High school students' engagement in planning investigations: findings from a longitudinal study in Spain
B.
Crujeiras-Pérez
*a and
M. P.
Jiménez-Aleixandre
b
aDepartamento de Didácticas Aplicadas, Facultade de Formación do Profesorado, Universidade de Santiago de Compostela, Spain. E-mail: beatriz.crujeiras@usc.es
bDepartamento de Didácticas Aplicadas, Facultade de Ciencias da Educación, Universidade de Santiago de Compostela, Spain. E-mail: marilarj.aleixandre@usc.es
First published on 13th October 2016
Abstract
This paper examines the process of high school students' planning investigations in the chemistry laboratory across two consecutive academic years in terms of their actions and their progress. The context is a set of five inquiry-based laboratory tasks in which participants (9th and 10th graders, 14–15 and 15–16 years of age) are required to plan and carry out investigations to solve contextualized problems. Data collection includes audio and video recordings of students' conversations and actions, as well as students' written products. For the analysis, two different rubrics have been developed, based on students' discourse and actions. Rubric 1, examining the students' actions related to planning investigations, consists of five categories that correspond to some of the operations assessed in the competency of assessing and designing scientific inquiry (OECD, PISA 2015 Draft Science Framework, 2013). Rubric 2, analyzing students' progress in planning investigations, consists of four levels of proficiency depending on: (a) the adequacy of their proposals to solve the issues addressed in each task and (b) their level of requirement of help from the teacher or not to complete the plan. The main findings point to a range of levels of achievement among groups and tasks, as well as in the type of scaffolding required from the teacher. Regarding the students' progression in the practice of planning, all groups evidence general progress from the beginning to the end of the study.
Introduction
Nowadays there is a consensus in considering that one of the main goals in science education should be to offer an education that develops students' understanding of both the canon of scientific knowledge and how scientific knowledge is constructed (e.g.Kelly, 2008; Osborne and Dillon, 2008; Duschl and Grandy, 2013). These recommendations propose to teach science through the practices used to establish, extend and refine knowledge (NRC, 2012). The term “practice” is the evolution of the notions “scientific method” and “inquiry” towards referring to students’ reasoning and acting scientifically. This new perspective involves a change in aims, moving from articulating regularities in reasoning and actions and using them to define what students should think and do to articulating what matters about science (Ford, 2015). Thus, scientific practices intend to situate the aspects of science into broader epistemic and discursive practices rather than only scientific process skills (Erduran and Dagher, 2014). This approach is in line with the guidelines included in the educational reforms in several countries (e.g.NRC, 2012; DfE, 2014; MECD, 2014) that recommend students' development of an appropriate conception of how scientific knowledge is constructed.Scientific practices for education have been proposed based on the analysis of professional scientists' work, characterized in three spheres of activity (NRC, 2012): investigation, evaluation and developing explanations. These three domains are unpacked into eight scientific practices: asking questions and defining problems, developing and using models, planning and carrying out investigations, analysing and interpreting data, using mathematics and computational thinking, constructing explanations and designing solutions, engaging in argument from evidence and obtaining, evaluating and communicating information. Furthermore, the three spheres of knowledge can be also associated with the three scientific competencies proposed in the PISA (Program for International Students Assessment) 2015 framework (OECD, 2013): evaluating and designing scientific inquiry; interpreting data and evidence scientifically and explaining phenomena scientifically, as summarized in Fig. 1.
 | ||
| Fig. 1 Relationship between scientific competencies and practices (adapted from Jiménez Aleixandre and Crujeiras, 2016). | ||
As represented in Fig. 1, at first sight scientific practices might seem to be more specific than scientific competencies, as they unpack the practices of professional science in more specific operations, proposing some that might be common to the three spheres of professional activity (using mathematical and computational thinking or obtaining, evaluating and communicating information). However, examining the 2015 PISA framework, it was found that there is some correspondence among the operations involved in the development of competencies and practices, such as controlling variables in order to obtain reliable results or looking for patterns in data.
Planning and carrying out investigations (PCOI) is one of the practices that can promote students' understanding about the nature of scientific work and its functioning. Thus in the US curriculum (NGSS, 2013) PCOI makes part of the eight scientific practices students should engage in. The National Curriculum in England (DfE, 2014) proposes a body of knowledge called “working scientifically” to be promoted across all scientific disciplines that involves experimental skills and strategies, with planning and carrying out investigations making part of it. In the Spanish curriculum (MECD, 2014) this practice is contemplated in a section called “scientific activity” that includes conducting scientific investigations and research projects as part of the content knowledge to be learned.
One of the recommendations proposed in the NRC (2012) framework is that students should engage themselves in the practices rather than merely learning about them; hence we consider that inquiry-based laboratory tasks constitute an appropriate resource to achieve this goal. According to Osborne (2014), the competency in assessing and designing scientific inquiry is developed by asking students to engage in the practice of designing empirical investigations to test hypotheses (planning investigations). However, as Osborne points out, yet this practice rarely takes place in classrooms. Our study seeks to add to the literature an examination of how high school students plan scientific investigations related to real life situations, and how they progress in the process of planning across two academic years.
The research questions that guide the investigation are as follows:
(1) How do students perform the operations involved in the practice of planning investigations?
(2) Which patterns of progress appear in students' designs across the study?
Theoretical framework
The role of laboratory in engaging students in scientific practices
Laboratory tasks have a crucial role in Chemistry Education since, when designed as investigations, they require students to apply content knowledge to solve practical problems. In the last few decades, there have been many studies in the literature that highlight the relevance of laboratory for Chemistry Education (e.g.Tobin, 1990; Hodson, 1998; Lunetta et al., 2008; Kelly, 2008; Högstrom et al., 2010). However, there is a gap between students' engagement in the laboratory and their learning outcomes (Hofstein and Mamlok-Naaman, 2007).Recently the role of laboratory in Education has been changed, from learning scientific skills and learning through inquiry to engaging in scientific practices (NRC, 2012) and developing scientific competencies (OECD, 2013). However, inquiry-based laboratory tasks continue to be relevant, as evidenced by the number of research projects framed in inquiry-based Science (such as Fibonacci, PROFILES or PRIMAS) funded by Horizon 2020 Agenda for Educational Research (European Commission, 2012) in the last few years.
Furthermore, this type of laboratory task puts students at the centre of the learning process (Kipnis and Hofstein, 2008), which is in line with “engaging in the practices rather than learning about them” proposed in the NRC framework (2012). Nevertheless, it needs to be noted that not all inquiry-based laboratory tasks are appropriate to engage students in scientific practices and competencies, as it depends on their structure and requirements. Inquiry-based laboratory tasks require an understanding of experimental design, appropriate means of measurement and data analysis and evaluation (Gott and Dugan, 1995). Other recommendations suggest that laboratory tasks should be designed as an authentic inquiry (Chinn and Malhotra, 2002; Lee and Songer, 2003), which means providing real life contexts relevant for students.
Taking into account the inquiry continuum (Windschitl, 2003), we consider that guided inquiry tasks, in which students are provided with a problem to investigate and they have to design how to investigate the issue prior to the experimentation, constitute a potential resource. Through this type of task, students develop knowledge that enables them to understand aspects of the material world through their own thinking, using critical and logical reasoning about the process and the evidence they get to address the issue investigated. Within the requirements of effective inquiry laboratory tasks to engage students in scientific practices, planning the investigation is considered as one of the most difficult aspects for students (Jones et al., 2000; Crujeiras and Jiménez-Aleixandre, 2015), as discussed in the next section.
The practice of planning and carrying out scientific investigations (PCOI)
Scientific investigations may be undertaken to describe a phenomenon or to test a theory or model for how the world works (NGSS, 2013). Planning and carrying out a systematic investigation that requires the identification of what is to be observed, measured and recorded is a major practice of scientists (Duschl and Bybee, 2014). The purpose of planning or designing investigations is to generate data that provide the evidence to support their conclusions (NRC, 2012). Hence, engaging students in planning and carrying out investigations involves establishing the purpose of research, predicting results and planning actions aimed at getting the best data to solve the issue investigated. More specifically, planning an investigation requires students to perform operations such as controlling variables, setting the materials and conditions for collecting data or proposing a procedure to investigate the issue.Duschl and Bybee (2014) propose a 5D model as an integrated approach to PCOI consisting of a group of practices embedded in five component elements of measurement and observation: (1) decide what and how to measure, observe and sample; (2) developing or selecting procedures to measure and collect data; (3) documenting and systematically recording results and observations; (4) devising representations for structuring data and patterns for observations; (5) determining if the data are good and can be used as evidence. As our study focuses on planning we consider the first two elements of the model.
This model regarding the scientific practice of PCOI is connected to the scientific competency of assessing and designing scientific inquiry through some common operations students should perform, such as proposing a way of exploring a given question scientifically, or describing and evaluating a range of ways that scientists use to ensure the reliability of data (OECD, 2013).
The benefits of planning for students' learning have been highlighted in the literature, as this practice involves them in scientific tasks that replicate real-world challenges and require solutions to solve them (Etkina et al., 2010). Apedoe and Ford (2010) emphasize the potential of making students design experiments to acquire an empirical attitude. Hmelo et al. (2000) propose that, through engaging in design, learners become more accountable for their learning. Schwartz and Martin (2004) point out that design activities help learners to activate knowledge and notice relevant features of phenomena or processes that enable them to take advantage of learning opportunities. Kolodner (2002) indicates that through this approach students learn to reflect upon and refine their reasoning ability. In line with this finding, Neber and Anton (2008) observe higher-order thinking in students when facing designing experiments.
Despite these benefits, laboratory tasks in which students are required to plan the investigation prior to the implementation are not as frequent as desired in high school chemistry lessons, perhaps due to the difficulties involved in the process. The main challenges are related to identifying the content knowledge relevant to solve the task (Girault et al., 2012; Crujeiras and Jiménez-Aleixandre, 2015); to propose complete and adequate designs, as students tend to provide ill-defined designs (Zimmerman, 2000), and to include familiar aspects that are not relevant for the design when planning the investigation (Krajcik et al., 1998).
It needs to be noted that within this approach the role of teachers is very important as it determines the learning process outcomes (Högstromn et al., 2010). According to Artigue et al. (2012) the role of teachers should be to guide students in developing the skills needed to carry out the investigation and the understanding of scientific knowledge through their own activity and reasoning.
In summary, although we recognise the difficulties students may experience in planning they should be immersed in this practice on a regular basis so that they can face these challenges. Students' engagement in inquiry-based laboratory tasks with increasing complexity in the planning phase could be a good chance to face these constraints. In this study, we have developed a longitudinal study across two academic years in order to examine if students' immersion in the practice of planning and carrying out investigations improves their ability to design investigations.
Methods
Research design
A longitudinal study is carried out in order to examine the students' actions related to planning investigations and their progression in this practice. The study was conducted during two consecutive academic years in a Chemistry classroom and consisted of five guided-inquiry laboratory tasks (two sessions each) in which students were required to plan and implement an investigation to solve the problems addressed each handout (see Appendix 1). The design of the tasks is summarized in Table 1.| Task | Content knowledge | Tips for planning | Operations to include in the design | |
|---|---|---|---|---|
| 9th grade | (1) To find out which toothpaste is ineffective in preventing tooth decay, through a simulation with clam shells (teeth) and HCl (mouth environment). | Chemical reaction Inhibition processes | Criteria for assessing the effectiveness of toothpaste Selecting the mass of shells and the volume of HCl Several methods for measuring gas release |
Proposing a procedure
Selecting materials and equipment Deciding measurement criteria Considering fair testing and reproducibility |
| (2) To identify and separate 5 substances (NaCl, C12H22O11, S8, Fe – iron chips – and C – graphite) that got mixed when an order got broken | Nature of substances Solubility Conductivity | A template with questions and decomposing the plan into two different phases: (1) separating the mixed substances, (2) identifying each substance |
Proposing a procedure
Selecting materials and equipment Deciding separation/identification criteria Considering reproducibility |
|
| 10th grade | (3) To identify which factory is responsible for polluting a river, through the analysis of water samples | Nature of substances Solubility Conductivity Chemical bonding |
Orientation tool aimed at identifying the relevant information provided in the handout |
Proposing a procedure
Selecting materials and equipment Deciding separation/identification criteria Considering reproducibility |
| (4) To identify the content of a forgotten flask in the laboratory (after working with KI, Al(OH)3, NaCl, NaHCO3, BaCl2 and HCl) | Inorganic reactivity pH | Orientation tool aimed at identifying the relevant information provided in the handout |
Proposing a procedure
Selecting materials and equipment Deciding identification criteria Considering reproducibility |
|
| (5) To find out who wrote an anonymous note that accused a student of copying the exam through chromatography | Polarity Organic solvents | Orientation tool for identifying the relevant information provided in the handout |
Proposing a procedure
Selecting materials and equipment Deciding identification criteria Considering fair testing and reproducibility |
|
It needs to be noted that each task involves different content knowledge, according to the lesson that was being taught at the moment of data collection. At first sight, it could be interpreted that tasks do not progress in complexity across the study, but they do if we consider not only the complexity of planning but also the amount of content knowledge to apply as well as the scaffolding provided to help students with planning. Thus, tasks in the first year of the study (9th grade) include more tips for planning, both from the teacher and the handout. The tasks in the second year (10th grade) do not include specific tips for planning, but an orientation tool aimed at interpreting the information provided in the handout. Furthermore, tasks 2 to 5 involve operations previous to planning, such as classifying the substances into their corresponding groups depending on their nature (task 2), depending on their appearance in water (task 3) or on their polarity (task 5).
Participants
The participants were 21 students in the 9th grade attending Physics and Chemistry classes in a rural high school, which was reduced to 10 students in the 10th grade. In Spain, scientific subjects are compulsory in the 9th grade but optional in the 10th grade that accounts for the loss of students. In this school there is only one class in each grade, which makes possible a longitudinal study otherwise, it would be quite difficult in our context. It needs to be highlighted that in public Spanish high schools there is not a tradition of teaching Chemistry through practical work, students do not carry out laboratory tasks on a regular basis, and when they do it they usually follow confirmatory experiences but not inquiry-based tasks. Hence, it is quite difficult to find a teacher who agrees to participate with his or her students in this type of research. In our case, the teacher has been collaborating with our research group for more than twenty years.Students work in five small groups (O, P, R, S, U) in the 9th grade, whereas in the 10th grade they were reduced to three, O′, P′ and U′, with some changes in the composition, as follows:
Group O/O′: in the 9th grade this group was formed by five students, but two of them did not enrol in Physics and Chemistry lessons, so in the 10th grade it was formed by the three remaining students, Ofelia, Olaia and Olga.
Group P/P′: in the 9th grade four students formed this group; two of them did not enrol in Physics and Chemistry. In the 10th grade group P′ was formed by two former group members, Paula and Pedro, and two additional students, Rosa, from group R, and Uxio, who was repeating the course. It was the group with more changes in members.
Group U/U′: in the 9th grade four students formed this group, but two of them did not enrol in Physics and Chemistry. In the 10th grade group U′ was formed by two former group members, Uxía and Úrsula, plus Sara, the only remaining student from group S.
Although student population is small and there are some changes in group composition across the two years, it represents the whole population of students in the school attaining Physics and Chemistry in both years. We consider that the loss of students in the second, a usual issue in Spanish education, does not affect student progression as those students who did not enrol in the subject were not engaged in solving the tasks in the first year, in other words, their contribution was not relevant to solve the tasks. In addition, we are aware that the sample is too small but our purpose in this preliminary study was not to obtain generalizable results, but understanding how students face to inquiry-based laboratory tasks when they are required to plan the investigations. It needs to be noted that the names used to refer to students are pseudonyms in order to protect their anonymity. Furthermore, both students and parents have been informed about both the goals of the study and the use that we would give to the data collected and they agreed to complete the informed consent documents.
Data collection
Data collection includes audio and video recordings of students' conversations and actions during the performance of the inquiry-based laboratory tasks and their written products (their experimental designs and the reports of each investigation).Tools for the analysis
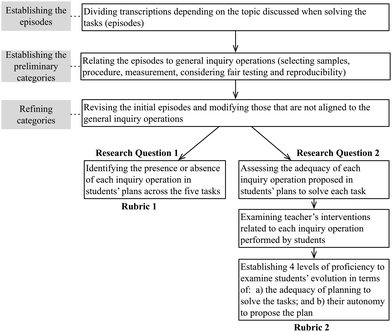
The unit of analysis is a small group as students perform the tasks in cooperative groups. The examination of data is framed in discourse analysis. Students' conversations were transcribed and coded. The teacher and students' utterances were unfolded by turns of speech (each intervention by participants in the talk) assigning one number to each intervention. Then we grouped turns identifying the episodes comprising one or more turns. Following Gee (2005), an episode is defined as one or several turns of speech related to the same topic or action. Once the episodes were established, the second phase of the analysis consisted of developing the preliminary categories. To do so, we have assigned an initial code to each episode drawing from the literature and comparing the assigned codes by both authors until refining the categories. This process was carried out for each research question and subjected to several cycles of analysis by the two authors until obtaining 90% of coincidence in the coding. The final products consist of two different rubrics, as summarized in Fig. 2.| Categories of the rubric | Description | 5D Model component | Scientific competency |
|---|---|---|---|
| (a) Selecting materials and equipment | Students decide the laboratory materials and equipment they will use in the investigations | ||
| (b) Deciding the measurement criteria | Students propose how to measure the magnitudes or variables involved in the investigation or the quantities they will use in each measurement | (1) Deciding what and how to measure, observe and sample | Deciding what things should be measured |
| (c) Proposing a procedure | Students indicate the operations and steps to follow in order to investigate the issue | (2) Developing or selecting procedures to measure and collect data | Proposing a way of exploring a given question scientifically |
| (d) Considering fair testing | Students take into account using the same criteria (quantities of substances, measurement method, etc.) for all samples, tests and experiments | Deciding what variables should be changed or controlled | |
| (e) Considering reproducibility | Students acknowledge the need for obtaining more than one value for each measurement in order to perform a reliable investigation | Deciding what action should be taken so that accurate data can be collected | |
Level 1: at this level, students propose an inadequate plan to solve the task, which means they cannot solve the task by implementing the planned design.
Level 2: students propose an adequate plan to solve the task but with the help of the teacher. At this level, students are able to plan an adequate investigation to solve the task because they have received support from the teacher during the planning process.
Level 3: students propose a partially adequate plan to solve the task by themselves. The proposed plan is considered as partially adequate because it enables students to investigate the issue but not to draw a complete conclusion.
Level 4: at this level, students are able to plan an adequate investigation to fully solve the task.
In short, the process of rubrics' development can be summarized as represented in Fig. 2.
Results and discussion
The results are discussed separately for each research question.Student's actions related to planning investigations
In this section, we first describe the type of operation students include in their designs according to rubric 1 and documented with examples from their conversations during the planning process. After that, we analyse the process through which students develop their designs.| Operation | O/O′ | P/P′ | U/U′ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | |
| (a) Selecting materials and equipment | ✓ | × | × | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| (b) Deciding measurement criteria | ✓ | × | × | × | × | ✓ | × | × | × | ✓ | ✓ | × | × | × | × |
| (c) Proposing a procedure | ✓ | ✓ | ✓ | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| (d) Considering fair testing | ✓ | × | ✓ | × | ✓ | × | × | × | × | × | × | × | × | × | × |
| (e) Considering reproducibility | ✓ | ✓ | × | ✓ | × | ✓ | × | × | × | × | × | × | × | ✓ | × |
Although we expect students to include all operations in their plans for the five tasks, there are operations that were not identified in their conversations and actions, as summarized in Table 3. Next, each operation is discussed separately and documented with examples from students' conversations. The selected examples are those considered as the most representative of the coding operations.
(a) Selecting materials and equipment. This operation is performed on all tasks by group U/U′ while in group P/P′ it is carried out on all tasks except number 2, and group O/O′ performs it only on tasks 1, 4 and 5. One example of how this operation is conducted is the following, corresponding to group P/P′ in task 1:
181 Pedro: So we have to ask for six pieces of shell
182 Paula: Six?
183 Pedro: Yes, two of them treated with toothpaste and four without treatment
184 Paula: But wouldn't it be four pieces treated and two without treatment?
185 Pedro: No
186 Paula: Two pieces treated with toothpaste x, the other two with toothpaste y and two without toothpaste
187 Pedro: What do you want two pieces treated with toothpaste x and y for?
188 Pilar: Because there are two different types of toothpaste to check
This excerpt is coded as selecting materials and equipment because students are discussing how many pieces of shell they are going to include in their design in order to assess the effectiveness of the two types of toothpaste.
(b) Deciding measurement criteria. This operation is identified in T1 for all groups and in T5 for group P/P′. A coding example for this operation regarding T1 is the following in group P/P′:
159 Paula: For measuring the gas what possibilities do we have?
160 Pedro: Using a graduate cylinder is complicated; does a balloon fit into it?
161 Paula: Yes
162 Pilar: But then it [the balloon] does not take the gas inside
163 Paula: The balloon?
164 Pedro: Why not? You don't understand! This is not about keeping the gas; the matter is inflating the balloon faster
165 Pilar: Ah ok!
In this conversation, students discuss the measurement criteria to assess the effectiveness of the two types of toothpaste, collecting the gas versus assessing the speed of inflation.
(c) Proposing a procedure. This operation is identified in all groups and tasks except in group P/P′ for T1. One instance of coding this operation is the following regarding group O/O′ in T4:
269 Olga: This [AgNO3] is only for identifying this [KI]. Well, but not as we said before. We proposed to differentiate this [KI] from this one [BaCl2], but its reaction produces the same [white precipitate] than this one [NaCl].
270 Olaia: Listen, first we have to identify if it [the transparent flask] is any of these two substances [KI or NaCl]
271 Olga: Yes
272 Olaia: Then we add this Argentum thing and after that we add ethanol in order to differentiate them.
In this excerpt, students are deciding how to identify the composition of the colourless solution contained in a flask by using some of the reactivity tips provided in the handout (see Appendix 1).
(d) Considering fair testing. This operation is only identified in group O/O′ in T1, T3 and T5, for instance, the following excerpt corresponding to T1:
255 Olaia: You have to use four [pieces of shell] two and two and they have to be equal [the same weight]
256 Ofelia: So one [piece] treated with toothpaste x and another with toothpaste y
257 Olaia: One piece has to be equal to the other, this for toothpaste x and the same for toothpaste y.
258 Olga: Yes.
259 Olaia: Otherwise, how do you do [compare them]?
As described in the conversation, students are considering using pieces of shells with the same weight in their plans, what we understand as considering fair testing. This appears again when they decide the volume of HCl to use in each trial.
(e) Considering reproducibility. This operation is identified in all groups, but in different tasks, O/O′ performs this operation in T1, T2 and T4. However the other two groups carry out this operation only in one task (T1 for P/P′ and T4 for U/U′). The following excerpt represents an instance of coding corresponding to O/O′ in T1:
132 Ofelia: Should we check it [each trial] twice?
133 Olivia: We can run several tests as the teacher said
[…]
136 Ofelia: So if the first and second [trial] match it is ok
In this excerpt, students consider repeating each trial, so it is coded as considering reproducibility.
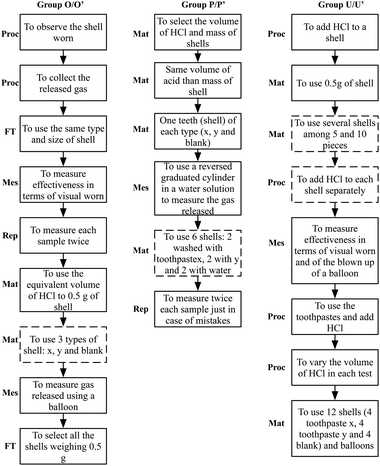
As represented in Fig. 3, students' actions are different among groups in number, type and order of appearance. Group O/O′ performs more different operations in number and type than the other two groups. They start their design by proposing a procedure, one qualitative (observing the shell worn) and another quantitative (collecting the released gas). However, their procedures are too general without specifying all the steps they need to perform. This group is the only one to consider fair testing criteria, regarding the type and size of shells. They also provide reproducibility criteria, proposing to run each test twice for each type of shell. However, they need teacher's help to select the type of shells to use, as they do not identify the three types of samples (shells washed with toothpaste x, with toothpaste y and the blank sample, a shell washed with water).
Group P/P′ perform few different operations (three), they do not propose either a procedure to follow or fair testing criteria, starting from selecting the materials they need to solve the task (proposing only shells and acid). After selecting the materials and the types of shells they will use, they propose using a graduated cylinder to collect the gas released in the reaction between shells and acid as measurement criteria. Then they propose an adequate number of samples to investigate the issue (six, two of each type) but with the help of the teacher. And finally, they explain why they use six shells in terms of reproducibility criteria.
Group U/U′ starts from proposing a not very precise procedure that is being completed across the process of planning with the help of the teacher, although they do not manage to propose an adequate one. They also need help to select the materials (several pieces of shells). This group proposes two methods for measuring gas release (observing the weathering of the shells and using a balloon) but they do not explain them. In addition, they do not consider either reproducibility criteria or fair testing, as at the end of the process, they propose to vary the volume of acid in each test.
It needs to be noted that all groups required help from the teacher during the planning phase, as represented in Fig. 3 by dashed lines. The three groups required scaffolding to select the appropriate materials and equipment and U/U′ for proposing some steps of the procedure, as well.
One strategy used by the teacher to scaffold students is the use of questions, as summarized in the following excerpt:
69 Teacher: So do you already know how to proceed? What are you going to do?
70 Uxía: Well, collecting the shells and the acid
71 Teacher: How many shells?
72 Úrsula: Several
73 Teacher: More or less than one thousand?
74 Úrsula: Less
75 Uxía: Five, well around five and ten
76 Teacher: And what else?
In this conversation, the teacher helps students with the selection of the materials through open and closed questions (turns 71 and 73 respectively). It needs to be highlighted that teacher's scaffolding was not directly required by students, but rather was the teacher who identified which group needed his help and when.
Regarding the other four tasks of the study, they were analysed following the same scheme, comparing students’ operations developed during each planning phase and examining the type of scaffolding provided by the teacher. Table 4 summarizes students' actions related to planning the five investigations and the input provided by the teacher (shaded) to help them with the process, if needed.
| O/O′ | P/P′ | U/U′ | ||
|---|---|---|---|---|
| T1 | s | Proposing different procedures: both qualitative and quantitative. Selecting measurement criteria (visual weathering of shells). Considering fair testing and reproducibility criteria | Identifying materials and equipment. Selecting quantities for each magnitude but imprecise. Selecting measurement criteria (reversed graduate cylinder to collect gas) | Proposing an ill-defined procedure. Selecting measurement criteria (visual weathering of shells, collecting gas released with a balloon) |
| t | Prompts for selecting the number of samples to assess effectiveness | Prompts for selecting the number of samples and for considering reproducibility | Prompts for selecting the mass and number of samples and for describing the procedure | |
| T2 | s | Proposing different procedures but incomplete. Using all the identification criteria provided to run the tests. Considering reproducibility criteria. | Proposing a procedure for identifying part of the substances composing the broken order | Selecting the materials needed. Proposing 1 ill-defined procedure explaining how to identify some substances |
| t | Prompts for using the identification tips provided in the handout | Tips for recalling the relevant knowledge to be used in the identification processes | Describing the use of identification criteria. Prompts for using the identification tips | |
| T3 | s | Considering fair testing. Proposing ill-defined procedures to identify the pollutants in the water | Selecting the materials needed. Proposing ill-defined procedures to identify the pollutants | Proposing ill-defined procedures. Selecting one solvent to identify one pollutant (CS2) |
| t | Posing open questions related to the identification processes | Posing open questions related to the identification processes | Posing open questions related to the identification processes | |
| T4 | s | Proposing 2 ill-defined procedures and 1 complete. Considering reproducibility criteria | Proposing 1 ill-defined procedure and 1 complete | Proposing 1 ill-defined procedure and 1 complete. Selecting the number of trials to perform. Considering reproducibility |
| t | Exemplifying how to run each identification test | Prompts for including all tests in the design | — | |
| T5 | s | Proposing the number of tests in terms of the data provided in the handout (colour of marker and type of font). Selecting the appropriate eluent in terms of the polarity. Describing the procedure to identify who wrote the note. Considering fair testing criteria | Selecting the appropriate eluent in terms of the polarity. Proposing the number of tests in terms of the data provided in the handout (colour of marker and type of font). Proposing an adequate procedure but ill-defined | Selecting the appropriate eluent in terms of the polarity. Proposing an adequate procedure but ill defined, including the number of samples and the required equipment, which overlaps with other operations. |
| t | — | — | — | |
As summarized in Table 4, students' actions are different among groups and tasks' contexts; the type of scaffolding required from the teacher also varies in all groups and tasks.
In tasks 1 and 5 students perform a wider range of different operations than in tasks 2, 3 and 4, which can be understood in terms of the nature of the tasks as they involve quantitative measurements and controlling more variables than other tasks.
In tasks 2, 3 and 4 all the groups focus on the procedure as the main operation included in their plans but to a different extent. For instance, group P/P′ includes the procedure as the only operation (in tasks 2 and 4) whereas group O/O′ considers also the identification criteria provided in the handout to run the tests (task 2), fair testing (task 3) and reproducibility criteria (tasks 2 and 4). Group U/U′ includes the materials they need to run the tests (tasks 2 and 3) or the number of tests to perform and reproducibility criteria (task 4).
Regarding teacher's scaffolding, the analysis also reveals differences among tasks and groups. It needs to be noted that the teacher was advised not to provide specific guidance to students during the planning process, only in those cases in which students would not be able to progress in the process of solving the tasks. The scaffolding strategies summarized in Table 3 can be grouped in terms of the more or less explicitness of the information provided to students: prompts, tips, exemplifications and descriptions (classified from the less to the most explicit strategy). Due to the nature of the study, the most appropriate scaffolding would be the prompts for engaging students in the operations involved in planning the investigations, such as the instance related to T1.
In general, regarding task 1 all groups need help to select the appropriate number of samples to assess the effectiveness of two types of toothpaste. In addition group P/P′ needs scaffolding to consider reproducibility criteria and group U/U′ to define the procedure. In task 2, only group U/U′ received direct help from the teacher aimed at describing how to use some identification criteria provided in the handout. In task 3 all groups received teacher's input, but in terms of open questions regarding some identification processes they need to include in their plan. For instance, questions aimed at checking electric conductivity to identify if there are substances dissolved in the river water, such as NaCl or sucrose that they do not identify at a glance. In task 4 two of the groups received teacher help, group O/O′ for specifying how to perform each identification test in the plan as they were only the identification criteria they were going to use (e.g. measuring the pH of the residuum and depending on the value they would perform the identification tests proposed in the handout for the specific pH) but they were not given an explanation as to how to perform these tests. And group P/P′ received help to include all the required tests in the procedure as they have only included some identification tests, but not measuring the pH which is the first step in the identification process. And in task 5 none of the groups received help from the teacher during the planning phase.
Students' progression in planning investigations
In this section, we categorize students' actions at different levels of proficiency. Fig. 4 represents the comparison of students' progression in planning investigations across the five tasks in terms of the four levels composing rubric 2. | ||
Fig. 4 Comparison of students' progress in planning investigations. Legend:    ; groups O/O′, P/P′ and U/U′ respectively; T1, T2, T3, T4 and T5: tasks 1, 2, 3, 4 and 5. ; groups O/O′, P/P′ and U/U′ respectively; T1, T2, T3, T4 and T5: tasks 1, 2, 3, 4 and 5. | ||
The progress across the study shows some common patterns, as there is improvement over time, but also some differences in each group. Group O/O′ progression is identified only at the end of the study (tasks 4 and 5). Their plans corresponding to the first three tasks are placed at level 1, as they do not allow them to solve the task. In task 4 this group progress to level 2 as they propose an adequate plan with the help of the teacher whereas in task 5 they advance to the high level (level 4) as their plan enables them to solve the task without teachers' help.
Group P/P′ progression is only at the last task (task 5). In tasks 1 to 4 they propose inadequate designs to solve the tasks, requiring a great deal of help from the teacher. However, in task 5 their plan is adequate in spite of not being completely defined.
Group U/U′ shows non-lineal progress at the beginning of the study, as they propose an adequate plan in task 2 but they go back to level 1 in task 3. However, from tasks 3 to 5 they progress from level 1 to 3 in task 4 proposing a plan that partially enables them to solve the task and in task 5 their plan enables them to solve the task, in both tasks without requiring teachers' help. It needs to be noted that their plan in task 4 is considered partially adequate as in this task students are required to consider two general issues in their planning (identifying the content of an unknown flask and selecting the best container to depose it depending on its composition) but they consider only the identification of the liquid in the flask.
In summary, substantial progress is identified in the last two tasks in two of the groups (O/O′ and U/U′), whereas in group P/P′ the progress appears at the end of the study. This difference could be influenced by the fact that group P/P′ is the one with more changes in members, since only two of the four members are present in the five tasks, recruiting a repeater student who was not familiarized with solving this type of task. It needs to be mentioned that the level of planning identified in students' performances did not affect to the completion of the tasks because the teacher revised each plan prior to the implementation in order to enable students to complete the investigations. However, teacher's help in planning did not guarantee good results, as experimental errors that lead students to obtain anomalous results made them draw inadequate conclusions for instance in tasks 1 and 3 for groups U/U′ and P/P′ respectively, as described in other studies (Jiménez-Aleixandre and Crujeiras, 2014).
Discussion and conclusions
This study is framed in the practice of planning and carrying out investigations (NRC, 2012) and in the competency of assessing and designing scientific inquiry (OECD, 2013). Both notions assess several operations such as deciding the types and quantities of data necessary to obtain reliable measures, considering the limitations about precision in data (for instance the number of tests to run, time or risks) and refining the design. The focus of the research is the process of planning the investigations, which can be considered as one of the most difficult operations involved in investigations and it is not as present as desired in high school chemistry lessons.In the literature, there are studies that examine students' general operations about planning investigations, from which the majority focus on undergraduate students (e.g.Etkina et al., 2006; Bugarcic et al., 2012). Other studies on high school students focus on particular aspects of planning, such as Hofstein et al.' (2004) in which the tasks require students to follow some direct steps to plan the investigation. Blonder et al. (2008) examine how high school students plan concrete aspects, related to using a particular technique, chromatography, but the task does not present a problem and is not set in a familiar context for students.
The difference between these studies and the one presented in our paper relies on the type of analysis. We examine all the operations involved in planning an investigation (proposing a procedure, selecting materials and equipment, proposing measurement criteria, considering fair testing and reproducibility criteria), as the aim of this research is to contribute to the knowledge about the processes involved in the design of investigations in which students have to solve a problem.
From the analysis of the operations involved in planning, there are some that students do not tend to include in their designs: deciding measurement criteria, considering fair testing and reproducibility. Perhaps this is due to their lack of experience in planning investigations, which points to the need for providing students with more opportunities to plan and carry out investigations so that they become familiar with this practice.
In addition, there are also differences among groups and tasks both in order of appearance and in the type of operation across the tasks. Thus, group O/O′ is the one who performs more different operations in all tasks, considering fair testing in their plans (in tasks 1, 3 and 5) without requiring help from the teacher. We consider this result as relevant that might indicate the effect of this sequence of tasks, as in other studies, like Hofstein et al.' (2004), they found that only 12th grade students familiar with planning investigations consider fair testing in their designs. Furthermore, there is only one operation that appears in all tasks (except in task 1 for group P/P′), which can be explained due to the consideration of the procedure as a central element in all investigations and essential for the implementation, as suggested by Girault et al. (2012).
The comparison of students' progression in planning investigations reveals some common patterns and also differences among groups. All groups progress in planning at the end of the study, which could be associated with the difficulty that this practice presents for students, as suggested in previous studies such as Jones et al.'s (2000).
Regarding the differences, the progress in group O/O′ begins in task 4, proposing adequate designs with the help of the teacher (level 2) and proposing adequate designs without teachers' help (level 4) in task 5. Group P/P′ evolves only in task 5 progressing from proposing inadequate designs (level 1) to proposing adequate designs without the teacher's help (level 4). Group U/U′ present a less clear pattern, as it progress in task 2 proposing an adequate design with the teacher's help. However, they go back to level 1 in task 3 proposing an inadequate design and they progress in the last two tasks proposing a partially adequate design in task 4 (level 3) and an adequate in task 5 (level 4) both without requiring teacher's help. It needs to be noted that U/U's regression in T3 could be related to the nature of the task, which requires students to consider a lot of aspects in their planning to identify the pollutants in the water sample and then to associate them with the corresponding factories. In addition, the level of scaffolding provided from the teacher could have been insufficient as he asked only open questions to support students during the planning process. The level of guidance provided by the orientation tool could have been also inadequate as it focuses on understanding the information provided in the handout to complete the task rather than on the planning process itself. These different behaviours in small groups could be interpreted by conducting an additional in-depth analysis of the type of teacher interventions provided by each group in each task. This is the next step in our research since other studies such as Blanchard's et al. (2010) suggest that the role of the teacher and their strategies influence the nature of inquiry-based learning.
It needs to be highlighted that students' plans, including those considered as adequate to solve the task, are little precise and ill-defined, which is in accordance with Zimmerman's (2000) revision, who found that students provide less information in their plans and they also do not propose systematic designs. We also agree with Krajcik et al. (1998) in considering that this fact could be due to the lack of familiarity with this practice as well as to the difficulties involved in the process of solving this type of task (Crujeiras and Jiménez-Aleixandre, 2015). Inquiry-based laboratory tasks designed as authentic problems enable students' engagement in the practices of Science but they are also challenging, as students have to apply content, procedural and epistemic knowledge to solve everyday life problems.
In light of the results we agree with Puntambekar and Kolodner (2005) as well as with Blanchard et al. (2010) in considering that students need scaffolding from the teacher to solve this type of task. We suggest that more support from the teacher prior and during the design phase is needed in order to improve the quality of students' designs. Reflecting on planning prior to the implementation could also enhance students' ability to design investigations, as according to the literature reflection improves students' ability to conduct inquiry-based experiments (Hofstein et al., 2004) and promotes meaningful learning about inquiry (Tobin, 1990; Yacoubian and BouJaoude, 2010). Even though we believe that students' engagement in scientific practices is developed through practice, perhaps they need previous knowledge on which aspects should include an experimental design as well as reflection about them. Engaging students in assessing scientific investigations, which forms part of scientific competency (OECD, 2013), could contribute to improve students' ability to plan investigations.
We are aware of the challenge that planning and carrying out investigations in chemistry lessons represents for teachers, as it requires developing strategies to make students learn about planning at the same time they plan the investigations, in other words, without setting aside the active role of students. Furthermore, we consider that students' effective engagement in this practice is a matter of time and familiarity, thus we suggest that students should be provided with opportunities to plan investigations on a regular basis and with increasing complexity to face the constraints involved in this practice.
Appendices
Appendix 1: handout excerpts
Part 1: designing the experiment
Design an experiment to find out which toothpaste is less effective. To do this you can use clamshell pieces (high-calcium carbonate material) as the tooth simulator and hydrochloric acid to simulate the environment created in the mouth after eating carbohydrates.
The following tips can help you to elaborate the design.
(a) Carbonate solution is a chemical reaction and it always takes place with gas release (carbon dioxide). The less effective the toothpaste is the faster the gas is released.
(b) You have to decide how to measure each material and also how to make the two things react, as the sample (clamshell piece) is a solid substance and the medium (hydrochloric acid) is a liquid. In addition, you have to decide how many clamshell pieces (in grams) and acid (in millilitres) you are going to use, taking into account that one tooth weights more or less 0.5 grams.
(c) You have to decide and justify which is the best option to measure gas release. For instance: watching the effervescence produced when the two substances are reacting, measuring the effervescence produced using a reversed test-tube in a water solution, measuring how much time does a balloon, put over the sample-tube in which the reaction is being produced, take to stand up or other options that you can think about.
(d) You have to establish how you are going to obtain the data that will allow you to make a decision about the results.
Other tips that can also help you with the design could be: what would you consider as evidence or what criteria would allow you to establish differences between the two types of toothpaste.
To help you with the planning phase you can consider the following data: among the five products there is a metal, two substances composed of molecules, one composed of ions and another one composed of a chain of atoms. In addition you can also use your knowledge about solubility, conductivity and magnetic properties.
The factories operating close to the river are:
– Factory 1: experts in producing sparklers. They use vegetal carbon and sulphur compounds.
– Factory 2: they produce salting cod using high-quality sea salt.
– Factory 3: they produce homemade sweets. They do not use artificial additives, only flour, water, sugar and animal fat.
– Factory 4: they produce the best countertops in town, specialists in granite and marble.
To solve the problem you need to design and implement an investigation in order to identify the factory or factories responsible for the pollution. You need to consider that some pollutants can be solid, dissolved or floating on water. To help you with the planning phase you can use your knowledge about solubility and conductivity of matter depending on their chemical bonding, for instance ionic substances conduct electricity when dissolved in water.
| Material | pH | Properties |
|---|---|---|
| KI | Neutral | It reacts with H2SO4 producing a white product. It reacts with AgNO3 producing a yellow precipitate |
| Al(OH)3 | Basic | It reacts with HCl producing a white product |
| HCl | Acid | It reacts with AgNO3 producing a white precipitate |
| BaCl2 | Neutral | It reacts with H2SO4 producing a white precipitate. The precipitate is dissolved when adding CH3CH2OH |
| NaCl | Neutral | It reacts with H2SO4 producing a white product. It reacts with AgNO3 producing a white precipitate |
| NaHCO3 | Basic | It reacts with H2SO4 producing gas release |
| Student | Type of handwriting | Marker brand | Marker colour |
|---|---|---|---|
| Sandra | Handwriting | Carioca | Black |
| Nacho | Handwriting | Staedler | Black |
| Lidia | Handwriting | Edding | Black |
| Roi | Handwriting | Bic | Blue |
| Rubén | Handwriting | Jovi | Black |
| Lara | Handwriting | Pilot | Black |
| Clara | Handwriting | Pentel | Red |
| Miguel | Handwriting | Staedler | Black |
| Carlos | Handwriting | Pilot | Black |
| Lois | Handwriting | Carioca | Blue |
After that the teacher uses his knowledge about paper chromatography to identify the author of the note. This technique enables us to obtain a detailed image of the ink components for each marker. Thus he took a small piece of the note and made the chromatography, obtaining the result you were given. To solve the problem you have to make a plan but first you should decide the most appropriate solvent to be used as eluent: (a) hexane; (b) ethanol, (c) water; (d) an ethanol–hexane mixture (50%); (e) a water–hexane mixture (50%); and (f) ethanol–water (50%). Please take into account that some ink components are polar substances, so the most polar the solvent is the faster is the elution process and therefore the separation is worst. On the other hand, if we use a non-polar eluent the process would be so slow that we would need a lot of time.
Appendix 2: laboratory implementation schedule for planning
In this section a schedule for planning based on questions is summarized. As each task requires specific tips, we include one example, corresponding to task 4:The answers to these questions could help you to plan the investigation:
(1) Which properties could you use to figure out the composition of the solution?
(2) What type of measures/observations are you going to make?
(3) What are you going to do (steps) to carry out the investigation?
(4) How many samples of the unknown solution do you need?
(5) Which materials and equipment do you need to investigate the issue?
(6) How many times are you going to repeat each measure/observation so that it will be reliable?
Appendix 3: teaching tips
In this section we propose some teaching tips for implementing these tasks in other classrooms| Task | Tip |
|---|---|
| 1 | Before starting the planning phase to present a similar reaction to students so that they can observe the effect of acid when reacting with carbonate elements |
| To model the different methods proposed to measure gas released produced in the reaction so that student can select the most appropriate or propose an alternative one | |
| To treat the shells with the toothpaste at least during 24 h otherwise the effect will be unappreciated | |
| To make sure students understand the simulation between shells and acid and are able to relate it to the real life | |
| To make sure students name each trial with the name of the corresponding sample (treated with toothpaste x, with toothpaste y or blank) to avoid obtaining anomalous results | |
| 2 | To include information about the nature of matter in the handout such as solubility, conductivity and magnetic nature of metals, ionic and molecular substances, as well as covalent chains |
| Before starting the planning phase, students should be encouraged to classify the products making part of the broken order into their corresponding groups (ionic, molecular and metallic substances or covalent chains). This would help them to link the type of product to the different properties presented in the handout | |
| As students have to perform two different operations: (1) separate and (2) identify substances. The plan can be subdivided into two parts: one regarding the separation of mixed substances and another for the identification process of the five substances once the three mixed have been separated. | |
| 3 | Students should be encouraged to think about the possible pollutants present in the river sample and their aggregation state by identifying the raw materials used in the factories. |
| To include information about the nature of matter in the handout such as solubility, conductivity and magnetic nature of substances depending of their chemical bonding, as well as about their reactivity. | |
| Before starting the planning phase, students should be encouraged to relate the possible pollutants to the identification tips provided in the handout. | |
| 4 | Before starting the planning phase, students should relate each substance to the container it should be deposed in, depending on their pH. |
| 5 | Before starting the planning phase, students should rank each solvent based on their polarity and select the most appropriate to carry out the ink chromatography. |
| Teacher should encourage students to use the data provided in the handout (colour of the ink used to write the anonymous note, colour of the markers and type of handwriting for each suspect student) instead of test all markers provided. | |
Acknowledgements
This work was supported by the Spanish Ministerio de Economía y Competitividad (MINECO), contract grant number: EDU2015-66643-C2-2-P. The authors thank the students and teacher who participated in the study.References
- Apedoe X. and Ford M., (2010), The empirical attitude, material practice and design activities, Sci. Educ., 19, 165–186.
- Artigue M., Dillon J., Harlen W. and Léna P., (2012), Learning through Inquiry: Background Resources for implementing Inquiry in Science and Mathematics at School, http://www.fibonacci-project.eu, accessed 4 June 2013.
- Blanchard M. R., Southerland S. E., Osborne J. W., Sampson V. D., Annetta L. A. and Granger E. M., (2010), Is inquiry possible in light of accountability?: a quantitative of the relative effectiveness of guided inquiry and verification laboratory instruction, Sci. Educ., 94, 577–610.
- Blonder R., Mamlok-Naaman R. and Hofstein A., (2008), Analyzing Inquiry questions of high-school students in a gas chromatography open-ended laboratory experiment, Chem. Educ. Res. Pract., 9, 250–258.
- Bugarcic A., Zimbardi K., Macaranas J. and Thorn P., (2012), An Inquiry-based Practical for a Large, Foundation-Level Undergraduate Laboratory that Enhances Student Understanding of Basic Cellular Concepts and Scientific Experimental Design, Biochem. Mol. Biol. Educ., 40(3), 174–180.
- Chinn C. A. and Malhotra B. A., (2002), Epistemologically authentic inquiry in schools: a theoretical framework for evaluating inquiry tasks, Sci. Educ., 86(2), 175–218.
- Crujeiras B. and Jiménez-Aleixandre M. P., (2015), Desafíos planteados por las actividades abiertas de indagación en el laboratorio: articulación de conocimientos teóricos y prácticos en las prácticas científicas, Enseñanza de las Ciencias, 33(1), 63–84.
- Department for Education (DfE), (2014), National curriculum in England, key stages 3 and 4 framework document.
- Duschl R. A. and Bybee R. W., (2014), Planning and carrying out investigations: an entry to learning and to teacher professional development around NGSS science and engineering practice, Int. J. STEM Educ., 1(12), 1–9.
- Duschl R. A. and Grandy R., (2013), Two views about explicitly teaching Nature of Science, Sci. Educ., 22, 2109–2139.
- Erduran S. and Dagher Z. (ed.), (2014), Reconceptualizing the nature of science for science education: scientific knowledge, practices and other family categories, Dordrecht, The Netherlands: Springer.
- Etkina E., Murphy S. and Zou X., (2006), Using introductory labs to engage students in experimental design, Am. J. Phys., 74(11), 979–986.
- Etkina E., Karelina A., Ruibal-Villasenor M., Rosengrant D., Jordan R. and Hmelo-Silver C., (2010), Design and reflection help students develop scientific abilities: learning in introductory physics laboratories, J. Learn. Sci., 19(1), 54–98.
- European Commission (EC), (2012), COM 211(811) Proposal for a council decision establishing the specific programme implementing horizon 2020 – the framework programme for research and innovation (2014–2020), Brussels: European Commission.
- Ford M. J., (2015), Educational implications of choosing “practice” to describe science in the next generation science standards, Sci. Educ., 99(6), 1041–1048.
- Gee J. P., (2005), An introduction to discourse analysis: theory and method, London: Routlegde.
- Girault I., D'Ham C., Ney M., Sánchez E. and Wajeman C., (2012), Characterizing the experimental procedure in science laboratories: a preliminary step towards students experimental design, Int. J. Sci. Educ., 34(6), 825–854.
- Gott R. and Dugan S., (1995), Investigative work in the Science curriculum, Buckingham: Open University Press.
- Hmelo C. E., Holton D. L. and Kolodner J. L., (2000), Designing to learn about complex systems, J. Learn. Sci., 9(3), 247–298.
- Hodson D., (1998), Is this really what scientists do? Seeking a more authentic in and beyond the school laboratory, in Wellington J. (ed.) Practical work in school science-which way now?, Padstow: Routledge, pp. 93–108.
- Hofstein A. and Mamlok-Naaman R., (2007), The laboratory in science education: the state of the art, Chem. Educ. Res. Pract., 8, 105–107.
- Hofstein A., Shore R. and Kipnis M., (2004), Providing high school chemistry students with opportunities to develop learning skills in an inquiry-type laboratory: a case study, Int. J. Sci. Educ., 26(1), 47–62.
- Högström P., Ottander C. and Benckert S., (2010), Lab work and learning in secondary school chemistry: the importance of teacher and student interaction, Res. Sci. Educ., 40, 505–523.
- Jiménez-Aleixandre M. P. and Crujeiras B., (2014), Interpreting Anomalous Primary Data in the Laboratory: Findings from a Longitudinal Study. Paper presented at the symposium promoting epistemic practices in science classrooms in AERA 2014 Annual Meeting, Philadelphia (USA).
- Jiménez-Aleixandre M. P. and Crujeiras B., (2016), Epistemic practices and Scientific practices in Science Education, in Taber K. S. and Akpan B. (ed.) Science Education: an international course companion, The Netherlands: Sense Publishers, pp. 69–80.
- Jones M. E., Gott R. and Jarman R., (2000), Investigations as part of the Key Stage 4 science curriculum in Northern Ireland, Educ. Res. Eval., 14, 23–37.
- Kelly G. J., (2008), Inquiry, activity and epistemic practice, in Duschl R. A. and Grandy R. E. (ed.) Teaching Scientific Inquiry, Rotterdam: Sense Publishers, pp. 99–117.
- Kipnis M. and Hofstein A., (2008), The inquiry laboratory as a source for development of metacognitive skills, Int. J. Sci. Math. Educ., 6, 601–627.
- Kolodner J. L., (2002), Facilitating the learning of design practices: lessons learned from an inquiry into science education, J. Ind. Teach. Educ., 39(3), 9–40.
- Krajcik J., Blumenfeld P. C., Marx R. W., Bass K. M. and Fredricks J., (1998), Inquiry in project-based science classrooms: initial attempts by middle school students, J. Learn. Sci., 7(3/4), 313–350.
- Lee H.-S. and Songer N. B., (2003), Making authentic science accessible to students, Int. J. Sci. Educ., 25, 923–948.
- Lunetta V. N., Hofstein A. and Clough M. P., (2008), Learning and teaching in the school science laboratory: an analysis of research theory and practice, in Abell S. K. and Lederman N. G. (ed.) Handbook of Research on Science Education, New York: Routledge, pp. 394–441.
- Ministerio de Educación Cultura y Deporte (MECD), (2014), Real Decreto 1105/2014, de 26 de diciembre, por el que se establece el currículo básico de la Educación Secundaria Obligatoria y del Bachillerato [Royal Decree 1105/2014, establishing the common curriculum for Secondary Education], Boletín Oficial del Estado, 3, 169–546.
- National Research Council (NRC), (2012), A framework for K12 Science Education: practices, crosscutting concepts and core ideas, Washington, DC: National Academy Press.
- Neber H. and Anton M., (2008), Promoting Pre-experimental Activities in High-school Chemistry: focusing on the role of students' epistemic questions, Int. J. Sci. Educ., 30(13), 1801–1821.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States, Washington, DC: The National Academies Press.
- Organisation for Economic Cooperation and Development (OECD), (2013), PISA 2015 Draft Science Framework, Paris, France: Organisation for Economic Cooperation and Development (OECD).
- Osborne J., (2014), Scientific practices and inquiry in the science classroom, in Lederman N. G. and Abell S. K. (ed.) Handbook of Research on Science Education, New York: Routledge, vol. II, pp. 579–599.
- Osborne J. and Dillon J., (2008), Science education in Europe: critical reflections, London: The Nuffield Foundation.
- Puntambekar S. and Kolodoner J. K., (2005), Toward implementing distributed scaffolding: helping students learn science from design, J. Res. Sci. Teach., 42(2), 185–271.
- Schwartz D. L. and Martin T., (2004), Inventing to prepare for future learning: the hidden efficiency of encouraging student production in statistics instruction, Cogn. Instr., 22(2), 129–184.
- Tobin K. G., (1990), Research on science laboratory activities; in pursuit of better questions and answers to improve learning, Sch. Sci. Math., 90, 403–418.
- Windschitl M., (2003), Inquiry projects in Science Teacher Education: what can investigative experiences reveal about teacher thinking and eventual classroom practice? Sci. Educ., 87, 112–143.
- Yacoubian H. A. and BouJaoude S., (2010), The effect of reflective discussions following inquiry-based laboratory activities on students' views of nature of science, J. Res. Sci. Teach., 47(10), 1229–1252.
- Zimmerman C., (2000), The development of scientific reasoning skills, Dev. Rev., 20, 99–149.
| This journal is © The Royal Society of Chemistry 2017 |