrate 1f with an assisting olefin was allowed to react under the same reaction conditions as those in Scheme 2, the cyclohexene product 2f was formed in 70% yield (Scheme 3a). However, when substrate 1fb, lacking the additional double bond, was subjected to the reaction conditions of Scheme 2 the corresponding six-membered ring product 2fb was not formed; instead 1fb was recovered in 88% (Scheme 3b). Importantly, we also observed the exclusive formation of 2h in 68% yield from substrate 1h (Scheme 3c). We also examined the reaction of a malonate-tethered substrate 1l, but the envisioned product 2l was not observed (Scheme 3d). These comparative experiments indicate that the assisting olefin of the substrate is an indispensable group for the formation of the palladium intermediate Int-4.
With these inspiring results in hand, we began to optimize the reaction conditions (for details, see the ESI, Table S1†). Solvent screening showed that 1,2-dichloroethane was the best solvent, in which the yield of 2a was 91% (Table S1,† entry 7). Other solvents such as THF, MeCN, and 1,4-dioxane also gave good yields (Table S1,† entries 1, 5, and 6). When the amount of BQ was increased to 1.5 equivalents, the yield of 2a was decreased to 78% (Table S1,† entry 8). The use of 2,6-dimethyl-BQ instead of BQ, gave a lower yield (Table S1,† entry 9). Catalyst screening showed that Pd(TFA)2 (TFA = trifluoroacetate) produced the corresponding cyclohexene derivative in only 36% yield together with 34% starting material recovered (Table S1,† entry 10).
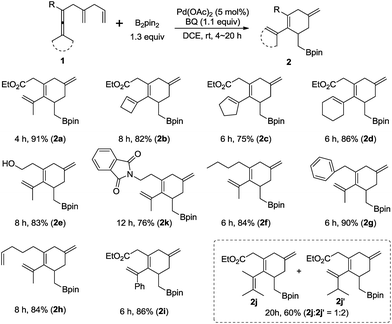
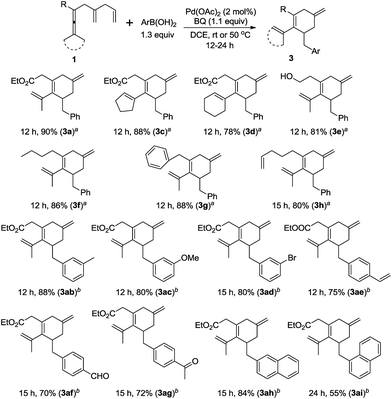
The substrate scope for the formation of cyclohexene boron compounds 212,13 was then studied under the optimized reaction conditions (Scheme 4): in addition to methyl substituents on the enallene moiety, cyclobutylidene, cyclopentylidene, and cyclohexylidene enallenes (1b, 1c, and 1d) also gave the corresponding products 2b, 2c, and 2d in good yields. To our delight, enallenes with functional groups, such as free hydroxyl in 1e and imide in lk, furnished cyclohexene derivatives 2e and 2k in 83% and 76% yield. Furthermore, the reaction tolerates R to be different alkyl groups in this reaction, e.g. n-butyl (1f), or benzyl (1g).14 It is worth noting that the product 2h was exclusively obtained in 84% yield. Finally, the reaction of a dissymmetric allene 1i, bearing Me and phenyl, or 1j, bearing Me and i-Pr, afforded 2i in 86% yield, and 2j and 2j′ in 60% yield, respectively. The ratio of 2j and 2j′ was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 due to the selective C–H bond cleavage, which occurred during allene attack forming Int-1 (see Scheme 1b). Notably, the reaction could be easily extended to a scale of 4.5 mmol of 1a (1.053 g) to afford the corresponding cyclohexene compound 2a (1.551 g, 90% yield).
2 due to the selective C–H bond cleavage, which occurred during allene attack forming Int-1 (see Scheme 1b). Notably, the reaction could be easily extended to a scale of 4.5 mmol of 1a (1.053 g) to afford the corresponding cyclohexene compound 2a (1.551 g, 90% yield).
After realization of the borylative carbocyclization, we next turned our attention to the arylating carbocyclization of enallenes. We were pleased to find that the arylative products15 could be obtained in good yields with a catalyst loading of 2 mol% Pd(OAc)2 (Scheme 5). The reaction of substrates with two methyls, cyclopentylidene, and cyclohexylidene, afforded the corresponding product 3a, 3c, and 3d in good yields. Interestingly, the substrate containing a free hydroxyl group could also be employed. Different alkyl substituents on the starting materials, such as n-butyl, benzyl, and 4-pentenyl groups were tolerated (3f–h). We also examined the scope of arylboronic acids, and electron-donating substituents such as 3-Me, and 3-MeO reacted smoothly under the standard conditions in good yields. Notably, the procedure tolerates a range of additional functional groups on the arylboronic acid, including bromo (3ad), vinyl (3ae), formyl (3af), and acetyl (3ag) groups, which is useful for further functionalization. Finally, it is worth noting that 2-naphthylboronic acid and 1-naphthylboronic acid also worked well, affording 3ah and 3ai in 84% and 55% yield, respectively.
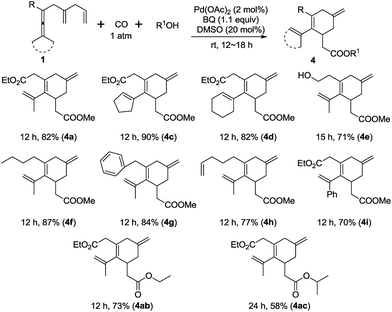
Interestingly, this new olefin-assisting strategy could also be applied to an oxidative carbonylating carbocyclization9c,f,g for the preparation of cyclohexene esters (Scheme 6).16 When the substrate 1a was treated with Pd(OAc)2 (2 mol%), and BQ (1.1 equiv.) under carbon monoxide (1 atm) in methanol at room temperature for 12 h the carbonylation product 4a was formed in 82% yield (Scheme 6). Under the optimal reaction conditions, cyclopentylidene and cyclohexylidene substrates 1c and 1d afforded 4c and 4d, respectively in good yields. The substrate bearing the free hydroxyl group (1e) also worked well. Different allenes with alkyl substituents such as n-butyl, benzyl, and 4-pentenyl group, were also tested and worked well in this reaction. The reaction of a dissymmetric allene 1i, bearing Me and phenyl, afforded 4i in 70% yield. Finally, the scope of the alcohol partners in the carbocyclization carbonylation reaction was explored, and in addition to MeOH, ethanol and isopropanol were shown to react smoothly to provide the desired esters in good yield.
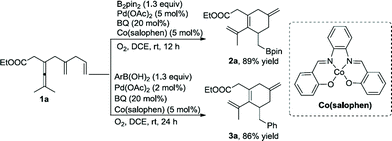
The biomimetic approach with the use of electron-transfer mediators (ETMs) is known to decrease the kinetic barrier for the reoxidation.17 In this aerobic approach the high kinetic barrier will be divided into several smaller units, and catalytic amounts of oxidant (BQ) would be enough to realize these transformations. When the reaction of 1a was treated with B2pin2 (1.3 equiv.), BQ (20 mol%), Pd(OAc)2 (5 mol%), and cobalt(salophen) (5 mol%) in the presence of O2 (1 atm), borylated product 2a was obtained in 89% yield. Phenylated product 3a was provided in 86% yield when PhB(OH)2 was used in place of B2pin2 (Scheme 7).
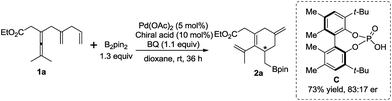
Preliminary attempt to develop an enantioselective carbocyclization–borylation of enallenes revealed that a reasonably good er value (83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17) was observed in the presence of catalytic amounts of Pd(OAc)2 and biphenol-type phosphoric acid C9d while poor enantiocontrol (55
17) was observed in the presence of catalytic amounts of Pd(OAc)2 and biphenol-type phosphoric acid C9d while poor enantiocontrol (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 er) was obtained with VAPOL phosphoric acid (Scheme 8).18,19
45 er) was obtained with VAPOL phosphoric acid (Scheme 8).18,19
Based on the experiments in Scheme 3 and the reaction outcome, a possible mechanism for the olefin-assisted palladium-catalyzed oxidative carbocyclization of enallenes via remote olefin insertion is given in Scheme 9. The reaction of palladium with enallene 1 bearing the assisting olefin forms vinylpalladium intermediate Int-1via allene attack involving allenic C–H bond cleavage, which is promoted by the coordination of allene and the assisting olefin to Pd(II).5,6 Then, the vinylpalladium intermediate Int-4 would be generated from Int-1via ligand exchange (from proximal olefin to remote olefin), instead of a direct olefin insertion to form cyclobutene complex Int-2.5 Intermediate Int-4 would undergo a remote olefin insertion to give cyclic intermediate Int-5. Subsequent transmetallation of Int-5 with B2pin2 or arylboronic acid, followed by reductive elimination would give the target cyclohexene derivatives 2 or 3. Under CO pressure in alcohol, Int-5 can undergo an alkoxy-carbonylation to provide product 4.
Conclusions
In conclusion, we have developed a highly selective olefin-assisted palladium-catalyzed oxidative carbocyclization of enallenes via remote olefin insertion for the selective formation of the cyclohexene skeleton. It was demonstrated that the assisting olefin moiety is essential for the formation of the cyclohexene derivatives. These reactions all show a broad substrate scope and good tolerance for various functional groups, and the catalyst loading could be decreased to 2 mol% in the arylative and carbonylative reactions with good to excellent yields. The biphenol-type chiral phosphoric acid was used in preliminary experiments of enantioselective carbocyclization–borylation of enallene. Further studies on the scope, synthetic application, and asymmetric variants of these reactions are currently carried out in our laboratory.Acknowledgements
Financial support from the European Research Council (ERC AdG 247014), The Swedish Research Council (621-2013-4653), The Berzelii Center EXSELENT, and the Knut and Alice Wallenberg Foundation is gratefully acknowledged.Notes and references
- For selected reviews involving carbocyclization reaction, see: (a) E. I. Negishi, C. Copéret, S. Ma, S. Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS; (b) M. Méndez and A. M. Echavarren, Eur. J. Org. Chem., 2002, 15 CrossRef; (c) B. M. Trost, F. D. Toste and A. B. Pinkerton, Chem. Rev., 2001, 101, 2067 CrossRef CAS PubMed; (d) C. Aubert, L. Fensterbank, V. Gandon and M. Malacria, Top. Organomet. Chem., 2006, 19, 259 CrossRef CAS; (e) D. H. Zhang, Z. Zhang and M. Shi, Chem. Commun., 2012, 48, 10271 RSC; (f) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed; (g) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed.
- For selected examples, see: (a) D. Green, I. Goldberg, Z. Stein, M. Ilan and Y. Kashman, Nat. Prod. Lett., 1992, 1, 193 CrossRef CAS; (b) S. H. Sennett, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 1992, 55, 1421 CrossRef CAS PubMed; (c) J. A. Enqusit Jr and B. M. Stoltz, Nature, 2008, 453, 1228 CrossRef PubMed; (d) D. L. Wright and C. R. Whitehead, Org. Prep. Proced. Int., 2000, 32, 307 CrossRef CAS.
- For recent reviews of transition-metal-catalyzed π-bond-assisted C–H bond activation, see: (a) P. Gandeepan and C.-H. Cheng, Chem.–Asian J., 2015, 10, 824 CrossRef CAS PubMed; (b) Y. Minami and T. Hiyama, Acc. Chem. Res., 2016, 49, 67 CrossRef CAS PubMed.
- For recent reviews of C–C activation, see: (a) C–C Bond Activation, in Topics in Current Chemistry, ed. G. Dong, Springer-Verlag, Berlin, 2014, vol. 346 Search PubMed; (b) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410 CrossRef CAS PubMed.
- Y. Qiu, B. Yang, C. Zhu and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2016, 55, 6520 CrossRef CAS PubMed.
- C. Zhu, B. Yang, T. Jiang and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2015, 54, 9066 CrossRef CAS PubMed.
- For selected examples involving remote olefin insertion, see: (a) A. Vasseur, J. Bruffaerts and I. Marek, Nat. Chem., 2016, 8, 209 CrossRef CAS PubMed; (b) J. W. Park, K. G. M. Kou, D. K. Kim and V. M. Dong, Chem. Sci., 2015, 6, 4479 RSC ; for selected examples involving alkene directing effects in palladium-catalyzed carbocyclization chemistry, see: ; (c) B. M. Trost and Y. Shi, J. Am. Chem. Soc., 1993, 115, 9421 CrossRef CAS; (d) B. M. Trost, G. J. Tanoury, M. Lautens, C. Chan and T. MacPherson, J. Am. Chem. Soc., 1994, 116, 4255 CrossRef CAS.
- For selected reviews involving palladium oxidative carbocyclization, see: (a) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (b) F. Dénès, A. Pérez-Luna and F. Chemla, Chem. Rev., 2010, 110, 2366 CrossRef PubMed; (c) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (d) Y. Deng, A. K. Å. Persson and J.-E. Bäckvall, Chem.–Eur. J., 2012, 18, 11498 CrossRef CAS PubMed.
- For selected examples of palladium oxidative carbocyclizaiton of enallenes for formation of five-membered rings, see: (a) A. K. Å. Persson, T. Jiang, M. Johnson and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2011, 50, 6155 CrossRef CAS PubMed; (b) T. Jiang, A. K. Å. Persson and J.-E. Bäckvall, Org. Lett., 2011, 13, 5838 CrossRef CAS PubMed; (c) C. M. R. Volla, J. Mazuela and J.-E. Bäckvall, Chem.–Eur. J., 2014, 20, 7608 CrossRef CAS PubMed; (d) T. Jiang, T. Bartholomeyzik, J. Mazuela, J. Willersinn and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2015, 54, 6024 CrossRef CAS PubMed; (e) J. Löfstedt, J. Franzén and J.-E. Bäckvall, J. Org. Chem., 2001, 66, 8015 CrossRef; (f) C. M. R. Volla and J.-E. Bäckvall, Org. Lett., 2014, 16, 4174 CrossRef CAS PubMed; (g) C. Zhu, B. Yang and J.-E. Bäckvall, J. Am. Chem. Soc., 2015, 137, 11868 CrossRef CAS PubMed.
- For selected examples of cycloisomerizations that lead to six-membered rings, see: (a) X. Han and R. A. Widenhoefer, Org. Lett., 2006, 8, 3801 CrossRef CAS PubMed; (b) M. A. Tarselli, A. R. Chianese, S. J. Lee and M. R. Gagné, Angew. Chem., Int. Ed., 2007, 46, 6670 CrossRef CAS PubMed; (c) F. Grau, A. Heumann and E. Duñach, Angew. Chem., Int. Ed., 2006, 45, 7285 CrossRef CAS PubMed; (d) Y. Qiu, J. Zhou, J. Li, C. Fu, Y. Guo, H. Wang and S. Ma, Chem.–Eur. J., 2015, 21, 15939 CrossRef CAS PubMed; (e) J. W. Park, Z. Chen and V. M. Dong, J. Am. Chem. Soc., 2016, 138, 3310 CrossRef CAS PubMed.
- Pd-catalyzed oxidative carbocyclization of bisallenes to cyclohexene derivatives have been realized in our laboratory: C. M. R. Volla and J.-E. Bäckvall, ACS Catal., 2016, 6, 6398 CrossRef CAS PubMed.
- For selected reviews involving the organoboronates, see: (a) J. Tsuji, Palladium Reagents and Catalysts, John Wiley & Sons, Chichester, England, 2004, pp. 289–310 Search PubMed; (b) D. S. Matteson, Chem. Rev., 1989, 89, 1535 CrossRef CAS; (c) D. S. Matteson, Acc. Chem. Res., 1988, 21, 294 CrossRef CAS; (d) G. C. Fu, Acc. Chem. Res., 2008, 41, 1555 CrossRef CAS PubMed.
- (a) H. C. Brown, R. Liotta and G. W. Kramer, J. Am. Chem. Soc., 1979, 101, 2966 CrossRef CAS; (b) J. Kister, A. C. DeBaillie, R. Lira and W. R. Roush, J. Am. Chem. Soc., 2009, 131, 14174 CrossRef CAS PubMed; (c) W. Yuan and S. Ma, Adv. Synth. Catal., 2012, 354, 1867 CrossRef CAS; (d) S. B. Thorpe, X. Guo and W. L. Santos, Chem. Commun., 2011, 47, 424 RSC; (e) F. Meng, B. Jung, F. Haeffner and A. H. Hoveyda, Org. Lett., 2013, 15, 1414 CrossRef CAS PubMed; (f) B. Jung and A. H. Hoveyda, J. Am. Chem. Soc., 2012, 134, 1490 CrossRef CAS PubMed; (g) K. Semba, T. Fujihara, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2013, 52, 12400 CrossRef CAS PubMed; (h) K. Semba, N. Bessho, T. Fujihara, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2014, 53, 9007 CrossRef CAS PubMed; (i) C. Zhu, B. Yang, Y. Qiu and J.-E. Bäckvall, Chem.–Eur. J., 2016, 22, 2939 CrossRef CAS PubMed.
- Allenes 1f, 1g, and 1h were prepared by iron-catalyzed cross coupling of propargyl acetate 5 and the appropriate Grignard reagent: S. N. Kessler and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2016, 55, 3734 CrossRef CAS PubMed
. - (a) Y. Deng, T. Bartholomeyzik, A. K. Å. Persson, J. Sun and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2012, 51, 2703 CrossRef CAS PubMed; (b) T. Bartholomeyzik, J. Mazuela, R. Pendrill, Y. Deng and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2014, 53, 8696 CrossRef CAS PubMed.
- For selected reviews on palladium catalyzed carbonylation, see: (a) T. Morimoto and K. Kakiuchi, Angew. Chem., Int. Ed., 2004, 43, 5580 CrossRef CAS PubMed; (b) A. Brennführer, H. NeuMann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114 CrossRef PubMed; (c) R. Grigg and S. P. Mutton, Tetrahedron, 2010, 66, 5515 CrossRef CAS; (d) X. F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113, 1 CrossRef CAS PubMed.
- (a) J. Piera and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2008, 47, 3506 CrossRef CAS PubMed; (b) J.-E. Bäckvall, R. B. Hopkins, H. Grennberg, M. Mader and A. K. Awasthi, J. Am. Chem. Soc., 1990, 112, 5160 CrossRef; (c) C. M. R. Volla and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2013, 52, 14209 CrossRef CAS PubMed.
- For selected examples, see: (a) G. Jiang and B. List, Angew. Chem., Int. Ed., 2011, 50, 9471 CrossRef CAS PubMed; (b) K. Ohmatsu, N. Imagawa and T. Ooi, Nat. Chem., 2014, 6, 47 CrossRef CAS PubMed; (c) Z. Chai and T. Rainey, J. Am. Chem. Soc., 2012, 134, 3615 CrossRef CAS PubMed; (d) P. Wang, H. Lin, Y. Zhai, Z. Han and L. Gong, Angew. Chem., Int. Ed., 2014, 53, 12218 CrossRef CAS PubMed.
- For selected reviews, see: (a) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518 CrossRef CAS PubMed; (b) R. J. Phipps, G. L. Hamilton and F. D. Toste, Nat. Chem., 2012, 4, 603 CrossRef CAS PubMed; (c) F. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef PubMed; (d) D. Chen, Z. Han, X. Zhou and L. Gong, Acc. Chem. Res., 2014, 47, 2365 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc02660e |
| This journal is © The Royal Society of Chemistry 2017 |