Investigating students' similarity judgments in organic chemistry
N.
Graulich
 *a and
G.
Bhattacharyya
b
*a and
G.
Bhattacharyya
b
aJustus-Liebig University Giessen, Institute of Chemistry Education, Heinrich-Buff Ring 17, 35392 Giessen, Germany. E-mail: Nicole.Graulich@didaktik.chemie.uni-giessen.de
bChemistry Department, Missouri State University, 901 S. National Ave., Springfield, MO 65897, USA
First published on 6th June 2017
Abstract
Organic chemistry is possibly the most visual science of all chemistry disciplines. The process of scientific inquiry in organic chemistry relies on external representations, such as Lewis structures, mechanisms, and electron arrows. Information about chemical properties or driving forces of mechanistic steps is not available through direct perception, and thus looking beyond the respresentation is challenging for learners. In this study, we investigated the categorization behavior of undergraduate students enrolled in an organic chemistry course when engaged in various categorization tasks involving electrophilic addition reactions to alkenes. The critical attribute a student chose to make a category out of a set of reactions was classified as perceptual or relational and gave insights into how students process and store information about reactions at an early level of expertise. Our results support the notion that students are prone to the surface level of representations and make sense of reactions depicted in a very minimalistic fashion. Implications for approaching this phenomenon in teaching are discussed.
Introduction
Organic chemistry is a heavily information- and diagram-laden discipline: “[…] more than other sciences, understanding chemistry relies on making sense of the invisible and untouchable” (Kozma and Russell, 1997, p. 949). The “invisible and untouchable” refer to the structural properties of chemical substances at the molecular level to explain the behavior of those substances at the macroscopic level – a connection we have referred to previously as the Central Dogma of Chemistry (DeFever et al., 2015). To express these connections, organic chemists use a diversity of models which are externally represented in an even greater diversity of symbolic languages. (For the purposes of this paper the term “representation” refers to external representations.) The ability to interpret a given representation beyond the explicit aspects of the image, therefore, is one of the major skills required to master organic chemistry (Bodner and Herron, 2002; Pretz et al., 2003).Prior research indicated that students in organic chemistry classes tend to struggle when working with these representations; even to the extent that skipping or ignoring parts of representations was a student problem-solving “strategy” frequently used (Ferguson and Bodner, 2008; Strickland et al., 2010; Grove et al., 2012b; de Arellano and Towns, 2014). While these and other studies began to create a picture of students' difficulties, much less is known about the information students process when viewing these representations and the meaning they attribute to them. Exemplarily, to what extent are students able to connect perceptual, or “surface level” attributes of a representation, such as recognizing a hydroxy group, with “deeper level”, implicit information, such as the polarity associated with that hydroxy group? Cooper and colleagues have addressed this question extensively in the context of stand-alone Lewis structures (Cooper et al., 2010). In the current research, we complement their work by investigating how students approach diagrammatic representations of entire chemical reactions.
One method to determine an individual’s perception and understanding of surface and deeper level information is to use categorization or classification tasks. The first study of this type was conducted in physics by Chi et al. (1981), who found that novices sorted various physics problems based on surface features, whereas experts were able to abstract deeper level information. More recently, two studies in general chemistry used categorization tasks to address these issues of meaning and perception. Stains and Talanquer (2007, 2008) compared the behaviors of undergraduate and graduate students while engaged in classifying different chemical representations at the submicroscopic, microscopic, and macroscopic level. They analyzed how often surface and deep level attributes were used in the classification tasks and determined that graduate students used more implicit information from the representations given than explicit ones for their classification. The most common approach used by undergraduates was a single attribute decision-making process, such as the concept that all bonded particles are compounds.
In the domain of organic chemistry, Domin et al. (2008) investigated the behavior of undergraduate students and experts while engaged in categorizing different cyclic or acyclic α-chloro derivatives of aldehydes and ketones. Consistent with Stains and Talanquer's findings, these authors found that students primarily categorized these compounds dichotomously by choosing a single surface level attribute, such as aldehyde/ketone, cyclic/acyclic, or halogenated/non-halogenated. In Stains and Talanquer's study, experts tended to build similar categories as novices, also focusing on functional groups, but made the decision based on more implicit considerations, such as reactivity of the functional group towards the addition of nucleophiles.
We report here on the extension of these studies into the realm of organic reactions. There was a two-fold purpose in using chemical reactions: firstly, this topic is used most frequently in organic chemistry classes. Secondly, it was hoped that this context may provide an opportunity to elicit “deeper” meanings. As prior knowledge is the key for subsequent learning (Cook, 2006), examining the nature of critical attributes chosen at an early undergraduate stage gives us not only an idea about how students process the information learned but also on what basis they may relate future content (Bodner, 1986).
Theoretical framework
Categorization is a fundamental cognitive process by which concepts are used to classify information (Anderson, 1991). Bruner asserted its importance by stating that to “perceive is to categorize, to conceptualize is to categorize, to learn is to form categories, to make decisions is to categorize” (Bruner, 1966). Similarity models, studied intensively for decades by cognitive scientists, focus on the internal or mental representation of concepts. The classical view proposed that concepts should be represented internally as a set of rules (Goldstone and Kersten, 2003). This rather formal model, however, was replaced over a period of time by prototype, exemplar and connectionist theories (Laurence and Margolis, 1999), which account for the way in which humans perceive and classify objects. The prototype model, for instance, considers a mental concept as an abstraction of the most prevalent characteristics common to the instances constituting that concept (Rosch and Mervis, 1975; Minda and Smith, 2011). A prototype or exemplar for a concept differs depending on one's experience. There is still no consensus model in cognitive science for the mental representation of concepts because each of these sets of models are unable to account for certain characteristics of categorization (Laurence and Margolis, 1999). We decided to circumvent this lack of agreement by adopting Gentner and Markman's structure-mapping framework (Gentner and Markman, 1997), because it describes a process that is consistent with all the similarity models. Regardless of which of the similarity models one favors, all of the postclassical models involve an initial process of comparison and structural alignments between the features of an object. The basis for their framework is that when people make similarity judgments they consider two different types of characteristics – those that are readily apparent (perceptual attributes) versus those that are “related” to an object, but which are not visually apparent (relational attributes) – and weigh them according to the task at hand. Gentner and Markman (1997) expressed this relationship in a graphical form similar to Fig. 1.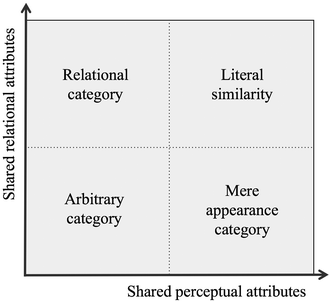 | ||
| Fig. 1 Degree of shared relational and perceptual attributes (cf.Gentner and Markman, 1997). | ||
We applied this framework to classify the nature of the similarities upon which the research participants formed their categories. A student, for example, who places the reactions of alkenes with Br2 or Cl2 in the same category because both reagents are molecular halogens, would be doing so based on recognizing visually similar attributes, i.e. building a mere appearance category using shared perceptual attributes. If the participant further noted that both reactions produced halonium-ion intermediates, the category would be classified as “literally similar”, since a shared relational attribute was also observed, i.e. recalling the mechanistic intermediate, which is a relational attribute. As another example, the judgment of a student who places an electrophilic aromatic substitution reaction next to a nucleophilic addition to an acyl chloride because both reactions are examples of addition–elimination mechanisms is based on shared relational attributes (same type of mechanism), i.e. building a relational category. More specific examples are offered in the description of the data analysis.
Relational categories are more valuable than mere appearance ones from a chemist's perspective, as it allows one to group reactions based on the causal effects that, for example, intermediates, reaction condition, or reagents exhibit on a mechanism. Those categories have a higher predictive value than those based on purely surface matches (Orgill and Bodner, 2005). Adopting Gentner and Markman's (1997) framework allows us to shed light on the nature of students' similarity judgments, from mere appearance to relational categories.
Methodology
Purpose of the study and research questions
The goal of this study was to investigate students' selection of attributes when comparing and contrasting organic reactions in categorization tasks. Our research design was guided by the following questions:– What types of cues, perceptual or relational attributes, do students use to make categories of reactions?
– How does the selection of attributes change when adding variations in perceptual attributes to the structures?
A qualitative research design was used to capture the selection of attributes in students' similarity judgment and to elicit their underlying thought process.
Setting and participants
The study was conducted in the Department of Chemistry of a large, research-oriented university in the southeastern United States. Twelve undergraduate students, five male and seven female, enrolled in an introductory organic chemistry course, were recruited on a voluntary basis and were given pseudonyms to maintain their anonymity. This course was intended for students taking their second year of chemistry (after general chemistry) and serves a broad population of Science and Engineering majors, including those with aspirations for professional degrees in Dentistry and Medicine. The gender distribution for the sample resembled that of the entire class, which comprised 61% women. All participants had passed the exam on addition reactions before the interviews and more than half of the volunteers in this study were expecting an A or a B in this course. The local institutional review board approved this study prior to recruitment and informed consent was obtained from all the participants.Instrument
Ferguson and Bodner (2008) reported in their study of Organic Chemistry II students' understanding of the electron-pushing formalism and electron-pushing mechanisms that the participants appeared to be most comfortable with the reactions of alkenes. Additionally, this topic was the first set of reactions taught in the course from which the participants were recruited. Finally, the chemistry of alkenes, as taught at this level, consists typically of reactions that tend to be similar at the surface level but vary significantly at the mechanistic level. We chose to develop the instrument using reactions of alkenes, because it would allow us to use a familiar topic to examine the extent to which students could look beyond superficial features of diagrams of reactions when sorting them.With the topic chosen, we had several considerations to make while creating the tasks. Firstly, the alkene(s) used as the starting material(s) would have to be asymmetric, i.e. different substituents needed to be bonded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C moiety, since many of the products of these reactions favor the more or less substituted side – the so-called Markovnikov's Rule. Secondly, we needed to choose a simple asymmetric alkene to minimize extraneous cognitive load (Paas et al., 2003). Thirdly, we needed to decide whether or not to include the stereochemical outcomes of reactions with dash(es) and wedge(s) in the product structure(s). Although stereochemical relationships of the products' substituents are important features for categorization, students in multiple studies either avoided or disregarded dashes and wedges in structures (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008). Based on these factors, we chose propene as the starting material and did not include dashes and wedges in any of the structures.
C moiety, since many of the products of these reactions favor the more or less substituted side – the so-called Markovnikov's Rule. Secondly, we needed to choose a simple asymmetric alkene to minimize extraneous cognitive load (Paas et al., 2003). Thirdly, we needed to decide whether or not to include the stereochemical outcomes of reactions with dash(es) and wedge(s) in the product structure(s). Although stereochemical relationships of the products' substituents are important features for categorization, students in multiple studies either avoided or disregarded dashes and wedges in structures (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008). Based on these factors, we chose propene as the starting material and did not include dashes and wedges in any of the structures.
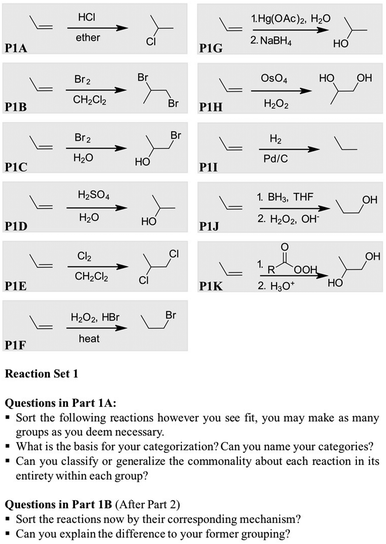
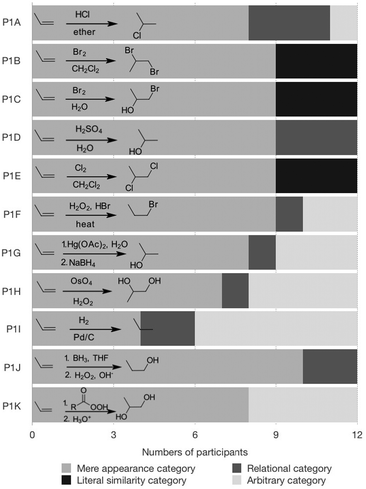
Regarding the specific reactions of alkenes, we needed a set that was large enough to make meaningful classification possible but not so big that it would result in cognitive overload. Furthermore, we wanted to use reactions that had similar surface level features but differed mechanistically. Fig. 2 contains the reaction inventory that we used for all the tasks. All the reactions and mechanisms had been covered explicitly during class lectures and exam, except for P1F, the NaBH4 step of P1G, and the H2O2 step of P1H.
Fig. 3 shows the chronology of distribution of tasks. After a warm-up task, which was only meant to activate the students' prior knowledge, four different parts were arranged. Part 1A and Part 1B used the same Reaction Set 1, but differed by the prompts used in the respective part (cf.Fig. 3). For the task in Part 1A, we asked the students to classify the reactions based on any attribute(s) and in as many categories as they desired.
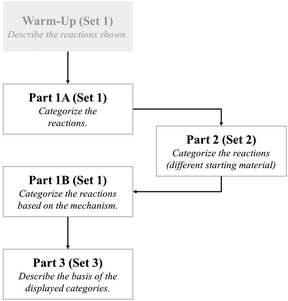
To see the stability of the categories formed in Part 1A, we gave the participants in Part 2 the same reactions as those in Reaction Set 1, but with a variety of starting materials (Fig. 4). Once again, the students were given complete discretion in the number of categories they formed and the bases for the classifications.
For the third task (Part 1B), we returned to the Reaction Set 1, but this time asked the students to categorize the reactions based on their mechanisms (cf.Fig. 2).
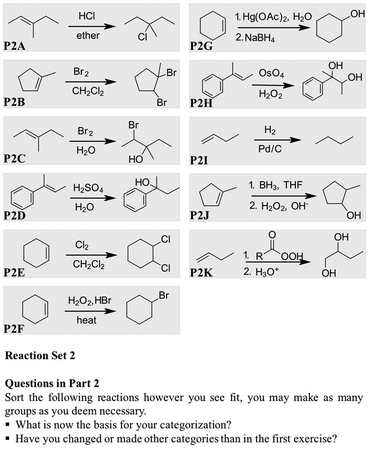
Since it was possible, or even likely, that students would group the reactions based on surface level features, we gave them a final task, shown in Fig. 5, in which we asked students to elaborate on the underlying similarity of each of the sets. We had chosen the reactions from the reaction inventory based on the least amount of shared perceptual and some degree of shared relational attributes.
The reactions of the first group, the syn-dihydroxylation (P3H), the hydrogenation (P3I) and the hydroboration (P3J), do not proceed through the typical carbocation pathway and are all syn-additions. Reaction P3H and P3J proceed through concerted mechanistic steps, whereas the metal-catalyzed hydrogenation is a stepwise process. The reactions were chosen to initiate students' reasoning on possible similarities and differences. The second group comprises reactions forming different ring-intermediates which, if reacting further, can lead to anti-additions. The bromination (P3B) and the oxymercuration (P3G) proceed through positively charged ring-intermediates, whereas the epoxidation leads to a neutral epoxide.
The instruments and interview protocol had been tested and evaluated in a preceding pilot study with eight undergraduates from the prior semester and five graduate students. The pilot study aimed at testing the length, prompts, and distribution of reaction sets used in the study. Changes were made regarding the prompts and an additional warm-up set was added to give the students time to adjust and recall their prior knowledge.
Data collection and analysis
The data were collected by semi-structured interviews lasting between 45 and 60 minutes. The reactions and interview questions associated with each task are shown in Fig. 2, 4, and 5, respectively. The reactions were presented in the form of cards using the program ExpoBoard, which records individuals' movement of the cards around on a virtual board by touching the screen. We began the interview with a warm-up task to help activate the students' prior knowledge and acclimatize them to ExpoBoard (cf.Fig. 3). During the interview the participants were asked to verbalize their thought processes to the greatest extent possible. These utterances and verbal exchanges between participant and interviewer were audio recorded. The students were also provided with paper and writing implements for any notes. Moreover, the students were told that the interviewer would answer any content-related questions. Finally, the interviewer wrote down observations during the interviews in the form of field notes.All interviews were transcribed verbatim and analyzed with the corresponding video segments and field notes. Using the categories from Gentner and Markman (1997) (cf.Fig. 1), the characteristic(s) – or critical attribute(s) – the students used to sort the reactions in each task were coded as “mere appearance”, “relational”, “literal similarity”, or “arbitrary”. When participants formed categories based only on perceptual attributes, such as the similarity of reagents or products, they did so using the mere appearance of the items. When participants invoked attributes not explicitly depicted by the reaction, such as movement of electrons or reaction intermediates, as the basis of their categories, they used relational attributes. Therefore, when a student grouped reactions based on the presence of one alcohol group in the product, it was coded as a mere appearance category. Whereas when a participant grouped the oxymercuration reaction (P1G) with a halogenation reaction (P1E or P1B) based on the formation of a three-membered ring intermediate, that category was coded as relational. A category where both perceptual and relational attributes were mentioned as the basis for sorting was classified as literally similar, for example, when participants mentioned the halogens as the product of the chlorination and bromination reaction and the formation of the halonium-ring in both reactions. Finally, the basis for classification was labeled an arbitrary category when participants placed reactions as “miscellaneous” or “on its own”, without mentioning any chemical reasons.
In addition to classifying the participants’ categories, we further analyzed the categorization strategies occurring and characteristics of their focus on similarity.
Results and discussions
Consistent with an entire body of literature beginning with the groundbreaking work of Chi et al. (1981), the participants in this study formed nearly 75% of their categories using perceptual attributes of the reactions, such as atoms constituting functional groups and distribution of substituents. It is important to note, that the overwhelming focus on surface level characteristics may have been, to some extent, an artifact of the tasks, since each reaction was a functional group transformation involving changes of atomic make-up and/or connectivities from reactants or products.1. The selection of attributes
The four different categories, based on Gentner and Markman's (1997) model with common examples for the corresponding attributes and the frequency of their choice in Part 1A of the interview, are summarized below:Mere appearance category (73%, high degree of perceptual attributes shared, low degree of relational attributes shared)
– product similarity based on functional group or regiochemistry (Markovnikov/anti-Markovnikov)
– reagent similarity: similarity between reagents used (acids and halogens)
Arbitrary category (13.5%, low degree of relational and perceptual attributes shared)
– reactions grouped alone, randomly, or based on unfamiliarity
Relational category (9%, high degree of relational attributes shared, low perceptual attributes shared)
– formation of carbocation
– concerted reaction
– ring intermediate
– syn-addition
Literal similarity (4.5%, high degree of relational and perceptual attributes shared)
– halogenation reactions (haloalkanes and halonium ions)
Fig. 6 shows the distribution of each reaction into the four categories. This diagram is not meant to convey any statistical meaning, but gives an overview of the participants' attribute selection at the end of their sorting in Part 1A. Note that only three participants made relational categories in Part 1A.
As shown in Fig. 6, the hydrogenation (P1I) was the one reaction that was classified in an arbitrary category by half of the participants. Alternatively, this reaction was grouped in mere appearance categories – with the halogenation reactions, since the reagents were all diatomic molecules or based on the product “non-alcohols” – or in a relational category based on the notion of “concerted, syn-addition” by two participants in Part 1A. Although the use of relational attributes is desired, it is perhaps ironic in this case, because halogenation is not an example of a syn-addition. The apparent difficulty to categorize the hydrogenation reaction may be expected since it does not generate a typical functional group or have a typical dipolar mechanism.
The reactions placed most frequently in a relational category, by three participants, were P1A and P1D (addition of HCl and acid-catalyzed hydration, respectively). However, the main mechanistic fact the participants recalled was the formation of the carbocation intermediate, as expressed in the following quote from Anna:
The carbocation ones (referring to P1A and P1D) are really simple, you just need two arrows to draw them, but these (referring to the oxymercuration, P1G, the syn-dihydroxylation, P1H, and the anti-dihydroxylation P1K), I wouldn't know where the electrons on the reagents go on the bond, like where the electrons come from, from which atom they would come from. With these (refers to formation of the carbocation), you only have two choices, the hydrogen or the chlorine.
The overall percentage of relational categories increased from 13.5% in Part 1A to 53% in Part 1B, though approximately two-thirds of the additional relational categories in Part 1B were either incomplete or erroneous. The categories made by most of the participants showed that if they did not spontaneously make at least one relational category in Parts 1A or 2, they were unlikely to create relational groups of reactions in Part 1B in which the task called for mechanism-based sorting.
Beside the attributes chosen to make a category, we looked at the resulting combinations; i.e. which reactions were mainly grouped together (cf.Fig. 7).
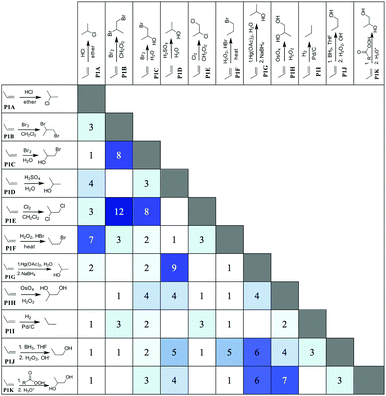 | ||
| Fig. 7 Frequency of reactions combined in similar categories (dark blue, very often; light blue, less often). | ||
In addition to the perceptual groupings expected, such as P1B and P1E (the halogenation reactions), other groupings revealed the participants' prioritization regarding salience. Eight of the students, for example, placed P1C (halohydrin formation) with the halogenation reactions, and the remainder grouped it with the reactions that produced alcohols. Unfortunately, the participants did not articulate the rationale behind choosing an alcohol substituent over a halogen and vice versa. In another example, P1D (acid-catalyzed hydration) was far more likely to be grouped with the other reactions that produced a Markovnikov alcohol product, P1G (oxymercuration), than with another acid-catalyzed reaction, P1A (addition of HCl). It seems evident just by looking at the combination of reactions in similar categories in Fig. 7 that the participants have the tendency to prioritize product similarity.
2. Categorization strategies
Since the participants used a similar approach – not necessarily the same categories – to sorting the reactions, we will focus on one student, Amanda, to illustrate this process and supplement data from others as it becomes relevant. As Amanda began to look at the reactions in Part 1A, she made two important observations. Firstly, she commented on the challenge posed by the sheer volume of the reactions that the students were expected to learn. After reviewing about half of the reactions, for example, she said:Because there are so many reactions, they get a little jumbled up in some ways. Sometimes it looks like hieroglyphics.
By the time she had finished looking over all of the reactions, Amanda lamented:
That's often part of the problem. There is just so much information that is thrown at you so fast in one day. We would not go over one or two; we would do up to five of these reactions. So, you have no other choice than to memorize as much as you can.
The second important observation Amanda made was the challenge posed by having to judge similarity in a symbolism that “looks like hieroglyphics.” One type of difficulty was judging the extent to which substituents would affect the reaction outcome. Consider the following comment:
And sometimes he'll (referring to the professor) show us the base reaction, but then when he shows examples and he adds other things in that slightly changes just enough where you’re not quite sure, if it is the same things again.
Another aspect for which making similarity judgments was challenging were the reagents. When attempting to distinguish the addition of HBr in the presence or absence of peroxide, Amanda commented:
See, this is what frustrates me sometimes. Because there are so many slight variations on it. I mean I'm sure that it's not the exact same thing (referring to the two reactions), but in my mind, as far as I can tell, the only thing that does anything is the HBr, ‘cause you end up with the bromine attached on the other side.
These comments illustrate that making similarity judgments, on which analogical reasoning is based, is not a trivial task. The characteristic of Amanda's classification scheme was that the critical attribute changed as she went further through the reactions. She described it concisely in the following quote:
I guess just as many similarities as I can find right now. You know, you look at the reagents and then at the product. That's kind of how I put these together. So, I put some together because they have the same reagent, but not the exact same mechanism.
Thus, Amanda placed P1A (addition of HCl) and P1F (addition of HBr in H2O2) together because both reactions had hydrohalic acids for reagents, to which she is referring in the following quote:
“As far as I can tell, these two are at least similar enough to be grouped. Obviously, there is something that's going on that is different.”
As such, she was willing to overlook the differences in mechanism and product regioselectivity to accommodate the familiar attributes of the two reactions. More interestingly, perhaps, she later put P1J (hydroboration/oxidation) in this group because it produced an anti-Markovnikov product, such as the addition of HBr in peroxides. The final group of the three reactions, P1A, P1F, and P1J, were based on two separate critical attributes.
As mentioned previously, most of the participants used a strategy with multiple critical attributes. Consider, for example, the following exchange with Ben:
I: “Can you describe what you are doing?”
Ben: “I'm just grouping them by similarity?”
I: “What kind of similarity?”
Ben: “Whatever is being added. So this is a separate reaction (referring to addition of H2), so I will place it by itself. But it is a diatomic molecule, so I will place it here, close to the halogens.”
I: “What kind of groups are you trying to make?”
Ben: “I try to find, probably, the reagents, but I think I'm going to instead of grouping them by that, I would group them based on whether they are concerted or not and whether (pause), I don't know, because all are very dissimilar. They are not the same reaction.”
Even in this short exchange, Ben changed his potential choices for critical attributes, going from products, to reagents, and then to mechanisms.
3. The focus on functionality change
Although the students' choice of attributes was mostly adapted during the categorization task, most of them ended up creating categories based on the functionality displayed (cf.Fig. 6). This perception of organic reactions as conveying change at the functional group was expressed in two different ways: (1) By recalling the regiochemistry of the functional group added (30%) and (2) by focusing on the functional group(s) of the product(s) (70%). The following excerpt from Nina exemplifies the first way of perceiving a change of functionality. Her categorization behavior shows the focus on the substituents. She started with referring to all reactions that, from her point of view, “add one substituent”:Here (referring to the hydrochlorination, P1A) we are adding on one side of the double bond and here too just on one side of the double bond (referring to the hydroxy group, P1D).
Then she grouped the reactions that “add two substituents” and further continued to adopt this strategy to the rest of the reactions:
And here (referring to the halogen substituents, P1B/P1E) we are adding the same to both sides of the double bond. […] Here we are adding only on one side (referring to the hydroxyl group, P1J). […] This one (referring to the anti-dihydroxylation, P1K) is adding on both sides but these are two alcohols, but I will put it in that group because it adds on both sides. This one (referring to the oxymercuration, P1G) is also on one side, so I'll put it here (she puts P1G in the “one substituent” group).
Her approach was to consider the total number of heteroatom substituents added. Like Nina, most of the participants expressed the perceived change in functionality by stating explicitly the functional group that the reactions produced and further compared the perceptual attributes of other reactions, that may fit, as exemplified by Anna's quote:
Anna: “I put these together (referring to the hydrogenation, P1I, and the halogenation reaction, P1E/P1B) just because these are halogenation problems and that (referring to the hydrogenation, P1I) reacts similar to it.
I: “Can you explain why?”
Anna: “It adds two hydrogens and these add two halogens.”
We could observe that the choice of perceptual attributes that a participant chooses seems to depend on the reagents given. When the match between reagents and product is easier, in the case of reaction P1E/P1B or P1I, they tend to mention what has been added. Whereas, if the match is not directly accessible, the product is chosen as the critical attribute, which is illustrated by the following quote:
Anna: “I put these together because they make alcohols (referring to the hydration, P1D, the hydroboration, P1J, and the oxymercuration, P1G). I put these together (referring to the hydrohalogenation, P1A, and the radical bromination, P1F) because these are additions of an acid and these two because they have two OH groups on it (referring to the anti-dihydroxylation, P1K, and the syn-dihydroxylation, P1H).
I: “You are mainly looking at?”
Anna: “I look at the product. In this group (referring to the halogenation and the hydrogenation, P1I) its kind of the same. And in this group (one OH group) I looked at the products. The other group has two alcohols on it. I don't remember how you would call it. And the other group is because of the reagent, one has HCl and the other HBr. And you have the same in the product (referring to the hydrohalogenation, P1A, and radical bromination, P1F).”
This creates an even stronger product focus when the reagents are not directly matchable to the substituent distribution in the product.
Decreasing the surface level similarity in Part 2 (Fig. 4) by adding rings and various chains in the starting materials did not influence the participants' choice of attributes notably or alter the major categories formed. This stability in categories in Part 1A and Part 2 further indicated that the participants were strongly focused on functional group transformation. Consider the following quote from Madison (Part 2):
I: “Is it more difficult for you to deal with more complex structures?”
Madison: “What was the first exercise?”
I: “We just had propene as the starting material?”
Madison: “Oh ok, I haven't paid attention to that. So, I don't think it makes a difference to me. I just see again the reagents and I didn't even notice that they were different. I was just focusing on the reagents and then seeing the starting material and seeing how it would add to give me the product.”
The observation that the participants tended to overlook the surface level disparity of the molecules in Part 2 and looked for the functional group displayed is one that recurred frequently throughout all the interviews. The stability of the students' categories across Parts 1 and 2 appears to be a proverbial good news – bad news situation. The good news is that the idea of a functional group as a collection of atoms exhibiting similar chemical behaviors appears to have been internalized by the students. As such, they are following a functional group approach perfectly. The not so good news is that students may be learn to ignore the effect of alkyl groups on reactions. The nature of the alkyl group becomes important when it is very bulky, as is the case with a tertiary-butyl group, or when the alkyl group changes to a phenyl or alkenyl group. Recognizing emergent properties in a molecule that result from the combination of adjacent functional groups is an important competence in mastering organic chemistry (Bhattacharyya, 2014).
Four out of the twelve participants tried to consider relational attributes in Part 1B by recalling knowledge about the involvement of a ring or a carbocation intermediate to categorize the reactions, but switched back to the surface level and created mere appearance categories. This tendency to consider perceptual over mechanistic attributes, even when asked to use the mechanism (Part 1B), is demonstrated in the following quote from Amanda. She initially put the oxymercuration reaction (P1G) next to the halogenation reactions (P1E/P1B), because of the ring intermediate and then struggled to use relational attributes for her grouping and grouped them rather by functional group.
I know that this one (referring to the oxymercuration, P1G) forms a ring, so it's kind of similar to that group (referring to the halogenation, P1E/P1B/P1C), but the product is the same to that one (referring to the hydration, P1D). (she moves the oxymercuration, P1G to the hydration, P1D) And I like to have these together that form one alcohol. Because I’ve learned that there are three ways to do that, so that's nice that these end up close together as well.
Even though she could recall mechanistic information, she seemed to be more confident at forming categories around perceptual attributes. As such, the students' “chemist-like” reasoning in focusing on the change of functionality was not connected with mechanistic considerations.
4. Students' distortion of relational attributes
The main relational attributes that the participants evoked involved either the entities of the mechanisms, for example, carbocations and rings, or terms as “syn- and concerted addition”. However, the students often confounded these terms to fit the perceptual attributes of the reactions, such as the product appearance. The following quote from Ben regarding the anti-dihydroxylation (P1K) exemplifies how the participants combined the notion concerted with the distribution of the substituents.It just adds two hydroxides. I'm not exactly sure. I think it was a complicated reaction with electrons moving around. I don't remember the mechanism, but I remember that it is supposed to do this. It was supposed to add two hydroxide groups. It's not concerted because it's not added to the same face.
Conversely, the participants used the presence of cis-products as a cue for a concerted process and were even able to recall the scientifically valid definition for the term. As an example, consider the following exchange with Drew:
Drew: “So, I know that the OsO4reaction is concerted and I believe that the reaction with mCPBA is concerted as well. So, they are just adding OH groups simultaneously on both sides of the bond. There are no intermediates.
I: “What do you mean by no intermediates?”
Drew: “That they are attaching the two OH at the same time on the same side. I just know that they have no intermediates.”
This result is consistent with previous work by Bhattacharyya and Bodner (2005) in which first-year graduate students used cis-products as cues for concerted processes. In that case, however, that association was not a valid assumption and it led the participants down an unproductive problem-solving trajectory, thereby demonstrating why confounding these terms leads students to problematic outcomes.
We observed another example of confounding terms in the context of carbocation rearrangements. Amanda used a product-oriented definition of a carbocation (Part 1B), as she related the notion of carbocation to the product appearance and reaction mechanism.
Amanda: “So, these two (referring to the hydration, P1D, and the hydrohalogenation, P1A), these are kind alike, because they are carbocation rearrangements.”
I: “What do you mean by carbocation rearrangement?”
Amanda: “That the Cl and the OH are attached here (referring to the secondary carbon) and the hydrogen is attached here (referring to the primary carbon). They are different to that group, the anti-Markovnikov group (referring to the hydroboration, P1J).”
I: “Can you explain why the Cl is attached here (referring to the secondary carbon) and not here (referring to the primary carbon)?”
Amanda: “I don't know. (pause) You always have to attach it to the most substituted carbon, like here (referring to P1D) or here (referring to P1A). Those are carbocation rearrangements. And here (referring to the hydroboration, P1J) you have to add it to the other carbon, because its anti-Markovnikov.”
The above results suggest that terms such as carbocation rearrangement or concerted lose their actual function to describe mechanistic steps and are linked to perceptual attributes, such as the substituent distribution. This overemphasis on functional group changes and connectivity by the participants seem to distort the accepted chemical meanings.
5. Recognizing relational categories
We purposefully designed Part 3 (cf.Fig. 5) to see if the participants could infer relational attributes by comparing reactions that are purposefully grouped together but that are dissimilar on the surface level.Although the reactions had very low perceptual similarity, the first approach of the majority of the participants was to determine the basis of the categories given by comparing the perceptual attributes, as shown in the quote from Anna, while describing group 1 in Part 3 (cf.Fig. 5).
Probably by the type of products it produces. One follows anti-Markovnikov, one is the hydrogenation with two Hs. They produce Hs and OHs. So, one (referring to the syn-dihydroxylation, P3H) and two (referring to the hydrogenation, P3I) have kind of the same product. And the third one just has a mixture of both, an H and OH atoms.
Furthermore, we could observe again the recall of mechanistic terms for the reactions displayed. Only three of the twelve participants mentioned “concerted and syn-addition” for the first group, and five participants mentioned “ring formation” for the second group of reactions. The explanations they gave about the relational attributes were scattered and remained a descriptive recall of each mechanism, as shown in Adam's quote:
Adam: “The second one, it's definitely the ring. The first one makes a ring, the mercury makes a ring and the last one, too.”
I: “Can you explain what is the difference and the similarity between the three rings that are formed?”
Adam: “It doesn't stay a ring. Whereas with these two (referring to the bromination, P3B, and the oxymercuration, P3G) something else comes in and breaks it. I guess this ring (referring to the epoxide, P3K) is more stable as a ring, as compare to these (referring to the bromination, P3B, and the oxymercuration, P3G), which would rather not be a ring.”
I: “Why do you think that?”
Adam: “Maybe because there is a ring here (referring to the epoxide, P3K) I don't know.”
However, the type of exercise in Part 3 seem to engage the participants to search for and compare relational attributes. The following quote from Justin illustrates how he proceeded with group 2. He made mere appearance categories in the prior parts and it was only in Part 3 that he mentioned relational attributes.
I'm trying to compare the way the bromine, the way the mercuration reaction and how you got an epoxide from this. It might just be the type of mechanism, the type of ion that is essentially forming (refers to the bromination). ‘Cause I know that the first one forms a bromonium ion and the way how the epoxide looks reminds me about the bromoniumion. One thing, they could have also done it by the stereochemistry, ‘cause I know this (refers to the bromination) creates trans products and this one (refers to the per acid reaction). If you have, I can't remember the order, if you have a cis product, then either the epoxide is trans or cis as well. (pause). So, the mercuration reaction might also follow the same trend, but I don't remember. So, it's either the stereochemistry or the intermediate, like the bromonium and the epoxide, how that forms.
The participants seem to recall the ring-formation as the common attribute in group 2 easily, however, they struggled to relate the presence of a ring-structure to the resulting distribution of the substituents in the product, for example, resulting in trans-products, or mentioned the charge differences in the halonium ring and the epoxide. Justin was the only participant who tried to incorporate the stereochemistry in his reasoning. The next stepping stone for the students would be to understand the mechanistic cause and effects of a ring-intermediate and its respective opening to generalize this knowledge in other problem situations.
Although the overall description of possible underlying relational attributes in Part 3 was, in most cases, more speculative than actually showing chemical reasoning, the analysis of the participants' quotes in Part 3 suggest that using contrasting cases, comparable to those tasks that Schwartz et al. (2011) used, might help scaffolding chemically relevant patterns. The participants were engaged in matching and comparing different aspects of the reactions, evaluated and tried to infer knowledge to other reactions.
Conclusions and implications
This focus on the functional change documented in this study seems totally rational from a chemist's perspective, as textbooks and organic chemistry curricula often follow the functional group approach. Given the variety of different mechanisms in the group of alkene addition reactions, it is not surprising that the cognitive load is reduced by focusing on the specific change at the atomic level with limited considerations how this structural change has occurred on the mechanistic level. It was rarely the implicit property of a reagent that determined change but rather the explicit combination of atoms that was considered as the source of change. Although the participants' concentration on the surface level is reported well, their application of that perception to their categorization offered interesting insights into their thought processes.The participants began to categorize the reactions as soon as they perceived an attribute that was common to at least some of the reactions. With the reactions left over from this first round, i.e. those lacking that first attribute, the participants searched for a second attribute, then, if needed a third, and so forth, until all the reactions were accounted for. Instead of observing a single classification scheme, we, in essence, observed multiple taxonomies in a single task, which is consistent with recent reports in the chemical education research literature of students' fragmented internal knowledge structures (Cooper et al., 2013; Anzovino and Bretz, 2015; DeFever et al., 2015).
Additionally, the mechanistic diversity of alkene reactions – dipolar, radical, cycloaddition, and heterogeneous catalysis – would make it very difficult to use a single organizing principle to complete these tasks. As such, our results indicate that helping students adopt a mechanistic approach to organic chemistry reactions may require more than teaching mechanisms; it may require a mechanistic organization. The latter point is consistent with work by Grove et al. (2012a), who found that only 40% of second-semester organic chemistry students spontaneously used mechanistic reasons when predicting products of reactions. The success rate, however, was only around 30% across all tasks.
The sequential application of attributes to the categorization tasks revealed other student habits that lead to unsuccessful problem-solving outcomes. The participants’ classification processes, for example, were linear, not recursive, so once they categorized a reaction, they did not attempt to revisit it using a subsequent attribute. This phenomenon of essentially ignoring or disregarding “inconvenient” information has been described previously (Ferguson and Bodner, 2008; Grove et al., 2012a). Another trait that could be detrimental to students' problem-solving success was their apparent lack of metacognition they demonstrated as they worked on these tasks. Martin and Schwartz (2009) showed that more successful problem solvers had a much longer latency before beginning their solutions than their less successful counterparts. The more successful participants used the latency period for studying the problem and creating a strategy to approach it.
The participants' classification schemes also indicated an overemphasis on functional group change between starting material and product. Consider, for example, the parts of a transformation that students appeared to commit to their long-term memories. Anna and Madison's comments indicate that students limit their memory of a reaction to the atoms that change between the structural representations of the starting material and product. Consequently, students in effect disregard the so-called “R-groups” associated with each functional group. This interpretation is coherent with the remarkable stability between Parts 1 and 2. This narrow focus is consistent with previous work by Bhattacharyya and Bodner (2005), who found that even graduate students often do not recognize groups such as esters as single reactive units; instead, they consider them to be ketones adjacent to ethers. Although the participants in this study did not explicitly explain the reasons for this strategy, we speculate that they, on the one hand, did so as an effort-reduction strategy (Shah and Oppenheimer, 2008) that helped them to memorize and recall reaction patterns easily without being distracted by additional surface features. On the other hand, one can assume that this behavior might as well be emphasized by how addition reactions are presented in textbooks or in class. As the only change occurs at the double bond, additional R-groups or cyclic structures are often omitted or abbreviated. This narrowed focus is only problematic when properties of functional groups change depending on the structural environment, for example in α,β-unsaturated carbonyls or ketal functions, which are normally taught in advanced level classes.
Furthermore, the students' use of Markovnikov's Rule became apparent. Almost every single participant used it in their categorization schemes. Use of this rule, once again, allowed students to limit their attention to surface level changes in structure rather than compelling them to approach such a task mechanistically. Certainly, when Markovnikov's Rule was formulated nearly three decades before the discovery of the electron (Kerber, 2002), it was a necessary tool for describing reactions of alkenes. However, it should have little prominence in a mechanistic treatment of organic chemistry beyond historical purposes and to bring a “common-name” classification of these reactions to the students' attention.
When looking at addition reactions, there are three major classes of reactions: polar, radical, and cycloadditions. Presenting so many different mechanistic categories at one functional group encourages students to develop a strong reliance on perceptual attributes, rather than developing the more important relational categories. Not only is recognizing change between structural representations of reactants and products an easier method of retaining information about reactions, but it also conveys implicitly a specific organizational structure of the subject. In asserting that there is a difference between teaching mechanisms and teaching mechanistically, we should promote a focus on mechanistic patterns and restructure the curriculum in organic chemistry, as proposed by Flynn and Ogilvie (2015). By organizing the content based on mechanistic processes and occurring intermediates, it is easier to anchor cause and effect relationships and to help students recall them in novel situations.
Related to this finding are the results from Part 3 that indicate the need for careful scaffolding, especially in the initial stages of learning. It is well-reported in the cognitive science literature that humans are innately seeking patterns (Stanovich, 2013). As expected, the research participants in this study found patterns among the reactions in Part 3. Unfortunately, the patterns were either scientifically irrelevant, when looking at the number of substituents added, or invalid, when using chemical terms inappropriately. The similarity and difference between the reactions presented in Part 3 may be more evident to a practicing organic chemist because he or she would be constrained by the scientific meaning of the structural representations. For students, on the other hand, the broader meaning they may attribute to the “lines and letters” of the structural representations would allow them to discern patterns that would be inherently disregarded by practicing organic chemists. As such, tasks in which we ask students to find patterns, especially mechanistic ones, should be more meaningfully designed or otherwise scaffolded in a way as to engage students to elaborate and compare mechanistic similarities or differences. Some participants in Part 3 were purposefully comparing the reactions given and seem to look beyond the surface level.
This study has raised several questions that we aim to answer in the future. First of all, it became clear that the students' reasoning was not guided by scientifically valid explanations. The mechanistic driving force for the reactions are often missing and, thus, the knowledge remains very factual. We will further investigate how to promote a meaningful understanding of organic chemical reactions and to externalize the reasoning process inherent to organic chemistry. Analyzing the factors and circumstances that influence a successful mechanistic reasoning will allow us to develop specific training methods for mechanistic reasoning.
Acknowledgements
The authors would like to thank the students of the organic chemistry class and their teacher for their willingness to participate in this study. The financial support for this work had been provided by the German Research Foundation.References
- Anderson J. R., (1991), The Adpative Nature of Human Categorization, Psychol. Rev., 98, 409–429.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15, 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bodner G. M., (1986), Constructivism: A Theory of Knowledge, J. Chem. Educ., 63, 873–878.
- Bodner G. M. and Herron J. D., (2002), Problem-solving in chemistry, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practise, Dordrecht: Kluwer, pp. 235–266.
- Bruner J. S., (1966), Towards a theory of instruction, Cambridge, MA: Harvard University Press.
- Chi M. T., Feltovich P. J. and Glaser R., (1981), Categorization and representation of physics problems by experts and novices, Cogn. Sci., 5, 121–152.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90, 1073–1091.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis Structures: An Investigation of Student Difficulties in Developing Representational Competence, J. Chem. Educ., 87, 869–874.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50, 699–721.
- de Arellano D. C.-R. and Towns M., (2014), Students understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental Rolodexing: Senior Chemistry Majors Understanding of Chemical and Physical Properties, J. Chem. Educ., 92, 415–426.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students' categorizations of organic compounds, Chem. Educ. Res. Pract., 9, 114–121.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: a mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92, 803–810.
- Gentner D. and Markman A. B., (1997), Structure mapping in analogy and similarity, Am. Psychol., 52, 45–56.
- Goldstone R. L. and Kersten A., (2003), Concepts and Categories, in Healy A. F. and Proctor R. W. (ed.), Comprehensive Handbook of Psychology, New York: Wiley, vol. 4, pp. 591–621.
- Grove N. P., Cooper M. M. and Cox E. L., (2012a), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89, 850–853.
- Grove N. P., Cooper M. M. and Rush K. M., (2012b), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89, 844–849.
- Kerber R. C., (2002), Markovnikov' Rule in History and Pedagogy, Found. Chem., 4, 61–72.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Laurence S. and Margolis E., (1999), Concepts and cognitive science, in Margolis E. and Laurence S. (ed.), Concepts: Core Readings, MA: MIT Press, pp. 3–81.
- Martin L. and Schwartz D. L., (2009), Prospective adaptation in the use of external representations, Cogn. Instruct., 27, 370–400.
- Minda J. P. and Smith J. D., (2011), Prototype models of categorization: basic formulation, predictions, and limitations, in Pothos E. M. and Wills A. J. (ed.), Formal approaches in categorization, New York: Cambridge University Press, pp. 40–64.
- Orgill M. and Bodner G., (2005), The role of analogies in chemistry teaching, in Chemists guide to effective teaching, Pienta N. J., Cooper M. M. and Greenbowe T. J. (ed.), Upper Saddle River, NJ: Pearson, Prentice Hall, pp. 90–105.
- Paas F., Renkl A. and Sweller J., (2003), Cognitive load theory and instructional design: recent developments, Educ. Psychol., 38, 1–4.
- Pretz J. E., Naples A. J. and Sternberg R. J., (2003), Recognizing, defining, and representing problems, in Davidson J. E. and Sternberg R. J. (ed.), The psychology of problem solving, New York: Cambridge University Press, pp. 3–30.
- Rosch E. and Mervis C. B., (1975), Family resemblances: studies in the internal structure of categories, Cogn. Psychol., 7, 573–605.
- Schwartz D. L., Chase C. C., Oppezzo M. A. and Chin D. B., (2011), Practicing versus inventing with contrasting cases: the effects of telling first on learning and transfer, J. Educ. Psychol., 103, 759.
- Shah A. K. and Oppenheimer D. M., (2008), Heuristics made easy: an effort-reduction framework, Psychol. Bull, 134, 207–222.
- Stains M. and Talanquer V., (2007), Classification of chemical substances using particulate representations of matter: an analysis of student thinking, Int. J. Sci. Educ., 29, 643–661.
- Stains M. and Talanquer V., (2008), Classification of chemical reactions: stages of expertise, J. Res. Sci. Teach., 45, 771–793.
- Stanovich K. E., (2013), How to think straight about psychology, Upper Saddle River, NJ: Pearson.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
| This journal is © The Royal Society of Chemistry 2017 |