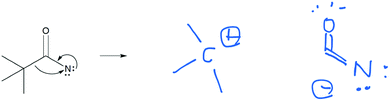Students’ interpretations of mechanistic language in organic chemistry before learning reactions
Kelli R.
Galloway
,
Carlee
Stoyanovich
and
Alison B.
Flynn
 *
*
Department of Chemistry & Biomolecular Sciences, University of Ottawa, Ottawa, Ontario, Canada. E-mail: alison.flynn@uOttawa.ca
First published on 16th February 2017
Abstract
Research on mechanistic thinking in organic chemistry has shown that students attribute little meaning to the electron-pushing (i.e., curved arrow) formalism. At the University of Ottawa, a new curriculum has been developed in which students are taught the electron-pushing formalism prior to instruction on specific reactions—this formalism is part of organic chemistry's language. Students then learn reactions according to the pattern of their governing mechanism and in order of increasing complexity. If students are fluent in organic chemistry's language, they should have lower cognitive load demands when learning new reactions, and be better positioned to connect the three levels of chemistry's triplet (i.e., Johnstone's triangle). We developed a qualitative research protocol to explore how students use and interpret the mechanistic language. Twenty-nine first-semester organic chemistry students were interviewed, in which they were asked to (1) explain a mechanism, given all the starting materials, intermediates, products, and electron-pushing arrows, (2) draw in arrows for a reaction mechanism, given the starting materials and products of each step, and (3) predict the product of a reaction step, given the starting materials and electron-pushing arrows for that step. To investigate the students’ ideas about mechanistic language rather than their knowledge of specific reactions, we selected reactions for the interview guide that had not yet been taught. Following transcription, we analyzed the interviews using constant comparative analysis to explore how students used and interpreted the mechanistic language. Four categories of student thinking emerged with electron movement underlying students’ thinking throughout the interviews. Herein, we discuss these categories, students’ interpretation of the symbolism, connections to learning theory, and implications for teaching, learning, and research.
Introduction
Evaluation of a transformed curriculum
The traditional approach to teaching university organic chemistry is organized by type of functional group. The organic chemistry sequence has evolved little over time despite consistent evidence of limited student learning. At the University of Ottawa, a patterns-of-mechanisms curriculum was designed and implemented (Flynn and Ogilvie, 2015). The basis for the redesign was grounded in prior literature on student learning in organic chemistry, namely the difficulties that students have understanding the electron-pushing formalism (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Kraft et al., 2010; Grove et al., 2012) and students’ lack of depth of understanding of organic chemistry concepts (Anderson and Bodner, 2007; Grove and Bretz, 2012; Bhattacharyya, 2014; Anzovino and Bretz, 2015, 2016). Within the new curriculum, students are taught the symbolic language of organic reaction mechanisms prior to learning about specific reactions. Then, course material is organized by the governing mechanistic pattern in order of increasing difficulty rather than by functional group or reaction name.Since fall 2012, organic chemistry at the University of Ottawa has been taught using this patterns-of-mechanisms curriculum. Now that the curriculum has been taught for several years, evidence needs to be gathered for how students are learning. Before and after the new curriculum was implemented, midterm and final exams were analyzed to ascertain how student performance was affected by the change. Initial results are showing that students are scoring higher on familiar and unfamiliar mechanism questions under the new curriculum (Webber and Flynn, 2017). To better understand how the new curriculum is affecting the students, the processes underlying student learning need to be studied. This article describes one study we conducted to investigate the influence of the patterns of mechanisms curriculum on student learning in organic chemistry, specifically related to students’ use of mechanistic language.
Research on learning the language of chemistry
Becoming proficient in the language of chemistry is an integral part of learning chemistry but is no small task (Osborne, 2002; Childs et al., 2015). The language of chemistry is complex and multifaceted due to: everyday words that have different meanings in a science context, words with unfamiliar Greek and Latin roots, specialized and technical vocabulary that is not practiced outside of school, and the need for greater accuracy in language due to the small nuances between words (Childs et al., 2015). If that were not enough, students must also learn the symbolic language of chemistry. The symbolic domain allows for chemists to communicate efficiently about macro- and microscopic processes, but for a novice, learning the symbols can feel like learning a foreign language (Taber, 2009, 2013; Talanquer, 2011). The same substance can be represented multiple ways highlighting different characteristics, each representation with its own strengths and limitations. Letters and shapes that are also used in the students’ everyday life now stand for new objects and processes in chemistry (e.g., Ph vs. pH vs. PH). Because chemistry symbols do not “simply store transparent information” (Liu and Taber, 2016, p. 448) but symbolize macro- and microscopic processes, the ambiguity of the symbolic language of chemistry confuses the learner (Taber, 2009).Research on student learning within the symbolic domain of chemistry has described the challenges that students face. Taskin and Bernholt (2012) reviewed the literature on student understandings of chemical formulas. Among other findings, the review discussed students’ difficulties with the function and syntax of chemical formulas and postulated that students may be relying more on chemical formulas as abbreviations rather than representing chemical composition. A study in Croatia explored student translation of text to chemical symbols using a pen and paper assessment (Vladušić et al., 2016). When students were asked to explain common chemistry symbols and rate their confidence in their responses, high confidence was reported even for symbols that students struggled to accurately explain (Vladušić et al., 2016). The researchers emphasized that instructors cannot make the assumption that students are able to understand chemical symbols and then are able to connect those symbols to the macro- and microscopic areas of chemistry (Vladušić et al., 2016). Cooper and colleagues examined students’ understanding of Lewis structures—chemical symbols that carry implicit meanings including chemical structure and shape, electron density, intermolecular forces, and reactivity. Beginning chemistry students must go through a lengthy process to reach the same conclusion that expert chemists can achieve in just moments (Cooper et al., 2012, 2013). Through multiple studies using both qualitative and quantitative methods, researchers found that students viewed drawing Lewis structures as a stand-alone task rather than a step toward interpreting physical and chemical information (Cooper et al., 2010, 2012, 2013). In a problem solving study, Bodner and Domin (2000) found that only students who were successful at solving problems chose to translate given representations given into a useable form. The researchers emphasized that until symbols represent physical reality for students, they are only “letters, numbers, and lines” (p. 27) on a page. Treagust et al. (2003) explored how grade 11 high school students understood the integration of the symbolic and the submicroscopic domains. The study found that students did not choose on their own to translate between types of representations and that the instructor's use of a symbolic representation to explain a submicroscopic concept was not always understood by the student the way the instructor intended. The authors suggested overtly discussing the relationship between the symbolic domain and the submicroscopic; in this way, the symbols can take on meaning rather than just being letters, lines, and dots on a page (Treagust et al., 2003). Becker et al. (2015) investigated the interplay across the macroscopic, submicroscopic, and symbolic domains of chemistry in a physical chemistry course. The findings showed that instructors used mainly symbolic representations with some connections to the macroscopic level and very few connections to the submicroscopic levels. The authors suggested that this isolated teaching could lead to confusion of how to connect the symbolic to the submicroscopic level.
Research on learning in organic chemistry
In organic chemistry, the symbolic language relies heavily on the curved arrows of the electron-pushing formalism (EPF); the EPF is a powerful tool to explain and explore reaction mechanisms. Outside of chemistry, a curved arrow typically represents where an object goes. Within chemistry, the curved arrows of the EPF represent the movement of electrons, i.e., how bonds are broken and formed in a reaction. Students are accustomed to thinking of arrows as representing the movement of objects, but the EPF represents the movement of electrons to and from objects (atoms). Unfortunately, the literature contains evidence that many students do not understand the EPF, organic mechanisms, or the underlying concepts, as we describe below.Research on student understanding of organic mechanisms has shown that the arrows in the EPF had little meaning to many students (Bhattacharyya and Bodner, 2005) and that many students added arrows as an afterthought (Grove et al., 2012). Bhattacharyya (2014) synthesized student difficulties with organic reaction mechanisms, and identified two major difficulties from his own research and from other researchers. First, students tended to resort to deterministic thinking where “chemical processes are believed to be driven by specific forces to yield specific products” (Bhattacharyya, 2014, p. 596). In this way, students sought to determine the products of a reaction based on a single idea such as seeking products with the lowest energy or highest stability while “ignoring the energetics of the [mechanistic] pathways” to arrive at the products (Bhattacharyya, 2014, p. 597). Second, students were unable to coordinate multivariate thinking resulting in either combining multiple properties into one or selecting only a single factor and ignoring other factors to explain a reaction (Bhattacharyya, 2014). In one example of this type of thinking, students were asked to estimate the pKa value of an amide's hydrogen atom. Students answered as if the hydrogen were on an amine, overlooking the carbonyl component of the amide (Flynn and Amellal, 2016). Thus, many students are not thinking deeply about reaction mechanisms but instead are drawing them with little to no meaning attached (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012; Bhattacharyya, 2014). Perhaps contributing to the difficulty in instruction, a recent study found that instructors had a variety of definitions for mechanistic reasoning using the EPF (Bhattacharyya, 2013). The article articulated many concepts that organic chemistry instructors found important for students to understand prior to learning organic mechanisms including electronegativity, drawing of Lewis structures, accounting for electrons, charge, acid–base theory, and nucleophiles and electrophiles (Bhattacharyya, 2013).
Research investigating student thinking in organic chemistry has shown that students have fragmented and disconnected ideas, can repeat definitions but cannot use them in context, and rely on rote memorization to get by in their degree program. In response to Bhattacharyya's research (2013) on organic chemistry instructors’ ideas about reaction mechanisms, Anzovino and Bretz (2015, 2016) investigated students’ ideas about nucleophiles and electrophiles. While students in their study could define the terms nucleophile and electrophile, students used structural features to identify nucleophiles and electrophiles in a reaction rather than talking about the function of the species. Bowen's (1990) study into how graduate students solve organic synthesis problems found that students did not view chemical principles as useful to solve the synthesis problems. Instead, the students used algorithmic and trial and error methods as long as it met their needs and only used physical and chemical properties when the tasks appeared to be ambiguous and non-straightforward.
Multiple studies have explored fourth-year (final-year) students’ understanding of organic chemistry. Rushton et al. (2008) interviewed 19 fourth-year chemistry or biochemistry majors using multiple-choice questions from the American Chemical Society (ACS) organic chemistry exam. Results showed that students were often confused about which model to use to answer a question and whether steric effects, inductive effects, feasibility of a reaction mechanism, or other factors were more important in a particular context. Additionally, some students showed an overemphasis on the products of reactions by considering the relative stability of the products presented as the multiple choice options rather than considering the mechanism itself, which demonstrated the students’ lack of understanding of the purposes of mechanisms as tools to decipher products of chemical reactions. In another study, DeFever et al. (2015) conducted a series of interviews with eight fourth-year chemistry majors. Among their findings, students could accurately discuss intermolecular forces and their role in polarity and solubility. But, students were not able to use their ideas about intermolecular forces and polarity to identify possible reaction sites within natural products. The students relied on isolated structural features, rather than thinking about the molecule as a whole. Studies on hydrogen bonding (Henderleiter et al., 2001) and organic acids and bases (Cartrette and Mayo, 2011) also found that students could reproduce definitions of terms but could not use them in context.
The aforementioned studies demonstrate students’ limited understanding of organic reaction mechanisms and related underlying concepts. The present study sought to explore how students interpret the symbolic language of reaction mechanisms after receiving explicit symbolism instruction, engaging in practice activities, and being assessed, but prior to learning specific reactions. Students are taught the symbolism first so that they can, in principle, become fluent in the electron-pushing formalism's language to allow for deeper understanding of reactions and their mechanisms (Flynn and Featherstone, 2017). Exam analysis has found that students rarely used reversed arrows or arrows starting at atoms on symbolism questions and generally had a high degree of success in such questions, except for questions that included an intramolecular step, rearrangement, or had implicitly-drawn atoms involved in a given step (Flynn and Featherstone, 2017). However, that study could not uncover how students reasoned with the EPF. The present study described in this article focused on how students used and reasoned with the EPF when asked to complete symbolism questions.
Theoretical framework
Chemistry's triplet
This work is guided largely by Johnstone's Triangle, or as it is more recently called, chemistry's triplet (Fig. 1). Johnstone discussed how chemistry is communicated across “three levels” (Johnstone, 1991). First identified as Descriptive, Representative, and Explanatory levels, the triplet now often refers to the macroscopic, symbolic, and submicroscopic levels of chemistry (Johnstone, 1991; Talanquer, 2011; Taber, 2013). Johnstone argued that expert chemists flow easily between each level, frequently using multiple levels at a time within a single dialogue (Johnstone, 2010). Additionally, arguments have been made that chemistry instruction is rarely structured to help students effectively translate across all levels (Talanquer, 2011). Too often emphasis is placed on one level without guidance for how to connect to the others; or worse, instructors move seamlessly between levels without pointing out to the students that they have shifted or how to make a shift (Johnstone, 2010). Helping students become aware of the three levels can prevent instruction from overloading and confusing students. Johnstone emphasized that instruction should explicitly target one level at a time to allow for development of competence in one area and then attempt to make connections to another (Johnstone, 2010).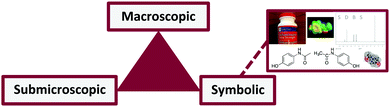 | ||
| Fig. 1 Chemistry's triplet. Reproduced from Flynn and Featherstone with permission from The Royal Society of Chemistry (2017). | ||
Talanquer (2011) and Taber (2013) have written perspectives and critiques on uses of the chemistry triplet in research and teaching. Both perspectives noted that perhaps the symbolic level cannot be understood separately from the macro- and submicroscopic levels. Taber (2013) argued that the symbolic/representational level “facilitates shifting between levels” (p. 161). In organic chemistry, the representation of mechanisms using the electron-pushing formalism offers an explanation for how molecules are interacting at the submicroscopic level to produce changes at the macroscopic level. Competence at the symbolic level includes accurately interpreting arrows, letters, charges, and lines to depict structures and processes at the submicroscopic level as well as accurately using the symbols to demonstrate how submicroscopic processes are reflected at the macroscopic level.
The EPF is a system of symbols used in organic chemistry to represent and communicate the submicroscopic process of electron movement in a reaction. The present study was designed to explore how students interpret the symbols of organic reaction mechanisms and connect them to the submicroscopic level.
Information processing theory and constructivism
The research is also guided by Information Processing Theory (Fig. 2). Our interpretation of this theory is informed by the writings of Johnstone (Johnstone and Selepeng, 2001; Johnstone, 2006), Mayer (2009, 2011, 2012), and Sweller (1994). Information Processing Theory (IPT) provides a way to model how people make sense of an experience. Briefly, people use their five senses consciously and subconsciously to first gather information about an experience. The information gained goes into the working memory. In the working memory, the information is held until a connection is made in the long-term memory in which the new information is stored, or if no connection is made, the new information is lost.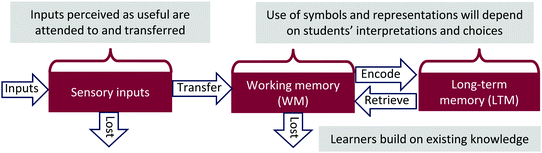 | ||
| Fig. 2 Information processing theory and meaningful learning. Adapted from Flynn and Featherstone (2017) with permission from The Royal Society of Chemistry. | ||
The IPT is supported by Ausubel and Novak's theory of meaningful learning and human constructivism (Ausubel, 1968; Novak, 1993, 2010; Bretz, 2001; Schunk, 2012). The three conditions that Ausubel first put forth for meaningful learning (Ausubel, 1968) can directly correspond to the IPT. First, the learner must have relevant prior knowledge. This condition corresponds to the learner's long-term memory and how knowledge is stored and organized. Second, the new material must be presented in a meaningful way. This condition corresponds to how and whether the students will perceive the new information and then hold it in the working memory. If the student is trying to hold too many ideas and concepts in his/her working memory, then the new information may either not have an opportunity to be considered or just not appear to be relevant. On the other hand, students with well-organized knowledge structures may be able to hold more information in their working memories because of their abilities to chunk (i.e., group) and consolidate new information. Finally, the student must choose to non-arbitrarily connect the new knowledge to the prior knowledge. New connections are only made when a learner chooses to incorporate the new information into the long-term memory. Meaningful connections are only made when a learner chooses to connect the new information into the long-term memory in a relevant, non-arbitrary way (Ausubel, 1968; Novak, 2010).
As this study explored how students interpret the symbolic language of mechanisms, we were interested in what features students deemed relevant, if students who understood the EPF were able to go conceptually deeper, and what information students pulled from their long-term memories to interpret the EPF.
Research questions
To explore how students use and interpret mechanistic language outside of the learning of specific reactions, this study set out to investigate the follow research questions:(1) How do undergraduate organic chemistry students work through symbolism of mechanisms questions?
(2) What meaning do undergraduate organic chemistry students assign to organic chemistry mechanistic symbols while solving symbolism questions?
We have defined a symbolism of mechanisms question as one in which students are given a reaction they have not yet been taught and are asked to: (1) draw the arrows of an organic reaction mechanism, given the reactants, intermediates, and products, (2) draw the products of an organic reaction mechanism, given the reactants and electron-pushing arrows, (3) draw the transition state structure, given the starting materials and products of a reaction step, or (4) draw the mechanism for the reverse reaction, giving the mechanism in the forward direction. The first two symbolism questions were used in this study and specific questions were designed to elicit students’ interpretation of the language of mechanisms. We developed a qualitative research protocol to investigate these questions, in which students worked through EPF questions using a think aloud procedure. As students worked through the symbolism questions, their discourse about the mechanistic language conveyed their understanding of the symbols. Thus, the goal of the study was to gather evidence for how students interpret the symbolic language of organic mechanisms as elicited through interview tasks.
Methods
Study context
At the University of Ottawa, students take a one-semester general chemistry course during the fall semester of their first year before beginning the organic chemistry sequence in the following winter/spring semester. Students typically take Organic Chemistry I (OCI) during the second semester of their first year and Organic Chemistry II (OCII) during the fall semester of their second year, consecutively. OCI is split into six sections (four English, two French) each taught by a different instructor (i.e., professor or lecturer). OCII is split into three sections (two English, one French). OCI has a mandatory bi-weekly laboratory component; OCII has an associated laboratory course that is mandatory for some students, depending on their program of study. Approximately six teaching assistants (TAs) lead optional discussion group sections (i.e., tutorials or recitation sessions). In the laboratory sections, each TA is responsible for 14–18 students. Approximately 1200 students go through the OC course sequence each year.In addition to the new curriculum (Flynn and Ogilvie, 2015), some sections of the courses are taught in a flipped format in which students watched videos prior to coming to class (Flynn, 2015). Then, in-class time is spent working on problems in small groups. For this study, students were recruited from an English section of OCI taught in the flipped format.
Data collection
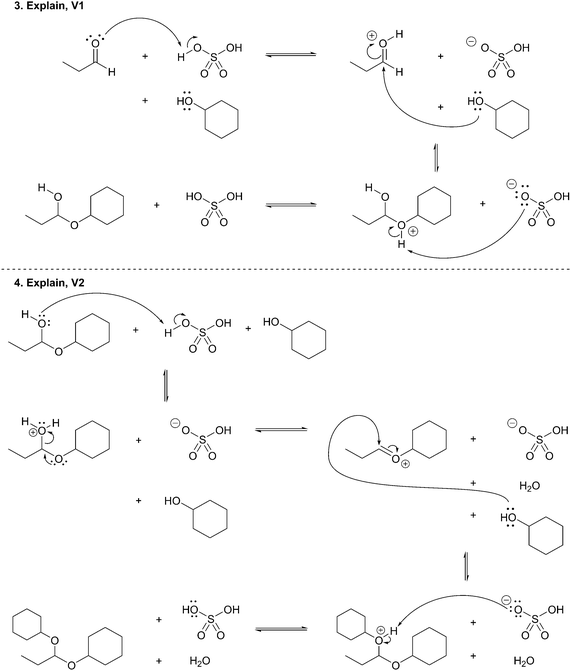
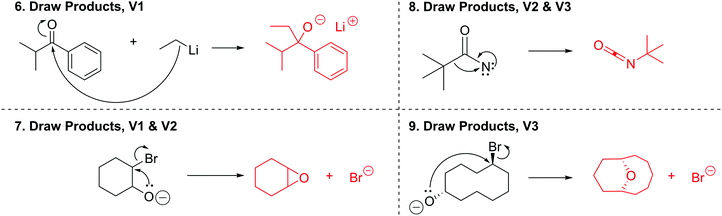
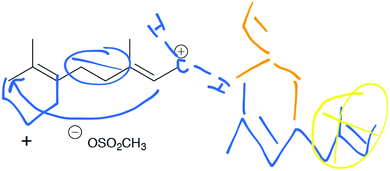
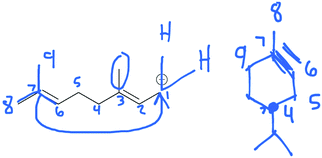
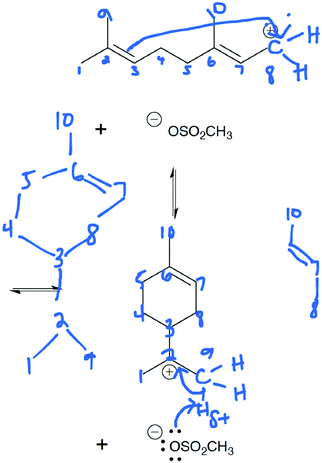
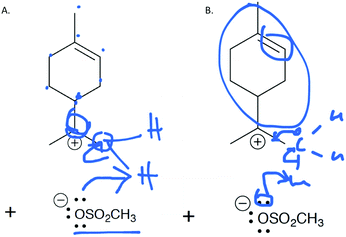
Following approval from the Office of Research Ethics and Integrity, students were recruited to participate in the study during the 2015 winter term. A convenience sampling method (Crewell, 2012) was used to recruit student volunteers from the first semester organic chemistry course where they were learning the language of the electron-pushing formalism for the first time. An announcement was made during class advertising for the study, and a handout was posted to the course management website with information about how students could volunteer to participate and a link to an online consent form. Students submitted their names and email addresses to give consent to volunteer to participate in the study. The electronic consent form informed students of the ways that their identities would be protected including that their responses would be confidential and anonymized, their names would be changed to pseudonyms, and that the data that collected in the study would be stored on a password protected computer. In addition, participants were informed of their right to withdraw at any time. Volunteers were contacted by email to schedule an interview. Because this study's goal was to explore how students understand and interpret the symbolic language of mechanisms, interviews were only scheduled for after students had received instruction on the symbolism of mechanisms (for more information on the initial instruction, see Flynn and Ogilvie, 2015). For the first few interviews, students had just begun learning organic acid–base reactions. Over the course of six weeks, 29 interviews were conducted by the second author, who was previously unknown to the participants.The interview tasks included the following four question types: (1) explain the reaction given a complete mechanism, including starting materials, intermediates, products, and electron-pushing arrows (Fig. 3 and 4); (2) draw the arrows given the reactants, intermediates, and products (Fig. 5); (3) draw the products given the reactants and curved arrows (Fig. 6); and (4) find errors in samples of student work for drawing arrows and drawing products (not shown). The reaction provided in each task was unfamiliar to the student to elicit interpretation of the mechanistic language, rather than memory of specific reactions. Thus, as students learned more reactions throughout the course of the semester, the interview guide had to be modified to replace reactions students had learned during the course with unfamiliar reactions. These modifications resulted in three versions of the interview guide (Fig. 3–6). Only the findings from the first three interview tasks (called Explain Mechanism, Draw Arrows, and Draw Products) are discussed in this article because questions of these types were included in each version of the interview, while question type 4 was not.
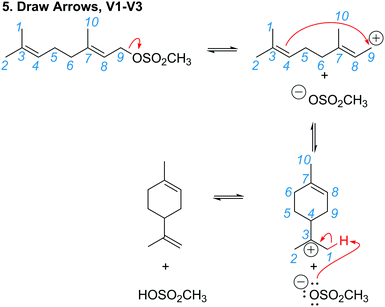 | ||
| Fig. 5 Versions 1, 2, and 3 of the interview guide had the same Draw Arrows task (student version in black). Answers shown in red; mapping added in blue italics to the manuscript. | ||
The length of the interviews averaged 28 minutes and ranged from 16 to 41 minutes. The interviews were audio recorded. The students completed the tasks using an iPad app called Notability, which recorded and synchronized the students’ drawings and audio.
The distribution of students per version of interview is listed Table 1. Not every student saw each task due to the version of the interview or because the student had to leave the interview early (one occasion).
| Question | Question type | V1 | V2 | V3 | Weeks of semester | N |
|---|---|---|---|---|---|---|
| 1 | Explain the mechanism (resonance) | X | X | 6–10 | 20 | |
| 2 | Explain the mechanism (resonance) | X | 10–11 | 9 | ||
| 3 | Explain the mechanism (hemi-acetal) | X | 6–7 | 5 | ||
| 4 | Explain the mechanism (acetal) | X | 9–10 | 15 | ||
| 5 | Draw the arrows (cationic cyclization) | X | X | X | 6–11 | 29 |
| 6 | Draw the products (intermolecular—organolithium and ketone) | X | 6–7 | 5 | ||
| 7 | Draw the products (intramolecular—epoxidation) | X | X | 6–10 | 20 | |
| 8 | Draw the products (intramolecular—Curtius rearrangement) | X | X | 9–11 | 14 | |
| 9 | Draw the products (intramolecular—ether formation) | X | 10–11 | 9 |
Sample description
The sample consisted of 29 OCI students (8 men, 21 women). Our ethics approval allowed for post hoc retrieval of program of study and collection of grades for the course, which revealed that the students were enrolled in a variety of programs mainly in either the Faculty of Science or Faculty of Health Sciences. The range of course grades for the participating students was 60% to 98% with an average of 78%. The course average for the sample was slightly higher than the course average of 72%.Data analysis
The interviews were transcribed verbatim, and annotations about the students’ drawings were included in the transcripts. The first author conducted all analyses in NVivo 11 for Mac. Analysis first began with open coding within question type across participants and then compared within participants across questions. Next, the constant comparative analysis method was used to move the analysis forward (Corbin and Strauss, 2008). Codes were refined, and questions were asked of the data including where are there intersections between the codes, where is there no overlap in codes where one would be expected, etc. This analysis allowed us to uncover patterns within students’ responses to the mechanism tasks. Through constant comparative analysis, categories were developed from the codes (Corbin and Strauss, 2008; Saldaña, 2013). These categories encompassed the big ideas that students’ discussed when working through the symbolism questions.We addressed validity in our study in a number of ways—analyzing the data with a constructivist paradigm (Creswell and Miller, 2000). We used the lens of researchers, repeatedly returning to our data to see if the codes, categories, and findings made sense. In doing so, we were mindful of the participants’ context. For example, the first five participants were interviewed very early in their first organic chemistry course and had only begun studying acid–base chemistry in an organic context. As such, we would not expect them to make the same connections as students later in their studies. We sought trustworthiness in our findings by analyzing the confirmability of our findings and seeking disconfirming cases. We independently found multiple examples of confirming data (participant quotes) to support our findings; the reader will find many supporting quotes herein as examples. The third author additionally sought disconfirming evidence in the transcripts—that is, quotes that would refute the claims herein; none were found. We sought authenticity as we gained improved understanding of our participants, their analysis processes, and meaning-making. Our improved understanding has stimulated action on our part from both research and instructional perspectives and will hopefully do the same for others. For the readers, we have provided thick descriptions of our findings, giving as much detail as possible (Geertz, 1973). These descriptions are meant to establish credibility through the lens of the reader to help transport them into the interview setting.
Results and discussion
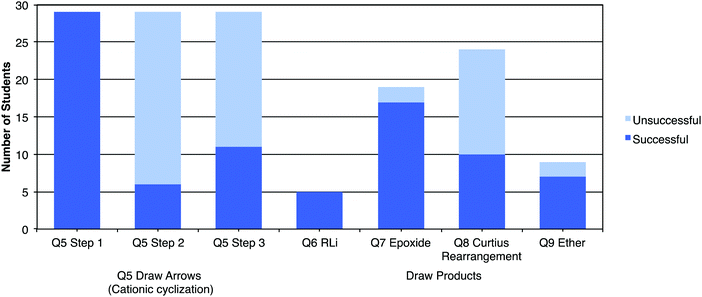
Students’ results on the Draw Arrows (Fig. 5) and Draw Products (Fig. 6) interview tasks is summarized in Fig. 7. All students were successful for the first step of the Draw Arrows task and for the organolithium Draw Products task. The SN2 reactions (labelled epoxide and ether formations) were the second most successful tasks with the majority of students being able to provide the correct products. Students struggled to give correct arrows for the second and third steps of the Draw Arrows task and the product of the Curtius rearrangement and only a small fraction of the students were successful on those tasks.To understand how students arrived at their answers for the interview tasks, students’ descriptions and explanations for each task were analyzed. Through the analysis process, four main categories emerged: (1) students’ approach to Mapping, (2) how students thought about and used Charges, (3) students breaking apart mechanisms into Stepwise processes, and (4) how students used Chemistry Reasoning in solving symbolic problems (Fig. 8). Underlying each of these categories is how students talked about Electron Movement while discussing the reaction for each task.
Electron movement
Frequently, students used electron movement to explain the mechanism, drawing of arrows, or the drawing of products. This section gives representative examples of how students talked about electron movement in general when talking about the mechanistic language, and the following categories continue to show examples of students using electron movement to work through the tasks.Students demonstrated knowledge that the curved arrowed depicted electron, not atom, movement. For example, Hannah explained a curved arrow:
Hannah: “Okay so it [curved arrow] goes from the bond so it's using those electrons in the bond.” (Draw Products)
Here, Hannah was able to explicitly talk about how the curved arrow represents electrons from a bond moving to form another bond. This explanation eventually led her to draw the correct product for the organolithium reaction. Ben also talked about electron movement while processing through the arrows of the same reaction:
Ben: “So there is electrons coming from the bond between the lithium and the carbon atom.” (Draw Products)
Ben saw the arrow from the lithium–carbon bond to the carbonyl and identified it as electrons moving from the bond. Jasmine explained her interpretation of the curved arrows for the epoxide formation:
Jasmine: “Um so uh the arrow that is moving from the bond between the carbon and the bromine is going to follow that um it's going to move electrons onto the Br um which is going to give it an overall negative charge and it's no longer going to be bonded to the ring. Um the oxygen uh will stay bonded to the original carbon and it will bond to the other carbon that was originally bonded to the Br because of the movement of electrons.” (Draw Products)
While working through the Draw Arrows task, Eve first identified the second step of the reaction as an intramolecular ring closing and then began talking about the electrons that must move in order to form the new bond:
Eve: “Yeah which means that um the electrons would move so the double bond would uh donate an electron pair to the positively charged carbon.” (Draw Arrows)
Eve acknowledged that electrons needed to move to form the new bond and then located the double bond that would donate electrons to the positively charged carbon atom. Eve's attention on electron movement preceded her drawing of the correct arrow for the step alluding to the idea that her thinking about electron movement set her to up draw the arrow properly.
Even students who were incorrect on some of the symbolism tasks considered electron movement in their reasoning. Consider how Mila talked through her decision to draw arrows for the third step of the reaction:
Mila: “And that lone pair is gonna form a bond with the other carbon to the right of it to form a double bond in the fourth structure, um oh yeah, that makes sense, and then the um the bottom right carbon is has too many um bonds attached to it now, so it has to kick off one of the hydrogens leaving that one open, um so it's gonna have an extra pair of electrons on that hydrogen which wait … I change my mind. … Um so instead of the bond breaking and going onto a hydrogen, the bond's gonna break and go onto the carbon so that this carbon is now neutral I guess. And then the lone pair from the oxygen is going to attach onto that hydrogen that just broke off of the carbon to form a bond and make the oxygen neutral.” (Draw Arrows)
Mila's ideas about where the curved arrows should be drawn were not chemically sound. She put a lone pair of electrons on a positively charged carbon atom and had a hydrogen atom leaving a carbon–hydrogen bond on its own (Fig. 9), but Mila was focused on drawing curved arrows from electron rich to electron poor areas. One could argue that Mila should have been able to use her prior chemistry knowledge to speak more accurately about the structure of the molecule; however, Mila's interpretation of the mechanistic symbols was being explored here, not her prior chemistry knowledge. The fact that she used the mechanism symbols in a more accurate way is evidence that she was trying to make sense of the given reaction. Perhaps as Mila continued to progress through the course, she was able to integrate her chemistry knowledge with her new knowledge of organic mechanisms.
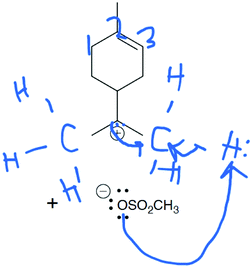 | ||
| Fig. 9 When drawing arrows in third step of the Draw Arrows task, Mila focussed on electron movement but made errors expanding the compounds’ structure. | ||
Within each task, students talked about electron movement. Even though not every instance of talk about electron transfer was discussed accurately (e.g., some students depicted moving the wrong pair of electrons or drew them to wrong atom or bond), the fact that there was discussion of electron movement is encouraging. Consider the following examples:
Scarlett: “Um the pair of electrons is being transferred to the nitrogen.” (Explain Mechanism)
Charlotte: “Um second this bond here, this carbon–carbon bond is going to donate its bonding electrons to the nitrogen.” (Draw Products)
Francine: “And you have the electrons being transferred to the carbon.” (Draw Products)
As these students had not yet received instruction on any of the reactions in the interview tasks, they would have had no knowledge of how the reactions proceed. Instead, they had to rely on their instruction on the language of mechanisms and any prior knowledge about bonding to decipher how the reactions proceeded. These students’ discussions of electron movement demonstrate that students were in fact thinking about electron movement and making explanatory connections to the representational level of the chemistry triplet (Talanquer, 2011; Taber, 2013) when talking through symbolism questions.
Mapping
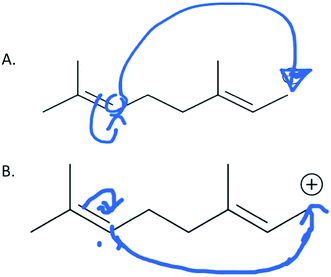
Mapping is a strategy to help keep track of atoms and electrons while drawing a mechanism. Although numbering atoms in the reactant and product tells the student (and others) that atoms were conserved in the reaction, mapping additionally equips students to track specific atoms and electrons from the starting materials to the product (Flynn, 2016). Students are taught mapping techniques for both Draw arrows and Draw products questions during the language of mechanism instruction of the course. During the interviews, students demonstrated attempts to map reactants onto products for Draw Arrows and Draw Products tasks. These mapping attempts revealed underlying ideas that students had about the mechanistic symbols, described below.The most difficult task given to the students in the interview was the second step of the draw arrows question with only 6 of the 29 students drawing in the correct arrow. For this step, students had to draw in the arrow for an intramolecular ring formation. How students chose to use mapping in this step revealed a tension between a focus on electron movement and a focus on connecting atoms together. Rachel began talking about which electrons could be used to form the ring and thinking about electron density which led to her map correctly:
Rachel: “Um since double bonds can donate electron density I'm going to try this and maybe see if that makes an actual structure. Um but that has to 2, 3, and this would form no wait. 3, 8, 7, that has a double bond. 6, 5, 4, I think that works.”
Rachel's notice of the double bond and its role in the reaction allowed her to easily draw a probable arrow. Rachel numbered the carbon atoms in the chain sequentially but started labeling the carbon atoms in the ring starting with carbons 2 and 3 as she realized that carbon 3 bonds with carbon 8. Upon drawing the arrow correctly, she saw that her choice to use the electrons from the double bond on the left side of the carbon chain made sense (Fig. 10).
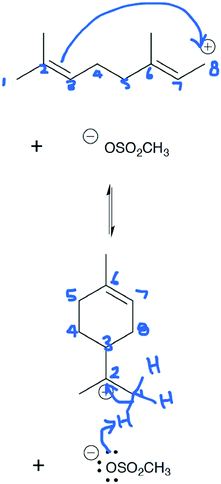 | ||
| Fig. 10 Rachel's mapping for Draw Arrows step 2 was correct (arrows for step 3 are also shown due to the format of the images). | ||
Other students focused on which atoms had to bond to match the ring in the next step of the reaction. Raisa's explanation of how the ring formed did not include anything about electrons. She began:
Raisa: “So you have the double bond, it's positive here [C8], so it moves down and that one goes like this so it has to be dun, dun, dun, dun, dun oo that's just going to I'm going to fix how that looks. And then those go away so you'll be just left with that.”
Raisa saw that the left portion of the carbon chain was similar to the top of the ring, so she concluded that the right side of the ring must have just folded in on itself (Fig. 11). To elicit ideas about electron movement, the interviewer asked Raisa what was actually bonding during the formation of the ring. Raisa responded:
Raisa: “Two carbons are bonding together…So the only thing I'm looking at is how they bond. Because I'm looking at it and these could bond together.”
Even when prompted, Raisa continued to focus on how the atoms in the chain must connect in order for the ring to form and did not go on to identify changes in electron positions.
Still, other students oscillated between focusing on electron movement and how the atoms must connect to form the ring. While acknowledging that electrons were needed to know where to draw the arrow, many students became side tracked with incorrect mapping. Like Raisa, many students were easily distracted with the similarities between the left side of the carbon chain, especially methyl C1 from Fig. 5 (called the first methyl group by the interviewer) and the top of the ring in the next intermediate—methyl C10 (Fig. 5; see Fig. 11 and 12 for examples). Consider these statements from Clara, Olivia, and Charlotte where each student compares the methyl group on the second carbon from the left (Fig. 5, C1) to the methyl group at the top of the ring in the next step (Fig. 5, C10):
Clara: “And I'm saying that number 2 is the methyl group [C10] in the attached to the ring.”
Olivia: “Ok. So this is this.”
Interviewer: “Ok so now we've highlighted the first methyl group [C1] and the methyl group off the carbon ring [C10] as the same.”
Olivia: “So because these two look like they are the same.”
Charlotte: “Ok um that looks like that [C1] could be the start of this like this part here [C10].”
Interviewer: “So the first methyl group?”
Charlotte: “Yeah the first methyl group [C1] looks like this group here [C10].”
Interviewer: “On top?”
Charlotte: “On top of the ring.”
By paying attention only to the superficial differences between the two structures, these students incorrectly made the assumption that the left side of the molecule stayed the same during this step in the reaction. With this assumption, students attempted to figure out how the atoms connected to form the ring. Adele made the same incorrect mapping mistake, and she followed through trying to map the carbon chain onto the ring (Fig. 12). This process created cognitive dissonance (Festinger, 1957) for Adele as she realized that something was not right:
Adele: “Uh I'm it's not lining up. I'm not sure where the ring would form what bond would make the ring and just trying to figure out where this Y shape would come from. … I'm going to try uh a different carbons forming the ring.”
Interviewer: “So remember to, when you figure it out, to draw in the arrow in the second structure that would form that ring.”
Adele: “Ok. And then I noticed that if this was the fourth carbon [ Fig. 5 , C6] on the bottom of the hexagon, it wouldn't make sense because it doesn't have –”
Interviewer: “Any substituents off of it?”
Adele: “Yeah. It only has one continuing part.”
The critical assumption about what stayed the same between the two structures proved faulty when following through. This cognitive dissonance provided an opportunity for the students to retract their steps and re-evaluate their process. In doing so, many students took the opportunities to consider how the electrons might move to form the ring. Adele continued:
Adele: “Uh I’m pretty stuck on this. I'm going to try an arrow here … Try and see if moving the bond would make sense.”
Interviewer: “And by moving the bond you mean onto the carbon 1 [ Fig. 5 , C9] with the positive charge?”
Adele: “Yeah moving the electrons around. Uh I think … hm … still don't think that would work.”
Even though she did not want to quite let go of her initial assumption, Adele did acknowledge the need for figuring out how the electrons moved. Only when considering the need for a tertiary carbon off the base of the ring did Adele finally give up her incorrect mapping and used the electrons from the leftmost double bond to participate in the reaction.
Like Adele, Olivia also first drew the electrons from the double bond on the right side of the ring as going to the carbocation. When Olivia realized that this step would create a positive charge on the wrong atom, she was able to give up her incorrect mapping assumption more easily than Adele:
Olivia: “Ok let's say ok the electrons go here.”
Interviewer: “Ok so we have the double bond closest to the positive charge breaking going to the positive bond, sorry, to the positive carbon.”
Olivia: “Ok and then this would become positively charged here at the hm –”
Interviewer: “At the, which would become positively charged? At the 6th carbon [ Fig. 5 , C7]?”
Olivia: “Yeah. But that doesn't make sense.”
As she considered her mistake, Olivia realized the flaw in her assumption:
Olivia: “Um I'm still trying to figure out how the chain sort of folded in so I'm trying to figure out oh ok, ok, ok. I think I was just looking at it, I was just kind of assuming it, ok, to be more linear. So I think it's the opposite actually. So this double bond –”
Interviewer: “And we mean the double bond furthest from the positive charge.”
Olivia: “Yeah. Um that should give up its electrons uh to this carbon which would make this part positive.”
Realizing her error motivated Olivia to consider different possibilities of how the ring could be formed. Then she was able to see the task from a different perspective (how Olivia completed this portion of the task will be described later in the article).
Another assumption that some students made was that the positive charge on the end of the carbon chain stayed on that same carbon in the next step. For instance, Nevaeh began talking about finding similarities between the two structures when she did not know where to start:
Nevaeh: “I would say I look at this, and I think, ok I don't, like, my instinct is not to, like, I can't figure out usually where it's start – the cyclic, so I look at the charge down here as my first hint because I know that there's a charge on that carbon up there.”
Nevaeh's assumption about the location of the charge led her to hold the previously discussed assumption about the leftmost double bond. When she followed through with mapping, she allowed herself to consider that the positive charge might be on a different carbon in the second structure:
Nevaeh: “That's my, this is gotta be 8 because that's where my plus is. Which means that um it's got 7 attached to it on one end but there's two other things that are over here. So maybe that's not actually where the carbon is. Maybe that's like not the same carbon.”
When her first mapping failed to work, Nevaeh began looking for other similarities between the structures, namely, for which carbons had methyl substituents. Still holding on to the assumption about the positively charged carbon, she said:
Nevaeh: “I'm trying to like match up now the carbons from the main chain with the ones that have the methyl groups. So 6 has a methyl group so maybe um if I do that that gives me 6 here which would give me 10 there. And then 7 is down here, 8 but now I don't have a plus on the 8. Huh.”
With this new insight, Nevaeh continued through with the realization that this mapping might work out:
Nevaeh: “Hm. Ok, well, in that case, I would go back this way, 5, 4, 3, ok, that makes sense because this is where some weird stuff is happening. And there's a double bond in 2 to 3. So, that could be 2, and that works because there's two things bonded to 2 so that totally works. So I'll pretend this is 9 and this is 1. And I don't think it would matter because it's tetrahedral so it can swing and stuff. Ok so, that's, I think this is right because, um, and, the plus from the carbon 8 probably just moved because this matches, so, um, now I have to figure out where the double moved to, so um it looks like, ok, this is not fair. Ok, 10 stays the same. So, something, like, it's gotta, I'm trying to, like, picture, so we know that this is the same, this is the 10, and the 6 is the same, 7 is the same, and 8, so it's gotta have happened, something happened to the 8 and that makes sense because the, yeah ok so it's uh yeah it's definitely where the double bond went to the 8 because of the plus the negative attracted to the plus and 3 is attached to 8 so that makes sense.”
Nevaeh seemed discouraged at first, unsure of how the mapping would lead her to the product. Then, she realized that the positive charge could now be on a different carbon atom in order to match the substituents on the carbon on the left side of the chain and the isopropyl group at the bottom of the ring. Nevaeh then supported this conclusion with her knowledge of how carbon chains can move. Becoming frustrated again with what happened to the double bond, Nevaeh finally realized that the double bond's electrons were attracted to the positively charged carbon atom. Only when Nevaeh considered electron movement within her mapping was she able to be successful in her response (Fig. 13). When students were able to integrate thinking about electron movement with mapping (such as Rachel, Adele, Olivia, and Nevaeh), they were more likely to be successful. When students focused only on trying to map without explicitly considering electron movement (such as Raisa Clara, and Charlotte), they were not as successful.
Charges
As charges were obvious and explicit pieces of reaction mechanisms, students frequently talked about charges throughout the interview during all tasks. Some students used charges as cues to know where electrons were going and leaving from in a reaction, but many students emphasized charge exclusively over electron movement.Adele: “There's an extra set of electrons so that's why there is a negative charge.” (Explain Mechanism)
Jasmine: “Well if I see a negative charge there's an extra pair of electrons.” (Explain Mechanism)
Sarah: “So if there's like a positive charge I know that there's less electrons than there would be if it was neutral.” (Explain Mechanism)
Within the context of each task, students implicitly defined charge by talking about the movement of electrons:
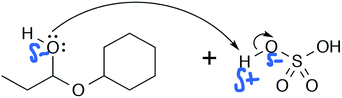
Heather: “The oxygen on the cyclohexane with the two oxygens donates an electron pair to the hydrogen on the sulfate [hydrogen sulfate] and then the bond between the hydrogen and oxygen breaks, so the oxygen gets two electrons, so it becomes negatively charged, and the oxygen that donated the electrons goes positive.” (Explain Mechanism)
Francine: “And then the double bond is being transferred like the pair of electrons to the carbon and you have an extra electron on it; that's why you have a negative charge.” (Explain Mechanism)
Ben: “It's getting a negative charge, so it's gaining electrons, so I draw an arrow there, and then you have the positive left on that last carbon.” (Draw Arrows)
Isaac: “Bromine bond is gonna break, uh bromine's gonna take the electrons, it's more electronegative in that case, so it's just kind be its own little product over here. Its gain of electrons, it's gonna be negatively charged.” (Draw Products)
Students’ connections of charge to the process of electron movement shows that these students were thinking about electron movement and integrating it in their understanding of the symbolism of mechanisms. In other instances, students used a formal charge calculation to know whether a charge was present. When Marianne was asked how she knew a negative charge was on a carbon, she replied:
Marianne: “Uh because the carbon has, uh supposed to have four bonds and uh so when the two electrons move to that carbon it's attached it's also like bonded to a hydrogen so then it has the one carbon bond and the other carbon bond and then the bond with the hydrogen so that's three bonds and carbon usually has four valence electrons so the four valence electrons minus the three bonds minus the two electrons gives you the negative 1.” (Explain Mechanism)
To determine the formal charge, Marianne first talked about electron movement to identify what was bonded to that carbon atom and then used a calculation. Adam calculated the formal charge on the bromine by implicitly accounting for the electron movement in the breaking of the carbon–bromine bond:
Adam: “So this is easy, the bromine is just unconnecting. So it's going to be a lone Br. It's going to be minus since it's going to have a full octet and Br is one of the seventh … so that means it should have seven minus eight is minus one.” (Draw Products)
Ben: “Then the positive charge is being, um, going away in the second step.” (Explain Mechanism)
Alessa: “The charges are moving from one of the functional groups to the other one.” (Explain Mechanism)
Ben and Alessa's descriptions gave the impression that they saw charge as an object that can be placed somewhere else rather than as a symbol to represent degrees of electron density. While figuring out where to draw the arrow to form the ring for the Draw Arrows task, Heather's explanation of the placement of the arrow reflected a similar idea:
Heather: “So the bond between the electrons and the last carbon [ Fig. 5 , C3] is made so the positive goes away.” (Draw Arrows)
Heather's treatment of the positive charge not only demonstrated the idea of charge being a contained object that could disappear or be placed somewhere else but also revealed the movement of charge as an effect of the electron movement. For Heather and many other students, a reaction or reaction step proceeded because a charge was present. Thus, the reaction step's purpose was to “take care of” the charge. Students used language indicating a cause and effect relationship between the presence of a charge and a reaction step:
Hannah: “A pair of electrons go into the upper nitrogen group in order to cancel out its positive formal charge.” (Explain Mechanism)
Sarah: “Because it has a negative charge, one of the electron pairs is attacking the hydrogen.” (Explain Mechanism)
By using words and phrases like “in order to” and “because of,” the students used charge as a reason for why electrons would be transferred. One idea could be the students use charge as an explicit cue to indicate where a reaction has taken place, as might a more experienced chemist. For a chemist, the cue reveals areas of a high or low electron density signalling reactive sites. However, these students are only beginning to use charge as indicators where a reaction will take place and are not yet mentioning electron density.
Rachel: “Um and since the oxygen in cyclohexanol now has a positive charge that pulls the electrons, uh, from the hydrogen that it's bonded to and then the extra electrons from the sulfuric acid attacks the H to neutralize all the charges and you get the final product with no charges.” (Explain Mechanism)
Seeing that there was a positive charge, Rachel explained that the sulfuric acid donated electrons to the hydrogen in order to neutralize that charge. Other students took the neutrality idea further as if it were a necessary goal for each reaction step. For instance, Charlotte was emphatic about neutrality:
Charlotte: “Um ok well it's gonna want to be as neutral as possible. Um and that's why I mean that's why that double bond donated electrons to the nitrogen on top.” (Explain Mechanism)
Charlotte's focus on the molecule becoming neutral was her reason that electrons would move to the nitrogen atom. As an early idea for what is happening in a reaction, Charlotte's reasoning could be a helpful stepping-stone before making links to energy and reactivity. Like Charlotte, Adam also connected neutrality with stability:
Adam: “And then the fourth one's really simple just because of the fact that they both have charges and you're just going to neutralize them. So basically the sulfuric acid's just going to get its H back. So the oxygen with its charge can't hold it as well. Um and then everyone's happy.”
Interviewer: “In the end? Ok.”
Adam: “Uh-huh.”
Interviewer: “Now what do you mean by 'everyone's happy in the product'?”
Adam: “There's no more charges.”
Interviewer: “No more charges. OK”
Adam: “So that means they are more stable which makes more sense that way.” (Explain Mechanism)
Adam declared the molecules happy in the end because they are neutral and stable.
Connor had a unique way of talking about the need to neutralize charges. When talking about forming the ring for the Draw Arrows task, Connor said:
Connor: “And it [meaning the transfer of electrons] would uh satisfy this carbon here and get rid of that charge.” (Draw Arrows; emphasis added by authors)
Connor continued when asked to explain his thinking about charges:
Connor: “I know that this carbon that I've numbered 8 [ Fig. 5 , C9] is going to be the most reactive of this set because it's not satisfied, and a carbon cation is very reactive.” (Draw Arrows; emphasis added by authors)
Instead of saying the transfer of electrons would cancel out or neutralize the charge, Connor described charged species as being unsatisfied and only satisfied when their charge was neutralized. Connor continued this train of thought through the Draw Products task as well:
Connor: “Um it [carbon with a positive charge] will be satisfied by the by one of the sets of lone pairs on the oxygen are going to flow over to it.” (Draw Products; emphasis added by authors)
Again, Connor expressed how a charged species was satisfied when electron transfer results in a neutral species. Although he did not explicitly refer to the atom's octet or to its orbitals, perhaps he was referring to the common expression of an atom's octet being satisfied. This choice of the word “satisfy” appeared to give human characteristics to the charged species.
The students further expanded the idea that neutralizing charges leads to greater stability during the Draw Arrows and Draw Products tasks. There was a general perspective that charges needed to be neutralized. Ivy explained how she used charges in the Draw Arrows task:
Ivy: “Well, the charges show that there's extra electrons or a deficiency of electrons which means these structures are unstable and so they're likely to form bonds in order to make a more stable structure.” (Draw Arrows)
Ivy indicated that the charges were unstable things that needed to be neutralized in order to become stable. Marianne talked about how electrons moved to positive charges to neutralize them:
Marianne: “When there's a positive charge it means that the electrons can move towards it because the electrons are negative. So they're gonna wanna follow around the positive charge. So I know it's easier for the double bond here to move towards the positive charge because then it neutralizes well it makes that carbon well neutral but then the carbon the double bleh the positive charge moves to another carbon, it has to get neutralized in another way…” (Draw Arrows)
Marianne demonstrated the idea that electrons can move to interact with positive charges. Other students expressed a need for both an explicit positive and negative charge for a reaction to occur. Mark said:
Mark: “I just always try to go from negative to positive. Keep it simple.” (Draw Arrows)
Students who relied on the presence of both a positive and negative charge to know how two species react faced a challenge when only a single charge was present. Olivia explained her reasoning behind her arrows for forming the ring:
Olivia: “Um well it seems that like in order like negative has to go to where it's positive like usually like when it's sharing electrons. I think that's the part I was having issues with for the second step here. Cause I wasn't, like I was trying to figure out how to get that negative charge in order to go towards the positive charge. And so –”
Interviewer: “So you knew we needed to create a negative charge?”
Olivia: “Mhm. I just didn't like when I was saying like how would I go like from this farthest carbon towards that I was trying to figure out how to create that [negative] charge in order to do it that way.” (Draw Arrows)
Rather than looking for areas of higher electron density that might react with the positive charge or mapping the atoms and electrons to see where the reaction did occur, Olivia first broke the double bond, creating a negative charge to react with the positive one. The creation of additional charges will be further described in the following section.
Finally, students rarely explicitly discussed the idea of partial charges. Even though partial charge was an idea discussed during class time, students appeared to rely heavily on formal charges rather than partial charges. A few students did talk about electrons from a double bond moving (see quote from Marianne above), but only one student explicitly used the idea of partial charge. Nevaeh incorporated partial charge into her explanations during each task throughout the interview, unprompted by the interviewer. Beginning with the acetal formation explanation (Q4), Nevaeh used partial charges to explain the symbolism:
Nevaeh: “Um alright so then what's happening is um this oxygen here has…um a partial negative charge and uh the hydrogen over there has a partial positive charge. So um this oxygen has two sets of lone pairs and so one of that sets, one of the set of lone pairs is attracted to the hydrogen because it has a partial charge and because negative is always attracted to positive.” (Explain Mechanism)
During her explanation, Nevaeh even drew the symbols for partial charge into the drawing (Fig. 14). Nevaeh continued the habit of drawing in partial charges for Draw Arrows and Draw Products tasks as well.
Stepwise
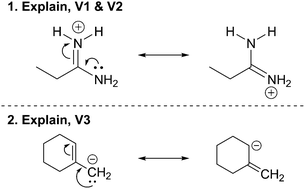
As students solved the mechanistic symbolism questions, they would often split up the elementary mechanistic steps and add additional steps that were not explicitly shown. Often, they drew extra structures that were less important resonance contributors of the one drawn in the question (without using the term “resonance”). Students seemed to think that some steps in the mechanism were not shown, and so they added them.The stepwise idea was first exhibited within students’ explanations of the resonance contributors in the Explain Mechanism tasks. Some students gave the central carbon atom a positive charge when electrons in the double bond move up to the nitrogen atom (Fig. 3, Q1). The new positive charge on the central carbon became the reason why the lone pair of electrons on the nitrogen would move to create a double bond between and carbon and nitrogen. As Raisa said:
Raisa: “And then because there's two lone pairs there's a lone pair on the NH 2 what happens is because carbon has a plus one charge now it has to go and equal it out.” (Explain Mechanism)
Although there was no positive charged shown on any carbon atoms in this task (Fig. 3A), Raisa's description of the mechanism arrows included a positively charged carbon atom. Isaac, too, included this perspective in his explanation:
Isaac: “Ok, so, on the first um structure here the positive charge on the nitrogen, since it's got a positive charge, the double bond is gonna head towards it and therefore bringing the electrons towards the nitrogen. That therefore leaves carbon with a positive charge. And then the electrons from the nitrogen on the NH 2 will be attracted to that positive charge and form a double bond there leaving the nitrogen with a positive charge.” (Explain Mechanism)
Isaac talked through the arrows one at a time as if the electrons moved sequentially rather than in a concerted fashion. In Isaac's explanation, the electrons moved from the carbon creating a positive charge, which then enabled the electrons from the second nitrogen to create the double bond with the carbon. These students did not describe these extra species as being resonance contributors or whether they pictured new discrete species forming, but seemed to be thinking of each arrow as occurring in a separate step.
Students continued explaining the arrows in an ordered and subsequent manner for the acetal formation as well (Fig. 4). Consider how Mila described the water leaving step of the reaction:
Mila: “Ok so first this is gonna go up there and form a bond there. Um so then this carbon is gonna become positive so because it's positive it has to take away another bond to make it neutral. So this bond is gonna go up there to the oxygen, which is already shows.” (Explain Mechanism)
Like Isaac, Mila talked about each arrow as happening one at a time, with a consequence for each one leading to the reason for the next. The electrons from the oxygen–carbon bond go to the oxygen leaving the carbon positive, and then because the carbon is positive, electrons from the second oxygen form a bond with the carbon.
The stepwise perspective was exemplified in the students’ explanations in the Draw Arrows task (Q5). Ten students drew arrows demonstrating a stepwise mechanism for step 2, step 3, or both steps 2 and 3 of the reaction (Table 2).
| Drew arrows stepwise | ||
|---|---|---|
| Step 2 | Step 3 | Both steps 2 and 3 |
| Heather | Hannah | Robert |
| Ruby | Sarah | Clara |
| Adam | Olivia | |
| Adele | Isaac | |
Ruby said she knew the double bond on the left of the carbon chain moved but expressed some confusion over what actually happened in the reaction:
Ruby: “I'm a little confused as to where this went. I always kinda get like mixed up with where when bonds break kind of um in terms of when they form the cyclohexane rings.”
Interviewer: “Yep. Which carbon connects to which?”
Ruby: “Yeah which carbon connects to which. So I've noticed that the carbon the double bond furthest from the ring uh sorry furthest from the positive charge is gone so it's gone somewhere I just don't know where so I'm gonna break that off um…let's see ok. Ok I'm not too sure how that one might have got right there but uh–”
Interviewer: “You can just try. Remember it's not about right or wrong. So just try.”
Ruby: “Ok I'm gonna just do that. Ok. I'm gonna do that. And that –”
Interviewer: “Ok so we've broken the double bond furthest from the positive and just put the electrons onto the third carbon in the chain [ Fig. 5 , C4].”
Ruby did not elaborate on why she drew the arrows as she did (Fig. 15A). Perhaps she found a way for the carbon chain to form the ring and decided to move on. Robert also drew stepwise arrows for the second step of the reaction and included an explanation using electron movement (Fig. 15B). Robert described his thought process:
Robert: “But I don’t know where I’m going to get the electrons, um so I’ll take the pencil and draw it here.”
Interviewer: “Sure, you do whatever you need to do.”
Robert: “So I’ve taken a pair of electrons off of the C, so I have three so I already had two hydrogens over here, [inaudible] this is the best step of this reaction.”
Interviewer: “Mhm.”
Robert: “Um I have to connect this one to that, I know that.”
Interviewer: “So you’re saying you have to connect the positive carbon [ Fig. 5 , C9] to the third carbon [ Fig. 5 , C4].”
Robert: “Yes.”
Interviewer: “Okay.”
Robert: “Uh so, I can only do that by basically connecting some kind of electron um so, oh boy, I would want to actually just go like that, but then I don’t show movement of electrons.”
Robert acknowledged his need to move electrons to form the ring, but he was not exactly sure how to make that work. A few moments later, he continued:
Robert: “Because if I were to take that double bond and move it to this carbon over here I think I would have a better response and that positive charge would still be there on that.”
Interviewer: “Okay so we’re going to move, so we’re going to put the double, we’re going to move the electrons from the double bond onto carbon 3 [ Fig. 5 , C4] now instead of carbon 2 [C3].”
Robert: “Yeah.”
Interviewer: “Okay great.”
Robert: “So they’re just going to go nicely here, yup so that's better, and then we have our two little lone pairs now here.”
Interviewer: “Okay.”
Robert: “And now we can take this and, there we go, and move them onto this carbon [C9] here creating our ring I guess you could say.”
Robert decided that he needed to move the electrons in the left double bond to the adjacent carbon [C4] to connect it to the positively charged carbon on the right side of the carbon chain. Robert's explanation made it seem like moving the electrons to the adjacent carbon first made the electrons available to move to the positive charge.
Without acknowledgement, each of the students who drew stepwise arrows for this step actually drew a less important resonance contributor of the reactant. These students demonstrated that they knew how the ring formed, which proved quite challenging for the majority of the students interviewed. Yet, these students also showed a limited understanding of the mechanistic language thinking that electrons first have to be available and explicit in order to be used in a reaction step.
For the third step of the Draw Arrows task, students talked about the electrons moving from the hydrogen–carbon bond to the carbon atom and then to the carbon–carbon bond (Fig. 16). Adele explained:
 | ||
| Fig. 16 Adele (A) and Adam (B) drew electrons moving one atom and bond at time for step 3 of the Draw Arrows task. | ||
Adele: “Because over here it has formed a double bond on the fourth picture and arrows go from electron to atom. This bond breaks and the electrons go back to the carbon.”
Interviewer: “Ok so the bond between the carbon–hydrogen breaks and goes on to the carbon [ Fig. 5 , C4].”
Adele: “And that the carbon will use those extra electrons to form the double bond with the center of the isopropyl and um get rid of its positive charge cause now all of them have full octets.”
Adele could only form the double bond once she had made the electrons available from the hydrogen–carbon bond (Fig. 16A). Adam gave a similar explanation but also wondered about just moving the electrons from one bond to the other. He said:
Adam: “Um so there's this lone pair here … And that's going to bond with the hydrogen. … And so this carbon is going to get the electrons from that because it is more electron negative. Um –”
Interviewer: “And from that we mean the bond between the carbon and hydrogen?”
Adam: “Yes. And then oh gosh, I know I skipped this one but this is how I see it. So the carbon is going to get the electrons so I'll just kinda draw them out. Um and then from there those electrons will form the double bond. So I guess technically you could just move, I don't know if that, I don't know which one's correct, if you could just move the bond that's breaking and put it –”
Interviewer: “Right to the carbon.”
Adam: “If that's going to be making it the double bond.”
Interviewer: “So by that you mean drawing the arrow from the carbon to hydrogen bond to the second carbon with the positive charge on it?”
Adam: “Yes. Hydrogen. So this one's breaking, say, I don't there's two possible answers, I'm not sure which one, I find this one's more logical, the first one.”
Interviewer: “To see it in the three steps.”
Adam: “Yeah um because that's what actually happens but it's probably way quicker.”
Although Adam saw both the stepwise and concerted arrows as possibilities, he came to the conclusion that the stepwise arrows were more logical and quicker. The more explicitly pictured electron movement made more sense to Adam; so therefore, he decided it would be the correct set of arrows (Fig. 16B).
For the Draw Products tasks, students used a stepwise perspective across each task. As in the Explain Mechanism tasks, students would talk through each arrow as if they happened one at a time, each with their own consequences leading to the next arrow. While only a few students used a stepwise explanation for the organolithium and Curtius rearrangement tasks, 12 of the 29 students exhibited stepwise thinking during the epoxide formation task. Often students spoke of bromine leaving the carbon first which then gave the carbon a positive charge. Once the carbon was positive, the negatively charged oxygen could bond with the positively charged carbon. Consider how Isaac, Raisa, and Mila described the epoxide formation:
Isaac: “So the carbon now, this carbon that was attached to the bromine it's going to be positively charged because it just lost it [Br].” (Draw Products)
Raisa: “Because the bromine went off the carbon became a positive, because it's a positive, the negative lone pair electrons went to bond to that positive carbon.” (Draw Products)
Mila: “Um and then that's gonna leave this carbon that was attached to the bromine positive so now the positive and the negative charge from the oxygen are going to attract and it's going to form a bond between one of the lone pairs of the oxygen and the carbon attached that was attached to the bromine.” (Draw Products)
Again, there was no positive charge shown on the carbon where the substitution took place. The students created the positive charge as a means to have something for the negatively charged oxygen to bond with. The majority of students were able to successfully draw the epoxide product demonstrating some understanding of how electrons are moving and without confusion over only one of the nonbonding electron pairs explicitly drawn on the oxygen, but their descriptions of the symbols while drawing the product suggested they were visualizing the reaction differently than depicted (i.e., stepwise versus concerted). Even students who were successful on all of the tasks used a stepwise explanation for this task. Ivy said:
Ivy: “And since the leaving of the bromine group will cause a carbocation to form the oxygen looks like it would donate its electrons happily to that carbon.” (Draw Products)
Nevaeh also talked about an extra intermediate forming, with a positive charge on the alpha carbon. She explained her reasoning:
Nevaeh: “Um and then I know that this is going, this carbon is going to be left with a positive charge because of its connection. It was partial positive to being with and then it will actually like in that split intermediate second it'll actually have a –”
Interviewer: “Positive charge?”
Nevaeh: “Yeah it'll become like a bit of a carbocation.”
Nevaeh described the partial charge as having carbocation character but actually being like a carbocation for a split second. It is unclear whether Nevaeh was intending to talk about transition states since she did not elaborate on her thinking.
In the examples presented here, the students appeared to lack the understanding that mechanistic arrows shown in one step represent concerted electron movement. By inserting a positively charged carbon into the reaction step, the students created a resonance contributor or an intermediate that was not actually present. Analysis of student exams on similar question types did not find that students were creating extra structures (Flynn and Featherstone, 2017). Without yet knowing the students’ true intentions for drawing the extra intermediate, in this study, the extra intermediate appears to be helpful for the students. While it is important to recognize when the EPF language communicates concerted versus stepwise reaction steps, drawing resonance structures and alternate reaction mechanisms may help students later in organic chemistry identify reactive sites or explore alternate reaction paths.
Chemistry reasoning
Throughout the interviews, some students intentionally tried to integrate their chemistry knowledge about how and why reactions proceed, with their making sense of the mechanism symbols, even though they did not have to do so to solve these questions. The choice to integrate their prior chemistry knowledge into the interview tasks when they were not required to do so shows these students’ desire to grow their conceptual frameworks of how they think about chemistry and, specifically, organic chemistry reactions. Students are taught the language of mechanisms prior to learning about specific reactions, but students still have their prior chemistry knowledge from high school, general chemistry, and the beginning units of OCI (e.g., structure, conformation, stereochemistry). Some students were able to apply their chemistry reasoning in a way that led them to be successful at the interview task. For other students, their chemistry reasoning created dissonance with their current understanding of mechanisms. Still, other students misapplied their chemistry knowledge leading them to make substantial errors in their interpretation of the mechanism symbols. This section will highlight the ways in which students attempted to use chemistry reasoning while working through the interview tasks.Charlotte: “Ok um so the lone pair on the oxygen uh on like the hydroxyl group is going to uh attract the hydrogen over here on the –”
Interviewer: “Sulfuric acid.”
Charlotte: “Sulfuric acid. Um because that hydrogen on the sulfuric acid is extremely acidic.”
Interviewer: “Ok now why do you say it's extremely acidic?”
Charlotte: “I think it's because of the resonance that can form on the sulfuric acid and I think like and it's in an equilibrium type thing so eventually something's going to shift and that hydrogen's going to be extremely acidic and it's going to want to leave the structure.” Explain Mechanism
Charlotte made sense of why the first step in the reaction would occur by recalling that sulfuric acid has an acidic proton. Similarly, Connor talked about why cyclohexanol would react with the intermediate in the third step of the acetal formation. Connor explained:
Connor: “Because of the resonance created between the double bond on the oxygen, it's able to now take on the double, er the lone pair from that cyclohexanol and those electrons shift to the electro-positive, um cause it's more electronegative it would rather that anyway. And nothing's happening with what's going on with the –”
Interviewer: “So when you say the oxygen is more electronegative, rather that, so it does not like the positive charge?”
Connor: “It, yeah, rather it is less in a positive state because it'll, it has more affinity for those electrons than the carbon that's up there so it's more likely to kinda hold on, hold them close to itself rather than the carbon.”
Connor used electronegativity to explain that the lone pair from the cyclohexanol could bond with the carbon atom. At the end of the task, Connor again used chemistry reasoning to talk more about the acetal formation reaction:
Connor: “Um I know it's just because um I guess protonating and deprotonating that deprotonating the sulfuric acid uh protonates this and makes it reactive so it gives it the ability for the cyclohexanol to bond with that group um so it's just in the presence of sulfuric acid that this would take place.” (Explain Mechanism)
Connor consistently applied his chemistry thinking throughout the interview tasks. During the Draw Arrows task, Connor struggled because his chemistry thinking was not sufficient to help him solve the problem. Connor initially noticed that there could be resonance on the right side of the carbon chain since the positive charge was beside a double bond (Fig. 17). Connor explained where this thought process came from:
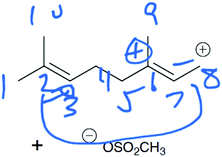 | ||
| Fig. 17 For step 2 of the Draw Arrows task, Connor fixated on resonance in the right side of the molecule. The drawing shows the bond he drew in on the far right side of the carbon chain and the would-be positive charge on the third carbon from the right (Fig. 5, C7). The drawing also shows the arrow he eventually drew in correct connectivity, but with a reversed arrow. | ||
Connor: “Um at first I, and so I looked at the, so I know that there's extra electrons in this group with the two double bonds, so first I looked at the closest double bond to it and I kinda worked through that, and I found that that wasn't giving me the structure that I was looking for, I don't know why, but that it was, I know, you would just kinda because kind of what we're talking about now in class that this positive would be better off in the tertiary structure between 9, 5, 7 on the 6 than it would be with the 8, so that's kinda where I'm focusing is here, but looking at the second structure, that would have to change, and so I'm just looking at the positive knowing that the second structure is going to be depend on what happens to this carbocation to make it happen.” (Draw Arrows)
Connor was using knowledge from his course to think about carbocations and their stability. His first strategy to draw the arrows was to make the molecule stable, and only when he looked ahead to the next structure was he able to consider how the electrons in the carbon chain would move to form the ring.
Heather: “So the 3 methyls on the left of the molecule don’t change at all. So there's the carbon that's there and it has 3 methyls still coming off of it, but it doesn’t have any hydrogens because it has the other bond still to it, but it breaks, so that one breaks, that makes the carbon there positive because there's no hydrogens and it just lost a bond and then that means there's a double bonded oxygen and the nitrogen, which has two lone pairs but it loses one to a bond. Loses one to a bond between nitrogen and carbon so that makes the double. So now the carbon that it's bonded to is neutral. It has 4 bonds. So it doesn’t get a charge. But the nitrogen lost a pair and it's now still gaining a pair from the bond that was between the carbon carbon. So it still has two lone pairs
Interviewer: “So it's got no charge?”
Heather: “No it does have a charge. It is… 6, it's only supposed to have 5 things around it so it's negative, which makes sense because it was neutral initially and it should still be neutral at the end.” (Draw Products)
Without offering an alternative, Heather broke the carbon–carbon bond and transplanted the electrons to the nitrogen atom (Fig. 18). In total, nine students drew products for the Curtius rearrangement (Q8) where the electrons from the carbon–carbon bond had been transplanted to the nitrogen resulting in two products. During the mechanism instruction, students were taught that electrons stay with one of the originating atoms, but this idea was still difficult for students to put into practice.
Connection to theory
The categories that emerged from the students’ responses reveal a range of thinking and interpretation of the symbolism of mechanisms. Applying the ideas from the Information Processing Model and Ausubel and Novak's Theory of Meaningful Learning offers insights into the students’ responses.The first part of the Information Processing Model involves the perception filter. Students attended to different features of the tasks including charge, electrons, and differences between reactants and products. For the students in this data set, the ones who were more successful explicitly talked about electron movement early in the task allowing for the connection to be made between success and attention on electron movement. For example, during the Draw Arrows step 2 task, Rachel considered electron movement from the beginning which helped her to map accurately from the beginning of the task. Students who were less successful often relied solely on three types of surface features: (1) Raisa focused on connecting atoms without mapping electrons; (2) Charlotte, Clara, Olivia, and Adele focused on structural similarities rather than electron movement; and (3) many students used charge alone to respond to a task. Students have different perception filters based upon their own prior experiences and prior knowledge (Novak, 2010). Thus, a student's organization of their prior knowledge in their long-term memory can also affect the features that they attend to (Schunk, 2012). Perhaps students with well-organized knowledge on the symbolism of mechanisms are able to more efficiently identify the relevant features needed to solve the task. Consequently, students with less organized prior knowledge could be less able to identify the relevant parts of each task necessary to provide an accurate answer.
The idea of how well the long-term memory is organized leads into the role of the student's working memory. Again, students who have more coherent knowledge frameworks could more easily consolidate new information (via “chunking”). By chunking, these students could possibly hold more information in their working memories than students who are unable to see patterns and consolidate new information into working chunks. In addition, students who were able to more often provide more accurate responses were often more open to possibilities than their one train of thought (such as Adele, Olivia, and Nevaeh). These students acknowledged their cognitive dissonance and took time to consider what else could be used to answer the task. Students who were less successful had a harder time when presented with evidence contradictory to the assumptions they were holding about the reactions. These students almost could not see the possibility for other options and were not able to decide what to do next. In these instances, perhaps these students had overloaded working memories. They could have been trying to think about too many different variables at one time which could have led either shutting down (nine students chose to skip the second step of the Draw Arrows task) or to use only the most obvious and explicit features of the reactions to respond.
Students’ inclination to provide a stepwise explanation could be an offloading strategy to deal with an overloaded working memory. Within the stepwise idea, both a positive and negative charge needed to be present, and one would be created if not explicitly shown. For many of these students, they would draw in either the new lone pair of electrons or talk about a new positive charge that would be present. Perhaps, they were unable to think about the electrons moving in a concerted fashion—unable to hold all the ideas about electron movement in their working memory at one time. Therefore, these students drew the movement of electrons one arrow at a time possibly to fulfill a need to detail each movement in order to understand the transition from products to reactants. Additional research is required to ascertain whether students believe the reaction actually proceeds in this stepwise way or if they are just using a problem solving method.
When the problem seemed too challenging or when the working memory became overloaded, students were faced with a choice of what to do next. There were a few students who stopped to think either early on in a problem or when confronted with conflicting information. In these instances, the student appeared to have an insight into how to solve the problem, suggesting that the student intentionally took the time to think. The choice to bring forth prior knowledge from the long-term memory to connect to new information is an essential component of meaningful learning. On the other hand, the students who chose to use only surface features to provide their responses perhaps demonstrated a choice not to incorporate their prior knowledge. We do not have enough information at this time to know whether students only had a surface feature understanding of symbolism in the long-term memory or if they decided not to think deeply about the tasks. Finally, some students gave up and did not complete the task. Perhaps for these students the challenge was too overwhelming to take time to consider all the information being held in the working memory or they felt pressure to think deeply within the interview setting of someone watching them work.
Conclusions
This study investigated student understanding of the symbolic language of mechanisms. Using a qualitative research protocol, 29 first-semester organic chemistry students were interviewed to explore how they interpreted the symbols of organic reaction mechanisms. When looking at the success of students on the tasks with a right answer (Draw Arrows and Draw Products), students were overall successful with drawing the arrows for the first step of the Draw Arrows task (removal of leaving group) and for drawing the products of the organolithium and epoxide formation reactions (Fig. 6). Students struggled to draw the correct arrows for the second and third steps of the Draw Arrows task and to draw the product of the Curtius rearrangement reaction. Analyzing the students’ responses revealed four categories summarizing the students’ use and interpretation of the symbols. First, students’ approaches to mapping had an effect on how they worked through the tasks. Students who chose to consider electrons at the beginning of the task demonstrated an interpretation of the symbolism (Fig. 10 and 13), rather than interpreting the structure only at face value looking at surface similarities between structures (Fig. 11 and 12). Second, some students relied on charge to work through the tasks. Some students used charged as a marker for reactivity and others talked about charge more as something that needed to be neutralized. Third, for some students, an overreliance on charge led them to draw additional charges to be able to explicitly show the neutralization of opposite charges, and thus, giving a stepwise explanation for how the reaction was occurring. Finally, students used their prior chemistry knowledge to work through the tasks. Even though not all the chemistry explanations were accurate, students attempted to interpret the symbols using chemistry ideas, demonstrating thought behind their responses beyond what was required of them to solve the tasks.Additionally, within each task, students talked about electron movement, demonstrating a connection being made between the symbolic and submicroscopic levels of the chemistry triplet. Students described electron-pushing arrows as moving electrons and talked through bonding breaking and forming using electron movement. This piece of evidence is crucial since these students have received explicit instruction on the symbolism of mechanisms without instruction on the specific reactions in the interview tasks. These students are at the beginning of their organic chemistry experience. Therefore, the attempt to think through the electron-pushing formalism as electron movement involved in bond-forming and breaking processes shows great strides for thinking about organic reaction mechanisms. Furthermore, students’ attempts to map, use of charges, and reasoning with relevant chemistry knowledge illustrates the potential for these students to continue to develop in successful problem solvers in chemistry.
This evidence continues to be promising for the patterns of mechanisms curriculum at the University of Ottawa. The goal of the present study was not to compare the University of Ottawa curriculum with a traditional organic chemistry curriculum but to explore how students interpret and use the symbols of organic reaction mechanisms in the context of reactions that are new to them. Our evidence shows that students are not using the EPF mechanically; rather, they are thinking about how to draw arrows and draw products based on the knowledge they have. For some students, this prior knowledge is about charges or how to map between reactant and product. For other students, they do have sufficient prior knowledge to have meaningful understanding of electron movement as they interpret and use the EPF. Further research is needed to examine how students’ interpretation of the symbolic language changes with more experience in organic chemistry.
Limitations
The results of this study should be interpreted within the context of the methodological choices made and constrictions based on the research design. All of the student volunteers were from one section of organic chemistry, so there is the possibility of an instructor effect. The other organic chemistry instructors at this institution use the same curriculum but could use slightly different terminology to discuss the mechanistic language. While the students from this study did use a variety of language on their own, future studies could attempt to diversify the course sections and institutions from which the sample is collected. Also, the students were volunteers and not based on purposeful selection except for their organic chemistry enrollment, so no claims for representativeness for the entire section or even course can be made. Even though the interview guide had to be modified as students learned reactions to keep the reactions new to the students, the majority of the interviews were conducted during a three-week time span during the semester (weeks 9–11). Students interviewed during the eleventh week of the semester had more experience with the EPF than students interviewed at any time prior; thus, they could have exhibited greater sophistication with the EPF. This study was not designed to measure the evolution of understanding of the EPF over a semester, but there may be individual differences present in how the students answered the questions due to their varying degrees of experience. The question remains as to how students develop language understanding of the symbols of mechanisms over time.Implications for teaching and learning
The evidence from this study is being used to evaluate the first component of the novel organic chemistry curriculum at the University of Ottawa (Flynn and Ogilvie, 2015). These students had already received instruction on the language of mechanisms and had opportunities to practice and receive feedback. In addition, the students had all the information needed to determine each answer. For example, starting materials and electron-pushing arrows were provided when students were asked to draw the products of each step. As such, the tasks given to the students should have been routine exercises, and yet, many of the students approached them as non-trivial questions for which both the answer and the process to determine the answer was not immediately obvious (Bodner and Domin, 2000). This work demonstrates many misunderstandings and challenges students have with the electron-pushing formalism and its associated meaning, even before students have to also learn the reactions themselves.The findings from this study demonstrate the variety of language students use to describe and talk about the symbols of mechanisms. Different students used different words to describe similar ideas but their different word choice could be influencing their understanding of organic reaction mechanisms. Instructors could reflect on what language is appropriate for beginning organic chemistry students to use and interpret. Then, instructors could lead a discussion with the students on the limitations of the language. As these are symbols to communicate submicroscopic behavior, when students start to acknowledge the limitations, they can also start to use the symbols more as a model instead of a picture of reality. Finally, students could then be expected to be precise with their language as well. Asking students to explain a mechanism in words on an assessment is one way to begin to elicit student thinking of symbolism (Cooper, 2015).
Using the Information Processing Model and Theory of Meaningful Learning to interpret the findings show that there could be multiple factors influencing how students’ interpret the symbols of mechanisms. Some students struggled to perceive relevant features or their working memories were overloaded, so they used only the most obvious and explicit features to work through the tasks. Students could have fragmented or misconnected knowledge structures in their long-term memories. Additionally, students could have the relevant prior knowledge but chose not to access it or did know how to do so. The results from this study point to a greater need to teach students how to approach problems, what to do when they do not know what to do, and other metacognitive monitoring techniques. Many students began working through problems using a strategy that would not lead them to a correct response. Some of those students saw the strategy through but then were faced with the decision of what to do when their strategy failed them. A few of those students were able to see their error and make a change. Some students did not follow through a strategy far enough to see the error they were making but made assumptions about the final answer. Instead of re-teaching content, students need to be taught how to see a strategy through to the end, to recognize when they are frustrated or confused, and what to do to move forward. Instructors have control over how new information is presented to students, but students must choose for themselves to actively participate.
Implications for research
The explanations that the students gave suggest future areas of research. These interviews have been the first to suggest that students think about mechanisms happening in a stepwise manner by moving the electrons one atom at a time and breaking or making one bond at a time. Additional research is needed to explore whether students believe reactions happen in this stepwise fashion in reality or whether the stepwise approach just represents a problem solving technique. The stepwise idea might disappear as the students learn more chemistry. Thus, longitudinal studies are needed to examine how students’ interpretation of the language of mechanisms evolves over time through a single semester and through multiple semesters of organic chemistry.Competing financial interests
The authors declare no competing final interest.Acknowledgements
This work was supported by the University of Ottawa. The authors thank the Flynn group for feedback on the research.References
- Anderson T. L. and Bodner G. M., (2007), What can we do about “Parker”? A case study of a good student who didn’t “get” organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students’ fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17, 1019–1029.
- Ausubel D. P., (1968), Educational Psychology: A Cognitive View, New York, NY: Holt, Rinehart, and Winston, Inc.
- Becker N., Stanford C., Towns M. and Cole R., (2015), Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16(4), 769–785.
- Bhattacharyya G., (2013), From source to sink: mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bodner G. M. and Domin D. S., (2000), Mental Models: The Role of Representations in Problem Solving in Chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Bowen C. W., (1990), Representational systems used by graduate students while problem solving in organic synthesis, J. Res. Sci. Teach., 27(4), 351–370.
- Bretz S. L., (2001), Novak's theory of education: human constructivism and meaningful learning, J. Chem. Educ., 78(8), 1107.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Childs P. E., Markic S. and Ryan M. C., (2015), The Role of Language in the Teaching and Learning of Chemistry, Chemistry Education: Best Practices, Opportunities and Trends, pp. 421–445.
- Cooper M. M., (2015), Why ask why? J. Chem. Educ., 92(8), 1273–1279.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87(8), 869–874.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 13, 195–200.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students’ understanding of structure–property relationships, J. Res. Sci. Teach., 50(6), 699–721.
- Corbin J. M. and Strauss A. L., (2008), Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 3rd edn, California: Sage, pp. 65–74.
- Crewell J. W., (2012), Educational Research, Boston, MA: Pearson, pp. 145–146.
- Creswell J. W. and Miller D. L., (2000), Determining validity in qualitative research, Theory Pract., 39(3), 124–130.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental rolodexing: senior chemistry majors’ understanding of chemical and physical properties, J. Chem. Educ., 92(3), 415–426.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Festinger L., (1957), A theory of cognitive dissonance, Evanston, IL: Row & Peterson.
- Flynn A. B., (2015), Structure and evaluation of flipped chemistry courses: organic and spectroscopy, large and small, first to third year, English and French, Chem. Educ. Res. Pract., 16, 198–211.
- Flynn A. B., (2016), OrgChem101 – Learning Lab, retrieved October 18, 2016, from http://orgchem101.com/.
- Flynn A. B. and Amellal D. G., (2016), Chemical information literacy: pKa values: Where do students go wrong? J. Chem. Educ., 93(1), 39–45.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students' strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18(1), 64–77.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: a mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92(5), 803–810.
- Geertz C., (1973), The interpretation of cultures: selected essays, New York: Basic Books, pp. 3–30.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13, 201–208.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89(7), 844–849.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78(8), 1126–1130.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7(2), 75–83.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7, 49–63.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Johnstone A. H. and Selepeng D., (2001), Methods and issues of teaching and learning, Chem. Educ. Res. Pract, 2(2), 19–29.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multivariate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Liu Y. and Taber K. S., (2016), Analysing symbolic expressions in secondary school chemistry: their functions and implications for pedagogy, Chem. Educ. Res. Pract., 17(3), 439–451.
- Mayer R. E., (2009), Information processing, in The Corsini Encyclopedia of Psychology, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 1–3.
- Mayer R. E., (2011), Learners as information processors: legacies and limitations of educational psychology's second, Educ. Psychol., 31(3/4), 151–161.
- Mayer R. E., (2012), Information processing, in APA Educational Psychology Handbook, Vol 1: Theories, Constructs, and Critical Issues, Washington: American Psychological Association, pp. 85–99.
- Novak J. D., (1993), Human constructivism: a unification of psychological and epistemological phenomena in meaning making, Inter. J. Pers. Const. Psych., 6, 167–193.
- Novak J. D., (2010), Learning, Creating, and Using Knowledge, New York, NY: Taylor & Francis Group.
- NVivo qualitative data analysis software, QSR International Pty Ltd, Version 11, 2016.
- Osborne J., (2002), Science Without Literacy: A Ship Without a Sail? Cambridge J. Educ., 32(2), 203–218.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students Introduction and theoretical framework, Chem. Educ. Res. Pract., 9, 122–130.
- Saldaña J., (2013), The Coding Manual for Qualitative Researchers, 2nd edn, Los Angeles, CA: Sage Publications.
- Schunk D. H., (2012), Learning theories: an educational perspective, 6th edn, Boston: Pearson Education.
- Sweller J., (1994), Cognitive load theory, learning difficulty, and instructional design, Learn. Instr., 4(4), 295–312.
- Taber K. S., (2009), Learning at the Symbolic Level, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Netherlands: Springer, pp. 75–105.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Taskin V. and Bernholt S., (2012), Students’ understanding of chemical formulae: a review of empirical research, Int. J. Sci. Educ., 36(1), 157–185.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Vladušić R., Bucat R. and Ožić M., (2016), Understanding of words and symbols by chemistry university students in Croatia, Chem. Educ. Res. Pract., 17(3), 474–488.
- Webber D. and Flynn A. B., (2017), Students’ mechanism proposals: familiar and unfamiliar reactions in courses taught with a mechanism-heavy curriculum and a patterns of mechanisms curriculum, manuscript in preparation.
| This journal is © The Royal Society of Chemistry 2017 |