Efficient synthesis of 5-(chloromethyl)furfural (CMF) from high fructose corn syrup (HFCS) using continuous flow processing†
T. M.
Kohl
 *,
B.
Bizet
,
P.
Kevan
,
C.
Sellwood
,
J.
Tsanaktsidis
and
C. H.
Hornung
*,
B.
Bizet
,
P.
Kevan
,
C.
Sellwood
,
J.
Tsanaktsidis
and
C. H.
Hornung

CSIRO Manufacturing Flagship, Bag 10, Clayton South, Victoria 3169, Australia. E-mail: thomas.kohl@csiro.au; Tel: +61 3 9545 7847
First published on 12th June 2017
Abstract
Using continuous flow processing the synthesis of 5-(chloromethyl)furfural (CMF) from both solid sugars and high fructose corn syrup (HFCS) was achieved. The use of HFCS allows for a convenient liquid sugar feedstock, which in turn through our improved three pump system, allows for production of CMF with no additional handling of stock solutions. Extensive reaction optimisation was also carried out with large increases in reaction efficiency achieved over existing batch processes.
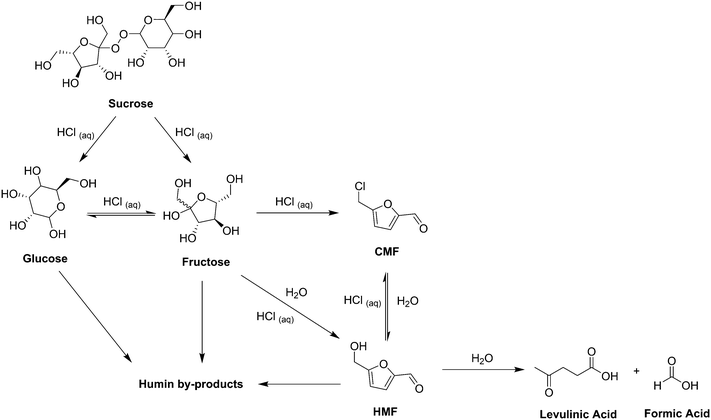
Introduction
Currently fossil fuels make up the vast majority of sources for chemical products, with almost 4% of oil produced worldwide used for chemical and plastic production.1 However, as environmental and political concerns over the use of fossil fuels increase it is expected that the feasibility of their exploitation will decrease in the future.2–5 In order to reduce the chemical industries dependence on oil and reduce environmental impacts it will be necessary to develop alternative, renewable, production chains. Over recent years it has been increasingly acknowledged that plant based raw materials (biomass) have the potential to replace a large proportion of fossil resources as feedstocks for industrial applications.6–8 Investment in this area has also increased with companies such as BASF, DOW, DuPont and DSM actively testing and in some cases developing products from natural resources.9 Furanic compounds have been widely studied as potential platform molecules upon which chemical biorefineries may be based.10,11 Much of this interest has been focussed around 5-hydroxymethylfurfural (HMF), which was first synthesised in 1895 (ref. 12 and 13) from the action of strong acids on sugars and incorporates important chemical handles, allowing for a wide variety of synthetic manipulation.14 However, despite this attention significant issues remain with the utilisation of HMF, mainly due to its high water solubility and poor chemical stability under the acidic reaction conditions under which it is formed.15 As such, the chlorinated derivative of HMF, 5-chloromethylfurfural (CMF), has emerged as a functionally equivalent but more synthetically practical platform molecule.15 The synthesis of CMF from both simple sugars and biomass has recently been extensively reviewed.16 Work in our lab in this area has focussed on adapting and optimising this synthesis for the scalable production of CMF using continuous flow processing.17 The small dimensions of the fluidic pathways in micro- and mesoscale flow reactors (channel diameters typically between 100 μm and 10 mm) result in a well-defined flow regime, high heat and mass transfer rates and a narrow residence time (τ) distribution.18,19 This level of control of reaction conditions can often lead to improvements in process safety, yield, selectivity and product quality over traditional batch reactors.20–23 Some of these benefits were observed in our previous work, with CMF yields of up to 81% from D-fructose achieved with residence times of 1.67 min at 100 °C, inside a 1 mm internal diameter (ID) tubular flow reactor. However the use of solid sugars limited the scalability of the reaction due to the need to dissolve the sugar in acid prior to reaction in order to make a stock solution suitable for pumping. This resulted in uncontrolled reaction of the sugar occurring prior to entry into the continuous reactor system, including formation of humics which can block the pumps. Humics consist of a group of poorly defined, structurally diverse and generally insoluble dark coloured solids.24,25 This work has since been improved as described herein with further optimisation of the reaction from solid sugar feedstocks along with the introduction of a 3-pump system allowing for processing of the liquid sugar feedstock high fructose corn syrup (HFCS).HFCS is a corn starch derivative used worldwide as a sweetener.26 The United States is the largest producer of HFCS where it has a net cost of production considerably less than domestic cane or beet sugar.27 Two classes of HFCS are manufactured through a 3 step enzymatic process, followed by fractionation to give HFCS 42 and HFCS 90, which contain 42% and 90% fructose respectively. A third HFCS class is also available, HFCS 55, which is produced by blending HFCS 42 and HFCS 90. All classes of HFCS contain ∼25% water.28 It is a clear, homogeneous and easy to handle feedstock which when coupled with its low cost could make it very economical for a series of high value added products, including pharmaceuticals, fine chemicals and speciality polymers. However, being derived from a food grade material, the use of HFCS as a chemical feedstock would directly compete with food production. Our experiments were set out to demonstrate the viability of this intensified continuous flow process based on a sugar syrup feedstock and HFCS was a simple model system for that purpose. It is conceivable that a less pure, non-food grade syrup could also be used for our process, derived from agricultural waste high in dissolvable and complex sugars, such as corn stover, sugar cane bagasse, sorghum bagasse, wood chips or others. Few efforts for the synthesis of HMF or CMF from HFCS have been reported in the literature. Early work by Szmant and Chundury describes the batch synthesis of CMF from both HFCS and D-fructose using a biphasic system with concentrated HCl and chlrorobenzene. Yields of up to 90% were reported after 1 h at 75 °C from HFCS 72 (custom blend).29 More recent work by Jeong describes the batch synthesis of HMF from HFCS 90 in 1,4-dioxane over Amberlyst-15 ion exchange resin in a single phase system. A maximum HMF yield of 80% (based on fructose content) was obtained at 100 °C after 3 h. Recycling of the solvent and catalyst was also demonstrated.30 This work was followed by Kim where HMF was again synthesised in a single phase system with a wood powder-derived, carbonaceous, solid acid in an ethylene glycol based solvent system.31 The continuous flow sugar syrup process presented herein demonstrates the first continuous production of CMF from HFCS and demonstrates large increases in reaction efficiency compared to existing batch processes.
Results and discussion
Continuous synthesis of CMF from solid sugar feedstocks
Reactions were carried out in a Vapourtec R2/R4 reactor system.32 Sugar stock solutions were prepared at a concentration of 1 g sugar per 10 ml of 37% HCl immediately prior to reaction. The sugar solution and organic solvent were then pumped at a defined flow rate via a mixing T-piece into a 10 ml PFA reactor coil where the reaction took place. The product stream was then directed into a second 2 ml reactor coil where it was allowed to cool prior to collection (Scheme 1). In our previous work an inline glass column (Omnitfit®) filled with a sand bed was used in order to filter out the solid, humic side-product formed during the reaction.17 However, this limited extended operation and scale up as the filter would eventually block. To address this limitation the filter has been removed from the reactor system and a custom made back pressure regulator (BPR) installed. This BPR was made from Hastelloy in order to withstand the acidic reaction medium and is suitable for operation in pressures ranging from 3.4 to 24.1 bar. It is an adjustable, spring loaded pressure relief valve, specifically designed by Cambridge Reactor Design (CRD) for our application.33 Due to its relatively large internal orifices, the unit is less susceptible to blockage caused by humics compared to conventional flow chemistry BPRs.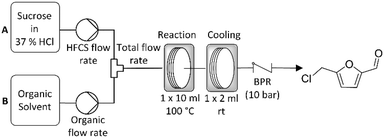 | ||
| Scheme 1 General reactor configuration used in the continuous synthesis of CMF from the solid sugar feedstocks sucrose and fructose. | ||
Our previous work was used to inform the initial reaction conditions.17 This work has been expanded upon here with a systematic investigation into the effect of the ratio of aqueous phase (sugar in HCl) to organic phase, as well as the effect of residence time and the organic solvent used (Table 1). Initial reaction optimisation was carried out using D-fructose at 100 °C, which was established during previous work as an optimal working temperature. A solvent screen was then performed targeting industrially acceptable solvents that would form a biphasic mixture with the acid/sugar stream. It was found that the yield of CMF was highly dependent on the organic solvent used, with cyclohexane and methylethylketone (MEK) giving much lower yields than methyl isobutyl ketone (MIBK), toluene and dichloroethane (DCE) under the same reaction conditions. The dependence of CMF yield on the organic solvent used is believed to be due to the partition coefficient of CMF in the acid/organic system. Efficient, continuous extraction and retention of CMF from the acidic, aqueous reaction medium is important in achieving high CMF yields. This is expected to not only aid in reaction kinetics by keeping the relative concentration of CMF in the aqueous phase low, but also protects the CMF once formed from further reaction onto a number undesired products, including humins (Scheme 2). The best performing solvent was DCE with a yield of 74% achieved, while toluene gave a fair yield of 60%. Despite a slightly lower yield toluene may remain a more attractive option for scale up of this reaction due to its greater acceptance industrially. However, whilst not ideal it may be possible to engineer the process to allow recovery of the organic solvent following reaction such that chlorinated solvents could be used to access the optimal CMF yields. The reaction was then optimised for the use of sucrose as the feedstock, here using dichloromethane (DCM) as an alternative to DCE due to the lower health risk posed.34 The lower boiling point of DCM was compensated for by increasing the system pressure using the adjustable BPR. It was expected that sucrose would take longer to react than fructose as it is a disaccharide. This was found to be the case as a maximum yield of CMF was achieved after a residence time of 2.5 min compared to 1 min when fructose was used. Further increasing the residence time beyond this point was found to have little or no effect. This is suspected to be due to competing reaction pathways leading to formation of CMF from sucrose and consumption of CMF to form humics. This is supported by observations of increased amounts of humic material both in the reactor tubing during reaction and in the BPR following reaction. Additionally it was found that the system pressure became more variable at longer residence times. This is also associated with the presence of humics and their accumulation in the BPR (see ESI† Fig. S1).
| Entry | Sugar | [HCl] (%) | Aq/org | Solvent | τ (min)a | CMF yield (%) |
|---|---|---|---|---|---|---|
| a τ = residence time. | ||||||
| 1 | D-fructose | 32 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
MEK | 1.0 | 3 |
| 2 | D-fructose | 32 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cyclohexane | 1.0 | 18 |
| 3 | D-fructose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
MIBK | 1.0 | 47 |
| 4 | D-fructose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene/MIBK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.0 | 49 |
| 5 | D-fructose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene | 1.0 | 60 |
| 6 | D-fructose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCE | 1.0 | 74 |
| 7 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
DCM | 1.5 | 36 |
| 8 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 1.5 | 34 |
| 9 | Sucrose | 37 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 1.5 | 44 |
| 10 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
DCM | 2.5 | 45 |
| 11 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 2.5 | 50 |
| 12 | Sucrose | 37 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 2.5 | 45 |
| 13 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
DCM | 3.5 | 44 |
| 14 | Sucrose | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 3.5 | 49 |
| 15 | Sucrose | 37 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DCM | 3.5 | 48 |
As can be seen from the reaction network presented in Scheme 2 there are a number of different competing reactions that are possible under the given reaction conditions. Sucrose is initially broken down into glucose and fructose under acidic conditions with an equilibrium existing under the same conditions between these two sugar species. Fructose may then react to give either CMF or HMF with CMF more highly favoured under concentrated acidic conditions using HCl(aq) while as the amount of water in the system is increased there is a tendency to favour the production of HMF. Again there is an equilibrium existing between these two species with CMF converting to HMF in the presence of water and HMF converting to CMF under highly acidic conditions. HMF may further react in aqueous conditions to give levulinic acid and formic acid, with levulinic acid representing an interesting sugar derived molecule in its own right. While the exact mechanism and structure remain unknown it is believed that humins may form under acidic conditions from any combination of glucose, fructose and HMF.
The effect of the aqueous-to-organic volumetric feed ratio (aq/org) was also investigated as shown in Fig. 1.
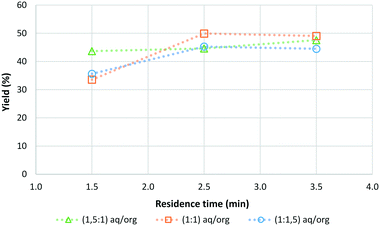 | ||
| Fig. 1 Reaction optimisation data for the continuous synthesis of CMF from sucrose including the influence of the volumetric feed ratio (aq/org) on the final yield (sucrose 1 g/10 mL, 100 °C). | ||
It was found that the ratio of aqueous to organics only had a minor effect on the yield of CMF for the investigated range. Across all 3 ratios tested the CMF yield peaked at a residence time of 2.5 min, with aqueous to organic ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 giving similar CMF yields. The observed minor effect of the aqueous to organic ratio on the CMF yield is consistent with what was found when the organic solvent type was varied. By increasing the relative amount of organic solvent in the system, more efficient extraction of CMF into the organic phase was achieved which as previously described protects the CMF already formed from further reaction to other undesired products resulting in an increase in yield. This effect does have a limit though and no benefit was found when the ratio of aqueous to organic phase was increased from 1
1.5 giving similar CMF yields. The observed minor effect of the aqueous to organic ratio on the CMF yield is consistent with what was found when the organic solvent type was varied. By increasing the relative amount of organic solvent in the system, more efficient extraction of CMF into the organic phase was achieved which as previously described protects the CMF already formed from further reaction to other undesired products resulting in an increase in yield. This effect does have a limit though and no benefit was found when the ratio of aqueous to organic phase was increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. It is expected that this is due to the relatively small interface between the aqueous and organic phases in the narrow reactor tubing, limiting mass transport from the aqueous phase to the organic phase even though the relative volume of organic phase has increased. However, the pressure data indicate that as the amount of organic solvent increases the pressure difference observed in the system decreases. This allows for more stable operation of the flow reactor and the processing of larger amounts of sugar (see ESI† Fig. S2). Through this work the optimum conditions for the synthesis of CMF from sucrose were determined to be at a temperature of 100 °C, with a 1
1.5. It is expected that this is due to the relatively small interface between the aqueous and organic phases in the narrow reactor tubing, limiting mass transport from the aqueous phase to the organic phase even though the relative volume of organic phase has increased. However, the pressure data indicate that as the amount of organic solvent increases the pressure difference observed in the system decreases. This allows for more stable operation of the flow reactor and the processing of larger amounts of sugar (see ESI† Fig. S2). Through this work the optimum conditions for the synthesis of CMF from sucrose were determined to be at a temperature of 100 °C, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio of aqueous phase to organic phase and a residence time of 2.5 min. Under these conditions CMF yields of up to 50% were achieved. Optimised conditions were also found for D-fructose where a temperature of 100 °C with a 1
1.5 ratio of aqueous phase to organic phase and a residence time of 2.5 min. Under these conditions CMF yields of up to 50% were achieved. Optimised conditions were also found for D-fructose where a temperature of 100 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of aqueous to organics, DCE as the organic solvent and a residence time of 1 min gave yields of up to 74%. As such the best yields were obtained using D-fructose as the feedstock.
1 ratio of aqueous to organics, DCE as the organic solvent and a residence time of 1 min gave yields of up to 74%. As such the best yields were obtained using D-fructose as the feedstock.
Optimised synthesis of CMF using HFCS as a liquid sugar feedstock
Whilst good results were achieved with solid sugar feedstocks the process was limited by the fact that a sugar in acid solution had to be prepared prior to reaction. As the sugar begins to react immediately upon addition of the acid this results in the sugar/acid reagent feed changing continuously during pumping. This limits the scale of the reaction that can be performed in a continuous reactor. As such we have proposed an improved three pump system where a liquid sugar syrup feed, namely high fructose corn syrup (HFCS), is used (Scheme 3). Here the HFCS is fed into the reactor using a separate pump (Knauer 80p) capable of delivering the viscous sugar solution. This is then combined in a T-piece with 32% HCl which is delivered by a second pump at a defined flow rate in order to give the desired 1 g/10 ml sugar concentration. This lower concentration of HCl was used over the 37% HCl used previously due to lower cost and ease of handling. The HFCS in acid solution then enters a 10 ml PFA coil where pre-mixing occurs. A variety of different temperatures were explored for this pre-mixing step, but holding the reaction coil at room temperature proved to be sufficient (see ESI† for details). The HFCS/acid solution was then directed into a second T-piece where DCM was introduced in order to give a biphasic mixture. This was then directed into a second 10 ml, PFA reactor coil where the reaction took place followed by a third coil where the product mixture was allowed to cool prior to collection.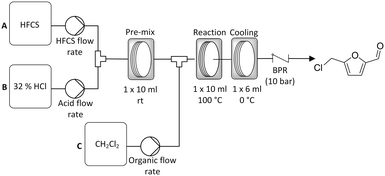 | ||
| Scheme 3 General reactor configuration used for the continuous synthesis of CMF from the liquid sugar feedstock high fructose corn syrup (HFCS). | ||
The reaction was then optimised targeting maximum CMF yield along with stable, continuous, operation of the reactor. Our previous work has indicated that fructose is the most reactive of these three sugar species and as such it was expected that HFCS 90 would give the highest yield of CMF. Therefore, initial reaction optimisation focussed on HFCS 90 with later reactions performed in order to compare HFCS 90 and 55.
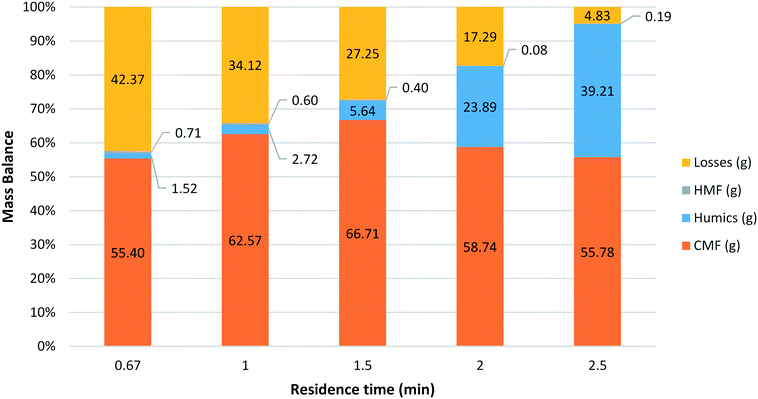
A series of reactions were carried out using HFCS 90 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous to organic phase ratio and a reactor temperature of 100 °C with the residence time varied from 40 s to 2.5 min (Fig. 2). For each of the experiments a mass balance was obtained; divided into the relative amounts of CMF, HMF, humics and losses. The amount of CMF and HMF present was determined based on the crude product mass and purity of each component seen in the GC-FID data. Humics were determined by direct measurement of mass according to the following procedure. Upon collection the product stream was filtered through a pre-weighed plug of celite 545® topped with sand. After processing HFCS 90 (5 ml) the reactor was washed with water and DCM, cleaning the filter of any soluble components. The filter was then removed from the system and dried overnight in an oven at 100 °C. After this time the filter was re-weighed in order to calculate the amount of humic material collected during the reaction. All mass not identified as CMF, HMF or humic matter was classified as ‘losses’ – unreacted sugars, other aqueous products not extracted with DCM and any humics lost inside the BPR or reactor system. A number of trends can clearly be seen in the mass balance data. First, mass fraction and in turn the yield of CMF is highest with a residence time of 1.5 min, with a molar yield of up to 85% obtained. Second, the amount of humic material produced increases as the residence time increases. The observed rise is not linear, as the amount of humic material increases rapidly beyond a residence time of 1.5 min. It is believed that while some CMF may be converted to produce humics at these longer residence times, a significant proportion of the additional humics come from the unaccounted material. This loss in CMF yield may be due to aqueous soluble materials such as HMF or levulinic acid that are not extracted efficiently into the organic phase and as a result are in contact with the acid for longer. As the residence time is increased the additional time that these species spend in the heated reactor zone in the acidic aqueous medium results in breakdown and on-reaction of these species to give humic material. The final trend observed is the extremely low amount of HMF isolated under these conditions. It appears that the amount of HMF produced has little or no relationship with the residence time in the reactor.
1 aqueous to organic phase ratio and a reactor temperature of 100 °C with the residence time varied from 40 s to 2.5 min (Fig. 2). For each of the experiments a mass balance was obtained; divided into the relative amounts of CMF, HMF, humics and losses. The amount of CMF and HMF present was determined based on the crude product mass and purity of each component seen in the GC-FID data. Humics were determined by direct measurement of mass according to the following procedure. Upon collection the product stream was filtered through a pre-weighed plug of celite 545® topped with sand. After processing HFCS 90 (5 ml) the reactor was washed with water and DCM, cleaning the filter of any soluble components. The filter was then removed from the system and dried overnight in an oven at 100 °C. After this time the filter was re-weighed in order to calculate the amount of humic material collected during the reaction. All mass not identified as CMF, HMF or humic matter was classified as ‘losses’ – unreacted sugars, other aqueous products not extracted with DCM and any humics lost inside the BPR or reactor system. A number of trends can clearly be seen in the mass balance data. First, mass fraction and in turn the yield of CMF is highest with a residence time of 1.5 min, with a molar yield of up to 85% obtained. Second, the amount of humic material produced increases as the residence time increases. The observed rise is not linear, as the amount of humic material increases rapidly beyond a residence time of 1.5 min. It is believed that while some CMF may be converted to produce humics at these longer residence times, a significant proportion of the additional humics come from the unaccounted material. This loss in CMF yield may be due to aqueous soluble materials such as HMF or levulinic acid that are not extracted efficiently into the organic phase and as a result are in contact with the acid for longer. As the residence time is increased the additional time that these species spend in the heated reactor zone in the acidic aqueous medium results in breakdown and on-reaction of these species to give humic material. The final trend observed is the extremely low amount of HMF isolated under these conditions. It appears that the amount of HMF produced has little or no relationship with the residence time in the reactor.
The optimum phase ratio was then investigated using the same methodology developed for the residence time investigations (Fig. 3). Here reactions were carried out at 100 °C with a residence time of 1.5 min. As was seen previously for sucrose the effect of changing the phase ratio was not very pronounced. The data show a peak around a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with comparable results on either side when a slight excess of aqueous or organic was present. The amount of humics and HMF produced was generally low with no clear trend for either species. Likewise the losses here appear to only be related to the amount of CMF produced.
1 with comparable results on either side when a slight excess of aqueous or organic was present. The amount of humics and HMF produced was generally low with no clear trend for either species. Likewise the losses here appear to only be related to the amount of CMF produced.
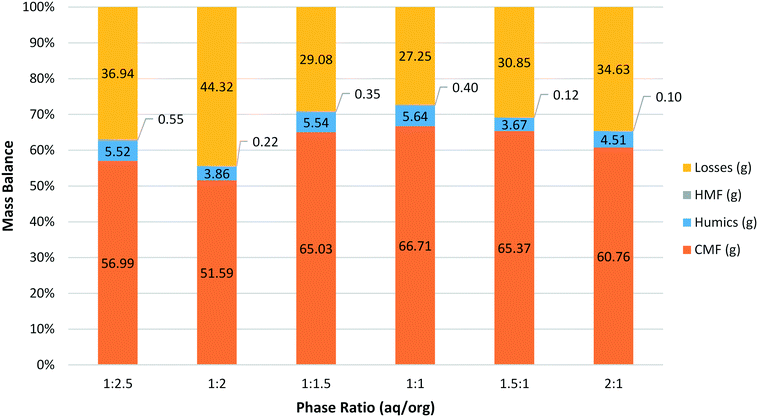 | ||
| Fig. 3 Mass balance for the synthesis of CMF from HFCS 90 at 100 °C with a residence time of 1.5 min varying the ratio of aqueous to organics (aq/org). | ||
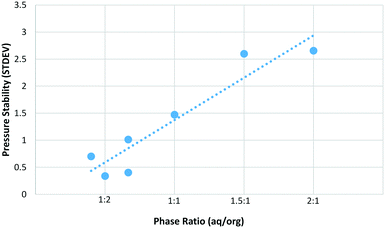
As can be seen in Fig. 3, the maximum yield was obtained with a phase ratio of between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1.5
1.5 and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Pressure stability also correlates well with phase ratio and shows a similar trend to that observed for sucrose (Fig. 4). As the relative amount of organic phase is increased the pressure performance of the reactor improves. There are likely two main factors contributing to this effect. First, the increased amount of organic solvent will better wash the reactor walls and BPR, reducing the likelihood of solid humic particles accumulating and leading to partial blockage of the reactor. Humics produced during the synthesis of HMF from sugars are also known to contain an organic soluble polymeric fraction.35,36 The nature of these humic materials is likely to be similar during the synthesis of CMF, as such it is expected that the organic soluble humic fraction is more efficiently dissolved when a greater proportion of organic solvent is used. This is linked to the first point in that the reactor walls and BPR will be less likely to accumulate solid particles due to the removal of this polymeric material. It is expected that this dissolved humic fraction precipitates from solution upon cooling and/or is retained on the Celite plug during filtration. As such, while a phase ratio of 1
1. Pressure stability also correlates well with phase ratio and shows a similar trend to that observed for sucrose (Fig. 4). As the relative amount of organic phase is increased the pressure performance of the reactor improves. There are likely two main factors contributing to this effect. First, the increased amount of organic solvent will better wash the reactor walls and BPR, reducing the likelihood of solid humic particles accumulating and leading to partial blockage of the reactor. Humics produced during the synthesis of HMF from sugars are also known to contain an organic soluble polymeric fraction.35,36 The nature of these humic materials is likely to be similar during the synthesis of CMF, as such it is expected that the organic soluble humic fraction is more efficiently dissolved when a greater proportion of organic solvent is used. This is linked to the first point in that the reactor walls and BPR will be less likely to accumulate solid particles due to the removal of this polymeric material. It is expected that this dissolved humic fraction precipitates from solution upon cooling and/or is retained on the Celite plug during filtration. As such, while a phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 may give the best yields it may be advantageous to use a slight excess of organic phase in order to increase the stability of the system and allow for longer, continuous operation of the reactor.
1 may give the best yields it may be advantageous to use a slight excess of organic phase in order to increase the stability of the system and allow for longer, continuous operation of the reactor.
Next, the effect of reaction temperature was explored ranging from 70 °C to 120 °C maintaining the phase ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and the residence time at 1.5 min (Fig. 5).
1.5 and the residence time at 1.5 min (Fig. 5).
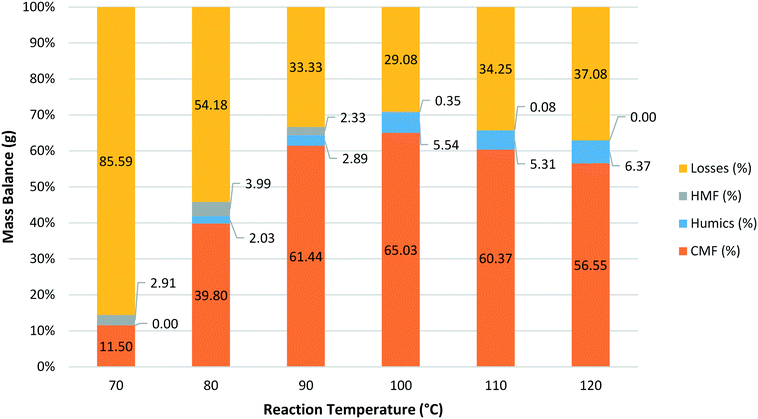 | ||
Fig. 5 Mass balance obtained for reactions at a range of different temperatures with a fixed residence time of 1.5 min and a phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. 1.5. | ||
A mass balance was again obtained for each reaction with the CMF yield peaking at 100 °C before decreasing at higher temperatures. The amount of humic material formed was found to increase with increasing reaction temperature but the effect was much smaller than that seen for residence time. An interesting trend was observed for HMF where the relative proportion of HMF to CMF was found to increase as the reaction temperature was lowered (Fig. 6).
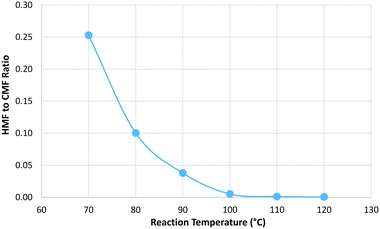 | ||
Fig. 6 Change in the relative proportion of HMF to CMF with reaction temperature where residence time was held constant at 1.5 min and a phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 was used. 1.5 was used. | ||
This suggests that there may be potential to tune the reaction conditions in order to favour the production of HMF by lowering the reaction temperature and increasing the residence time. Alternatively, by keeping the reaction temperature elevated to 100 °C or above it is possible to tune the reaction to be almost totally selective for CMF.
From these results it has been demonstrated that a continuous 3-stream process may be used to synthesise CMF from HFCS 90. The reaction has also been optimised with reaction temperature of 100 °C, residence time of 1.5 min and phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 determined to be optimal. With these conditions for the synthesis of CMF from HFCS 90 in hand the lower grade HFCS 55 was then tested for comparison (Table 2). As expected, HFCS 55 gave lower yields under the same reaction conditions due to the lower fructose content, with a yield of 67% obtained compared to 85% for HFCS 90. In HFCS 55 the lower fructose content is predominantly replaced by additional glucose and a small amount of extra sucrose. From our work it has already been shown that the disaccharide sucrose which is made up of one fructose unit and one glucose unit reacts much more slowly than a pure sample of fructose. As such, for HFCS 55 it was expected that by increasing the reaction time it may be possible to allow the glucose time to react without excessive degradation of CMF already formed from the fast reacting fructose. Interestingly, a comparison of HFCS 90 and HFCS 55 under the same conditions with a 2.5 min residence time gave almost identical yields. The result for HFCS 55 stayed essentially the same while the CMF yield from HFCS 90 dropped from 85% to 66%. This suggests that the CMF from fructose is reacting to form humics and other side products faster than it is produced from the additional glucose in HFCS 55. As such, HFCS 90 remains the optimal liquid sugar source for this process. The process was then run continuously for 3 h (tR) using the optimised conditions in order to test the stability of the reactor over an extended period of operation. In entry 5 a total mass of 23.24 g of CMF was obtained after 3 h of collection at steady state conditions giving a yield of 68%. However, during this reaction the pressure fluctuation within the reactor became too great due to the accumulation of humics in the BPR. As a result the temperature was lowered to 90 °C and the phase ratio changed to 1
1.5 determined to be optimal. With these conditions for the synthesis of CMF from HFCS 90 in hand the lower grade HFCS 55 was then tested for comparison (Table 2). As expected, HFCS 55 gave lower yields under the same reaction conditions due to the lower fructose content, with a yield of 67% obtained compared to 85% for HFCS 90. In HFCS 55 the lower fructose content is predominantly replaced by additional glucose and a small amount of extra sucrose. From our work it has already been shown that the disaccharide sucrose which is made up of one fructose unit and one glucose unit reacts much more slowly than a pure sample of fructose. As such, for HFCS 55 it was expected that by increasing the reaction time it may be possible to allow the glucose time to react without excessive degradation of CMF already formed from the fast reacting fructose. Interestingly, a comparison of HFCS 90 and HFCS 55 under the same conditions with a 2.5 min residence time gave almost identical yields. The result for HFCS 55 stayed essentially the same while the CMF yield from HFCS 90 dropped from 85% to 66%. This suggests that the CMF from fructose is reacting to form humics and other side products faster than it is produced from the additional glucose in HFCS 55. As such, HFCS 90 remains the optimal liquid sugar source for this process. The process was then run continuously for 3 h (tR) using the optimised conditions in order to test the stability of the reactor over an extended period of operation. In entry 5 a total mass of 23.24 g of CMF was obtained after 3 h of collection at steady state conditions giving a yield of 68%. However, during this reaction the pressure fluctuation within the reactor became too great due to the accumulation of humics in the BPR. As a result the temperature was lowered to 90 °C and the phase ratio changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and operation was continued. This change in process conditions likely resulted in the reduced yield. Following on from this result an additional experiment was performed here returning to the optimal phase ratio of 1
2 and operation was continued. This change in process conditions likely resulted in the reduced yield. Following on from this result an additional experiment was performed here returning to the optimal phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 but a temperature of 80 °C. Here the pressure remained significantly more stable in the reactor and the test was successfully complete with 3 h of operation resulting in 18.47 g of CMF with a yield of 47%. This compares well with the previous small scale experiment carried out using these conditions (entry 7) which gave a yield of 50%. While this compromise is not ideal the stability of the reactor during this extended operation was promising and it could conceivably be run for an even longer time in order to generate tens or hundreds of grams of CMF on demand. Given the small reactor dimensions the space–time yields obtained in this system are excellent. The space–time yield is defined as the amount of product obtained per hour for one litre of reactor volume. Under our ideal conditions (entry 1) a space–time yield of 31 g L−1 h−1 was achieved, while under the less efficient, but more pressure stable conditions used for extended operation the space–time yield was 17 g L−1 h−1. This compares very favourably with similar reactions performed in batch from sucrose (1.02 × 10−4 g L−1 h−1), D-fructose (3.25 × 10−3 g L−1 h−1) and HFCS 72 (3.80 × 10−3 g L−1 h−1).29,37 As such our continuous process represents a process efficiency gain of 4500–300
1.5 but a temperature of 80 °C. Here the pressure remained significantly more stable in the reactor and the test was successfully complete with 3 h of operation resulting in 18.47 g of CMF with a yield of 47%. This compares well with the previous small scale experiment carried out using these conditions (entry 7) which gave a yield of 50%. While this compromise is not ideal the stability of the reactor during this extended operation was promising and it could conceivably be run for an even longer time in order to generate tens or hundreds of grams of CMF on demand. Given the small reactor dimensions the space–time yields obtained in this system are excellent. The space–time yield is defined as the amount of product obtained per hour for one litre of reactor volume. Under our ideal conditions (entry 1) a space–time yield of 31 g L−1 h−1 was achieved, while under the less efficient, but more pressure stable conditions used for extended operation the space–time yield was 17 g L−1 h−1. This compares very favourably with similar reactions performed in batch from sucrose (1.02 × 10−4 g L−1 h−1), D-fructose (3.25 × 10−3 g L−1 h−1) and HFCS 72 (3.80 × 10−3 g L−1 h−1).29,37 As such our continuous process represents a process efficiency gain of 4500–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 over current batch processing methods.
000 over current batch processing methods.
| Entry | HFCS grade | Aq/org | T (°C) | τ (min) | CMF mass (g) | t R (min) | Space–time yield (g L−1 h−1) | Yield CMF (%) |
|---|---|---|---|---|---|---|---|---|
| a Experimental conditions were adjusted during the run due to pressure build up in the reactor system. The temperature was decreased to 90 °C and the ratio of aqueous to organic lowered to 0.5 in order to combat the pressure fluctuations. | ||||||||
| 1 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
100 | 1.5 | 3.5536 | 19 | 31 | 85 |
| 2 | 55 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
100 | 1.5 | 2.8491 | 19 | 25 | 67 |
| 3 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
100 | 2.5 | 2.7518 | 31 | 15 | 66 |
| 4 | 55 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
100 | 2.5 | 2.7358 | 31 | 15 | 65 |
| 5a | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
100 | 1.5 | 23.24 | 180 | 22 | 68 |
| 6 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
80 | 1.5 | 18.47 | 180 | 17 | 47 |
| 7 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
80 | 1.5 | 4.1259 | 38 | 18 | 50 |
Conclusions
Herein we have demonstrated a new, intensified continuous flow approach for the synthesis of 5-(chloromethyl)furfural (CMF) from liquid and solid sugar feedstocks. Especially the continuous 3-stream process using a sugar syrup such as HFCS 90 shows great potential for scale-up to production. With the optimal conditions for this process of 100 °C, a residence time of 1.5 min and a volumetric phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, it was many orders of magnitude more efficient than published batch processes, resulting in an improvement in space time yield of more than 4500. Complications with blockage of the reactor system due to the formation of solid humic side-product were identified and would need to be addressed in a production setting. We believe that filters and other engineering solutions, commonly used in industry, could be implemented here to manage the humics issue. CMF is an interesting platform molecule that can be synthesised directly from an appropriate sugar feedstock in very short reaction times when using concentrated HCl at elevated temperatures. Excellent process control is imperative for this approach as the harsh conditions would otherwise lead to fast decomposition of the product. We believe that the two phase liquid–liquid process in combination with a continuous flow reactor achieves this much needed control of the reaction environment and presents an immensely intensified alternative to slower, milder, cooler and more dilute batch operations.
1.5, it was many orders of magnitude more efficient than published batch processes, resulting in an improvement in space time yield of more than 4500. Complications with blockage of the reactor system due to the formation of solid humic side-product were identified and would need to be addressed in a production setting. We believe that filters and other engineering solutions, commonly used in industry, could be implemented here to manage the humics issue. CMF is an interesting platform molecule that can be synthesised directly from an appropriate sugar feedstock in very short reaction times when using concentrated HCl at elevated temperatures. Excellent process control is imperative for this approach as the harsh conditions would otherwise lead to fast decomposition of the product. We believe that the two phase liquid–liquid process in combination with a continuous flow reactor achieves this much needed control of the reaction environment and presents an immensely intensified alternative to slower, milder, cooler and more dilute batch operations.
References
- P. Nossin, in Fifth International Conference on Renewable Resources and Biorefineries, 2009 Search PubMed.
- D. L. Greene, J. L. Hopson and J. Li, Energy Policy, 2006, 34, 515–531 CrossRef.
- R. W. Bentley, S. A. Mannan and S. J. Wheeler, Energy Policy, 2007, 35, 6364–6382 CrossRef.
- Intergovernmental Panel on Climate Change, Climate Change 2007 - The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC, Cambridge University Press, 2007 Search PubMed.
- Climate change 2007: mitigation of climate change: contribution of Working Group III to the Fourth assessment report of the Intergovernmental Panel on Climate Change, ed. B. Metz and Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge; New York, 2007 Search PubMed.
- F. Cherubini, Energy Convers. Manage., 2010, 51, 1412–1421 CrossRef CAS.
- A. V. Bridgwater, Chem. Eng. J., 2003, 91, 87–102 CrossRef CAS.
- E. de Jong and G. Jungmeier, in Industrial Biorefineries & White Biotechnology, Elsevier, 2015, pp. 3–33 Search PubMed.
- European Commission, Towards a European knowledge-based bioeconomy. Workshop conclusion on the use of plant biotechnology for the production of industrial bio-based products, York University 2004, https://publications.europa.eu/s/cVwH, (accessed December 2016) Search PubMed.
- D. J. Hayes, S. Fitzpatrick, M. H. B. Hayes and J. R. H. Ross, in Biorefineries-Industrial Processes and Products, ed. B. Kamm, P. R. Gruber and M. Kamm, Wiley-VCH Verlag GmbH, 2005, pp. 139–164 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- G. Dull, Chem.-Ztg., 1895, 19, 216 Search PubMed.
- J. Kiermayer, Chem.-Ztg., 1895, 19, 1003 CAS.
- B. Kamm, M. Kamm, M. Schmidt, T. Hirth and M. Schulze, in Biorefineries-Industrial Processes and Products, ed. B. Kamm, P. R. Gruber and M. Kamm, Wiley-VCH Verlag GmbH, 2005, pp. 97–149 Search PubMed.
- M. Mascal, ChemSusChem, 2015, 8, 3391–3395 CrossRef CAS PubMed.
- M. Mascal and S. Dutta, in Selective Catalysis for Renewable Feedstocks and Chemicals, ed. K. M. Nicholas, Springer International Publishing, 2014, pp. 41–83 Search PubMed.
- M. Brasholz, K. von Känel, C. H. Hornung, S. Saubern and J. Tsanaktsidis, Green Chem., 2011, 13, 1114 RSC.
- W. Ehrfeld, V. Hessel and H. Löwe, Microreactors: new technology for modern chemistry, Wiley-VCH, Weinheim; New York, 1st edn, 2000 Search PubMed.
- Chemical micro process engineering: processing and plants, ed. V. Hessel, Wiley-VCH, Weinheim, 2005 Search PubMed.
- B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688–6728 CrossRef CAS PubMed.
- T. N. Glasnov and C. O. Kappe, Chem. – Eur. J., 2011, 17, 11956–11968 CrossRef CAS PubMed.
- B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300–2318 CrossRef CAS PubMed.
- L. Malet-Sanz and F. Susanne, J. Med. Chem., 2012, 55, 4062–4098 CrossRef CAS PubMed.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS.
- S. K. R. Patil, J. Heltzel and C. R. F. Lund, Energy Fuels, 2012, 26, 5281–5293 CrossRef CAS.
- S. Vuilleumier, Am. J. Clin. Nutr., 1993, 58, 733S–736S CAS.
- A. Zargaraan, L. Kamaliroosta, A. S. Yaghoubi and L. Mirmoghtadaie, Nutr. Food Sci. Res., 2016, 3, 3–11 Search PubMed.
- L. O. Nabors, Alternative sweeteners, CRC Press, Boca Raton, FL, 2012 Search PubMed.
- H. H. Szmant and D. D. Chundury, J. Chem. Technol. Biotechnol., 1981, 31, 205–212 CrossRef CAS.
- J. Jeong, C. A. Antonyraj, S. Shin, S. Kim, B. Kim, K.-Y. Lee and J. K. Cho, J. Ind. Eng. Chem., 2013, 19, 1106–1111 CrossRef CAS.
- B. Kim, C. A. Antonyraj, Y. J. Kim, B. Kim, S. Shin, S. Kim, K.-Y. Lee and J. K. Cho, Ind. Eng. Chem. Res., 2014, 53, 4633–4641 CrossRef CAS.
- International Flow Chemistry Equipment|Vapourtec Ltd, https://www.vapourtec.com/, (accessed December 2016).
- Reactors for both batch and flow chemistry, combined hot and cold plate stirrers, http://www.cambridgereactordesign.com/, (accessed December 2016).
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- B. F. M. Kuster, Starch/Staerke, 1990, 42, 314–321 CrossRef CAS.
- H. Ma, F. Wang, Y. Yu, L. Wang and X. Li, Ind. Eng. Chem. Res., 2015, 54, 2657–2666 CrossRef CAS.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2, 859–861 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and experimental details; analytical methods and spectra. See DOI: 10.1039/c7re00039a |
| This journal is © The Royal Society of Chemistry 2017 |