The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations
Resa M.
Kelly
 *a,
Sevil
Akaygun
b,
Sarah J. R.
Hansen
c and
Adrian
Villalta-Cerdas
d
*a,
Sevil
Akaygun
b,
Sarah J. R.
Hansen
c and
Adrian
Villalta-Cerdas
d
aChemistry Department, San José State University, San José, CA 95192, USA. E-mail: resa.kelly@sjsu.edu
bDepartment of Mathematics and Science Education, Bogazici University, Istanbul, 34342, Turkey
cDepartment of Chemistry, Columbia University, New York, NY 10027, USA
dDepartment of Chemistry, Sam Houston State University, Huntsville, TX 77340, USA
First published on 28th April 2017
Abstract
In this qualitative study, we examined how a group of seventeen first semester General Chemistry students responded when they were shown contrasting molecular animations of a reduction–oxidation (redox) reaction between solid copper and aqueous silver nitrate for which they first viewed a video of the actual experiment. The animations contrasted in that they portrayed different reaction mechanisms for the redox reaction. One animation was scientifically accurate and reflected an electron exchange mechanism, while the other was purposefully inaccurate and represented a physical exchange between the ions. Students were instructed to critique each animation for its fit with the experimental evidence and to ultimately choose the animation that they felt best depicted the molecular level of the chemical reaction. Analyses showed that most students identified that the electron exchange animation was the more scientifically accurate animation; however, approximately half of the students revised their drawings to fit with the inaccurate physical exchange animation. In addition, nearly all students thought that both animations were correct and useful for understanding salient information about the redox reaction. The results indicate that when students are shown contrasting animations of varying accuracy they make errors in deciding how the animations are supported and refuted by the evidence, but the treatment is effective. Contrasting animations promote students to think deeply about how animations fit with experimental evidence and is a promising way to engage students to think deeply about animations.
Introduction
“We are not taught to ask questions. We are taught to learn it and do well on tests, rather than understand it. …Everything is always given to you. This is what is happening…, but we don’t know why.” – S10Making decisions is a normal part of daily life. As instructors, we must make decisions about the best educational experiences to provide for students to challenge their cognitive abilities and help them develop into scientifically literate citizens. We assume that the more students know about the factors involved in their decisions, the better their decisions will be. Unfortunately, as noted in the opening quote by student S10, some students view their learning experience as void of inquiry with an emphasis on information that is best memorized for mastery. Students must not only have information, they must know how to use information and what inferences it does or does not support (Thorndike, 1997). In this study, we explored how students responded to a visualization task that consisted of viewing a video of a redox reaction with two molecular animations that conflicted in their depiction of the redox reaction mechanism. Unbeknownst to the students, one of the animations in the task was inherently flawed in its depiction while the other was scientifically accurate. The students were charged with critiquing the animations for flaws, and they were asked to decide which of the two animations best fit with the experimental evidence and best represented the reaction mechanism.
The practice of using errors to assess student understanding is not new. It is a common practice of multiple choice test construction, in which a question is posed and answers to the item include a best answer and several distractors. However, there is a paucity of research studies that have examined the use of errors in teaching chemistry. One exception is a study by Coppolla and Pontrello (2014) introducing the structured use of errors for posting exam solutions in an Organic Chemistry course called the “exam error check.” They posted one key with two different sets of solutions (Option 1 and Option 2) for all quiz, examination and practice problem items. One solution was consistent with the scientifically accepted answers while the other contained instructor generated errors. The students were never told which of the solutions was correct, but they were required to post comments to explain the solution inconsistencies. Their findings indicated that most students found the task to be helpful however, students felt it required more effort to study than having a single answer key. Another example of the use of errors in instruction was found in physics education research. Muller (2008) reported that when a participant viewed a video of two students discussing Newton's first and second laws, in which one student provided a wrong explanation, it caused the students viewing the video to express confusion. In addition, students reported having less confidence in their understanding, but interestingly, they had improved test performance.
This study offers new insight, into the practice of presenting students with molecular level animations that contain errors. We examine how students respond to two animations of a redox event in which one of the animations presents a scientifically acceptable explanation consistent with experimental evidence in contrast to another animation that presents an inaccurate representation of the same reaction mechanism. We also examine which animation students believe is a better representation of molecular level events.
Animations taught with macroscopic events
Animations that portray the molecular level of chemical reactions have consistently been shown to be useful for presenting the abstract nature of matter and assisting students’ conceptual development (Kozma and Russell, 1997; Ardac and Akaygun, 2004; Tasker and Dalton, 2006; Marbach-Ad et al., 2008). Perhaps the greatest attribute of animations is that they represent the dynamic, interactive and multi-particulate nature of chemical reactions explicitly “and assist students in imagining the nature of physical evidence at the submicroscopic level” (Tasker and Dalton, 2006). Several studies have specifically examined how atomic level visualizations partnered with macroscopic phenomena in the form of demonstrations and laboratory activities have been particularly effective in improving student achievement and representational competence in chemistry (Velázquez-Marcano et al., 2004; Tasker and Dalton, 2006; Ryoo and Linn, 2014). For example, Tasker and Dalton (2006) reported that students developed more vivid mental imagery of reaction phenomena and had greater confidence in their images when they viewed a VisChem animation partnered with viewing the intricate crystalline nature of silver crystals on a copper surface. To uncover the attributes that led to effective learning from VisChem animations, Tasker and Dalton conducted a multiple regression analysis in a post-test design study. They found that the highest post-test scores were obtained by students with high prior knowledge and high disembedding ability, meaning students had the ability to discern details in visual displays. Interestingly, while students with high prior-knowledge scored the highest on their post-test understanding, students with low prior knowledge who had high disembedding ability had the greatest measured gains. They recommended highlighting key features in animations to ensure that students can extract visual information.Kelly and Jones (2007, 2008) examined how students’ understanding of sodium chloride dissolution was affected by viewing two different molecular animations of the same event. Their findings revealed that students developed enhanced conceptual understanding from viewing both animations, but their learning was uneven as some students retained misconceptions or even developed new ones (Kelly and Jones, 2007). In addition, students reverted to their past representations when they were presented with a different context in which an aqueous solution of sodium chloride was involved in a precipitation reaction (Kelly and Jones, 2008). This was an early indication that animations used as an explanation were less effectual than hoped.
In another study, focusing on the connection between macroscopic and submicroscopic levels, Velázquez-Marcano et al. (2004) reported that students’ understanding of dynamic fluid equilibrium improved after they viewed either a video demonstration or an atomic level animation. However, students’ understanding improved the most when both video demonstration and animation were shown regardless of order. Velázquez-Marcano et al. contend that students were challenged to accept video evidence that contradicted their understanding of how things work and animations assisted with understanding the macroscopic evidence.
Kelly and Akaygun (2016) reported that when students viewed molecular visualizations in the scaffolded context of a tutorial, they recognized variation between their mental models and the tutorial models very well, and the students could recall explicit structural and mechanistic differences. However, they noted that despite this improvement, students may not fully understand how or why these atomic level representations and mechanisms account for macroscopic evidence. Animations assist students in making sense of experimental evidence, but sometimes students have difficulty understanding that the animations are representing the submicroscopic level of the macroscopic events. They fail to grasp the connection between the two levels. The effectiveness of animations depends on whether learners have sufficient cognitive resources to perceive and process the essential information in dynamic visualizations (Plass et al., 2009). Students may not fully comprehend the limits of models, and they need help to explore their limitations (Ye and Lewis, 2014).
Insights into the learning process
Chemistry has been deemed to be a challenging subject by many chemistry learners primarily due to the complexity of there being three ‘levels’ at which the learning of chemistry operates (Taber, 2013). In 1993, Alex Johnstone first raised awareness of the inherent complexity associated with students’ ability to master the interplay between three components of chemistry, later termed “the chemistry triplet” by other researchers (Talanquer, 2011; Taber, 2013). The components were: the submicroscopic level of atomic and molecular species and their interactions, the macroscopic level of the experimental lab setting with its tangible and visible results, and the representational level of symbols and equations. Johnstone observed that students did not seem to master chemistry to the same extent at all three levels due to the overwhelming task of thinking about very different types of things simultaneously (Taber, 2013).Talanquer (2011) provided a descriptive summary and analysis of different adaptations and reinterpretations on the paradigmatic triplet relationship in chemistry and science education. He provided evidence that the chemistry triplet had been further characterized at each level and, of significance to this study, provided deeper characterization of the submicroscopic and macroscopic levels. The submicroscopic level was further divided into three conceptual levels: the molar (bulk properties), the molecular (dynamic interaction of atoms and molecules, which is the primary purpose of the visualizations in this study) and the electrical (subatomic components). In addition, there were various formalisms for representing atomic and molecular structures and lattices relating to different aspects of our structural models (for example, bonding and charge distribution) (Taber, 2013). Similar to the submicroscopic level, the macro level was also adapted and took on various meanings, one perspective described macro as “bulk properties of matter (pH, temperature, pressure, density and concentration).” Another perspective, more relevant to this study, described the macro level as the “actual phenomenon and the concepts used to describe them” (Taber, 2013). As separate levels these components are challenging, but to explore how the macro level can be explained with molecular level models is extremely challenging and requires considerable practice on the part of students to build coherent and integrated mental models of phenomena.
Øyehaug and Holt (2013) conducted a longitudinal study to investigate how students’ understanding of matter and chemical reactions evolved over two years in a variety of lesson contexts. Their study suggests that learning results when students reorganize ideas and connections in productive ways; however, part of the reorganization involves forming wrong conclusions. Øyehaug and Holt (2013) advise that students will communicate incomplete and fragmented understanding, because there is inherent complexity associated with how they reorganize their understanding of matter and chemical reactions. The learning processes involve adding and integrating ideas, but also differentiating them from each other. Sometimes students promote an idea by connecting how a concept applies to other areas. Students also tend to coalesce ideas, a process in which students merge two ideas such as how atoms are involved in reactions and in cells. Different contexts such as interviews, teaching situations and subject contexts, can also affect students’ ability to express their understanding and how they organize the relationship between ideas. “Conceptual change seems to evolve from disjointed sets of context dependent ideas toward a more integrated cohesive perspective” (Øyehaug and Holt, 2013). In relation to this study, the animations depict the reaction mechanism differently, thus assuming viewers recognize this variation as they critique the models, the activity may create a disjointed context as students try to decide which animation makes most sense based on its fit with experimental evidence. While the task may be confusing to students, the confusion and struggle may assist students toward developing their understanding of the reaction.
In general, students’ cognitive processing of the macroscopic level differs from their processing of submicroscopic content (Al-Balushi and Al-Harthy, 2015). Understanding the macroscopic level is perceived as less abstract for students than learning about the submicroscopic level, since it is tangible and can be seen, touched and smelt. Al-Balushi and Al-Harthy (2015) reported that when reading passages about macroscopic content, students’ minds wandered less than when they read submicroscopic content and this was attributed to the macro level: being less abstract, requiring less spatial reasoning, and being less demanding on cognitive load. Students tended to estimate size and scale at the macro level more precisely than they did at the submicro level and there was less denial of entity existence. However, Taber (2013) contends substances commonly used in chemistry labs are already a major abstraction from students’ real-life experience. For example, when students observe chemical reactions they must learn how the reaction results in a change into a different substance that has different properties. Thus, the conceptual demand is high even at the macro level although perhaps not as demanding as the submicroscopic level which is considerably more abstract.
The submicroscopic level requires students to trust in the existence of different unobservable theoretical entities (atoms, ions and molecules) to make sense of their behaviour and interaction to explain observable phenomena (Taber, 2013; Al-Balushi and Al-Harthy, 2015). Kelly (2014) investigated how students revised atomic level pictures depicting the conductivity properties of aqueous solutions after viewing several atomic level visualizations of solutions tested for electrical conductivity. She also observed that students had difficulty conceptualizing the particulate nature of matter and misconceptions were difficult for students to let go. However, she noticed that students incorporated several aspects of the visualizations, especially when they recognized variance between their understanding and that which was depicted in the visualizations. Similar to Øyehaug and Holt (2013), she noticed that students demonstrated imperfect understanding as they progressed. Most students were influenced by very general characteristics such as structural features of the substances that were animated and the general movement of particles.
A component that is critical for constructing understanding from animations is the way students are asked to engage with the animations. Thus, it is important to consider how learning and interaction occurs and how to characterize it. Active learning can be defined by the level of student's engagement with learning materials, which in turn can be operationalized by the overt behaviors students undertake while learning. Chi and Wylie (2014) characterized and differentiated overt learning behaviours into four behavioral modes: passive, active, constructive and interactive. The passive mode of engagement was defined as learners being oriented toward and receiving information from the instructional materials without overtly doing anything else related to learning. For example, when students watch a video without doing anything else; they are passively learning. When students exert some form of overt motoric action or physical manipulation while learning it is classified as an active mode of engagement. For example, when a student manipulates an animation by pausing, replaying, or fast-forwarding this is termed active manipulation. Constructive behaviors are defined as those in which learners generate or produce additional externalized outputs or products beyond what was provided in the learning materials. For example, having students explain or draw their understanding of concepts that they viewed in an animation or getting them to compare their prior knowledge to what they see in the animation are examples of constructive behaviors. The final mode of engagement is “interaction” and this is defined by dialogues in which the student contributes to a group constructively. There is a sufficient degree of turn taking when a student debates with a peer about the justification of the video or when students discuss similarities and differences between two animations. In this study, the treatment possessed active, constructive and interactive aspects and we explored how students’ understanding through oral and drawn explanations was affected in their effort to choose the animation that best fit with experimental evidence.
Redox understanding
Understanding oxidation–reduction reactions poses a significant challenge to students, and students can have considerable difficulty conceptualizing the nature of this broad group of reactions (Allsop and George, 1982; Garnett and Treagust, 1992; DeJong et al., 1995; Schmidt and Volke, 2003; Stains and Talanquer, 2008; Österlund and Ekborg, 2009). Several chemistry textbooks focus on a wide range of physical properties associated with redox reactions before introducing oxidation states to identify redox reactions. In addition, vocabulary terms are introduced to describe oxidizing and reducing agents, which makes it challenging for students who have not achieved the same language competence as instructors (Österlund et al., 2010). Lending insight into how students worked with redox reactions, Stains and Talanquer (2008) reported that students focused on charge to identify a reaction as a redox reaction and students struggled with the meaning of oxidation states and oxidation numbers.Bandriet and Bretz (2014) in reporting on the development of their Redox Concept Inventory (ROXCI) conducted an exhaustive literature search and observed that much of the literature focuses on eliciting students’ understandings at the symbolic level with chemical equations and symbols. Through qualitative studies that they completed to design ROXCI, they identified six major misconception themes with the following descriptions: “(1) oxidation numbers – the application and understanding of oxidation numbers, (2) surface features – using surface features of a chemical equation to identify whether or not a reaction is redox, (3) electron transfer – the role of electron transfer in a redox reaction, (4) spectator ions – the role of spectator ions in single-replacement reactions, (5) dynamics reaction process – the dynamic nature of particles, (6) electrostatics and bonding–bonding, charge attractions, or replacing charges between charged species in redox reactions” (Bandriet and Bretz, 2014). In general, learning about redox chemistry is laden with challenges that may make it difficult for students to comprehend information presented in molecular level animations.
Rosenthal and Sanger (2012) described how second semester general chemistry students responded to two animations depicting the oxidation–reduction reaction between solid copper and aqueous silver nitrate after students first were shown a demonstration of the reaction. One animation was a simplistic animation, designed by Michael Sanger, that depicted two silver ions colliding with a copper atom and exchanging electrons. No water molecules were depicted, except the solution color was blue which was consistent with the iconic representation of water. In addition, nitrate ions floated about in the open space. The second animation was a more complicated and sophisticated version of the same reaction event and incidentally was also the electron exchange animation that was used in this study. It was designed by Roy Tasker as part of the VisChem project. The animation showed how the silver ion was transported toward the copper lattice by water molecules, an electron cloud exchange occurred resulting in neutralizing the silver ion, while a distant copper ion was formed and attracted by water molecules into solution. Rosenthal and Sanger reported that students had difficulty interpreting the two different animations even though the animations provided different perspectives of the same event. They observed that students who viewed the more simplified animation gave better explanations for redox reactions than those who viewed the complex VisChem animation. Several misconceptions were identified that may have resulted from students misinterpreting information in the animations, such as confusing water molecules for nitrate ions and confusing ions with neutral atoms. Rosenthal and Sanger (2012) contended that the VisChem animation was difficult for students to comprehend, and details of the complex animation were likely distracting. In 2013, Rosenthal and Sanger published another study using the same animations, but this time they studied the sequence of viewing the simple animation before the complex animation and the complicated animation before the simplistic one. They found significantly greater improvement when students viewed the simplified animation prior to the complicated one and they attributed this improvement to the simplified animation serving as an instructional cue. In general, they noticed that students were challenged to connect macroscopic evidence with the animations.
This study builds on Rosenthal and Sanger's work; however, it differs in two major ways. First, we replaced the use of a live demonstration activity with a video showcasing additional experimental information. For example, the salt solutions, aqueous silver nitrate and copper(II) nitrate, were made and tested for electrical conductance, as was pure water. A copper wire was added to each solution. The solutions were tested for electrical conductance at the end of the experiment after the wires were removed. Second, students were tasked with critiquing two contrasting animations, one unbeknownst to the students contained errors, to determine the animation that best fit with the evidence. This change gave students incentive for viewing the animations as they were required to use the experimental evidence to decide which animation was best. This study varied from Rosenthal & Sanger's study as both animations in their study served as scientifically accurate animations.
Theoretical framework
Variation theory
The theory that guided this work was phenomenography and specifically, variation theory which has its roots in phenomenography (Bussey et al., 2013; Kelly, 2014). As a result, both are briefly defined for the way they framed this study. Phenomenography is “a method for mapping the qualitatively different ways in which people experience, conceptualize perceive and understand various aspects of, and phenomena in, the world around them” (Marton, 1986). Phenomenographers try to characterize how things appear to people, because people both perceive and experience things differently. In this study, we studied how students perceived and experienced molecular level animations that differed from each other. The animations under investigation were designed to contrast with each other mechanistically in terms of how the reaction happened. However, they also had some structural features in common, such as the color and size of the atoms and ions. With variation theory, the goal was to learn how students would take in information from these two animations and how they would detect the similarities and differences between them, and ultimately how they would use their connection to experimental evidence to decide which animation was the more scientifically accurate of the two. A goal of variation theory is to explain how people can experience the same phenomenon differently and how that knowledge can be used to improve learning from animations (Bussey et al., 2013). The presence of variation between the animations was intended to make it obvious that the animations were noticeably different in their reaction mechanisms, drawing attention to the mechanisms. The students should have wondered why in one animation there was an exchange of electron cloud, while in the other animation, the atoms seemed to physically exchange. Variation theory contends that the act of noticing is related to three key processes that include: awareness of differences, being able to discern what the differences are, and being simultaneously aware of multiple critical features. Under investigation was how students would be able to discern several aspects of the animations under investigation based on the variation they detected.Learning theory and engagement
The design of the visualization task, consisting of the video and animations, was constructed differently from the theoretical framework that shaped the study. It was developed using a constructivist learning theory lens, in which learning from activity is constructed by the student. In constructivism, when people gain knowledge they are trying to make sense of new information based on their previous ideas and understanding, and ultimately knowledge resides “inside the mind of the learner” (Bodner, 1986). Since past studies revealed that learning from animations could be uneven and that students often only modified structural changes and general movement after viewing them, we were motivated to seek a way to use animations that required deeper reflection of the animated events (Kelly, 2014; Kelly and Akaygun, 2016). Thus, we conceived the idea that animations would be placed in contrast to each other, one of the animations would have errors, and it would be the student's responsibility to determine which animation was best through its fit with experimental evidence. We believed that a task that required students to deeply reflect on the animated processes had the potential to lead to conceptual change. Posner et al. (1982) defined conceptual change as the “process by which people's central, organizing concepts change from one set of concepts to another set, incompatible with the first”. Changes in knowledge that students experience can occur at various grain sizes, meaning that some students may make substantial gains that are drastically different from their initial understanding while others may make more subtle changes through the refinement of existing ideas (Chi, 2008). In our study, we focused on knowledge changes that students made to their understanding of the submicroscopic level of a redox reaction as they tried to decide which animation was a best fit with the experimental evidence. Specifically, we examined new inferences students made after the learning activity. Inferences were revealed through actions of revising and repairing hand drawn representations and reflection about why changes were made.Research question
The main goal of this study was to examine how students charged with critiquing conflicting animations were affected by the task and how it affected their pictorial representation of the reaction. The following research question guided the study:• How does viewing and reflecting on conflicting animations affect students’ understanding of a reduction–oxidation (redox) reaction?
Method
This Institutional Review Board (IRB) approved study took place at a comprehensive public university in the western United States. There were 17 students (10 females and 7 males) whose ethnicity were consistent with the university's demographics, in which 32% were Asian, 23% were Hispanic, 22% were white (non-Hispanic) and the remainder reflected a mixture of ethnicities. The students were enrolled in their first semester of general chemistry and were invited to participate through oral announcement. Students whose schedules permitted them to participate were each interviewed one time for approximately two hours per session. The interviews took place over the course of a month from November to December. At the start of the study, all the students had completed laboratories that investigated the conductivity of a variety of substances using a hand-held conductivity tester. They had completed a lab on the activity series of metals, and they were taught how to balance simple reduction–oxidation (redox) reactions, also referred to as single replacement reactions and complex redox reactions using the half-reaction method.During the sessions, students viewed a video of an experiment. The video was made by Resa Kelly as she conducted the experiment. The iPad 2 camera was positioned on a stand approximately 1 meter from the experimental setup and faced it directly. In the video, two solutions: aqueous silver nitrate and aqueous copper(II) nitrate were made from their dry salts mixed with pure water. The pure water was tested for electrical conduction using a handheld conductivity tester and the resulting aqueous salt solutions were also tested. Next, a copper wire coil was added to each beaker to soak in the solutions. During this time, the camera was zoomed in to focus only on the test tube undergoing the reaction. After approximately eight minutes, the camera was zoomed out to capture the full experimental setup, and the wires were removed. The solutions were once again tested for electrical conduction. Lastly, the copper wire that had reacted with aqueous silver nitrate was scraped of its outer coating and copper metal was revealed underneath. The video was approximately fifteen minutes long and the students viewed it only once. They were allowed to control the video as they desired, but most simply let the video play without interruption. The students were permitted to take notes during the video, but most did not pause the video to do so.
After viewing the video the students were asked to orally describe what they saw. Then they wrote a list of the events or main features that were represented in the video. Next, students were shown three still images from the video: (1) before the reaction had time to occur, (2) after 8 minutes had passed, (3) after the wire was removed from the solution to stop the reaction. They were given a worksheet with blank boxes to construct atomic level pictures of the segmented reaction events. They reflected on their drawings and constructed a written list of the features they represented. Students were interviewed to gain a richer sense of their understanding. During the semi-structured interview students were asked the following questions: What features do you feel are supported by the experimental evidence? Did you construct any features that might not be supported by the experimental evidence? How did you decide to include or exclude these features in your drawings? Additional questions were asked to probe for student understanding as necessitated by each individual interview.
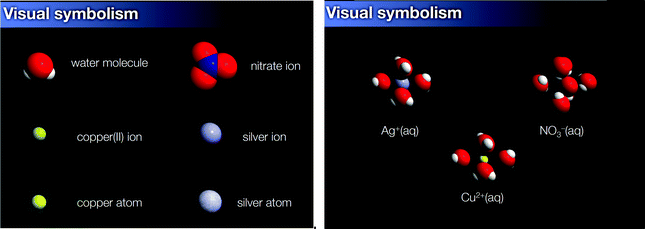
After the video session, students viewed two conflicting animations separately and they were asked to compare and contrast the animation features to the list of features they represented prior to viewing the animations. This part of the study was analysed separately (Kelly, 2017). During the interview, students were asked to consider if the animations were valid representations of the reaction event based on their fit with the video of experimental evidence. Each animation was accompanied by a key (Appendix I) identifying the species in each animation and the keys were reviewed with each student before the animations were played. The animations differed in how they portrayed the reaction mechanism and consequently, the features unique to these mechanisms were identified (Table 1) (Kelly and Hansen, 2017). The “inaccurate” animation represented the reaction mechanism, without narration, as a physical exchange and from this point forward will be referred to as the Physical Exchange Animation (PEA). Specifically, it depicted the following incorrect features: the reactant, silver nitrate, was represented as two molecules that appeared to move quickly and with direction with the nitrate end of the molecule directed toward the lattice. The collision appeared to cause the molecule to break apart. The two, freed nitrates bonded to a copper atom (exchange) on the lattice surface (Fig. 1). The animation did not distinguish neutral atoms from ions. Lastly, a molecule of copper(II) nitrate went into solution. Water molecules were not involved in the reaction and there was no interaction between the water molecules and the other species. Only two water molecules were shown moving through space, which was consistent with how students often represented water molecules in reactions (Kelly, 2014). The only accurate features were the depiction of the copper lattice and the silver atoms adhering to the copper surface.
| Electron exchange animation | Physical exchange animation | |
|---|---|---|
| Depiction of aqueous silver nitrate | Ions, as noted by the absence of valence electron clouds, are separated and surrounded by water molecules | The atoms are intact as a formula unit or could be perceived as a molecule. There is no discernible way to distinguish that the atoms are ions. (incorrect model) |
| Depiction of copper lattice | 3-Dimensional lattice, comprised of multiple rows of yellow copper ions, each surrounded by valence electron clouds to imply that they are neutral. The atoms are spaced so that their valence electron clouds touch each other. | 2-Dimensional lattice, comprised of multiple rows of yellow copper spheres. The atoms have no space between them and touch. |
| Depiction of solvent water | Water molecules surround the copper lattice and the aqueous ions. They fill the solvent space and move randomly and chaotically. | The solvent is portrayed as grey empty space with only two separate water molecules depicted. They pass by the lattice unaffected. (incorrect model) |
| Reaction mechanism |
• Hydrated silver ions move toward the copper lattice.
• A neutral silver ion gains some of the electron cloud from the copper lattice to form a neutral silver atom. • Water molecules hydrating the silver ion move away from the neutral silver atom. • Additional ion of silver gains “one electron's worth of electron cloud” at another part of the lattice. • Elsewhere on the lattice, as the silver ions are being reduced, a copper atom loses its valence electron cloud. • Water molecules attract to and surround the newly formed copper(II) ion then enter the solution. |
• Two silver nitrate molecules move rapidly toward the copper lattice.
• The nitrate ends of the molecules collide with the copper surface first and then the compound breaks apart. • The two nitrates bond to a copper atom. • The copper(II) nitrate moves away from the lattice. • The two silver atoms left behind, adhere next to each other on the copper surface. |
| Depiction of silver build-up | 3-Dimensional crystal of grey silver ions surrounded by valence electron clouds that touch. The silver atoms bond to the copper surface and to each other. | Only two grey silver atoms are shown to adhere to the copper surface and each other. The representation is 2-dimensional. |
| Depiction of aqueous solution following the reaction | The solution consists primarily of water molecules, randomly moving. Occasionally, a nitrate ion is shown surrounded by water molecules and moves through the solution, uninvolved in the reaction. Hydrated copper ions enter the solution from the lattice. | A single copper(II) nitrate molecule enters the solution. One water molecule moves in the solution and by the lattice unaffected. The solution is mostly grey empty space. |
| Macroscopic connection | None was depicted in the animation |
The animation begins with a single test tube filled with a coiled copper wire submerged in a clear and colorless aqueous solution before it zooms into the submicroscopic level.
The animation ends with a single test tube filled with a copper coil now covered with a blackish-grey substance, submerged in a clear and blue aqueous solution. |
| Length of the animation | 3 minutes and 53 seconds | 21 seconds |
 | ||
| Fig. 1 An image from the incorrect Physical Exchange Animation (PEA) showing two nitrate ions bonding to a copper atom after they break from their bond to silver atoms (Kelly and Hansen, 2017). | ||
The “accurate” animation represented the reaction as an electron exchange from neutral copper atoms to silver ions and from this point forward will be referred to as the Electron Exchange Animation (EEA; Fig. 2). The animation began with a view of the copper lattice described by the narrator as “vibrating copper ions in a sea of valence electrons.” A hydrated silver ion approached the copper lattice where the ion was described as gaining some of the electron cloud from the copper lattice to form a neutral silver atom. The electron transfer was shown as a transfer of a white opaque aura (the valence electron cloud) from copper atoms (yellow) to the silver ion (gray) (Fig. 2). A valence electron cloud was used in place of physically representing electrons, because electrons would not be visible under the scale restraints of the atoms that were depicted.
 | ||
| Fig. 2 An image from the accurate Electron Exchange Animation (EEA) showing the exchange of electron cloud from copper atom to silver ion (Kelly and Hansen, 2017). | ||
The hydrating water molecules that surrounded the silver ion moved away. This sequence was repeated for another silver ion. The animation showed and described that at another part of the lattice, water molecules hydrated a copper ion and removed it from the lattice. The narrator stated, “in effect, a copper ion leaves behind its share of two electrons in the electron cloud.” The narrator instructed the viewer to notice that the copper atom lost electrons at the exact same time that the silver ion gained an electron, but at different parts of the lattice. The viewer was forewarned to look for the nitrate ion that would move by and the narration stated that the nitrate ion was a spectator ion. The viewer had to infer that a spectator ion was a non-reacting species. The reaction between the silver ions and copper lattice repeated, and the narrator asked that the viewer focus on the competition between the water molecules and copper's electron cloud for the positively charged silver ion. The animation made this look like a “tug of war” between the water molecules and copper's electron cloud. In the final stage of the animation, the narrator asked the viewer to notice that a silver crystal formed on the surface of the copper lattice while copper ions left the lattice. The narrator further explained that the silver ions bonded to a growing silver crystal as well as to the copper surface and an explanation for why silver built up on the copper surface was provided.
The first ten students were shown the PEA prior to the EEA, while the last seven students viewed the EEA first. This was done to examine whether the order in which the animations were shown would affect students’ response to the animations; however, it was found to have no effect. In the final phase of the study, students were asked: “How do you determine which animation is a better representation of the reaction event? Do you believe one animation is a better animation? Then students were given the opportunity to redraw their atomic level understanding of the redox reaction involving solid copper reacting with aqueous silver nitrate to examine how the different animations influenced the features they depicted. In this manuscript, we focused on the analysis of students’ understanding before and after the animation treatment.
Data analysis
The data analysis began by examining students’ oral, written and drawn accounts of the redox reaction presented in the video. During the molecular level drawing task students were asked to describe their understanding of the reaction in three stages: (1) at the start of the reaction, before the reactants had time to react, (2) after 8 minutes or during the reaction, and (3) at the end of the reaction, when the copper wire was removed from the solutions. The first author (Kelly) coded the molecular level descriptions using a constant-comparative method (Glaser and Strauss, 1967) whereby students’ oral, written and drawn accounts were compared for similar properties. Next, students’ atomic level descriptions were coded against the animation features that were represented commonly in both animations, and against the features that varied between the animations. In order to organize the data for coding, animation features were sequentially listed in the first column of three data tables (Tables 3–5) and each student was listed, by code name, horizontally in the top row of the table. If the students’ description matched with the animation feature, a “c” was placed in the table to indicate complete understanding. This gave us insight into how closely aligned students’ understanding was to the animations before they viewed them. However, in a few cases this was sometimes challenging as students might provide an explanation that was too simplified to be considered an exact match or they might exhibit incomplete or wrong ideas. For example, if a student orally described that electrons were transferred between nitrogen and oxygen, but not between silver ions and copper atoms, we would recognize that at the very least they were aware that electrons were transferred. In these cases, a lighter shade of cell color and the letter “p” representing partial understanding was recorded in our tables to identify that understanding was not a perfect match with our codes. Next, the videos and transcriptions were shared with the second author (Akaygun) who then independently coded the data against the animation characteristics constructed by Kelly. The authors discussed their coding discrepancies and resolved their differences to reach 97.4% agreement. In cases where the researchers disagreed an average of their codes was used and this was also recorded as a “p” and with a lighter shade of cell color.Results and discussion
Analysis of students’ understanding before viewing contrasting animations
A comparison of students’ molecular level explanations of the reaction was performed to develop understanding of students’ molecular level conceptions prior to viewing the contrasting animations. Reoccurring regularities in students’ drawn and oral explanations revealed four challenges students had with conceptualizing the molecular level: (1) difficulty in representing the molecular level without the macroscopic level, (2) failing to distinguish between neutral atoms, ions, ionic compounds or molecules in drawn representations, (3) unrealistic representation of spacing between chemical species, (4) inability to identify chemical species that caused the macroscopic changes.Students found it very difficult to draw the molecular level and expressed that it was challenging in their oral comments. Many students (13 of 17) drew pictures that emphasized macroscopic features of the reaction even though they were asked specifically to draw the molecular level. For example, many students (10 of 17) drew circles or dots strewn along a coiled wire, matching the look of the coiled wire shown in the preceding video, to represent the atomic level of copper. When students were asked to account for why they felt it necessary to draw this macroscopic connection, some indicated that they did not know what the molecular level would look like and they needed the macroscopic level to establish the connection.
I mean I don’t really know what an atom would look like in the middle of a reaction like it's changing into a different form…you wouldn’t really be able to recognize it if I drew just one atom next to another. – S11
It's hard to visualize it. I feel like it's not something that you usually picture so it doesn’t remind you of anything that you know. You can’t associate it with anything. It's just its own thing. – S1
I wasn’t quite sure how to show my understanding of everything without including that (macroscopic level). – S16
The difficulty students had conceptualizing the molecular level was also revealed through students’ drawn representations of chemical species and the spacing between them. Many participants drew single circles or dots to represent silver nitrate as one particle and they spaced these scattered throughout the solution. Most students did not distinguish between atoms, ions or molecules, and they represented the molecular level as a jigsaw puzzle of circles that together made the macroscopic picture (Fig. 3). For some, expressing the nuances of atoms, ions and molecules was deemed unnecessary, because these particles were extremely small.
 | ||
| Fig. 3 Examples of students’ (S5, S11, and S2) drawings made before viewing the animations showing their emphasis on the macroscopic level. | ||
When I think of atoms I think of really small things, a dot is kind of like the smallest thing you could use to represent an atom…when you think about dots you just think about small things and that's the same thing with atoms and so that's how I came up with it. – S6
Many students (10 of 17) had an unrealistic understanding of spacing between solute and solvent species. The challenge students had mentally picturing the molecular level was consistent with findings reported in previous studies and may signify that students lacked practice with conceptualizing scale.
In general, students found it very difficult to imagine what the molecular level looked like because of the abstract nature of having to draw what they could not see, but there was the additional challenge of figuring out what the substances were that caused the reaction. In the case of silver forming on the surface of the copper, students recognized that the substance on the wire was chemically different from the copper, even if they were unsure of its chemical identity.
I didn’t really know what was happening, just that it forms a weird substance after, but I just thought that maybe the copper and the ions from the copper just reacted with the chemicals around it. – S9
The challenge to ascertain what formed on the surface of the wire was evident in that a group of seven students conveyed, in both their written and oral observations that the reaction between silver nitrate and the copper wire was due to the silver nitrate adhering to the copper metal (Table 2). Excerpts and pictures from two students, S6 (Fig. 4) and S7 (Fig. 5), who expressed these beliefs and who were representative of the group are provided.
| Student | Written descriptions of molecular representations |
|---|---|
| S4 | An unknown compound or element is reacting with Cu and sticking between the gaps. |
| S5 | I began to draw the black circles (silver nitrate molecules) completely on the copper molecules (orange) to represent what rxn is taking place and to make sense of why the wire turned black. I then stopped surrounding the wire with free floating black circles because all of the silver nitrate molecules coated the copper molecules |
| S6 | Silver nitrate atoms interacting with copper atoms, with the wire removed from test tube: silver nitrate atoms bonded with the copper atoms. |
| S9 | The chemicals already in the tube start attaching itself to the copper molecules. Reaction occurs the atoms in the copper stick to atoms of the chemical. Copper is removed with the other atoms still attached to it |
| S10 | AgNO3 molec. and Cu mingle together, start of reaction. Cu and AgNO3 molec. moving fast, reaction is occurring. Cu and AgNO3 molec. moving closely together, towards the outside of the Cu wire (pack). Cu and AgNO3 molec. are moving, but tightly together. |
| S11 | The solution is reacting with the copper wire, copper atoms are being released into solution and new substance is forming on copper, represented by orange dots. Substance has completely covered copper wire, more atoms of copper and substance released into solution. Solution contains atoms of nitrogen, silver, hydrogen, oxygen, but not copper or substance. Wire, removed still contains atoms of solution. |
| S12 | The AgNO3 atoms are attaching to bits of the copper atoms and they are taking off atoms from the copper wire structure, thus making it thinner and making more copper atoms float inside the solution. |
S6: You could see that something happened, copper changed, so my picture shows that the silver nitrate atoms actually bonded with the copper atoms.
R: How did they get there?
S6: I guess there has to be some kind of force that would make them attract to the copper atoms.
R: Any idea what that force is?
S6: I don’t have the terminology. I don’t know. I guess it's just the reaction that copper has with silver nitrate.
S7: The silver nitrate reacted with the spiral and at the end you could scrape off the black that built up during the experiment. I believe that the spiral and the silver nitrate formed during the experiment. In my head, I’m thinking that it got attached to the spiral because it couldn’t go anywhere else. …I don’t know if it's actually like that…I don’t really have evidence for these pictures.
In addition, students were challenged to determine a way to represent the solution's color change finding it challenging to account for why the color change occurred.
When you scraped off the outer part, like I don’t know, I mean I know that's from the chemical reaction, but I am not sure how that formed is a big one. …. Also, the color change of the solution, I don’t know if it was the reaction between copper and the water or in the liquid when it dissolved copper, but I don’t know how it turned from clear to blue. – S10
Copper nitrate when dissolved in water will give you kind of a bluish color. But I don’t really think I have a way of drawing that unless I color some blue in there to show it's blue. I don’t really know if there is a way to illustrate color change on a molecular level. – S16
This initial analysis provided a rich description of the challenges students had unpacking the macroscopic reaction event to assist with constructing their submicroscopic level representations. Most did not appear to relate what they had learned about redox reactions to assist with the drawing task even though they were told that the video showed a redox reaction. Instead, students mostly pictured the macroscopic level as comprised of smaller particles with the understanding that bonds must have formed or forces drew the atoms together.
Analysis of students’ understanding after viewing contrasting animations
A comparison of students’ molecular level explanations and hand drawn revisions of the redox reaction was performed to develop understanding of students’ molecular level conceptions made after they viewed the contrasting animations. This comparison was done in the same manner as the “Before Viewing Contrasting Animations” analysis was performed. Reoccurring regularities in students’ drawn and oral explanations revealed similar conceptualization challenges: (1) evidence of macroscopic level entities in molecular level pictures, (2) lack of specificity in the representation of chemical species, (3) inappropriate depiction of spacing between chemical species. (4) inability to identify the chemical makeup of products formed.The findings revealed that students (14 of 17) continued to emphasize macroscopic features of the reaction in their revised pictures. The macroscopic level was shown in the PEA at the beginning and end of the animation as a zoom in and zoom out feature and this may have reinforced the tendency to include this connection. In addition, some students found it challenging to understand what the more complicated EEA depicted without a macroscopic connection. Student, S15, stated, “For someone who doesn’t know…they don’t know what they are looking at. They are just seeing a bunch of tennis balls in a box.” Thus, having the macroscopic level with the molecular level may help students make the connection between the levels, which has consistently been shown to be a challenging endeavour. (Talanquer, 2011; Taber, 2013)
The struggle to represent detailed atomic and molecular species seemed to decrease given that more students incorporated details, such as the nitrogen and three oxygen atoms that makeup a nitrate ion or the oxygen and two hydrogen atoms that makeup a water molecule. Most of the students (13 of 17) drew more complex atomic structures instead of dots or single circles in their revised pictures. Only five students continued to represent single dots or circles for polyatomic ions and compounds in their revisions. Some students (5 of 17) incorporated valence electron clouds, a detail they adapted from viewing the EEA (Fig. 6).
 | ||
| Fig. 6 Examples of students’ (S1, S9) drawings made after viewing the animations, showing valence electron clouds and improved submicroscopic details. | ||
Students’ representation of spacing between atoms in the copper lattice improved greatly with all students representing spacing that reflected either touching atoms or little space between the atoms. It was difficult to tell if students continued to struggle with the spacing of solvent and solute as many students simplified their representations by leaving out solvent water molecules to focus on the reactant and product species as modelled in the PEA.
Lastly, students’ ability to identify the chemical species produced in the reaction improved as most students drew silver atoms that formed on the copper wire. Only a few students remained uncertain with some indicating that they still believed a silver compound adhered to the copper. However, the chemical species that caused the blue solution color remained a challenge with only six students identifying that copper ions contributed to the blue color. Other suggestions for the chemical identity of the blue solution were: copper without distinguishing that they were ions (2 of 17), copper(II) nitrate (2 of 17), a mixture of species (4 of 17), silver nitrate (1 of 17) and two students did not notice the blue color. A deeper analysis of students’ ability to identify the chemical species as connected to the depiction of the species in the contrasting animations follows as we delve deeper into how students align their understanding to fit with these animations.
Study of students’ alignment to and variation from the animations
As a reminder, the treatment consisted of two contrasting animations that had some structural features in common, but differed in their proposed reaction mechanism, general complexity of species, and in length of time (Table 1). A central focus of the analysis was to discern how students’ understanding aligned with and varied from the animation features before and after viewing the animations. As a result, we examined how features that were common to both animations as well as how features that were unique to each animation were detected in students’ oral and drawn explanations. It also allowed us to examine how students who initially had more alignment with one of the two animations or who had very little in common with either animation revised their drawings after viewing the animations.Response to features common to both animations
We first examined how students responded to features that both animations represented by examining how prevalent these features were in students’ oral and drawn explanations before and after students viewed the contrasting animations (Table 3). Prior to viewing the animations, only four students drew and/or described the copper lattice similar to the animations’ depiction of the lattice as made up of an orderly arrangement of multiple copper atoms with little or no space between the atoms. This finding was consistent with our initial observation that most students had difficulty with scale, and were challenged to represent the molecular level of a solid that, in this case, was strewn into a coil. After viewing the animations, every student represented the lattice in a manner that was consistent with the animations.The second feature, common to both animations, was that silver atoms bonded to the copper surface (Table 3). It was depicted by approximately half (9 of 17) of the students prior to viewing the animations. A few students (3 of 9) had attributes that indicated uncertainty that the build-up was due solely to silver atom formation and consequently, they were coded as partially meeting this code. The bridge between the macroscopic observation, that a substance built-up on the wire, and the submicroscopic level, that the substance was made of silver atoms or silver containing compounds, was made by half of the students prior to viewing the treatment. After viewing the animations, the number of students representing silver atoms in accordance with the animations increased to 16 of 17 students. A few students were unsure of the substance responsible for the darkened appearance of the wire and indicated that they were guessing. For example, S2 believed it was silver nitrate that bonded (S2) to the surface; another student (S12), who earned a partial rating, believed that some copper atoms were darker than they were before and they mixed with silver atoms (Fig. 7).
 | ||
| Fig. 7 S12's revised drawing showing copper atoms with a ring around them to indicate that a dark colored substance formed on the wire. | ||
R: I noticed that you represented copper atoms with a ring around them, what is that?
S12: That is just copper, but it's darker than it was before and the red stuff (referring to drawing) is the silver.
Another participant, S15, also conveyed confusion about the silver that formed.
The fact that silver was collected around the coil, and then, I don’t know how the color is like that. I guess it's the natural color, but I guess it's different. For me silver would be more greyish silver, but it's not. I’m not too sure as to why it reacted like that. – S15
Lastly, each animation represented the presence of water, although to differing levels of complexity (Table 3). Nearly half of the students represented water molecules in their pictures prior to viewing the animations, a sign that many students were aware of water's molecular level presence. After viewing the animations nearly every student included water molecules in their revisions, indicating that more students were compelled to include this molecular species after viewing the animations.
To summarize, our findings indicate that students adapted their pictures to fit with features that were reinforced by both animations. They seemed to trust that the features represented by both animations were scientifically accurate, since they were represented in both animations. However, students may continue to harbour uncertainty if the representation does not, in their opinion, fully account for the macroscopic evidence or if it does not fit with a student's prior knowledge.
Response to incorrect features unique to the PEA
A comparison of students’ molecular level explanations to five incorrect features unique to the PEA was made to better understand how students’ initial alignment with or lack of alignment was affected by the viewing process (Table 4). The label “incorrect” was used to recognize that these features were purposefully provided as errors to be inconsistent with and distinct from the more scientifically accurate animation (EEA) whose features were labelled as “correct”. Students’ features made prior to viewing the contrasting animations were examined first. The results indicated that many students (10 of 17) held the misconception that the reactant species, silver nitrate, was a molecule. Representing aqueous ionic compounds as molecules is consistent with observations reported in the literature and supports that this is a common misconception (Kelly et al., 2010). After viewing the PEA, 8 of the 10 students who initially represented silver nitrate as an intact compound or molecule persisted in retaining the representation (Fig. 8). Thus, we recognized that this was a very robust misconception, and unfortunately, many students were unclear of how the conductivity test gave evidence that ions were present in solution. Thus, this macroscopic evidence was of little assistance to help students dispel this misconception. For example, S12 described that he learned that metals conducted electricity and applied this logic to make sense of the PEA. When asked if the solution depicted in the PEA would conduct, S12 responded: | ||
| Fig. 8 Examples of students (S5 and S6) who continued to represent silver nitrate as an intact compound or molecule. | ||
It's closely related to metals, like I know metals conduct so if you have solution with a metal dissociated inside a solution. I think overall the solution would be more conductive. – S12
Of interest, S12 noted that metals conducted when dissociated and in the PEA, the nitrates detached from the silver upon collision with the copper lattice, thus S12 likely rationalized that at that moment, the metal was dissociated. However, students were challenged by statements such as, “metals are good conductors” as evidenced by S2's comment.
I don’t know where, in a textbook, it said that there is among metals, there is a pool of electrons which is why metals are good conductors, so I am trying to picture this pool of electrons, but it's like, if there's molecules everywhere, where can these electrons bundle up? – S2
In some cases, students made sense of the conductivity test, but they were unable to grasp how it would assist them in modifying their belief that silver nitrate existed as a molecule, a misconception that appears to be strengthened by students’ experience with balancing and writing chemical equations. For example, Student S5 shared,
I would assume that they’re attached. When you are talking about compounds and you’re putting them together in equations, you’re assuming that when they’re together, they are together. – S5
However, S5 also tried to account for why the conductivity would change in her revised drawings. She compared the video evidence in which the aqueous solutions of silver nitrate and copper(II) nitrate were tested prior to adding the copper wire with how the solutions tested for conductivity at the end of the experiment.
S5: I wanted to show all of the things that are happening in solution as well to explain why conductivity went up.
R: Can you explain to me how do your pictures reinforce that conductivity went up?
S5: The second animation (EEA) showed me that it also gave off a copper atom. The first animation (PEA) did not show me that. That's why in the other solution (from the video), the copper nitrate solution was ten. When you measured your silver nitrate, it went up to ten because copper replaced itself.
S5 believed that the replacement of the silver by the copper caused the conductivity to go up, but she also believed that free copper atoms resulted (Fig. 9) as noticed in her drawings.
 | ||
| Fig. 9 An example of how S5 adapted his revised picture to show how silver nitrate broke apart leaving the silver atoms on the surface and a mixture of water, copper and nitrate ions in solution. | ||
The second incorrect PEA feature that of silver and nitrate breaking apart after physical contact, was represented by very few students prior to viewing the animation as many students believed that the entire molecule adhered to the surface of the copper lattice. After seeing the PEA, the breaking conception soared with several students (10 of 17) adapting this feature. All students who believed that silver nitrate was a molecule also adapted the animation's depiction that the molecule broke apart (Fig. 6).
Next, we examined how students responded to the incorrect PEA reaction mechanism in which the nitrates break from silver then attract a copper atom and move into solution. Prior to viewing the animations, a small group of students (5 of 17) represented a reaction mechanism that was similar to the PEA. After students viewed the animations, the number of students representing the physical exchange mechanism increased to nine students. However, only one of the five students who initially depicted this mechanism maintained this representation. The other four students, who initially held this mechanistic view either modified their representation to align more with the EEA or in the case of S2, developed an alternative mechanism. The group of eight students who adapted to fit with the PEA mechanism consisted of students who did not favour either of the animation reaction mechanisms prior to viewing the animations.
The fourth incorrect characteristic of the PEA, the depiction that copper(II) nitrate left the lattice and went into solution was also analysed for its occurrence in students’ explanations. It was represented by only four students prior to viewing the animations, the same students (S7, S8, S15, & S16) who initially believed there was a physical exchange mechanism occurring. After viewing the animations these four students let go of this representation; however, five new students adapted this PEA feature. Students: S7, S9, S10, S11, and S12, revised their oral and drawn explanations to fit nearly exclusively with the PEA. An example of S11's revised drawing demonstrates the adaption to the PEA (Fig. 10).
The last characteristic incorrect feature of the PEA analysed, was its overly simplistic depiction of solvent water molecules as uninvolved in the reaction, but simply present. Initially, five students similarly depicted water molecules as present and uninvolved. However, after viewing the animations, the number of students who represented water in this manner increased to 11 of 17 students. This was another unfortunate case of how some students misused the macroscopic evidence from the video which showed that water, when tested for electrical conductance, did not conduct electricity nor did it conduct after the copper wire was removed. Some students interpreted this lack of electrical conductance to mean that water must not be involved in the reaction.
I don’t think like water changed itself because it would have brought, I think it would have cancelled out some of the conductivity, like when it showed on the meter it didn’t go down or anything, it was mixed together. Like once the copper wire went in, water would have reacted, it would have brought the conductivity less (conductivity of the aqueous salt solutions remained high), because water doesn’t have any conductivity. – S2
In other cases, some students expressed difficulty in accepting that the solvent water could be part of the chemical process occurring in a redox reaction since it was not part of the overall balanced equation.
Water is involved in kind of like covering it up, but it's not involved in the reaction. Wait, this video (EEA) says that it is (replays animation). That's kind of what trips me up. They show that the water is kind of like competing with copper over the silver ion. The water doesn’t really do anything in the reaction other than that, because for most equations that I’ve looked at water doesn’t play a role, because water doesn’t dissociate in water. That's why I feel like it's not clear to me. – S4
These quotes and pictures show that when students have limited prior recollection of redox reactions or balanced equations they tend to adapt toward the PEA features. In general, these students try to account for the macroscopic features as best they can prior to viewing the animations, but they have considerable gaps in their understanding. This accounts for the simplistic atomic level details and the belief that there is an accumulation of material on the wire. Viewing the PEA may cause these students to recall single replacement reactions, which in turn may influence them to adapt to the features represented in the PEA.
Response to correct features unique to the EEA
A comparison of students’ molecular level explanations to six correct features unique to the EEA was made to better understand how students’ initial alignment with or lack of alignment from this animation was affected by the viewing process (Table 5). Students’ features made prior to viewing the contrasting animations were examined first. The results revealed that the only EEA feature represented by more than two students, before viewing the contrasting animations, was the electron exchange mechanism. Only two students recognized that electron exchange occurred between silver ions and copper atoms, while three students understood that there was an exchange of electrons, but they were either incorrect or unclear about which species exchanged the electrons. The other EEA animation features that were represented by no more than two students prior to viewing the animation were: hydrated silver ions approaching the copper surface, water molecules moving away from a neutral silver atom, nitrate ions not involved in the reaction, water molecules hydrating copper ions and moving them into solution and the presence of hydrated copper ions in solution (Table 5).After viewing the animation, a characteristic that most students (9 of 17) adapted was the representation that nitrate was a spectator ion that did not react. Students more in sync with the EEA often mentioned that they recalled learning about nitrate as a spectator ion when they learned about solubility rules, thus they were more convinced by this animation's depiction of nitrate as a spectator. For example,
…and also in classes they teach you that nitrates dissociate in the solubility rules, and it was confusing (when viewing the PEA) as to why this remained a molecule, and why it just wouldn’t break apart. – S13
I was like from solubility rules and what I already know in chemistry that it's not a compound. – S17
Another feature of the EEA that many students adapted in their revisions was the electron exchange mechanism between silver ions and copper atoms. Of the eight students who revised their explanations to depict this mechanism, three of the students represented it prior to seeing the animation and remained convinced that it was most scientifically accurate. Another set of three students initially represented a mechanism consistent with the physical exchange mechanism and revised their explanations to fit with the electron exchange mechanism (Fig. 11), and only two students initially held a mechanistic view that favoured neither animation prior to viewing the animations and then changed to fit with the EEA.
The role of water in the redox reaction was strongly depicted in the EEA and several students revised their explanations to better represent water's attraction to silver ions and copper ions (Fig. 11). Specifically, six students adapted their pictures to show hydrated silver ions moving toward the copper surface and five students represented hydrated copper ions moving away from the lattice. However, only one student (S8) revised their explanation to show that water molecules moved away from neutral silver ions and many students commented that copper ions were hydrated in solution, but they did not draw the hydration sphere, thus they were labelled as having partial representation of this feature (Table 5).
In general, students who adapted their pictures to fit with the EEA animation typically could recall being taught features, such as spectator ions, and electron exchange and this helped to convince them of the scientific accuracy of the animation. Students were less willing to adapt their revisions to express the detailed role of water in the reaction, which may speak to their belief that water is unimportant or that this is a level of detail that is unnecessary to represent.
Which animation did students prefer and how did they account for their selection?
Prior to revising their drawn representation of the reaction, students were reminded that the two animations were different and they were asked: “How do you determine which animation is a better representation of the reaction event?”, “Do you believe one is a better representation?” Slightly more than half (9 of 17 students) expressed preference for the EEA, stating that the EEA animation was “more accurate”, “explained what was really going on”, and in general, it had more details. Only one student, S6, reported that the EEA was better based on an incorrect observation that the copper ions were drawn away from the lattice by nitrates and not by water molecules. In addition, S6 revised her drawings to completely match the PEA, but when asked which animation was better she chose the EEA.If you actually went back to the experimental video… when you added the copper onto the silver nitrate and you actually zoomed in and saw what happened with the individual atoms you would see the same thing that you saw in the first animation (PEA), but then you would see the second animation video (EEA) if you actually took the first animation video and zoomed in a little bit more where you could see the cloud around the copper atoms and then it would also slow down… I believe the second animation is more accurate. The first animation is just a more general way of seeing how it would actually look in real time speed. – S6
Fewer students (5 of 17) chose the PEA as the best animation. Those that preferred it reasoned that it had better graphics, was “easier to understand” and it was “less complicated.”
I liked the first animation (PEA), it wasn’t much talking. It was more, I don’t even think it talked, but I was more concentrated. I took a lot more from it even though it was shorter. When the girl kept talking (narration in the EEA) I kept getting more overwhelmed because I was trying to understand what she was saying. For this animation(PEA) it was more simple. It was straight to the point on what was happening. – S5
Four of these five students indicated that the PEA was more like the physical and chemical reaction equation and easier to accept. Some students felt that the experimental evidence fit better with the PEA. For example, S12 stated:
…then on the atomic level I think it was better too, because at the beginning I saw that the atoms collided super-fast. From that I could understand if something is coming fast then also kind of breaks off and just the physical aspect is there. – S12
After analysing the students’ explanations made before and after the contrasting animation treatment, two groups of students emerged. One group consisted of four students who made revisions that showed stronger alignment with the EEA over the PEA as noted by the many EEA features that they chose to emphasize in their picture revisions. These students recognized at least four of the six major correct features depicted in the EEA in their revisions and had at most only one attribute associated with the PEA. In contrast, there was also a group of students whose revisions favoured the PEA. These students recognized all five of the incorrect features emphasized in this animation in their revisions and they had at most, only one attribute consistent with the EEA. As a result, we systematically grouped these students as Pro-EEA and Pro-PEA and drew theoretical comparisons to stimulate thinking about the characteristics that led these students to their revision decisions.
The group of students designated Pro-EEA had prior knowledge that was consistent with either the electron exchange mechanism (S1 and S17) or with the physical exchange mechanism (S8 and S16), but all four students labelled and accounted for all species symbolically. Having mechanistic ideas with detailed atomic level connections to electrons, ions and symbols indicated that these students may have been better at adapting their revisions to fit with features they observed in the animations. An interesting finding was that all four students recognized that the EEA was much more detailed in expressing how and what happened during the reaction. Even though all four students adapted their revised pictures to match with the EEA, all four were certain that both animations were useful for understanding the reaction. Student S17 summarized it best when he stated that the PEA was a simplified view of the reaction that would be useful for teaching the molecular equation. From this insight, it was obvious that S17 was confused by how symbolic representations represent the submicroscopic level. At this point in his understanding, he believed that the PEA represented the equation, while the EEA represented the submicroscopic level.
Pro-PEA Students struggled to describe the atomic level prior to seeing the animations. They were unable to discern atoms from ions or molecules in their pictures and had trouble accounting for why a reaction would occur mechanistically. It was very challenging for this group of students to describe their understanding. After viewing the animations, these students were confused by the complicated EEA and they found it easier to understand the PEA. In addition, this group of students expressed uncertainty with the macroscopic results. For example, S10 was confused by the blue solution color and the fact that neither animation showed explicitly what caused the blue color. S9 expressed that the conductivity test showed that water did not conduct and she assumed that this meant it would not react. This belief caused her to favor the PEA. S11 had trouble understanding how the copper wire could still exist and reasoned that it should have dissolved based on the animation. This made both animations confusing, but ultimately the PEA was easier to understand because it was more simplistic in its design. In addition, this group also expressed that both animations supported each other, and they chose to follow the model that was easier to understand.
Conclusions
Meaningful learning from conflicting animations requires learners to make connections between their own ideas, the experimental evidence and the information depicted in the animations. Our findings revealed that students were challenged to comprehend both the macroscopic level and submicroscopic level, and how these levels were related. Prior to viewing the contrasting visualizations, students conveyed uncertainty about how to represent a submicroscopic picture without macroscopic cues in their pictures. They lacked the ability to represent detailed molecular level species and often did not distinguish atoms from ions or molecules nor did they represent the appropriate spacing between atoms in a lattice or atoms in solution. Many students were unable to identify the chemical makeup of the products. Fewer students recalled equations or how electrons were exchanged in redox reactions, and those who recalled equations or electrons being transferred had limited success in using this information to form their initial pictures. After the treatment was introduced and students had the opportunity to revise their pictures, we observed that approximately half of the students revised their drawings to fit with the inaccurate animation, PEA animation. They incorporated at least three of five of the incorrect PEA characteristics into their oral and drawn explanations. Fewer students revised their pictures to incorporate the correct EEA features. Only two students did not revise their pictures to favor either animation. If a student began with an understanding more aligned with the EEA, they tended to retain this view. However, students who expressed no real connection to either animation did not revise their pictures to favor the EEA, and all made revisions to be consistent with the PEA. In general, the PEA was described by the students as easier to understand. In contrast, the EEA was very detailed and complicated. Students had difficulty unpacking its complexity, a finding consistent with Rosenthal and Sanger (2012, 2013) regarding this animation.The conclusion we can draw from this comparison of student understanding before and after viewing the contrasting animation, was that when students were better able to recall their understanding of basic chemistry concepts, particularly of reactions, such as single replacement and redox chemistry, and if they could make sense of the macroscopic experimental evidence, they had greater success both in choosing the best animation and in adapting their revised pictures to have more of the EEA features. However, when students were unable to tap into their prior knowledge or if they had little relevant prior knowledge and they were confused by the macroscopic experimental evidence, they were less successful in choosing the best animation and adapting their revised pictures to have more of the EEA features. These students adapted their pictures to fit almost exclusively with the PEA because they could draw and explain it, despite many believing that the EEA was a better, more detailed animation. Regardless of the animation that the students selected or favored in their revisions, what was most important was that students had the opportunity to apply information from the experimental evidence and their prior knowledge to explore and critique the animations they viewed.
We observed that sometimes the learner's views or understanding did not fit well with scientific evidence because they did not understand the evidence and perhaps they were untrained to apply how macroscopic evidence related to the submicroscopic level. For example, we observed that students had problems understanding all aspects of the macroscopic evidence. They sometimes drew incorrect conclusions. Students typically did not understand the purpose of the electrical conductivity test although all students had completed a lab in which conductivity testers were used to assist with writing net ionic equations. Neither animation explicitly portrayed how the species would respond when tested for electrical conduction and most students were unable to discern what this evidence meant. Some students shared that they thought that only metal containing compounds conducted due to the presence of the metal, because they knew metals were good electrical conductors. Some deduced that since pure water did not conduct, then water must not have any involvement in the reaction and they reasoned the EEA must be incorrect. A further experimental challenge was that in the video the silver appeared to be very dark which was inconsistent with students’ perception of silver as a shiny light grey metal. In addition, many students did not understand how solution color change would be represented at the molecular level. Some could deduce that copper had something to do with it, but there was variation in how they perceived copper was involved, whether as ions or atoms in solution, or part of the compound copper(II) nitrate.
The findings indicate that having students practice the art of critiquing contrasting animation models is useful. It helped students learn how experimental evidence was connected to molecular level events, and students tended to think more deeply about what the animations conveyed. In addition, it helped researchers and instructors learn how students thought about the molecular level. This study indicated that students were enticed by the simpler animation and if they had a weak understanding of redox and were unable to understand how the experimental evidence supported or refuted the animation, they accepted that the simpler model was better. Students who could interpret the connection between animations and experimental evidence and who had stronger chemistry recall were able to incorporate more details related to the mechanism into their revised pictures. In general, students in this study were reluctant to dismiss an animation as wrong and most thought that both animations were useful for better understanding redox reactions. In part this was because the simple animation represented the look of a single replacement reaction, which is a common way to introduce students to redox reactions and unfortunately, students have difficulty understanding how symbolic equations relate to the molecular level (Kelly et al., 2010; Rosenthal and Sanger, 2012, 2013).
Limitations
The primary limitation of this study, as we have previously documented was that the animations were different from each other in appearance and process, and as a result the emphasis on different mechanisms was not as pronounced as it could be were the animations identical apart from the mechanism (Kelly, 2016). The PEA was purposefully meant to look more simplistic and wrong, yet at the time we tried to keep the color scheme and the look of the chemical species very similar to the EEA. The PEA was also designed specifically for this study, but it was much quicker, lasting less than 30 seconds and it did not have narration. The EEA animation was made with older software, it had narration that explained what was happening and it highlighted features and replayed sections of the animation where students were known to miss details. Interestingly, one would expect that students might realize that this long and very detailed animation was, in fact, the more accurate of the two animations; however, that was not always the case.Implications
This study suggests that animation designers may need to construct animations that draw more explicit connections between animated features and macroscopic evidence or instructors may need to work on this connection in their lessons. For example, it may be necessary to provide clues that hydrated copper ions contribute to the blue colored solution by making the ions blue in color. While this is inaccurate, students may need this overt connection to the macroscopic level as a scaffold while they are learning to build connections between the submicroscopic and macroscopic levels.Students often did not find the video of experimental evidence to be as straight forward as the researchers had hoped, but several students made very natural suggestions for inquiring more deeply about what was happening experimentally. For example, one student S10 wondered if there was a temperature change occurring during the reaction. Having the student replicate the experiment and test the temperature may help students like S10 better understand how temperature was or was not involved. Another student, S4, recognized that the conductivity of the solutions was not tested during the reaction and he wanted to know what would happen if conductivity was tested throughout. Another student, S5, wondered if the animations were correct and copper was leaving the lattice. S5 wanted to know if the copper wire would disintegrate. Having animations in contrast may naturally lead students to inquiry investigations, which could be conducted in laboratory courses, in which they explore through experimentation whether their selected animation is scientifically accurate or inaccurate.
We recommend instructors try the contrasting animation activity after they have taught students about redox reactions so that students have some background upon which to draw when viewing the animations. It would also be highly advisable to have taught students about conductivity prior to introducing the activity to assist in helping students make sense of the electrical conductivity evidence. The activity could be done as part of a lecture, in which students are first shown the video of experimental evidence then construct their own molecular model of the reaction event before they view, discuss the contrasting animations and vote on the animation that they feel best fits the evidence (Kelly, 2016). The activity could also be done as a pre-lab exercise that could lead to inquiry investigations related to the experiment. However the activity is used, the process empowers students to examine how scientific evidence can be used to support or refute animations. The way students critique animations can seem challenging, as students sometimes make wrong decisions or draw inappropriate connections, but the act of having students articulate their animation preference based on their understanding and reasoning is inherently valuable. When conflicting animations are presented to students, they are confronted with different submicroscopic explanations, and they must resolve which animation is best. We believe that even if students select the wrong animation, they are learning how to critique models in comparison to evidence, which is fundamental for understanding the nature of science. In addition, with the contrasting animation challenge, students are being taught to think deeply about what the animations convey. They are exercising their ability to recall their prior knowledge and to examine how the experimental evidence fits with the animation models. Students tell us this is not easy and that they want to know the answer. They tell us if they were not told the answer, they would try to find the answer and that may be the most convincing evidence of all.
Appendix I
Keys that were shown to students prior to viewing the electron exchange animation.Key that was shown to students prior to viewing the physical exchange animation.
Acknowledgements
The authors wish to acknowledge that the National Science Foundation under Grant No. 1525557 supported this work. Any opinions, findings, and conclusions or recommendations expressed in this paper are those of the authors and do not necessarily reflect the views of the National Science Foundation.Notes and references
- Al-Balushi S. M. and Al-Harthy I. S., (2015), Students’ mind wandering in macroscopic and submicroscopic textual narrations and its relationship with their reading comprehension, Chem. Educ. Res. Pract., 16, 680–688.
- Allsop R. T. and George N. H., (1982), Redox in Nuffield advanced chemistry, Educ. Chem., 10, 57–59.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia- based instruction that emphasizes molecular representations on students’ understanding of chemical change, J. Res. Sci. Teach., 41, 317–337.
- Bandriet A. R. and Bretz S. L., (2014), The development of the redox concept inventory as a measure of students’ symbolic and particulate redox understandings and confidence, J. Chem. Educ., 91(8), 1132–1144.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63, 873.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14, 9–22.
- Chi M. T. H., (2008), Three types of conceptual change: belief revision, mental model transformation, and categorical shift, in Vosniadou S. (ed.), Handbook of research on conceptual change, Hillsdale, NJ: Erlbaum.
- Chi M. T. H. and Wylie R., (2014), The ICAP framework: linking cognitive engagement to active learning outcomes, Educ. Psychol., 49(4), 219–243.
- Coppolla B. P. and Pontrello J. K., (2014), Using errors to teach through a two-staged, structured review: peer-reviewed quizzes and “what's wrong with me?”, J. Chem. Educ., 91(12) 2148–2154.
- De Jong O., Acampo J. and Verdonk A., (1995), Problems in teaching the topic of redox reactions: actions and conceptions of chemistry teachers, J. Res. Sci. Teach., 29, 121–142.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29, 1079–1099.
- Glaser B. G. and Strauss A. L., (1967), The Discovery of Grounded Theory, Chicago: Aldaline.
- Kelly R. M., (2014), Using variation theory with metacognitive monitoring to develop insights into how student learn from molecular visualizations, J. Chem. Educ., 91(8), 1152–1161.
- Kelly R. M., (2016), Exploring the instructional use of contrasting molecular animations of a redox reaction, in Schultz M., Schmid S. and Holme T. (ed.), Technology and assessment strategies for improving student learning in chemistry, Washington, DC: ACS, pp. 117–136.
- Kelly R. M., (2017), Learning from contrasting molecular animations with a metacognitive monitoring activity, Educ. Quim., DOI: 10.1016/j.eq.2017.02.003.
- Kelly R. M. and Akaygun S., (2016), Insights into how students learn the difference between a weak acid and a strong acid from cartoon tutorials employing visualizations, J. Chem. Educ., 93(6), 1010–1019.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kelly R. M. and Hansen S. J. R., (2017), Exploring the design and use of molecular animations that conflict for understanding chemical reactions, Quim. Nova, DOI: 10.21577/0100-4042.20170043.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students’ explanations, J. Sci. Educ. Technol., 16, 413–429.
- Kelly R. M. and Jones L. L., (2008), Investigating students’ ability to transfer ideas learned from molecular animations to the dissolution process, J. Chem. Educ., 85, 303–309.
- Kozma R. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Marbach-Ad G., Rotbain Y. and Stavy R., (2008), Using computer animation and illustration activities to improve high school students’ achievement in molecular genetics, J. Res. Sci. Teach., 45(3), 273–292.
- Marton F., (1986), Phenomenography – a research approach to investigating different understandings of reality, J. Thought, 21, 28–49.
- Muller D. A., (2008), Designing effective multimedia for physics education. School of Physics, University of Sydney, retrieved from: http://www.physics.usyd.edu.au/super/theses/PhD(Muller).pdf.
- Øyehaug A. B. and Holt A., (2013), Students’ understanding of the nature of matter and chemical reactions – a longitudinal study of conceptual restructuring, Chem. Educ. Res. Pract., 14, 450–467.
- Österlund L.-L. and Ekborg M., (2009), Students’ understanding of redox reactions in three situations, Nordina, 5, 115–127.
- Österlund L.-L., Berg A. and Ekborg M., (2010), Redox models in chemistry textbooks for the upper secondary school: friend or foe? Chem. Educ. Res. Pract., 11, 182–192.
- Plass J. L., Homer B. D. and Hayward E. O., (2009), Design factors for educationally effective animations and simulations, J. Comput. High Educ., 21(1), 31–61.
- Posner G. J., Strike, K. A. Hewson P. W., and Gertzog, W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66(2), 211–227.
- Rosenthal D. P. and Sanger M. J., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation-reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Rosenthal D. P. and Sanger M. J., (2013), How does viewing one computer animation affect students’ interpretations of another animation depicting the same oxidation-reduction reaction? Chem. Educ. Res. Pract., 14, 286–296.
- Ryoo K. and Linn M. C., (2014), Designing guidance for interpreting dynamic visualizations: Schmidt generating versus reading explanations, J. Res. Sci. Teach., 51(2), 147–174.
- Schmidt H. J. and Volke D., (2003), Shift of meaning and students’ alternative concepts, Int. J. Sci. Educ., 25, 1409–1424.
- Stains M. and Talanquer V., (2008), Classification of chemical reactions: stages of expertise, J. Res. Sci. Teach., 45, 771–793.
- Taber K., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7(2), 141–159.
- Thorndike R. M., (1997), Measurement and Evaluation in Psychology and Education, 6th edn, Columbus, OH: Prentice Hall.
- Velázquez-Marcano A., Williamson V. M., Ashkenazi G., Tasker R. and Williamson K. C., (2004), The use of video demonstration and particulate animation in general chemistry, J. Sci. Educ. Technol., 13, 315–323.
- Ye L. and Lewis S. E., (2014), Looking for links: examining student responses in creative exercises for evidence of linking chemistry concepts, Chem. Educ. Res. Pract., 15, 576–586.
| This journal is © The Royal Society of Chemistry 2017 |