Science writing heuristics embedded in green chemistry: a tool to nurture environmental literacy among pre-university students
Sheila
Shamuganathan
a and
Mageswary
Karpudewan
 *b
*b
aPenang Matriculation College, Penang, Malaysia
bUniversiti Sains Malaysia – School of Educational Studies Penang, Penang, Malaysia. E-mail: kmageswary@usm.my; mageswary_karpudewan@yahoo.com
First published on 24th February 2017
Abstract
Existing studies report on the importance of instilling environmental literacy among students from an early stage of schooling to enable them to adopt more pro-environmental behaviors in the near future. This quasi-experimental study was designed to compare the level of environmental literacy among two groups of students: the experimental group (N = 120) was taught using science writing heuristics embedded in a green chemistry curriculum (SWH-GC) and the comparison group (N = 90) was taught using a green chemistry curriculum. For the purpose of this study an environmental literacy model that consisted of pro-environmental attitudes, beliefs, knowledge and behavior was employed. ANCOVA with the pre-test as the covariate showed statistically significant differences in all four of the constructs that constitute environmental literacy, with the experimental group taught using SWH-GC exhibiting higher mean values. Interview findings with randomly selected students from both groups further reinforced the quantitative findings. Both quantitative and qualitative findings suggest that after the treatment, the experimental group students were keen to read about the environment, believed their actions would bring changes, were more knowledgeable regarding how to keep streams and lakes clean, and were more engaged in recycling activities. The implications are finally discussed.
Introduction
Chemicals, chemistry and chemists are generally believed to be the underlying reasons for many environmental problems. Following this assertion, Clark and Button (2011) claimed that chemistry has a key role to play in achieving the societal, economic and environmental objectives of sustainable development. Hence, Clark et al. (2006) called for students at the tertiary level to be imparted with the knowledge of sustainable chemistry, since they are the future citizens who will be involved and engaged in making important decisions for the advancement of society and in sustaining the healthy and prosperous livelihood of the nation. The literature in the field of chemistry education suggests that one possible way of reorienting the teaching and learning of chemistry is to address sustainability through integrating green chemistry into classroom practices (Burmeister et al., 2012; Eilks and Rauch, 2012). In the past, green chemistry has been used as a teaching and learning tool in various contexts across many levels. For instance, green chemistry has been implemented as a life-cycle analysis tool (Juntunen and Aksela, 2013, 2014), while green chemistry principles have been integrated in specific cases (Kennedy, 2016) as classroom activities (Gross, 2012; Prescott, 2013; Parrish, 2014) and as laboratory experiments (Sharma et al., 2012; Graham et al., 2014; Morsch et al., 2014; Purcell et al., 2016). In the laboratory setting the experiments were either designed by the researchers or adopted from elsewhere and integrated into teaching using various active learning approaches. For the purpose of this study science writing heuristics was used as a tool to present the green chemistry experiments. Science writing heuristics embedded with green chemistry (SWH-GC) experiments were employed as a laboratory-based pedagogy and its effectiveness in improving the environmental literacy of the students compared to students who learnt the same concepts using solely green chemistry experiments was evaluated. The notion that interdisciplinary teaching and learning is required to nurture environmental literacy (EL) (Newell, 2006) stipulates that SWH-GC would be a possible means.Theoretical background
Environmental literacy (EL)
EL is an ambiguous and evolving term with various definitions provided by many writers. For instance, an environmentally literate individual must have sufficient knowledge, skills and attitudes to understand the interconnectedness of nature, people and the surroundings in making informed decisions about their daily undertakings (Elder, 2003). In a different study, EL is presented as a set of understandings, skills, attitudes, and habits that empower individuals to act in a positive way to protect the environment (Roth et al., 1982). Roth has expanded the dimensions and included values, personal investment, responsibility and active involvement in later research (Roth, 1992). In a different study collaboratively with Disinger, EL is perceived as an ability to interpret the health of an environmental system and then to take actions to improve, restore or maintain those systems (Disinger and Roth, 1992). This study hypothesizes EL as involving pro-environmental knowledge, attitudes, beliefs and behaviors (Disinger and Roth, 1992). The importance of the behavioral component in EL was further highlighted with the claim that an environmentally literate person should be able to impart the actual skills through appropriate behaviors (Coyle, 2005).Based on these four constructs (pro-environmental attitudes, beliefs, knowledge and behavior), a model that describes the EL of Malaysian pre-university students has been developed using a covariance-based structural equation modelling (CB-SEM) approach (Shamuganathan and Karpudewan, 2015). The measurement model suggests that improved attitudes, beliefs, and knowledge will consequently result in the improvement in a range of pro-environmental behaviors such as recycling, consuming environmentally safe products, buying environmentally friendly products and reporting environmental problems. For the purpose of this study, this model was used to explain the EL of the pre-university students who participated in this study as they will be engaged in making informed decisions concerning the environment.
Green chemistry
Green chemistry or sustainable chemistry focuses on protecting the natural environment through the efficient use of non-hazardous natural resources and thus eliminating or reducing the use of hazardous materials (Anastas and Warner, 1998). Integration of green chemistry into education creates the opportunity for future generations to be trained in how to achieve sustainable living. In the chemical industry, knowledge on pollution prevention among chemists was identified as being useful in designing more benign manufacturing processes (Hjeresen et al., 2000). Green chemistry is one viable approach to train chemists on pollution prevention. To this end, various approaches have been used to permeate green chemistry in the educational context.Green chemistry experiments and activities developed using socio-constructivist approaches were employed as a strategy to teach and learn chemistry for secondary level students (Karpudewan et al., 2012a). In a different study, green chemistry experiments were also introduced as a laboratory-based pedagogy in the pre-service teachers' curriculum (Karpudewan et al., 2012b). The activities or pedagogy involved learning about real-life issues during chemistry lessons, emphasizing working collaboratively in small groups, debating, making informed decisions, and solving the real world authentic problems posed to them. At the undergraduate level, students used real world examples in learning about green chemistry, explored the literature and communicated the finding with others, investigated the application of green chemistry towards creating ‘greener minds’ among the students and at the end of the course students created a mini-proposal on greening projects (Parrish, 2007). For non-majors, green chemistry principles were embedded into the discussions using active learning strategies such as problem solving and interactive lectures; the discussions coherently resulted in learning of other chemistry concepts (Prescott, 2013).
Green chemistry is also used as a medium to include societal perspectives into chemistry lessons. Issues relevant to green chemistry, for instance alternative fuels and bioplastics, were presented as controversial socio-scientific issues in the secondary chemistry classroom (Mamlok-Naaman et al., 2015). Additionally, green chemistry has also been infused while teaching about life cycle analysis of a product as a socio-scientific issue (Juntunen and Aksela, 2013, 2014). The product life cycle analysis project was introduced as a new educational socio-scientific approach to teach students about socio-scientific argumentation. In sum, green chemistry was introduced into education using various platforms including laboratory experiments, classroom activities and discussion of socio-scientific issues. In addition to the aforementioned means, through this study, science writing heuristics is suggested as another possible way to introduce green chemistry into the educational context.
Science writing heuristics embedded in green chemistry (SWH-GC)
The laboratory is an integral part of chemistry teaching and learning and many benefits are accrued when students engage in practical work. However, the traditional laboratory format that requires the students to follow recipe-like procedures hinders meaningful learning and students fail to acquire the actual benefits of the practical work (Greenbowe and Burke, 2008). Inquiry-based chemistry experiments, which are not only about conducting investigations, and collecting and gathering data, but also emphasize posing questions, proposing solutions for problems, and communicating the results, encouraging active involvement of students (Szalay and Toth, 2016), were suggested as an effective approach to promote chemistry learning (Peleg et al., 2017). In an attempt to encourage inquiry in the science laboratory, the use of science writing heuristics (SWH) was introduced (Wallace et al., 2004; Burke et al., 2006b; Akkus et al., 2007; Greenbowe et al., 2007). SWH is an approach grounded on socio-constructivist theory. Social constructivism emphasises the social contexts of learning. Knowledge is mutually built in social contexts when the students interact with one another, share their ideas and collaboratively generate a shared understanding (Jonassen, 1999). The characteristics of social constructivism are reflected in the introduction of SWH as a teaching technique that guides students to continuously negotiate and discuss with their peers to clarify meanings while preparing a laboratory report (Greenbowe and Burke, 2008). This happens when the students are engaged in constructing and testing questions, justifying their claims with evidence and comparing their ideas with others while being engaged in the laboratory work with the guide from the SWH student template. At the same time, teachers employ a pedagogical approach that enhances argumentation and negotiation with the guide of the SWH teacher template that combines inquiry and collaborative learning (Keys et al., 1999).Implementation of the SWH approach has resulted in various positive outcomes. These include improving the understanding of the concepts (Rudd et al., 2001a, 2001b; Burke et al., 2006a, 2006b; Cronje et al., 2013), ecological literacy (Balgopal and Wallace, 2009) and scientific literacy (Nam et al., 2011). Improved understanding occurs because the SWH approach enables the students to make better connections between the lecture and laboratory materials (Burke et al., 2005). This approach also enables students to be engaged in higher-order thinking skills (analysis and synthesis) to demonstrate their understanding of the concepts (Hand et al., 2004). Following the aforementioned positive outcomes and the nature of SWH that encourages argumentation, negotiation and discussion, the use of SWH corroborates well with the nature of green chemistry which also necessitates argumentation and discussions over real life issues. In this study SWH embedded green chemistry (SWH-GC) experiments were developed and employed to improve the EL of the students. Fig. 1 shows an example of a SWH-GC teacher and student template used for the laboratory activity to determine the heat of combustion of paraffin and soy wax. The original SWH teacher and student template proposed by Keys et al. (1999) was adapted and embedded with the green chemistry experiment. The integration was performed across all the stages from pre-laboratory instruction to identifying prior knowledge to exploration of the findings in the teacher template and for the students to establish early questions as well as to reflect on the outcome of the laboratory work.
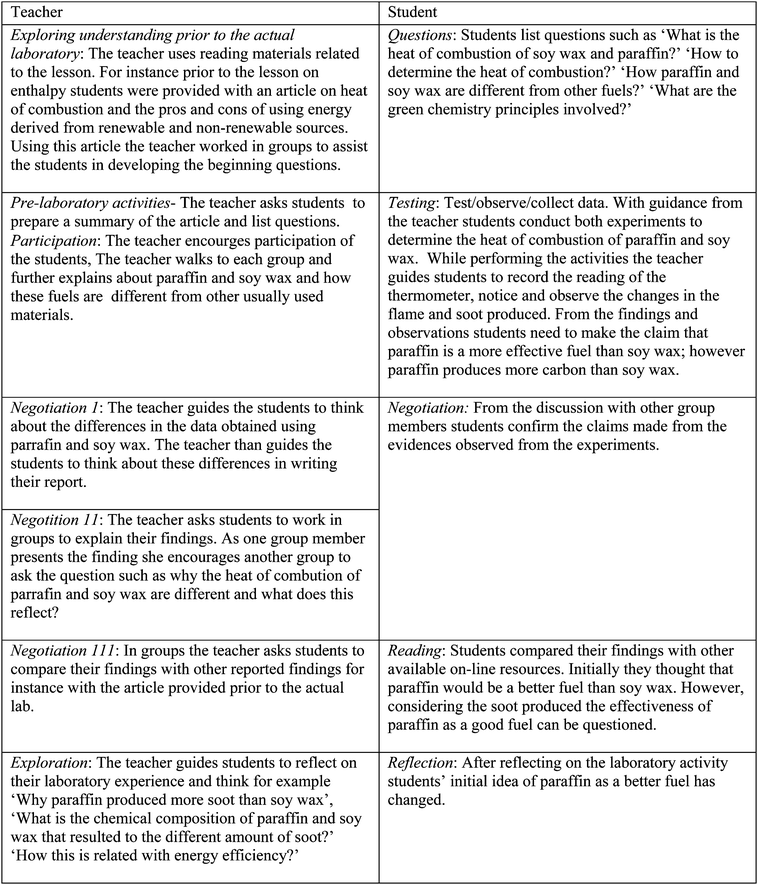 | ||
| Fig. 1 SWH-GC teacher and student template for the lesson on enthalpy: determining heat of combustion of paraffin and soy wax. | ||
Methods
Sample
The sample for this study consisted of matriculation students enrolled in the 2014/2015 academic session. The students were enrolled into matriculation studies after completing their secondary education. Matriculation studies are equivalent to pre-university level, as students after completing matriculation courses will further their studies to the undergraduate level at local universities. At the time of the study, the participating student were in semester 2. In semester 2 of the pre-university course, it is compulsory for students to study physical and organic chemistry for a duration of 11 months. A total of 210 students (130 females and 80 males) with an average age of 18 years from five different classes participated in this study. Out of the five classes three classes were randomly assigned as the experimental group (N = 120) and the remaining two classes were assigned as the comparison group (N = 90). Students were placed in different classes based on the choice of subjects they intend to study, either physical, biology or computer studies. Chemistry and mathematics are compulsory subjects for students in these five classes. Since the division is based on the choice of subject, the abilities of the students are similar across the five classes. Additionally, only students that obtained good grades in physics, chemistry, biology and mathematics in the secondary school leaving examination were provided with the opportunity to study in the matriculation colleges. As such students enrolled in matriculation colleges possess a similar level of achievement.Ethical precautions
Prior to the study, permission was obtained from the Evaluation Planning Research Department, Ministry of Education, to perform the study at the matriculation college. Upon receiving approval detailed information about the research, particularly about the treatment, was shared with the director of the college. This is to inform the director that the study would not disturb the normal educational activity in the college. With the permission from the director of the college, the participants were informed of the study. The participants were told that involvement in the study would require them to provide responses to questionnaires and that some of them would be interviewed. They were also told that they have the option either to participate or not in the study. Additionally, to ensure that the comparison group students were not deprived from the benefits of the treatment employed to teach the experimental group the comparison group students were provided with the opportunity to experience SWH-GC after the data collection session (Taber, 2014).Research design
In this study, a quasi-experimental design involving two groups was employed. According to Shadish et al. (2002), a quasi-experimental study is the most powerful method available for assessing intervention effectiveness. Quantitative data were collected from both groups using the Questionnaire on Environmental Literacy (QEL) before and after the treatment. Quantitative data collection was followed by qualitative interviews. The purpose of the interviews was to obtain further insights into the quantitative findings. The study was carried out for a duration of 8 weeks. In the first week prior to the treatment the researcher delivered a presentation for 60 minutes to introduce green chemistry and its principles, industrial applications and some examples of green chemistry in the academic context. This was followed by the administration of the QEL as the pre-test. After the pre-test, a focus group interview was conducted with 12 students from the experimental and control groups who had volunteered to be interviewed.During the treatment from week 2 to week 7, lessons on matter, stoichiometry, enthalpy of combustion, rate of reaction, state of matter, solubility and synthetic polymers were taught using the SWH-GC curriculum for the experimental group. For the comparison group these lessons were taught using the green chemistry curriculum. In week 8, the QEL was administered to both groups as a post-test and followed by focus group interviews. The same 12 students who had participated in the first interview were involved in the second interview.
Treatment
Using the laboratory activity on “Polylactic Acid (PLA)” adapted from Gurney (2008) we will illustrate the differences between the treatment received by the experimental and comparison groups in the following section.During the lab work, the teacher further encouraged students' participation using prompting questions such as ‘If you use more shredded PLA cup pieces how would it affect the product?’, ‘If you use bigger PLA cup pieces how would the reaction change?’, ‘What do you notice with the increase in the volume of sodium hydroxide?’ and ‘How would you present your findings?’ While responding to these questions, the students simultaneously developed claims that hydrolysis of PLA produces lactic acid soap and identified evidence for their claims based on the observation. They discussed their findings with other group members and referred to other available resources on recycling of plastics. Finally, students reflected on how their initial idea that plastic cannot be recycled has changed by turning PLA cups into soap. Ultimately after completing the activity, students prepared a report using the following format: questions, test, observation, claims, evidence, and reflection.
Instrument
The items in the QEL were originally obtained from the Environment Attitude Inventory (EAI) (Milfont and Duckitt, 2010). The EAI has been used in a different study and the items were reported as valid and reliable to measure environmental attitudes, beliefs, knowledge and behavior (Rodríguez-Barreiro et al., 2013). The original version consisted of 19 items. For the purpose of this study, following the CB-SEM analysis, items loaded above 0.5; items with adequate convergent validity (AVE) exceeded 0.5 and adequate internal validity CR above 0.7 are more reliable in the context of this study and were retained (Hair et al., 2009). The outcome of the CB-SEM analysis resulted in the QEL used in this study having nine items. Items with lower factor loading were removed. One of the possible reasons for items to obtain a lower factor loading in the CB-SEM analysis could be that the students had difficulty in fully understanding the real intention of the item (Byrne, 2010). Hair et al. (2009) recommended that a minimum of three items are required to provide adequate identification for the construct. However, according to Hayduk and Littvay (2012), two items are often sufficient, but three indicators may occasionally be helpful. Hence, two items for each attitude, belief and knowledge reported in this study were considered sufficient.
The interview responses were qualitatively analyzed. The responses were constantly inspected and compared and inductively analyzed. A total of four chemistry lecturers and three experts in environmental studies were involved. After multiple times of inspection and comparison a consensus was reached among the experts and the teachers' data were categorized according to attitudes, beliefs, knowledge and behavior. The inter-rater reliability was determined using Cohen's kappa agreement (Cohen, 1960) and the value 0.85 obtained indicates a good degree of agreement between the raters (Landis and Koch, 1977). The responses from each respondent were further compared between the first and second interviews to identify any changes.
Results
This study was designed to measure the effect of the treatment on students' EL. Students' EL was measured in terms of attitudes, beliefs, knowledge and behavior. As such four null hypotheses comparing attitudes, beliefs, knowledge and behavior mean scores between the experimental and comparison groups were tested. Mean and median are suggested as commend measures of central tendency. Median is suitable to be used with smaller sample size and data with outliers. For the purpose of this study, mean is more appropriate as this study involves larger sample size and the data are not skewed (Hair et al., 2009). The qualitative interview findings provided further insights into the quantitative findings.Quantitative findings on the effect of treatment on EL
The first hypothesis stated that there would be no difference in the attitudes of the post-test score between the experimental and comparison groups. This hypothesis was rejected (F(1,204) = 62.416, p < 0.00025) as the experimental group (Mexp = 4.83, SDexp = 0.26) outperformed the comparison group (Mcom = 4.13, SDcom = 0.75) suggesting that the experimental group students' attitudes improved after the treatment. The second hypothesis compared the students' beliefs between the comparison and the experimental groups. The significantly different (F(1,204) = 25.27, p < 0.00025) higher mean score of the experimental group (Mexp = 4.83, SDexp = 0.26) compared to that of the comparison group (Mcom = 4.13, SDcom = 0.75) suggested that the experimental group students developed higher level of beliefs after the treatment. In terms of knowledge, the experimental group exhibited a higher increase in the mean score in the post-test (Mexp = 5.39; SDexp = 0.79) compared to the comparison group (Mcom = 4.23; SDcom = 0.78) and the differences appeared significant (F(1,204) = 33.27, p = <0.00025). This finding suggested that the null hypothesis that there was no significant difference between the groups in the post-test after controlling the pre-test scores was not upheld. The fourth hypothesis compared the students' behavior. The experimental group students were reported to exhibit a higher mean score for behavior (Mexp = 4.88; SDexp = 0.79) in the post-test compared to the comparison group (Mcom = 4.23; SDcom = 0.78) and the difference between these scores was significant (F(1,204) = 32.13, p < 0.00025).Qualitative findings
| 1st interview | 2nd interview | |
|---|---|---|
| S1 | ||
| Experimental group | I am not interested in reading about environmental issues because I cannot do anything about it. I only read materials relevant to my studies. | I want to read more on endangered species so that I can gain more knowledge. This is because I think I appreciate the environment more if I have the knowledge. Now I understand environmental issues are wider than just the 3 Rs. By reading too I can learn and broaden my view on how to take care of the environment. |
| S3 | ||
| Comparison group | I don't read on environmental issues because I don't have the time. | I will read about environmental issues only when I am given related assignments. Lately I have not read about environmental issues because I have a lot of tutorials to be completed. |
| 1st interview | 2nd interview | |
|---|---|---|
| S4 | ||
| Experimental group | More trees are being chopped down and replaced with houses. I know that the environmental problem is definitely getting more serious and everyone must protect the environment. | The air pollution is bad because the number of cars on the road has increased and these vehicles contribute to carbon monoxide and nitrogen oxide gases, which cause acid rain. The government is also promoting hybrid cars to reduce air pollution. Besides that, renewable energy such as solar energy, wind energy and nuclear energy which are cleaner for the environment with no pollution should be used as alternative energy sources. |
| S2 | ||
| Control group | The pollution level is bad. The rivers are polluted. The amount of resources such as fish is decreasing over the years. | Lately there has been severe pollution in our country. Now having hazy days is common especially in industrial areas. The temperature of the Earth has increased over the years too. |
| 1st interview | 2nd interview | |
|---|---|---|
| S8 | ||
| Experimental group | I carry my own shopping bag which is made of cloth to avoid paying for plastic bags. | I understand that using the dryer to dry my clothes after washing does not help in any way in the conservation of energy. Now I dry the clothes in the open air to reduce the usage of electrical energy and separate my waste before throwing it. I buy environmentally friendly items. When exchanging gifts or cards, I like to buy greeting cards which are made from recycled paper. |
| S5 | ||
| Comparison group | I recycle my papers; I use both sides of the paper before throwing it away into the recycling bin. I carry refillable water bottle when I go for classes and do not buy mineral water bottles. The unwanted stuff, I throw them into the recycling bin provided by the college. | I do play my part in helping to save the environment by throwing the chemicals after each experiment into the waste bins as advised by the lecturers in my college. Besides that I carry my own water bottles and I reuse my bottles because it is safe. |
| 1st interview | 2nd interview | |
|---|---|---|
| S6 | ||
| Experimental group | Lakes and streams create beautiful sceneries therefore we have to keep them beautiful. Lakes and streams become dirty when we throw unwanted things into them. | During the monsoon season, excess fertilizers and pesticides flow into streams. These fertilizers and pesticides consist of excessive nutrients such as nitrogen and phosphorus that can speed up the growth of plants in the water. High level of nutrients causes the rivers to be polluted with algae. Decomposition of algae reduces the quantity of dissolved oxygen in the lakes. Therefore fishes and other aquatic life die. |
| S7 | ||
| Comparison group | If we keep the lakes and streams clean, more tourists would like to visit these places. Plastic bags and empty bottles which are thrown into the lakes or streams kill the aquatic life. | We must protect the lakes and streams because they are habitats for aquatic plants and marine animals. Lakes and streams create beautiful sceneries so we have to keep them beautiful but become dirty when we throw unwanted things. |
Discussion and conclusion
The quantitative ANCOVA and qualitative interview findings suggest that students who learned using the SWH-GC curriculum exhibited better improvement in attitudes, beliefs, knowledge and behavior compared to students who learned using the green chemistry curriculum. The positive outcome noticed among both groups corroborates with the notion that engaging students in the socio-constructivist approach permitted them collaboratively and individually to construct knowledge (Jonassen, 1991, 1999) and take ownership of their learning by creating multiple interpretations of the subject matter. The constructivist teaching assisted in building their knowledge (Von Glasersfeld, 1995). The findings of this study are consistent with various other studies that reported positive results following teaching using a constructivist approach to teach about climate change (Pruneau et al., 2010; Shepardson et al., 2011), improving understanding and reducing misconceptions (Treagust et al., 2011; Porter et al., 2012), improving attitudes toward the environment (Karpudewan et al., 2012b), and students' behavior (Boyes et al., 2009; Karpudewan et al., 2012b) and beliefs (Ernst, 2014).On the other hand, students who learned using the SWH-GC curriculum exhibited more positive attitudes, beliefs, knowledge and behavior. This probably was because the integration of SWH with green chemistry activities further enhanced the students' involvement in the laboratory activities. Similar to other previous studies, the SWH-GC student template required the students to frame questions, propose methods to address these questions, and carry out appropriate investigations. Students frequently negotiated their claims and evidence for their claims with peers by referring to other external resources (Keys et al., 1999; Greenbowe et al., 2007). The higher level cognitive questions posed by the teacher in the SWH-GC approach encouraged students to think deeper about the subject matter resulting in the students performing better than the others in the comparison group (Hand et al., 2004). For example, respondents in the present study were required to compare the combustion of soy wax (54 kJ g−1) with the heat of paraffin (41.50 kJ g−1) and support it with data as evidence on why soy wax has more advantages than petroleum-based paraffin. These kinds of activities helped respondents construct arguments embedded in scientific inquiry, particularly written forms of arguments which resulted in improving their behavior.
In this study EL is framed in terms of attitudes, beliefs, knowledge and behaviour. The model of EL depicts that enhancing EL is about improving pro-environment behaviours (Shamuganathan and Karpudewan, 2015). The CB-SEM analysis of the model indicated that an improvement in behaviour occurs with an improvement in attitudes, beliefs and knowledge. As the findings of this study suggest, the SWH-GC approach improved students' attitudes, beliefs, knowledge and behaviour, The SWH-GC approach would be one possible approach to improve the EL of the students.
Although the findings of this study suggest that SWH-GC is a possible approach to enhance EL, further study is warranted involving a larger sample size to generalize the findings. The quasi-experimental design using ANCOVA employed in this study claimed to be the most appropriate design in measuring the effectiveness of any treatment as ANCOVA is able to rule out other possible hypotheses that might interfere with the findings and improve the external validity (Shadish et al., 2002); however, this study exhibits several limitations. The interaction effect between the students from the experimental and control groups is controlled. Therefore, whether diffusion between the students is one of the causes for the differences observed was not ruled out. For this purpose, further study is warranted.
Acknowledgements
This work was supported by Universiti Sains Malaysia's University Individual (RUI) Grant [1001/PGURU/816279].References
- Akkus R., Gunel M. and Hand B., (2007), Comparing an Inquiry-based Approach known as the Science Writing Heuristic to Traditional Science Teaching Practices: Are there differences? Int. J. Sci. Educ., 29(14), 1745–1765.
- Anastas P. T. and Warner J. C., (1998), Green Chemistry: Theory and Practice, USA: Oxford University Press.
- Balgopal M. M. and Wallace A. M., (2009), Decisions and dilemmas: using writing to learn activities to increase ecological literacy, J. Environ. Educ., 40(3), 13–26.
- Boyes E., Skamp K. and Stanisstreet M., (2009), Australian secondary students' views about global warming: beliefs about actions, and willingness to act, Res. Sci. Educ., 39(5), 661–680.
- Burke K. A., Hand B., Poock J. and Greenbowe T. J., (2005), Using the Science Writing Heuristic: Training Chemistry Teaching Assistants, J. Coll. Sci. Teach., 35(1), 36–42.
- Burke C. S., Stagl K. C., Salas E., Pierce L. and Kendall D., (2006a), Understanding team adaptation: a conceptual analysis and model, J. Appl. Psychol., 91(6), 1189–1207.
- Burke K. A., Greenbowe T. J. and Hand B., (2006b), Implementing the science writing heuristic in the chemistry laboratory, J. Chem. Educ., 83(7), 1032–1038.
- Burmeister M., Rauch F. and Eilks. I., (2012), Education for Sustainable Development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2), 59–68.
- Byrne M. B., (2010), Structural equation modelling with AMOS: basic concepts, applications and programming, New York: Routledge.
- Clark B. and Button C., (2011), Sustainability transdisciplinary education model: interface of arts, science, and community (STEM), Int. J. Sustain. High. Educ., 12(1), 41–54.
- Clark J. H., Budarin V., Deswarte F. E., Hardy J. J., Kerton F. M., Hunt A. J. and Rodriguez A., (2006), Green chemistry and the biorefinery: a partnership for a sustainable future, Green Chem., 8(10), 853–860.
- Cohen J., (1960), A coefficient of agreement for nominal scales, Educ. Psychol. Meas., 20, 37–46.
- Coyle K., (2005), Environmental literacy in America: what ten years of NEETF/Roper research and related studies say about environmental literacy in the US, accessed January 15, 2015 at http://www.neefusa.org.
- Cronje R., Murray K., Rohlinger K. S. and Wellnitz T., (2013), Using the Science Writing Heuristic to Improve Undergraduate Writing in Biology, Int. J. Sci. Educ., 35(16), 2718–2731.
- Disinger J. F. and Roth C. E., (1992), Environmental Literacy, accessed January 15, 2015 at http://files.eric.ed.gov/fulltext/ED351201.pdf.
- Eilks I. and Rauch F., (2012), Sustainable development and green chemistry in chemistry education, Chem. Educ. Res. Pract., 13(2), 57–58.
- Elder J. L., (2003), A field guide to environmental literacy: making strategic investments in environmental education, North America: Environmental Education Coalition.
- Ernst J., (2014), Early childhood educators' use of natural outdoor settings as learning environments: an exploratory study of beliefs, practices, and barriers, Environ. Educ. Res., 20(16), 735–752.
- Graham K. J., Jones T. N., Schaller C. P. and McIntee E. J., (2014), Implementing a Student-Designed Green Chemistry Laboratory Project in Organic Chemistry, J. Chem. Educ., 91(11), 1895–1900.
- Greenbowe T. J. and Burke K. A., (2008), Instruction by using the writing heuristic. Science inquiry, argument and language: a case for the science writing heuristic, Rotterdam, The Netherlands: Sense Publishers.
- Greenbowe T. J., Poock J. R., Burke K. A. and Hand B., (2007), Using the science writing heuristic in the general chemistry laboratory to improve students' academic performance, J. Chem. Educ., 84(8), 1371–1380.
- Gross E. M., (2012), Green Chemistry and Sustainability: An Undergraduate Course for Science and Nonscience Majors, J. Chem. Educ., 90(4), 429–431.
- Gurney R., (2008), Hydrolysis of post-consumer polylactic acid waste, accessed on January 15, 2015 at http://greenchem.uoregon.edu/Pages/Overview.php?WhereFrom=ResultsAll&ID=102.
- Hair Jr J. F., Black W. C., Babin B. J. and Anderson R. E., (2009), Multivariate data analysis, Upper Saddle River, New Jersey, US: Pearson Prentice Hall.
- Hand B., Wallace C. W. and Yang E. M., (2004), Using a Science Writing Heuristic to enhance learning outcomes from laboratory activities in seventh-grade science: quantitative and qualitative aspects, Int. J. Sci. Educ., 26(2), 131–149.
- Hayduk L. A. and Littvay L., (2012), Should researchers use single indicators, best indicators, or multiple indicators in structural equation models? BMC Med. Res. Methodol., 12, 159.
- Hjeresen D. L., Boese J. M. and Schutt D. L., (2000), Green chemistry and education, J. Chem. Educ., 77(12), 1543.
- Jonassen D. H., (1991), Objectivism versus constructivism: Do we need a new philosophical paradigm? Educ. Technol. Res. Dev., 39(3), 5–14.
- Jonassen D. H., (1999), Designing constructivist learning environments, in Reigeluth C. M. (ed.), Instructional design theories and models, a new paradigm of instructional theory, New Jersey: Lawrence Erlbaum Associates, pp. 215–239.
- Juntunen M. and Aksela M. K., (2013), Life-Cycle Analysis and Inquiry-Based Learning in Chemistry Teaching, Sci. Educ. Int., 24(2), 150–166.
- Juntunen M. K. and Aksela M. K., (2014), Improving students' argumentation skills through a product life-cycle analysis project in chemistry education, Chem. Educ. Res. Pract., 15(4), 639–649.
- Karpudewan M., Ismail Z. and Roth W. M., (2012a), Ensuring sustainability of tomorrow through green chemistry integrated with sustainable development concepts (SDCs), Chem. Educ. Res. Pract., 13(2), 120–127.
- Karpudewan M., Ismail Z. and Roth W. M., (2012b), Promoting pro-environmental attitudes and reported behaviors of Malaysian pre-service teachers using green chemistry experiments, Environ. Educ. Res., 18(3), 375–389.
- Kennedy S. A., (2016), Design of a dynamic undergraduate green chemistry course, J. Chem. Educ., 93(4), 645–649.
- Keys C. W., Hand B., Prain V. and Collins S., (1999), Using the science writing heuristic as a tool for learning from laboratory investigations in secondary science, J. Res. Sci. Teach., 36(10), 1065–1084.
- Landis J. R. and Koch G. G., (1977), A one-way components of variance model for categorical data, Biometrics, 33(4), 671–679.
- Mamlok-Naaman R., Katchevich D., Malka Y., Burmeister M., Feierabend T. and Eilks I., (2015), Learning about sustainable development in socio-scientific issues-based chemistry lessons on fuels and bioplastics, in Vania Z. and Mammino L. (ed.), World Wide Trend on Green Chemistry, UK: Royal Society of Chemistry, pp. 45–60.
- Milfont L. T. and Duckitt J., (2010), The environmental attitude inventory: a valid and reliable measure to assess the structure of environmental attitude, J. Environ. Psychol., 30(1), 80–94.
- Morsch L. A., Deak L., Tiburzi D., Schuster H. and Meyer B., (2014), Green Aqueous Wittig Reaction: Teaching Green Chemistry in Organic Teaching Laboratories, J. Chem. Educ., 91(4), 611–614.
- Nam J., Choi A. and Hand B., (2011), Implementation of the science writing heuristic (SWH) approach in 8th grade science classrooms, Int. J. Sci. Math. Educ., 9(5), 1111–1133.
- Newell T. S., (2006), Rethinking information literacy learning environments: a study to examine the effectiveness of two learning approaches, US: The University of Wisconsin-Madison.
- Onwuegbuzie A. J. and Leech N. L., (2007), A call for qualitative power analyses, Qual. Quant., 41(1), 105–121.
- Parrish A., (2007), Towards greening our mind. A new special topic course, J. Chem. Educ., 84(2), 245–247.
- Parrish A., (2014), Teaching green and sustainable chemistry: a revised one semester course based on inspirations and challenges, J. Chem. Educ., 91(7), 1084–1086.
- Peleg R., Yayon M., Katchevich D., Mamlok-Naaman R., Fortus D., Eilks I. and Hofstein A., (2017), Teachers' views on implementing storytelling as a way to motivate inquiry learning in high-school chemistry teaching, Chem. Educ. Res. Pract., DOI: 10.1039/C6RP00215C.
- Porter D., Weaver A. J. and Raptis H., (2012), Assessing students' learning about fundamental concepts of climate change under two different conditions, Environ. Educ. Res., 18(5), 665–686.
- Prescott S., (2013), Green goggles: designing and teaching a general chemistry course to non-majors using a green chemistry approach, J. Chem. Educ., 90(4), 423–428.
- Pruneau D., Gravel H., Bourque W. and Langis J., (2010), Experimentation with a socio-constructivist process for climate change education, Environ. Educ. Res., 9(4), 429–446.
- Purcell S. C., Prithvi P., Yingxin L., Rivera E. J., Latisha P. U., Smallwood L. M., Kerstiens A. G., Armstrong L. B., Robak M. T., Baranger A. M. and Michelle. D.C., (2016), Extraction and Antibacterial Properties of Thyme Leaf Extracts: Authentic Practice of Green Chemistry, J. Chem. Educ., 93(8), 1422–1427.
- Rodríguez-Barreiro L. M., Fernández-Manzanal R., Serra L. M., Carrasquer J., Murillo M. B., Morales M. J., Calvo J. M. and Valle J. D., (2013), Approach to a causal model between attitudes and environmental behaviour. A graduate case study, J. Clean. Prod., 48, 116–125.
- Roth C., (1992), Environmental literacy: its roots, evolution, and directions in the 1990s, accessed January 15, 2015 at http://files.eric.ed.gov/fulltext/ED348235.pdf.
- Roth L. H., Lidz C. W., Meisel A., Soloff P. H., Kaufman K., Spiker D. G. and Foster F. G., (1982), Competency to decide about treatment or research: an overview of some empirical data, Int. J. Law Psychiatry, 5(1), 29–50.
- Royte E., (2006), Corn Plastic to the Rescue, accessed January 15, 2015 at http://web.mit.edu/3.064/www/slides/Smithsonian_corn_plastic.
- Rudd II J. A., Greenbowe T. J. and Hand. B., (2001a), Recrafting the general chemistry laboratory report, J. Coll. Sci. Teach., 31(4), 230–234.
- Rudd II J. A., Greenbowe T. J., Hand B. and Legg M. J., (2001b), Using the science writing heuristic to move toward an inquiry-based laboratory curriculum: an example from physical equilibrium, J. Chem. Educ., 78(12), 1680–1686.
- Shadish W. R., Cook T. D. and Campbell D. T., (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston, MA: Houghton-Mifflin.
- Shamuganathan S. and Karpudewan M., (2015), Modelling Environmental literacy of Malaysian pre university students, Int. J. Environ. Sci. Educ., 10(5), 757–771.
- Sharma R. K., Gulati S. and Mehta S., (2012), Preparation of gold nanoparticles using tea: a green chemistry experiment, J. Chem. Educ., 89(10), 1316–1318.
- Shepardson D. P., Niyogi D., Choi S. and Charusombat U., (2011), Students' conceptions about the greenhouse effect, global warming, and climate change, Climatic Change, 104(3), 481–507.
- Szalay L. and Toth Z., (2016), An active inquiry-based approach of traditional ‘step-by-step’ experiments, Chem. Educ. Res. Pract., 17, 923–961.
- Taber S. K., (2014), Ethical considerations of chemistry education research involving human subjects, Chem. Educ. Res. Pract., 15, 109–113.
- Treagust D. F., Chandrasegaran A. L., Zain A. N., Ong E. T., Karpudewan M. and Halim L., (2011), Evaluation of an intervention instructional program to facilitate understanding of basic particle concepts among students enrolled in several levels of study, Chem. Educ. Res. Pract., 12(2), 251–261.
- Von Glasersfeld E., (1995), Radical Constructivism: A Way of Knowing and Learning. Studies in Mathematics Education Series: 6, Bristol, PA: Falmer Press.
- Wallace C. S., Hand B. and Yang E. M., (2004), The science writing heuristic: using writing as a tool for learning in the laboratory, in Saul E. W. (ed.), Crossing borders in literacy and science instruction: perspectives on theory and practice, Newark, DE: International Reading Association & National Science Teachers Association, pp. 355–368.
| This journal is © The Royal Society of Chemistry 2017 |
