Argumentation to foster pre-service science teachers’ knowledge, competency, and attitude on the domains of chemical literacy of acids and bases
C.
Cigdemoglu
*a,
H. O.
Arslan
b and
A.
Cam
c
aDepartment of Educational Sciences, Atilim University, Ankara, Turkey. E-mail: ceyhan.cigdemoglu@atilim.edu.tr; Fax: +90 312 5868091; Tel: +90 312 5868673
bDepartment of Mathematics and Science Education, Yuzuncu Yil University, Van, Turkey. E-mail: harikaozge@gmail.com; Tel: +90 444 5065
cDepartment of Elementary Science Education, Mugla Sitki Kocman University, Mugla, Turkey. E-mail: aylincam@mu.edu.tr; Tel: +90 252 2113118
First published on 13th December 2016
Abstract
Argumentative practices have the potential to contribute to scientific literacy. However, these practices are not widely incorporated in science classrooms and so their effect on the domains of literacy is still not revealed. Therefore, this study proposes to reveal the effect of argumentation on the three domains of chemical literacy related to the concepts of acids and bases. The study participants comprised 29 freshman pre-service science teachers’ enrolled in a General Chemistry-II course. Argumentation practices were implemented over six weeks. Open-ended contextual chemical literacy items were developed to assess the differences in the chemical literacy domains and the items were administered before and right after the intervention. The responses to the chemical literacy items were scored with a rubric and three scores were calculated: knowledge, competency, and attitudes. Paired sample t-tests were used to compare the mean scores. All the intervention sessions were video recorded, and three of them were analyzed according to three criteria: the presence of arguments, the frequency of arguments, and the levels of the arguments. The findings revealed that the argumentation practices contributed to the pre-service teachers’ chemical literacy skills, mostly to their knowledge and competencies when compared to their attitudes. Moreover, distinct differences in the quality of argumentation levels were observed over the six weeks.
Introduction
In today's complex and rapidly changing world, it is inevitable to focus on the educational practices that could improve students’ scientific literacy skills. Many educational organizations now accept that achieving scientific literacy helps students to make effective knowledge-based decisions (American Association for the Advancement of Science [AAAS], 1989; National Research Council [NRC], 1996; Organization for Economic Cooperation and Development [OECD], 2006) such that they will not be alienated in this science and technology dominated world (BouJaoude, 2002). But how can we help our students achieve scientific literacy? Glynn and Muth (1994) stated that it is neither through teaching more science facts nor increasing laboratory work; instead, they stress the importance of “mind-on” activities rather than utilizing only “hands-on” activities. Supporting this statement, AAAS (1989) emphasizes that assisting students to comprehend the nature of science (NOS) is a central component in achieving scientific literacy as well. Although the term ‘scientific literacy’ covers various descriptions, the commonly held opinion is that it has at least three explicit strands: “knowledge of science concepts and ideas; some understanding of the processes of scientific enquiry and the nature of the knowledge produced; and some awareness of the influence on scientific work of the social context within which it is conducted and, conversely, of the influence on daily life and personal and social decisions of scientific ideas and practices” (Ratcliffe and Millar, 2009, p. 946). Furthermore, almost each scientific literacy description focuses on the importance of the ability to understand and explain the phenomena using a clear language, reading, and writing ability to evaluate the information, communicate ideas to others, and apply scientific knowledge and reasoning skills to daily-life situations and decision-making processes. Most of such ‘scientific literacy’ aspirations structure curriculum developments, learning materials, and assessment practices, so that, when the content and instruction of science-related courses are facilitated with such competencies, scientific literacy may be fostered (Shwartz et al., 2005; Roberts, 2007; Cigdemoglu and Geban, 2015).The definition of chemical literacy (CL) takes its roots from the scientific literacy definition. In the current study, CL is defined in light of two main theoretical frameworks. The first is provided by Shwartz et al. (2005, 2006), where a profound definition for chemical literacy is constructed with a wide consensus among scientists, educators, and high-school chemistry teachers. The second theoretical framework utilized is the one given by the Program for International Student Assessment, PISA, (OECD, 2006) for scientific literacy. In fact, both of these descriptions arise from the scientific literacy definition suggested by Bybee (1997). According to Shwartz et al. (2006), chemical literacy includes four components. The first component covers chemical content knowledge, which describes how a chemically literate student should understand: (a) general chemical ideas, including scientific investigations, how to generalize findings, and how to use knowledge to serve other disciplines in order to explain phenomena; (b) the characteristics (key ideas) of chemistry, such that they can explain the macroscopic level by means of molecular structures, explain processes, reactions, energy changes, the structures of living systems, and the contribution of scientific language to chemistry. The second component is about ‘chemistry in context’, which states that chemically literate students should be able to use chemistry knowledge for explaining everyday situations, should understand daily-life chemistry, be able to make effective decisions, become involved in social arguments on chemistry-related issues, and see the relatedness of innovations in chemistry and sociology. The third component is about higher-order learning skills, which refer to asking questions, investigating relevant information when required, and evaluating the pros/cons of debates. The last component covers the ‘affective aspects’, that is, a literate person should have a fair and rational perspective of chemistry and its applications. Furthermore, such individuals should show an interest in chemistry issues, specifically in non-formal environments, such as the mass media (Shwartz et al., 2006).
The PISA (OECD, 2006) framework proposed a model for science assessment that was developed to reveal to what extent 15-year-old students exhibit features of scientific literacy. The framework takes an everyday context involving science and technology as the starting point and creates a learning environment in which students are able to make a decision or choice. In this process, students are required to identify scientific issues, understand the underlying science, and use evidence competently. Their scientific knowledge and attitudes toward science influence their competencies (OECD, 2006). To further elaborate, scientific knowledge is both what you know about the natural world (science content knowledge/knowledge of science) and what you know about science as a form of knowledge and enquiry (knowledge about science) (Ratcliffe and Millar, 2009). The same authors also state that attitudes are important because students’ responses to scientific issues represent their interest in these issues, how supportive they are of the scientific approach, and their sense of responsibility for the situation. In addition, they claim that appropriate teaching materials and curricula to promote scientific literacy are needed to support this framework. To assess students’ chemical literacy, a similar framework with PISA (OECD, 2006) may be utilized.
Chemistry, as a branch of applied science, teaching also explicitly aims to establish a high level of scientific and chemical literacy for all students. Since students find chemistry difficult to learn (Osborne and Dillon, 2008) and chemistry curricula are perceived to have some critical problems (Gilbert, 2006), traditional chemistry teaching can struggle to improve chemical literacy (CL). Attaining chemical and scientific literacy may therefore necessitate the introduction of new curricula (Fensham, 2002), the training of citizens for society (Bader, 2003), and differentiating the instruction of science-related courses (Shwartz et al., 2005; Albe, 2008; Cigdemoglu and Geban, 2015). Shwartz et al. (2005) stated that when such courses are professionalized with science-technology-and-society movements, they contribute to social, technical, and personal aspects toward improving science literacy. On the other hand, Cavagnetto (2010) stressed the importance of a steady increase in argument-based interventions to foster scientific literacy since it allows developing communications skills, metacognitive awareness, and critical thinking. In both, chemistry lessons include discussion platforms in which social interactions and evaluation skills are promoted. Such skills are essential to reflect the interplay of science and technology with society, ecology, economy, and with students’ own desires, needs, and interests (Bybee, 1997; Fensham, 2002; Marks and Eliks, 2009). These interactions are perceived as key issues for a well-developed multidimensional scientific literacy (Bybee, 1997) and chemical literacy (Shwartz et al., 2005).
According to Jimenez-Aleixandre and Erduran (2008), discourse practices through which students attempt to construct, support, evaluate, or validate a claim by evidence-based reasoning should really be referred to as ‘argumentation’ in science learning contexts. Simon et al. (2006) claim that such discourses encourage students to use scientific theory, data, and evidence to oppose or confirm claims. Aydeniz and Dogan (2016) stress that the engagement in argument is not only a process that includes claims, evidence, and reasoning, but also a process in which students persuade their peers of the validity of their arguments. A considerable number of studies have investigated the impact of argumentation practices to improve students’ understanding of scientific concepts (Driver et al., 2000; Kaya, 2013), to remedy misconceptions (Taasoobshirazi et al., 2008), to advance higher-order thinking skills (Eskin and Bekiroglu, 2009), and, thus, to promote students’ scientific literacy (Driver et al., 2000; Duschl and Osborne, 2002; Cavagnetto, 2010). The need and relevance of emphasizing decision-making through argumentation is a process that contributes to students’ scientific literacy level by creating a learning environment in which learners’ minds are ‘on’ and their critical thinking skills are ‘poked’.
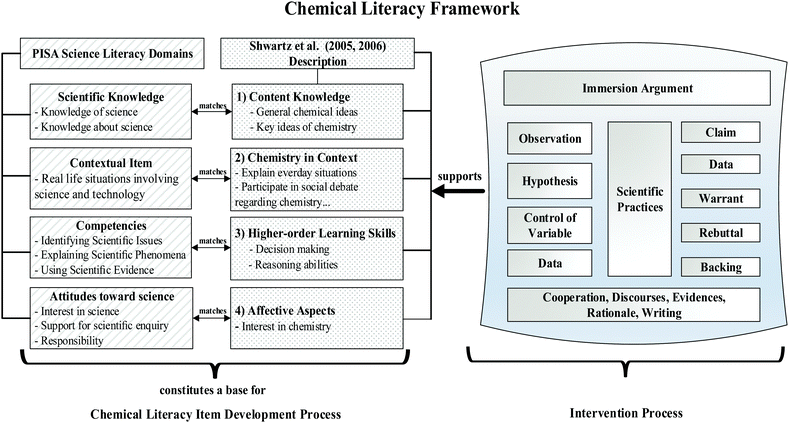
In argument-based instruction, scientific arguments become a leading framework for the teaching and learning of concepts by emphasizing science not as an experimental verification, but, rather, as a process of scientific argumentation and explanations (Erduran et al., 2006; Zembal-Saul, 2009). In such practices, no longer is conceptual repetition or factual accumulation the focal point, instead, the focus is on constructing concepts through scientific argumentation (Erduran et al., 2006; Cavagnetto, 2010). This feature makes the process ambitious in terms of increasing students’ reasoning skills and achievements. According to Heng et al. (2015), scientific argument is core in knowledge construction and students need to propose, support, criticize, evaluate, and refine ideas about concepts as well as to use scientific theories and evidence to confirm their claims. Cavagnetto (2010) examined the argument literature to reveal how this instruction fosters scientific literacy, and highlighted three orientations. The first is “understanding the interaction of science and society to learn scientific argument (socio-scientific), the second is immersion for learning scientific argument (immersion), and the last is understanding the structure to learn scientific argument (structure)” (Cavagnetto, 2010, p. 350). The study concludes that, at some level, these three orientations foster students’ literacy skills; however, in immersion orientation, scientific practices are fully addressed, so this seems to have the highest potential for increasing scientific literacy. In the present study, the immersion argument is referred to as an inquiry-based learning environment embedded in argumentation. In this kind of argument instruction, students find the opportunity to work with basic elements of science, such as the control of variables, errors, and data transformations. In spite of such a claim, further studies are required to establish their own framework for fostering scientific literacy. Therefore, this study aims to develop its own framework and to implement it to reveal its effect on the knowledge, competency, and attitude aspects of CL. The framework used in this study is given in Fig. 1. As seen in Fig. 1, both PISA's (reported in OECD, 2006) and Shwartz's et al. (2005, 2006) frameworks overlap, and the immersion argument has strong facilities to support the chemical literacy domains.
This study focuses on the concepts of acids and bases, as an example of one of the topics that chemistry students usually have difficulty in comprehending (Abu Hassan and Tan, 2009; Heng et al., 2014) and in transferring their knowledge of such to other concepts and real-life applications. Similar to others, these concepts occupy a considerable part of chemistry teaching in elementary, secondary, and related tertiary levels. The concepts of acids and bases have wide applications in daily life, especially household chemicals, media news, such as the news of acid rain, and in industry, all of which makes the concepts familiar to students, and hence worthy of research. Heng et al. (2015) studied argument-based instruction on acids and bases concepts and compared students’ individual and group performances. A student having a deep understanding of acid/base concepts can utilize propositional networks that are sufficiently developed to allow them to explain observed phenomena and to predict the behavior of new phenomena. Studies, however, reveal that many students actually have difficulties in understanding these concepts (Abu Hassan and Tan, 2009; Sendur et al., 2010; Tarhan and Sesen, 2010; Heng et al., 2014). However, little is speculated on how students’ other skills can be fostered. Therefore, the aim of the present study was to reveal the effect of immersion-type argumentation intervention on the development of pre-service science teachers’ chemical literacy dimensions. Specifically, the research questions addressed in the present study were:
– Is there a significant difference in the pre- and post-test content knowledge scores of pre-service science teachers?
– Is there a significant difference in the pre- and post-test competency scores of pre-service science teachers?
– Is there a significant difference in the pre- and post-test attitude scores of pre-service science teachers?
– What is the evidence for the effect of immersion argumentation on pre-service science teachers’ content knowledge and the competency domains of chemical literacy in acid/base concepts?
Method
A one group pre-test/post-test experimental research design was utilized by comparing the effects of an immersion-type argumentation on pre-service science teachers’ (PSTs) chemical literacy domains before and after intervention. Freshman PSTs were selected. The intervention lasted six weeks. Before and after the implementation, the participants took the same open-ended chemical literacy item sets as a pre-test and post-test.Although there are potential validity threats (or risks), such as history, instrument decay, data collector characteristics, data collector bias, attitude of subjects, testing, and implementation, in a one group pre-post design (Fraenkel and Wallen, 2000), the researchers tried to control these. The history threat relates to unplanned events that might make students remember the concepts, but in the present study, no such unplanned events were reported by the instructor or observer. To control instrument decay and instrument bias, a rubric was used and all the researchers were involved in the scoring procedure. As the data collector characteristic is another threat in the study validity, all the data were collected by a single researcher under standard procedures. The attitude of the subjects’ threat involves two aspects: the attitudes to the intervention and the attitudes to the content. This study controlled the second aspect since the attitude of the PSTs to the acid/base concepts was already treated as a dependent variable. The chemical literacy item sets used in the study included five open-ended questions and several sub-questions that tested not only content knowledge, but also competency and attitudes, therefore the related concepts needed to be comprehended deeply in order to answer the items; thus, simply remembering each item would be hard and the effect of the testing threat would consequently be small. The implementation threat occurs when different individuals are assigned to implement activities. In this study, only the course instructor implemented the method, so this threat was also controlled. Having one group prepost-test design, as an exploratory approach, is an effective approach for deciding whether the possible explanation is worth carrying out further investigations.
Sample
Freshman PSTs enrolled in a section of a General Chemistry-II course in the spring semester of 2015 at a well-known state university of Turkey were selected as the participants. At the beginning of the study, the participants signed a consent form explaining the rationale of the study and guaranteeing that any data collected from or about the participants were held in confidence and the names of the participants would never be used in any publications. Their rights to withdraw from the study at any point without prejudice were emphasized.Although 71 PSTs took the course and confirmed their participation, 15 of them were absent at the pre-test, while 27 of the remaining were absent at the post-test. Finally, the remaining 29 (18 females, 11 males) freshman PSTs’ responses were analyzed. The age range of the participants was between 20 and 22 years old. The instructor had four years of experience in this course as well as research in the field of argument-based instruction.
The instrument: the chemical literacy item sets
The chemical literacy skills of the participants were assessed using open-ended chemical literacy item sets. There were five contextual items: three items sets were constructed by one of the researchers (Çam and Geban, 2016); and two of them were adopted from PISA 2006 science assessment questions according to the PISA 2006 framework. The contextual nature of the items involved both a task embodying scientific facts and one covering PSTs’ daily-life experiences. Table 1 briefly summarizes the names and domains of each item set and the min/max points obtained from these items. The contexts used in the items were olive trees, the stomach, a teapot, tooth decay, and acid rain. The fourth and fifth ones were adopted from PISA 2006 questions.| Contextual item setsa | ||||||
|---|---|---|---|---|---|---|
| Domains | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Max. points |
| a The minimum point is zero for each contextual item set. Item 1: olive trees, Item 2: stomach, Item 3: teapot, Item 4: tooth decay, Item 5: acid rain. | ||||||
| Knowledge | 10 | 14 | 4 | 16 | 12 | 56 |
| Competency | 10 | 14 | 4 | 16 | 12 | 56 |
| Attitude | 10 | 14 | 4 | 16 | 12 | 56 |
| Maximum points | 30 | 42 | 12 | 48 | 36 | 168 |
Fig. 1 also includes the framework of the PISA 2006 questions, which were set out in context and included the knowledge, competency, and attitude dimensions of scientific literacy. Similar with the PISA 2006 items, our item sets were contextual, and each item set helped for collecting data related to the PSTs” knowledge, competency, and attitudes toward acids and bases concepts. A sample item is provided in Appendix A.
The first item, olive trees, was related to the concepts of acidity, basicity, and neutrality as well as the determination of pH and the meaning of the pH scale. Based on the background knowledge given in the question, the PSTs were expected to decide the pH of certain soil types and on the appropriate soil type for planting olive trees. The second item, the stomach, assessed PSTs’ knowledge on the properties of acids and bases, whether acids/bases are dangerous or not, and their strengths. The context of the third item, teapot, covered the formation of lime (calcium carbonate), the ways to eliminate it (e.g. neutralization reactions), and the reaction of acids and calcium carbonate. The fourth item, tooth decay, asked about the chemical reactions involved in the deformation of teeth, how acids weaken teeth, and what components of tooth paste can be included for reducing tooth decay. The last item, acid rain, addressed the chemical reaction involved in the formation of such rain, the source of acidity, and the chemical reaction between marble and acid.
The instrument was reviewed by faculty members majoring in chemistry education in terms of the content, construct, and validity. They examined the consistency of the learning outcomes of the chapter with the knowledge that the items proposed to measure, then the match between the items and the developed framework. Both the researchers and the faculty members categorized the items concerning the chemical literacy dimensions based on the framework. The inter-rater reliability among these coders was a 90% overall agreement (Appendix B). In addition, the items were also distributed to another PST group not belonging to this research to check whether the items were understandable. According to the lessons learned in the reviews and the results of the pilot study, the final form of the Chemical Literacy Items was constructed. The items were tested at the beginning and the end of the intervention.
The intervention
The immersion-type argumentation intervention was implemented covering acid/base concepts three times a week over six weeks (50 minutes, 3 × 6 = 18 sessions in total). Throughout the intervention, the instructor facilitated arguments through the use of scaffolds, such as prompts, to create a learning environment that utilized group collaboration, and addressed common misconceptions related to the concepts. The concepts of acids and bases included Arrhenius’, Brønsted–Lowry's, and Lewis’ theories, the self-ionization of water, strong/weak acids–bases, and pH. The sub-topics of the chapter included the definitions for acids and bases, their properties, and titrations.PSTs were trained about argumentation and how to use argumentation practices before the intervention started. The instructor, experienced in this field, explained the meanings of the notions of claim, data, warrant, backing, and qualifier. Then, a sample argumentation practice, “solving mystery deaths” (Kıngır et al., 2011), was conducted with the PSTs to exemplify the notions of claim, data, warrant, backing, and qualifier. The content of the sample argumentation was not related to any chemistry concepts. A sample argumentation scheme (Table 2) on acids and bases concepts was prepared and distributed to the participants in order to clarify how argumentation practices, including the notions of claim, data, warrant, rebuttal, and backing should be.
| Scheme | Context of the argument: properties of acids and bases |
|---|---|
| Claim | Acids are harmful. |
| Warrant | Acid rain can damage plants, animals, and buildings. Also, they can irritate our body. |
| Questions/probes | What are lemons or tomatoes made of? Do you know how aqua fortis influences our body? |
| Backing/rebuttal | One day, my mother erroneously spilled aqua fortis on her hand, it irritated her hand. |
| Claim | Acids are beneficial. |
| Warrant | Acid in the stomach helps our foods to digest, and most vitamins are acids and so they are beneficial. |
| Backing/rebuttal | I know that some cleaning products are acids, I read their labels. Thus, they are harmful. |
| Questions/probes | How could you say whether acids are harmful or beneficial? |
| Conclusion | Some acids are harmful, such as cleaning products, but others are beneficial, such as lemon. |
During the intervention, different argumentation practices about acid/base concepts were used. These practices were developed based on immersion-type argumentation as described by Cavagnetto (2010). Table 3 briefly summarizes the concepts, related argumentation practices, and immersion orientation of these practices.
| Acids and bases topics | Argumentation practices | Immersion orientation |
|---|---|---|
| Arrhenius’, Brønsted–Lowry's, and Lewis’ theories | What is an acid? What is a base? What about NH3? | Development of acid–base theories, NOS practices in class. |
|
Self-ionization of H2O
Examples of acids and bases Properties of acids and bases |
Is water an acid or base?
Which is more acidic, lemon or lime? Does the acidity of fruit change when it is dried? |
Inquiry in laboratory, group work, lab report writing. |
| Strong/weak acids/bases | Which is the stronger acid H2O or NH3? Compare the strength of acidity of ammonia, acetic acid, sodium hydroxide, and water. | Misconceptions, cognitive conflict, conceptual change, NOS practice. |
| Acid–base titrations and pH scale | “Mystery alkaline solution” – Is it possible to treat bee sting with baking powder? What is the reasoning behind this treatment? Whether factory disposals of sulfuric acid are treated with lime or not, what is the reason for this? | Inquiry-based investigations in laboratory, cooperation, in-class group discussions, misconceptions, lab report writing. |
| Lewis acids/bases | Which proton is more acidic: HCO–O–H or CH3–O–H? | In-class investigative contexts, misconception, cognitive conflict. |
Another argumentation practice is provided here as a sample activity implemented in the laboratory. The activity was called ‘Mystery Alkali Solution’. The activity concerned the notion of neutralization and was adopted from Heng et al. (2015). In the activity, the PSTs were given a bottle labeled as ‘strong mystery alkali’ and five other solutions: W, X, Y, V, and Z. They were required to remove the strong corrosive property of the mystery alkali solution. The PSTs were informed that one of these solutions had the potential to remove the strong corrosive property of the mystery alkali solution. They worked in groups and carried out several tests on the W, X, Y, V, and Z solutions to find out some basic properties of these solutions in order to conclude which solution could remove the corrosive property of the mystery alkali solution.
Ten groups, each consisting of three PSTs, were formed and each solution was examined by two groups. The groups were expected to determine the color of blue litmus paper and phenolphthalein with their solution, then the same following the reaction of the solution with metal and carbonate, and finally to find pH of their solution through titration. At the end of the laboratory investigations, Table 4 was created with the results. The table was used to help the PSTs to write a laboratory report about their attempts to remove the strong corrosive property of the mystery solution. As a guided inquiry, in the laboratory sessions the PSTs were provided with topics, questions, and materials; however, the instructor allowed them to develop their own procedure. In this investigative context, the PSTs collected and analyzed data and came to conclusions about their solution, and then consulted with the other group working on the same solution to finalize their conclusions. The laboratory sessions were intellectually demanding scientific reasoning by requiring the PSTs to set their own experimental designs and to hypothesize their own questions. Such processes were socially constructed to support their investigations through experiencing the process of gathering scientific evidence. The laboratory report (see Fig. 3) included the conclusion drawn from the data collected (Q1), the data used to support the conclusion (Q2), the reasons behind how the students linked the data to support their conclusion (Q3), and the relationship between the data and the conclusion (Q4).
| Solution | Color of blue litmus paper | Color of phenolphthalein | Reaction with metal | Reaction with carbonate | pH |
|---|---|---|---|---|---|
| W | Red | Colorless | H2(g) is produced | CO2(g) is produced | 5 |
| X | Red | Colorless | H2(g) is produced | CO2(g) is produced | 1 |
| Y | No change | Pink | No change | No change | 13 |
| V | No change | Pink | No change | No change | 8 |
| Z | No change | Colorless | No change | No change | 7 |
Treatment verification
Some argumentation practices were adopted from literature, while some were developed based on the course objectives. Two faculty members experienced in argument-based interventions examined the activities and made revisions to the course materials. One of them observed two argumentation sessions and verified the presence of argumentation practice in these sessions.Data analysis
| Item | Knowledge score (2) | Competency score (2) | Attitude score (2) |
|---|---|---|---|
| a Numbers in parentheses are the max points that could be obtained. | |||
| Olive trees (1.a) | Correct answer (2) | Full competent (2) | Highly interested (2) |
| Partially correct (1) | Partially competent (1) | Moderately interested (1.5) | |
| Wrong answer (0) | No competency (0) | Lowly interested (1) | |
| Not interested (0.5) | |||
After the evaluation process, each PST had three scores: content knowledge, competency, and attitude. In order to examine whether there was a significant difference between the pre- and post-test scores, paired sample t-tests were conducted with the help of PAWS 18 (Predictive Analytics Software).
Analysis of the qualitative data
Results and discussion
The missing data analysis started with investigating whether there was a pattern between the groups of students who completed both the pre- and post-tests and those in the group who did not. First, an independent sample t-test was conducted to compare the post-test scores of 15 students (number of students who did not take the pre-test) and the scores of those who completed the post-test (29 students). Participants with missing values were coded as 0 and the rest as 1, but there was no evidence to claim a statistically significant difference between the missing and present participants in terms of content knowledge scores [t(42) = −0.166, p > 0.05], competency scores [t(42) = 9.290, p > 0.05], and attitude scores [t(42) = 0.291, p > 0.05], and, therefore, the post-test scores of these students with missing values were automatically excluded from the data instead of replacing their scores with the mean as Tabachnick and Fidell (2007) described. The same procedure was applied for the missing data in the post-tests, and an independent t-test was performed to compare the 27 PSTs’ (who were absent in the post-test) pre-test scores and 29 PSTs (who have both test scores). The results indicated that there was no significant difference between the pre-test scores in terms of the content knowledge scores [t(54) = −0.155, p > 0.05], competency scores [t(54) = 0.466, p > 0.05], and attitude scores [t(54) = 10.537, p > 0.05]. Therefore, these students with missing date were also excluded.Analysis of CL items
Each PST had three scores: knowledge, competency, and attitude based on the chemical literacy framework. Table 6 indicates the mean scores of the PSTs for each item and each domain before and after the implementation period. The highest increase in chemical literacy domains was observed in the knowledge scores. Since the knowledge domain is the most predominant constitute of the literacy description, it suggests it is not possible to promote literacy skills without a promotion in the content knowledge of students (PISA, 2006; Bybee et al., 2009; Dawson and Venville, 2009). Due to the nature of the PISA questions and our chemical literacy questions, the text provided as background information in the questions included almost the correct answer. Therefore, the PSTs’ mean score on the pre-test and post-test were close to each other.| CL Item | Pre-test | Post-test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| K | C | A | Overall score | K | C | A | Overall score | ||
| a Note: K: knowledge, C: competency, A: attitude. | |||||||||
| Olive trees | Max | 10 | 10 | 10 | 30 | 10 | 10 | 10 | 30 |
| Mean | 7.31 | 5.17 | 9.08 | 21.56 | 7.40 | 5.90 | 9.01 | 22.31 | |
| Stomach | Max | 14 | 14 | 14 | 42 | 14 | 14 | 14 | 42 |
| Mean | 6.82 | 4.52 | 11.10 | 22.44 | 8.13 | 5.66 | 11.20 | 24.99 | |
| Teapot | Max | 4 | 4 | 4 | 12 | 4 | 4 | 4 | 12 |
| Mean | 1.72 | 0.46 | 3.36 | 5.54 | 3.16 | 1.31 | 3.23 | 7.7 | |
| Tooth decay | Max | 16 | 16 | 16 | 48 | 16 | 16 | 16 | 48 |
| Mean | 9.93 | 8.25 | 14.23 | 32.41 | 10.83 | 7.93 | 13.95 | 32.71 | |
| Acid rain | Max | 12 | 12 | 12 | 36 | 12 | 12 | 12 | 36 |
| Mean | 5.32 | 4.57 | 11.14 | 21.03 | 5.34 | 5.23 | 11.06 | 21.63 | |
| Total max | 56 | 56 | 56 | 168 | 56 | 56 | 56 | 168 | |
| Overall mean | 31.10 | 22.97 | 48.91 | 102.98 | 34.86 | 26.03 | 48.45 | 109.34 | |
The post-competency mean scores were also increased compared to the pre-competency mean scores through the intervention. This shows that the immersion argumentation practices supported the PSTs’ competency skills by helping them to identify scientific issues, explain phenomena scientifically, and use scientific evidence. The smallest change was observed between the PTSs’ pre-attitude mean scores and post-attitude mean scores. The investigative contexts here did not support the affective domain as much as the knowledge and competency domains.
Next, we investigated whether the differences between the pre-test and post-test mean scores of the PTSs’ were significant through the paired-samples t-test. Before the statistical analysis, the normality of the distribution was checked. The results indicated that there was a significant difference between the pre-test and post-test scores in terms of content knowledge [t(28) = −3.961]. Table 7 presents the statistical values.
| Mean | SD | t | p | |
|---|---|---|---|---|
| Pre-test | 31.10 | 5.90 | −3.961 | 0.000 |
| Post-test | 34.86 | 6.68 |
The result concerning the impact of argumentation practices on the PSTs’ knowledge are consistent with the findings of the studies in the literature reporting a positive effect of intervention on knowledge construction (Driver et al., 2000; Zohar and Nemet, 2002; Cross et al., 2008; Venville and Dawson, 2010; Kaya, 2013). In any intervention, a gain in knowledge is expected, but this study claims that the learning environment supported knowledge construction through an immersion argument supporting experimental design, data interpretation, and evidence-based knowledge claims.
The second analyses were conducted to reveal whether there was a significant difference in terms of PSTs’ competency scores before and after the intervention. According to the results, the mean post-competency scores were significantly higher than the pre-competency scores [t(28) = −2.550]. Table 8 presents the statistical results.
| Mean | SD | t | p | |
|---|---|---|---|---|
| Pre-test | 22.97 | 5.72 | −2.550 | 0.017 |
| Post-test | 26.03 | 7.52 |
In this study to test their CL items, the competencies of the PSTs were scored according to their responses, including scientific reasoning, the justification for their reasons, decision-making, higher-order thinking, and their ability to explain everyday situations. The findings of this study indicated that the three key scientific competencies: identifying scientific issues, explaining phenomena scientifically, and using scientific evidence, as described by the PISA 2006 feature, could inevitably be improved by implementing immersion argument practices.
Some sample excerpts are provided below to demonstrate the PSTs’ progress during the intervention on some of the CL items. For instance, related to the tooth decay item, one of the sub-questions was:
H+ + OH− ⇌ H2O. What happens to the OH− ions when an acid is added to the given equation? Explain.
One of the PST's responses from the pre-test was:
When acid is added, the reaction system becomes acidic and OH − ions’ concentration is the same.
The same PST's post-test response was:
When we add acid to the formation of water equilibrium equation, H + ions increases and OH − ions decreased. Or we can say that neutralization occurs. By the neutralization of OH − with H + , the mineral formation gets slower. Thus, the tooth lost minerals gradually.
The improvement in this response is explicit; the answer in the pre-test includes a partially correct response; however, in the post-test, not only is the knowledge correct but also full competency is observed.
Related to the stomach item, the PSTs were expected to answer the sub-question: What are the properties of chemicals used in the treatment of gastritis? One PST responded in the pre-test as follows:
The medicine should be able adjust the pH of the stomach
The same PST answered the question in the post-test as follows:
Medicine should be designed for diminishing acid secretion in the stomach or the medicine could have the property of lowering acidity.
The difference in the answer portrays that the PST's competency level in terms of the appropriate use of scientific terms and explaining phenomena scientifically has improved through the intervention. In general, the PSTs’ responses to the post-test items contained more reasoning skills when compared to their pre-test responses, and distinct differences were also observed in the PSTs’ explanations regarding the knowledge and competency domains of chemical literacy before and after the intervention. The contribution of argument practices on the above-mentioned scientific competencies in this study is in accord with the related literature (Driver et al., 2000; Duschl and Osborne, 2002; Shwartz et al., 2005, 2006; PISA, 2006; Sadler and Zeidler, 2009).
Next, analyses were conducted to reveal whether there was a significant difference between the pre-test and post-test scores in terms of attitudes. According to the results, there was no significant impact of the immersion argument practices on the mean attitude scores of the PSTs, [t(28) = 0.315]. Table 9 shows the statistical values.
| Mean | SD | t | p | |
|---|---|---|---|---|
| Pre-test | 48.91 | 6.93 | 0.315 | 0.755 |
| Post-test | 48.45 | 8.75 |
Almost no change was observed between the pre-attitudes and post-attitudes. As Berg (2005) suggested that when the intervention is learner-centered, opportunities for collaborative work is provided, exchange among studies ideas occur, the instructor is accessed when needed, chemistry concepts are connected to other subjects and daily-life situations, and when enough time to complete the tasks is allocated, students’ attitudes and motivation change in a positive manner for the university chemistry settings. Although such elements were verified in this study, the gain in PSTs’ attitudes was not satisfactory. Four possible reasons might account for the almost unchanged attitude scores: (1) the duration of the intervention did not support the PSTs’ affective domain efficiently and such a short-term intervention period (six weeks) might be an obstacle to changing their attitudes positively. (2) In contrast, longer treatment sessions (50 minutes each and a block of two 50 minutes for labs) might get the PSTs to focus on the whole session but lack the capability to persuade their beliefs since only sessions “of less than 30 minutes in length can change attitudes” (Kobella, 1989). (3) Chemical literacy items might be perceived to have a high cognitive demand and require sufficient time to address. (4) The contexts of the items might not capture PSTs’ attention sufficiently to provide a positive gain in their attitude scores.
Qualitative support for chemical literacy skills
The data obtained from the in-class video records and PSTs’ written responses to the laboratory reports were examined to support the data obtained from the chemical literacy items. Analysis of the video records showed distinct differences in the nature and quality of the PSTs’ argumentation levels and understanding of the concepts in different weeks of the instruction. The improvement in the argumentation level of the PSTs’ over the weeks is given in Table 10 and Fig. 2.| Argument levels | Week 2 | Week 4 | Week 6 |
|---|---|---|---|
| Number of argument levels (frequency) | Number of argument levels (frequency) | Number of argument levels (frequency) | |
| Claim | 5 (42%) | 3 (17%) | 1 (5%) |
| Claim & data | 2 (17%) | 4 (24%) | 2 (9%) |
| Basic argument | 4 (33%) | 6 (35%) | 11 (50%) |
| Higher level argument | 1 (8%) | 4 (24%) | 8 (36%) |
| Total | 12 | 17 | 22 |
In the last week of the intervention, even as the frequency of both the claim and claim and data decreased, the frequency of the basic arguments and higher level arguments increased. That result is predictable because of the fact that there is a consensus among most of the science education researchers about the fostering effect of argumentation practices on learners’ argumentation skills (Osborne et al., 2004; Kaya, 2013; Heng et al., 2015) and knowledge construction (Jimenez-Aleixandre et al., 2000; Duschl and Osborne, 2002; Cross et al., 2008; Kaya, 2013). Duschl and Osborne emphasized that “learning environments that involve dialog with teachers and between peers provide opportunities for learners to share, critique, think with and add to a common knowledge base” (2002, p. 47). When the irreplaceable place of content knowledge in literacy description is considered, its improvement is undeniable for fostering literacy skills. The enhancement in the level of argumentation and the increase in the frequency of the arguments verify that the learning environment assured the right conditions for an improvement in the PSTs’ chemical literacy skills.
Throughout the course of the intervention, the PSTs shared their ideas with their peers and the instructor and had the opportunity to extend their existing knowledge and to construct new knowledge while engaging in in-class argumentation practices. For instance, the PSTs were asked to compare the strength of the acidity of ammonia and water, and some PSTs’ arguments are provided in Table 11.
| Argument components | Context of the argument: the strength of acid bases |
|---|---|
| a Note: all the names in the parenthesis are pseudonyms. | |
| Claim | Ammonia is a stronger acid than water (multiple PSTs) |
| Question/probe | Why? What is your reason? (Instructor) |
| Data | It has only one lone pair (Adam) |
| Warrant | The single lone pair of electrons in ammonia indicates the presence of a Brønsted–Lowry acid (Laurel) |
| Data | Ammonia can donate more hydrogen ions (Kimberly) |
| Question/probe | What is the meaning of donating more H+ ions? (Instructor) |
| Warrant | In a molecule, as the number of hydrogen atoms increases, its acidity becomes stronger (Kimberly) |
| Question/probe | Compare the acidity of HCl and NH3 (Instructor) |
| Claim | Water is a stronger acid (multiple PSTs) |
| Question/probe | Why? Justify your reason? (Instructor) |
| Rebuttal | The number of H atoms in HCl is less than in NH3 but HCl is a stronger acid (Evelyn) |
| Data | Oxygen in water is more electronegative than nitrogen in ammonia (Laurel and Isabel) |
| Data | Water is a proton donor so it is a stronger acid than NH3 (Gina) |
| Question/probe | How does electronegativity affect the strength of acids or bases? (Instructor) |
| Claim | It is about the definition of Lewis acid/bases (multiple PSTs) |
| Warrant | Water can more easily donate a proton (Evelyn) because of the oxygen |
| Rebuttal | Water has a double lone pair so it is more acidic than ammonia (Gina) |
| Backing/rebuttal | If a reaction occurs between water and ammonia, ammonia will donate its electrons to water, which means it will accept hydrogen from water (Instructor) |
| Question/probe | What is a proton (H+ ion) acceptor? (Instructor) |
| Data | According to Brønsted–Lowery, a base is a proton (H+ ion) acceptor (Gina) |
| Warrant | NH3 is a base according to Brønsted–Lowery, because it is a proton acceptor (Evelyn) |
| Backing | If NH3 is a base according to Brønsted–Lowery, then this means water is more acidic that NH3. (Gina) |
In this discourse, PSTs’ wrong statements were probed to obtain any correct conceptual knowledge left by removing their misconceptions, for example;
Kimberly: As the number of hydrogen atoms increases, its acidity becomes stronger
Probe: Compare acidity of HCl and NH 3 ? (Instructor)
Evelyn: Number of H atoms in HCl is less than number of H atoms in NH 3 but HCl is stronger acid.
Their arguments reveal that their decisions are sometimes a simple evaluation of existing conceptions/misconceptions or an interpretation of given knowledge, but sometimes, rather than recalling existing knowledge, the participants transferred their knowledge of chemistry to a given situation. In line with the findings of Heng et al. (2015), when discourses include misconceptions, the argumentation schemes became disorganized. The use of prompts and rebuttals given at a sub-micro level can enable misconceptions to be replaced by correct chemical knowledge. For example, PSTs used chemical evidence to refute their friends’ warrants:
Adam: Ammonia has one lone pair (I mean it is more acid)
Gina: Water has double lone pair so it is more acidic than ammonia
Probing these dialogs enabled an understanding of their knowledge of micro-level chemistry along with developing PSTs’ decision-making processes. In some cases, when PSTs stated their claims and warrants, the instructor probed their answers to create cognitive conflict and to help them rebut their wrong claims and to provide backing for their right claims. At the end of the test comparison of the strength of the acidity of ammonia and water, the PSTs were able to explain these concepts more scientifically. Arriving at the correct conceptual knowledge was usually guaranteed when the PSTs were able to generate scientific rebuttals and backings, which symbolized higher argument levels (Von Aufschnaiter et al., 2008). Additionally, as seen in Table 11, the PSTs encountered how the definitions of acids and bases were expanded once more. Their first level of science dialogs was carried out using the Arrhenius model and Brønsted–Lowery model; whereas here it was broadened to include the Lewis model.
Further evidence was collected from the argument practices that took place during the laboratory investigations. The sessions were designed to include guided-inquiry elements to improve the PSTs’ chemical literacy skills. They collaborated with their group members to complete Table 4. The results obtained from the PSTs’ performances in the laboratory showed more intellectually demanding scientific reasoning abilities when compared to the in-class argument practices. By setting their experimental designs and hypothesizing their questions, they were able to draw more valid conclusions with genuine evidence.
A sample record of the PSTs’ work indicates their argument dialog, which includes evidence for their hypothesis during complementing Table 4.
If solution Y is an acid, our litmus paper will turn red. Ohh, the litmus paper did not turn into red. Thus, solution Y is not an acid.
This excerpt is an example of the experimental verification of a hypothesis in scientific reasoning. Such a result is aligned with the findings of Walker and Sampson (2013), who state that argument-driven chemistry is needed in the laboratory to develop valid conclusions with evidence. In our study, lab sessions were socially constructed to support the PSTs to experience the process of scientific investigation. Heng et al. (2014) state that the “collaborative development of explanation and justification in group argumentation advances students’ conceptual understanding” (p. 514), thus leading them to use better argumentation components, and so contributing to them developing better chemical literacy skills.
Another argumentation record is provided below from a group of PSTs during a laboratory session related to the “mystery alkaline solution” after completing Table 4.
Evelyn: Now, our mission is to remove corrosive property of this mystery alkali solution.
Adam: I think; first we must determine whether alkaline solution is acidic or basic.
Evelyn: No, alkali means basic solution so we must use acid, is it right? (Reasoning)
Bianca: Remember, blue litmus becomes red if the solution is acid, let's test it (Data)
Evelyn: (takes a small sample and immerses blue litmus), ohh!! You see no color change!
Adam: No color change means the mystery alkali solution is a base (Evidence)
Bianca: Yes, the table verifies this too, is it a strong base or weak base? (She examines the table)
Evelyn: (looks at the table) See, data on the table does not inform us how we decide whether it is strong or weak base. (Claim, Reasoning, Evidence)
Adam: (looks at the table), upps… the test results for weak and strong bases are the same. (Claim, Data)
Bianca: We know the solution is ether weak or strong base, we can remove corrosiveness by using an acid because they neutralize each other (Claim, Evidence, Reasoning)
Adam: I think water can also remove the corrosive nature of the mystery alkaline solution (Claim)
Evelyn: If we use water, we will decrease the corrosiveness instead of removing it (Reasoning)
Bianca: That is right; it is a base solution; water can only dilute it. (Reasoning)
Evelyn: We need a solution that neutralizes the mystery solution. (Reasoning)
Adam: Ok! we need an acidic solution (Claim)
Bianca: But we have two acidic solutions; W and X, which will better remove corrosiveness? (Reasoning)
Evelyn: pH 5 is less acidic when compared to pH 1 (Claim, Data).
Bianca: Do you mean pH 1 is more strong acid?
Adam: Yes, she (Evelyn) says pH 1 is stronger acid.
Evelyn: Remember the titration reactions, if we use a strong acid, it will neutralize all of the alkaline solution regardless of being strong or a weak base (Claim, Data, Evidence, Reasoning)
Bianca: Got it, we have to use X solution for titration.
In such discourses, the students experienced stating their claims, deciding relevant information, sharing ideas, identifying scientific issues, explaining phenomena scientifically, using scientific evidence, and reasoning. These PSTs interactions forced them all to have a better understanding and higher competencies. The dialog among the PSTs was more fruitful when they discussed a topic that they were a little bit familiar through experiments or that they had prior knowledge of. Our results align with Cross et al. (2008), who state that individuals tend to feel more comfortable and be more competent in arguing about concepts when they are sufficiently knowledgeable about that subject. The PSTs had already performed some experiments to fill the related table (Table 4), so they were more competent in this discourse compared to other discourses with no prior experience. Analyses of the whole class and group work arguments were different in terms of the argumentation quality, which is in accordance with the findings of Heng et al. (2014), who observed better argumentations with students’ in-group work. Although the responses were fruitful in our study for the sample excerpt, in some discourse practices, the participants struggled with stating the warrants, giving evidence, and making reasoning. Being unfamiliar with such student-centered approaches, the participants’ poor reasoning capabilities might be one reason for such results (Jimenez-Aleixandre and Erduran, 2008; Dawson and Venville, 2009).
The responses obtained from the laboratory reports were also analyzed to verify the quality of arguments in order to promote PSTs’ chemical literacy skills, specifically their competencies. After each investigation, the PSTs cooperated to answer the questions in the lab report through argumentation. The instructor guided whole class discourses and examined the findings of the investigations. Fig. 3 shows a sample of the responses to the questions on the ‘mystery alkali solution’ laboratory report from Group A.
Writing reports included claims about the knowledge acquired through laboratory experiments and provided evidence to support those claims. For example;
We use blue litmus paper, the color does not change, that means alkaline solution is a base.
The statement is an indication of recognizing the issues that are possible to probe through investigation. In report writing, PSTs experienced applying their knowledge in a specific situation, interpreting issues scientifically, and providing scientific evidence to make conclusions. However, some of the PSTs had trouble in identifying scientific issues and rebutting counterarguments. In addition, generating claims, reasoning, and evidence were less observed in PSTs’ reports. Because students usually write cook-book-oriented lab reports, they lack experience in supporting claims and providing evidence. In line with Walker and Sampson (2013), our lab reports indicated that the PSTs’ written arguments were usually not as strong as their oral argumentation. According to Berland and McNeill (2010), if audiences present during an argument episode, students feel more forced to construct rich and convincing arguments. Therefore, extending in-class discourses with in-laboratory arguments and report writing comprehensively feed the continuity and diversity of argumentation. However, both the lack of different instructional support (Berland and Reiser, 2009) and the lack of exposure to such activities (Heng et al., 2014) are perceived to prevent developing better skills. The lab reports also indicated that high-level responses were observed more when scientific data sets are provided for the participants.
Although some in-class and in-laboratory practices had limited improvement, a moderate positive change was observed in supporting claims, providing evidence, and developing high-level arguments throughout the intervention. All the records indicated that student–student and student–instructor interactions that permit cooperation in knowledge construction and competencies supported improving chemical literacy skills.
Conclusions
This study was an attempt to reveal the contribution of immersion argument practices to the knowledge, competencies, and attitude dimensions of chemical literacy. Diversity among argument instructions necessitates investigations that most foster students’ gaining and improving chemical literacy skills (Cavagnetto, 2010). Although some research stresses the impact of argument studies on developing scientific literacy (Bybee et al., 2009; Dawson and Venville, 2009; Driver et al., 2000), no specific work has yet been conducted to determine the effect of the argumentation on the dimensions of chemical or scientific literacy. Therefore, by investigating the effect of such an intervention on each specific dimension of chemical literacy, this study reveals which literacy domain (knowledge, competency, or attitude) is more developed for acid–base concepts. The findings indicated that greater enhancements occurred in the knowledge and competency domains when compared to attitudes.In this orientation, an immersion argument embedding inquiry activities was used to teach acid–base concepts; the arguments were also emerged throughout the inquiry because the PSTs were able to question the content, hypothesize experiments, interpret the obtained data, and construct and defend the evidence-based knowledge claims (Cavagnetto, 2010). All of which are expected to improve the knowledge, competency, and attitude components of chemical literacy. The utilized immersion argument contributed to the knowledge construction and understanding of the main principles of acids and bases, and thus supported the PSTs’ skills in transferring their knowledge to other concepts, so that their overall competencies were developed. Providing an argument over a real-life situation created a learning environment in which students scrutinized what they know about acid–base concepts. This ‘minds on’ process supported knowledge construction, and the students were able to propose, criticize, evaluate, and refine ideas about acids/bases and to use scientific theories and evidence to confirm their claims, as Heng et al. (2015) also proposed. Furthermore, the process included communication with peers and an instructor, thus this provided opportunities for pre-service teachers to share, critique, and think with others (Duschl and Osborne, 2002), in order to construct their knowledge and promote their competencies and attitudes. The development in the attitude dimension is not as explicit as compared to knowledge and competency, which may imply that the PSTs had a high level of attitude at the beginning. Additionally, the scientific argument environment we used may not have been perceived as attractive for all our PSTs, which in turn may have resulted in little change in the affective component of their chemical literacy.
Throughout the study, scientific arguments were facilitated through scaffolds, like prompts and group works, through contexts that involved related concepts. The created learning environment supported the PSTs’ understanding by enhancing their use of scientific theories, data, and evidence to oppose or confirm claims. As Aydeniz and Dogan (2016) stressed, such an engagement in argument is also a process in which students persuade their peers of the validity of their arguments. This persuasion of others occurs only when knowledge construction proceeds meaningfully, and the use of scientific language abilities is supported. Scientific practices develop language and thus metacognition and critical reasoning abilities, and when these abilities are promoted, scientific literacy or, as in our case, chemical literacy is fostered (Duschl and Osborne, 2002; Cavagnetto, 2010). Additionally, in line with Eskin and Berkiroglu (2009), we can conclude that argument instruction contributed to higher-order thinking skills, and allowed the creation of classroom climates that provided the opportunity to foster chemical competencies. This inquiry approach is key to increasing student's scientific literacy by developing their scientific argumentation capabilities too (Duschl and Osborne, 2002; Zembal-Saul, 2009).
As for study limitations, the conclusions drawn from this study are confined to acid–base concepts. Since only one topic was studied over six weeks, the progression may also be topic-dependent. Although, the study allowed us to evaluate the development in knowledge and competency at the end of six weeks, we observed that these skills were constantly improving over the weeks and they might have been even more increased with a longer lasting intervention. We guess that if the intervention were longer, most probably, the PSTs’ literacy domains would increase more since the quality arguments increased over the weeks too. As to another limitation, a stronger research design (experimental-control group) would make the conclusions stronger.
Further studies may utilize a stronger experimental design. Other argument interventions might also have an opportunity to develop students’ chemical literacy; whereby different argument instructions could enhance our understanding of this intervention in pursuit of chemical literacy. For example, socio-scientific issues in class can attract students’ interest more than an immersion argument (Osborne et al., 2004). More experimental work is needed to make further conclusions on what kind of argument instruction contributes to which chemical literacy domain more. Further work could also reveal useful distinctions among argument orientations on chemical literacy concerning other chemistry concepts as well. Although the literature (Kuhn, 1991; Zohar and Nemet, 2002; Venville and Dawson, 2010) states that argumentation skills can be improved even in the short term, we suggest further studies verify their treatment on this issue.
Appendix A: a sample item of chemical literacy
Olive trees
Adam wanted to plant olive trees in his garden. However, despite planting the trees in the garden and sufficiently watering the plants, the trees did not grow in spite of them getting enough sunlight and watering. The surrounding neighborhoods suggested Adam grow blueberries instead of olive trees in that region. Blueberries are usually grown in neighboring gardens. Adam decided to set up a research group to understand how best to cultivate olive trees in the garden.Adam decided to utilize this class as a research group. Each group will work individually on these issues, and then Adam will select the best solution among the responses.
Background knowledge
Soils may be acidic, basic, or neutral. Different plant species are grown in different areas, such as regions taking too much rainfall and drought regions. Olive plants grow well in soils with pH = 8.5 and blueberries in soils with pH = 3.5. However, the acidity of the soil can be changed with a variety of soil fertilization methods. For example, if soil is too acidic for a specific plant, then an amount of slaked lime (Ca(OH)2) can be added to increase the pH of the soil. Whereas, in order to reduce the pH of the soil, gypsum (CaSO4), or organic substances are used.Questions
1. Is the soil in Adam's region acidic, neutral, or basic? Explain your reasons with justifications.2. Suggest a method to determine the pH of the soil.
3. What is the climate of the region where Adam lives?
4. Based on scientific findings, do you think Adam will be able to grow olive trees on his land? Why?
5. If Adam had to support the olive tree in the ground, what is required in order to help it grow?
| ATTITUDE | |||||
|---|---|---|---|---|---|
| Up to what extent are you interested in the following? | |||||
| Please tick only one box in each row. | |||||
| I am highly interested | I am moderately interested | I am lowly interested | I am not interested | ||
| (1) | To know a species is acidic, basic, or neutral | □2 | □1.5 | □1 | □0.5 |
| (2) | To propose methods to determine the pH | □2 | □1.5 | □1 | □0.5 |
| (3) | To make inferences based on the data available | □2 | □1.5 | □1 | □0.5 |
| (4) | To make decisions from the given information | □2 | □1.5 | □1 | □0.5 |
| (5) | To make recommendations for changing the pH | □2 | □1.5 | □1 | □0.5 |
Appendix B
The sub-questions of the chemical literacy items and the domains of chemical literacy are measured by these questions| Item sets | Sub-questions | Related chemical literacy domains based on Shwartz et al. (2005, 2006) | Related chemical literacy domains based on PISA, 2006 |
|---|---|---|---|
| Note: CK: content knowledge, CC: chemistry in context. | |||
| The first contextual item set: olive trees | 1 | CK/general chemical ideas/ knowledge to explain phenomena CC/understanding of daily-life chemistry, decision processes Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes |
| 2 | CK/key ideas/explaining the macroscopic level with molecular structures CC/understanding daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 3 | CK/key ideas/explaining the macroscopic level with molecular structures CC/knowledge of chemistry in explaining everyday situations Higher-order learning skills/investigating relevant information Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 4 | CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 5 | CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| The second contextual item set: stomach | 1 | CK/general chemical ideas/knowledge to explain phenomena in other fields CC/understanding of daily-life chemistry, decision processes Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes |
| 2 | CK/key ideas/explaining life and the structures of living systems CC/knowledge of chemistry in explaining everyday situations Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 3 | CC/knowledge of chemistry in explaining everyday situations CC/understanding daily-life chemistry, decision processes Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 4 | CK/key ideas/explaining the macroscopic level with molecular structures CC/understanding of daily-life chemistry, decision processes Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 5 | CK/key ideas/dynamics of processes and reactions CC/knowledge of chemistry in explaining everyday situations Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 6 | CK/general chemical ideas/generalizing findings CK/key ideas/explaining the macroscopic level with molecular structures Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 7 | CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| The third contextual item set: teapot | 1 | CK/general chemical ideas/proposing theories to explain the world CK/key ideas/explaining the macroscopic level with molecular structures CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes |
| 2 | CK/general chemical ideas/generalizing findings CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| The fourth contextual item set: tooth decay | 1 | CK/general chemical ideas/generalizing findings CK/general chemical ideas/knowledge to explain phenomena in other fields CK/key ideas/explaining the macroscopic level with molecular structures CK/key ideas/explaining life by the structures of living systems CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes |
| 2 | CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 3 | CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 4 | CK/general chemical ideas/generalizing findings CK/key ideas/seeks dynamics of reactions Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 5 | CK/general chemical ideas/generalizing findings CK/key ideas/explaining life by the structures of living systems CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 6 | CK/key ideas/explaining life by the structures of living systems CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 7 | CK/general chemical ideas/generalizing findings CK/general chemical ideas/knowledge to explain phenomena in other fields CK/key ideas/explaining the macroscopic level with molecular structures CK/key ideas/explaining life by the structures of living systems CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 8 | CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes CC/understanding of daily-life chemistry, involvement in social argumentation Higher-order learning skills/investigating relevant information when required Higher-order learning skills/evaluating pros/cons of debates Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| The fifth contextual item set: acid rains | 1 | CK/general chemical ideas/knowledge to explain phenomena in other fields CK/key ideas/explaining life by chemical processes CC/knowledge of chemistry in explaining everyday situations Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes |
| 2 | CC/knowledge of chemistry in explaining everyday situations Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 3 | CK/general chemical ideas/knowledge to explain phenomena in other fields CK/key ideas/explaining the macroscopic level with molecular structures CC/knowledge of chemistry in explaining everyday situations CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 4 | CK/key ideas/seeking dynamics of reactions and processes CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/investigating relevant information when required Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 5 | CC/understanding of daily-life chemistry, involvement in social argumentation CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/evaluating pros/cons of debates Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
| 6 | CC/understanding of daily-life chemistry, involvement in social argumentation CC/understanding of daily-life chemistry, decision processes Higher-order learning skills/evaluating pros/cons of debates Affective aspects/interest in the issues of chemistry | Knowledge Competencies Attitudes | |
Acknowledgements
The entire instrument can be obtained from the corresponding author.References
- Abu Hassan K. and Tan C. T., (2009), Understanding and application of acids and bases concepts in daily life among form four science students in Johor Bahru, Journal Sains dan Matematik, 1(1), 22–29.
- Albe V., (2008), When scientific knowledge, daily life experience, epistemological and social considerations intersect: students’ argumentation in group discussions on a socio-scientific issue, Res. Sci. Educ., 38(1), 67–90.
- American Association for the Advancement of Science, (1989), Project 2061. Science for all Americans, Washington, DC: American Association for the Advancement of Science.
- Çam A. and Geban Ö., (2016), Effectiveness of case-based learning instruction on pre-service teachers' chemistry motivation and attitudes toward chemistry, Res. Sci. Technol. Educ., 1–14.
- Aydeniz M. and Dogan A., (2016), Exploring the impact of argumentation on pre-service science teachers’ conceptual understanding of chemical equilibrium, Chem. Educ. Res. Pract., 17, 111–119.
- Bader B., (2003), Interpretation d'une controverse scientifique: strategies argumentatives d'adolescentes et d'adolescents Quebecois, Canadian J. Math., Sci. Technol. Educ., 3(2), 231–250.
- Berg C. A. R., (2005), Factors related to observed attitude change toward learning chemistry among university students, Chem. Educ. Res. Pract., 6(1), 1–18.
- Berland L. K. and McNeill K. L., (2010), A learning progression for scientific argumentation: understanding student work and designing supportive instructional contexts, Sci. Educ., 94(5), 765–793.
- Berland L. K. and Reiser B., (2009), Making sense of argumentation and explanation, Sci. Educ., 93(1), 26–55.
- BouJaoude S., (2002), Balance of scientific literacy themes in science curricula: the case of Lebanon, Int. J. Sci. Educ., 24(2), 139–156.
- Bybee R. W., (1997), Achieving scientific literacy: from purposes to practice, Portsmouth, NH: Heinemann.
- Bybee R., McCrae B. and Laurie R., (2009), PISA 2006: an assessment of scientific literacy, J. Res. Sci. Teach., 46(8), 865–883.
- Cavagnetto A. R., (2010), Argument to foster scientific literacy: a review of argument interventions in K-12 contexts, Rev. Educ. Res., 80, 336–371.
- Cigdemoglu C. and Geban O., (2015), Improving students’ chemical literacy level on thermochemical and thermodynamics concepts through context-based approach, Chem. Educ. Res. Pract., 16, 302–317.
- Cross D., Taasoobshirazi G., Hendricks S. and Hickey D. T., (2008), Argumentation: a strategy for improving achievement and revealing scientific identities, Int. J. Sci. Educ., 30(6), 837–861.
- Dawson V. and Venville G. J., (2009), High-school Students’ Informal Reasoning and Argumentation about Biotechnology: an indicator of scientific literacy? Int. J. Sci. Educ., 31(11), 1421–1445.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84, 287–312.
- Duschl R. A. and Osborne J., (2002), Supporting and promoting argumentation discourse in science education, Stud. Sci. Educ., 38, 39–72.
- Erduran S., Ardac D. and Yakmaci-Guzel B., (2006), Learning to teach argumentation: case studies of pre-service secondary science teachers, Eur. J. Math., Sci. Technol. Educ., 2(2), 1–14.
- Eskin H. and Bekiroglu F. O., (2009), Investigation of a pattern between students' engagement in argumentation and their science content knowledge: a case study, Eur. J. Math., Sci. Technol. Educ., 5(1), 63–70.
- Fensham P. J., (2002), Time to change drivers for scientific literacy, Canadian J. Sci., Math. Technol. Educ., 2, 9–24.
- Fraenkel J. R. and Wallen N. E., (2000), How to design and evaluate research in education, New York: McGraw-Hill.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Glynn S. M. and Muth K. D., (1994), Reading and writing to learn science: achieving scientific literacy, J. Res. Sci. Teach., 31(9), 1057–1073.
- Heng L. L., Surif J. and Seng C. H., (2014), Individual versus group argumentation: student's performance in a Malaysian context, Int. Educ. Stud., 7(7), 109–124.
- Heng L. L., Surif J. and Seng C. H., (2015), Malaysian Students' Scientific Argumentation: Do groups perform better than individuals? Int. J. Sci. Educ., 37(3), 505–528.
- Jimenez-Aleixandre M. P. and Erduran S., (2008), Argumentation in science education: an overview, in Erduran S. and Jimenez-Aleixandre M. P. (ed.), Argumentation in Science Education: Perspectives from Classroom-Based Research, Dordrecht: Springer.
- Jimenez-Aleixandre M., Rodriguez A. and Duschl R., (2000), ‘Doing the lesson’ or ‘doing science’: argument in high school genetics, Sci. Educ., 84(6), 757–792.
- Kaya E., (2013), Argumentation practices in classroom: pre-service teachers’ conceptual understanding of chemical equilibrium, Int. J. Sci. Educ., 35(7), 1139–1158.
- Kıngır S., Geban O. and Gnel M., (2011), Ogrencilerin kimya derslerinde argumantasyon tabanlı bilim ogrenme yaklaşımının uygulanmasına ilişkin gorusleri, Journal of Selçuk Unıversıty Ahmet Kelesoglu Educatıon Faculty, 32, 15–28.
- Kobella T. R., (1989), Changing and measuring attitudes in the science classroom, in Research Matters to the Science Teacher, retrieved from http://www.narst.org/publications/research/attitude.cfm.
- Kuhn D., (1991), The skills of argument, Cambridge: Cambridge University Press.
- Marks R. and Eilks I., (2009), Promoting scientific literacy using a socio-critical and problem-oriented approach to chemistry teaching: concept, examples, experiences, Int. J. Environ. Sci. Educ., 4(3), 231–245.
- National Research Council (NRC), (1996), National science education standards, Washington, DC: National Academy Press.
- OECD, (2006), Assessing scientific, reading and mathematical literacy: a framework for PISA 2006, Paris: OECD Publishing.
- Osborne J. and Dillon J., (2008), Science education in Europe: critical reflections, vol. 13, London: The Nuffield Foundation.
- Osborne J., Erduran S. and Simon S., (2004), Enhancing the quality of argumentation in school science, J. Res. Sci. Teach., 41, 994–1020.
- Ratcliffe M. and Millar R., (2009), Teaching for understanding of science in context: evidence from the pilot trials of the Twenty First Century Science courses, J. Res. Sci. Teach., 46(8), 945–959.
- Roberts D., (2007), Scientific literacy/science literacy: threats and opportunities, in Abell S. K. and Lederman N. G. (ed.), Handbook of research on science education, Mahwah, New Jersey: Lawrence Erlbaum Associates, pp. 729–780.
- Sadler T. D. and Zeidler D. L., (2009), Scientific literacy, PISA and socioscientific discourse: assessment for progressive aims of science education, J. Res. Sci. Teach., 46(8), 909–921.
- Sendur G., Ozbayrak O. and Uyulgan M. A., (2010), A study of determination of pre-service chemistry teachers’ understanding about acids and bases, Proc. Soc. Behav. Sci., 3, 52–56.
- Shwartz Y., Ben-Zvi R. and Hofstein A., (2005), The importance of involving high-school chemistry teachers in the process of defining the operational meaning of ‘chemical literacy’, Int. J. Sci. Educ., 27(3), 323–344.
- Shwartz Y., Bez-Zvi R. and Hofstein A., (2006), The use of scientific literacy taxonomy for assessing the development of chemical literacy among high-school students, Chem. Educ. Res. Pract., 7(4), 203–225.
- Simon S., Erduran S. and Osborne J., (2006), Learning to teach argumentation: research and development in the science classroom, Int. J. Sci. Educ., 28(2–3), 235–260.
- Taasoobshirazi G., Hendricks S. and Hickey D. T., (2008), Argumentation: a strategy for improving achievement and revealing scientific identities, Int. J. Sci. Educ., 30(6), 837–861.
- Tabachnick B. G. and Fidell L. S., (2007), Using multivariate statistics, Boston: Pearson Education, Inc.
- Tarhan L. and Sesen B. A., (2010), Investigation the effectiveness of laboratory works related to “Acids and bases” on learning achievements and attitudes toward laboratory, Proc. Soc. Behav. Sci., 2, 2631–2636.
- Venville G. J. and Dawson V. M., (2010), The impact of a classroom intervention on grade 10 students’ argumentation skills, informal reasoning, and conceptual understanding of science, J. Res. Sci. Teach., 47(8), 952–977.
- Von Aufschnaiter C., Erduran S., Osborne J. and Simon S., (2008), Arguing to learn and learning to argue: case studies of how students’ argumentation relates to their scientific knowledge, J. Res. Sci. Teach., 45(1), 101–131.
- Walker J. P. and Sampson V., (2013), Learning to Argue and Arguing to Learn: Argument Driven Inquiry as a Way to Help Undergraduate Chemistry Students Learn How to Construct Arguments and Engage in Argumentation During a Laboratory Course, J. Res. Sci. Teach., 50(5), 561–596.
- Zembal-Saul C., (2009), Learning to teach elementary science as argument, Sci. Educ., 93(4), 687–719.
- Zohar A. and Nemet F., (2002), Fostering students’ knowledge and argumentation skills through dilemmas in human genetics, J. Res. Sci. Teach., 39(1), 35–62.
| This journal is © The Royal Society of Chemistry 2017 |