Open Access Article
Open Access ArticleA colorimetric and far-red fluorescent probe for the highly sensitive detection of silver(I)†
Yong-jun Wang*a,
Jing-gong Liu*b,
Hui-ya Tana,
Jin-wu Yan *a and
Lei Zhang*a
*a and
Lei Zhang*a
aSchool of Biology and Biological Engineering, South China University of Technology, Guangzhou, P. R. China. E-mail: yjw@scut.edu.cn; lzhangce@scut.edu.cn; Tel: +86 20 39380678
bThe Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou, 510006, P. R. China
First published on 7th December 2017
Abstract
Herein, a potential colorimetric and far-red fluorescent probe DCPS-1 for Ag+ was rationally developed based on a dicyanomethylene-4H-chromene chromophore. Its prominent and distinct changes in both color and fluorescence enable the on-site and naked-eye detection of Ag+ easily and quickly, accompanied with excellent sensitivity. No absorption and fluorescence response could be observed towards other metal ions. The fluorescence intensity of this probe showed a linear response to Ag+ in the concentration range of 0–10 μM with a detection limit of 21.1 nM. In addition, this probe had low toxicity to cells and gave distinct fluorescence changes in cell imaging. All these results demonstrate its promising application prospects for Ag+ sensing in both environmental and biology fields.
Introduction
With the development of modern science and technology, heavy and transition metals have been widely applied in many fields such as chemistry, electrical and electronic materials, biology and environmental science.1–5 Gold, platinum, palladium and silver play a very vital role in modern society. Gold, platinum and palladium are precious metals; they are widely used in the chemical industry, materials science, the biomedical field, jewellery, etc. However, these precious metal ions have some negative effects. They can damage the liver, kidneys and cause gene alteration, autoimmune disorders, a variety of cellular dysfunction processes etc.6–10Silver, as a very important precious mental, plays an important role in many fields such as ornaments, glass, fine chemicals, and nano-materials, as well as in monetary manufacturing. However, with the development of modern industry, more and more Ag+ is released which makes water sources polluted and further affects the food and agriculture.11,12 Silver also has some negative biological effects, such as silver ions inactivate sulfhydryl enzymes, bind with various metabolites and organ failure.13–15 Besides, Ag-exposure to the silver environment for a long time can lead to anemia, heart dilation, poor growth, embryotoxicity, and even cirrhosis of the liver.16,17 Blood silver and urine silver excretion are useful indices of human silver exposure, silver ion is not only known to be a cumulative toxin, but also interact with and displace essential metal ions like Ca2+ and Zn2+ in hydroxyapatite in bone.18 Also, silver ion shows a rich biological chemistry, serving as a widely used antibacterial agent, and a transcriptional initiator in plants and mammals.19 Therefore, the need for a highly sensitive and selective detection above ions in vivo is very significant.
Due to the continual concern over silver in the environment and health, several available methods have been used for the detection of Ag+, such as atomic absorption spectroscopy (AAS), inductively coupled plasma/mass spectrometry (ICP-MS), inductively coupled plasma/atomic emission spectrometry (ICP-AES). However, these traditional methods suffer from time and money-consuming, and nonpocketable.20,21 Therefore, optical fluorescent probes, including colorimetric or fluorescent probe for sensitive and selective detection of heavy and transition metal ions have received significant attention in recent years.22–25 Especially the fluorescent probes with far-red to near-infrared (NIR) emission are extremely appropriate for bioimaging because of deep penetration and minimum autofluorescence.26,27 Fluorescent probes have displayed outstanding properties such as simplicity, high sensitivity, and high detection limits compared with the traditional methods. Therefore, the development of fluorescent probe for the detection of Ag+ with high selectivity and sensitivity is urgently desirable. In particular, colorimetric and far-red fluorescent dual probes with favourable sensitivity, selectivity and cost-efficiency are in urgent demand for practical applications.28,29
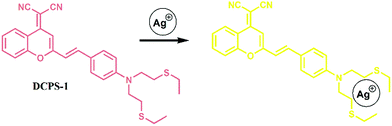
The mechanism of these fluorescent probes for the selective detection of Ag+ is that Ag+ shows a high tendency to coordinate with soft Lewis base such as sulfur containing ligand due to the soft Lewis acid character. As a part of work for Ag+ recognition, we designed a dicyanomethylene-4H-chromene-based fluorescence probe (DCPS-1) for the selective detection of Ag+, this probe would form complex with Ag+, accompanied with color change and turn-off far-red emission (Scheme 1). Some fluorescent probes based on the turn-off mechanism have been reported in recent years.30–34
Results and discussion
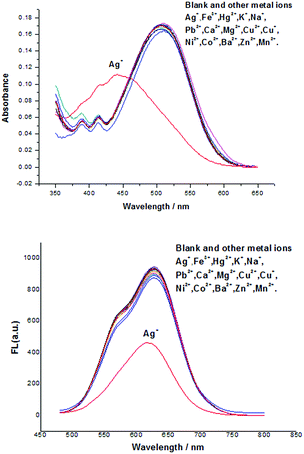
The designed probe DCPS-1 was prepared by the reaction of (2-methyl-4H-1-benzopyran-4-ylidene)-propanedinitrile and 4-[bis[2-(ethylthio)ethyl]amino]-benzaldehyde, and the detailed synthetic procedure is described in Scheme S1 in the ESI.† With probe DCPS-1 in hand, we first performed UV-vis and fluorescence experiments to test the selectivity of probe DCPS-1 (5 μM) for Ag+ (25 μM), while 10 equivalent various other metal ions (Fe3+, Hg2+, K+, Na+, Pb2+, Ca2+, Mg2+, Cu2+, Cu+, Ni2+, Co2+, Ba2+, Zn2+ and Mn2+) in the MeOH solution respectively. As shown in Fig. 1, as shown in Fig. 1A, the absorption band of probe DCPS-1 took place a blue shift from 510 nm to 441 nm when Ag+ was added, while there was no significant difference of absorption band when other mental ions were added and the color of probe DCPS-1 solution changed from red to yellow obviously when Ag+ was added, while the color of probe DCPS-1 solution without any change when other mental ions were added (Fig. 2).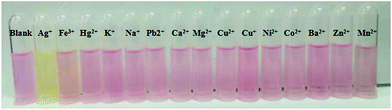 | ||
| Fig. 2 The color changes of DCPS-1 solution (5 μM) when a various mental ions (10 equiv.) were added in MeOH solution at room temperature. | ||
We further assessed the possible interferences by other ions, competitive experiments were conducted, as shown in Fig. S12,† there was no significant variation in fluorescence intensity of DCPS-1–Ag+ solution was observed when other ions were added or not. The above experimental results shown that the probe DCPS-1 have a high selectivity toward to Ag+ than other mental ions, so this probe DCPS-1 is suitable for naked-eye detection of Ag+ with highly selectivity.
Then the time-dependent absorption and fluorescence intensity changes of probe DCPS-1 (5 μM) after adding 5 equivalent of Ag+ were tested. As shown in Fig. 3, the absorption band of probe DCPS-1 disappeared soon at 510 nm after adding Ag+, and a new absorption band appeared at 441 nm, which subdued quickly with incubation time and reached the minimum values after incubation for 30 min at room temperature. In the fluorescence spectra, the emission band of probe DCPS-1 at 628 nm disappeared immediately upon adding Ag+, and a new emission band appeared at approximately 620 nm, over incubation time, the intensity was almost the same, showing that the time for the probe DCPS-1 to form a complex with Ag+ was very short, and this characteristic it is very favorable for us to detect Ag+ qualitatively and quantitatively. It is also noteworthy that the emission of complex reached the far-red to NIR range, which is very attractive for intracellular sensing and imaging.
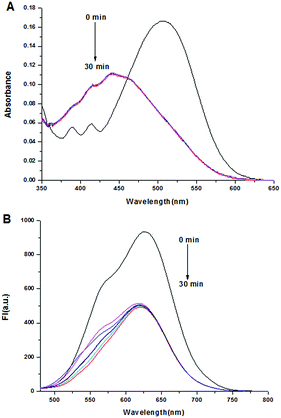 | ||
| Fig. 3 UV-vis (A) and fluorescence spectral (B) of probe DCPS-1 (5 μM) against time in the presence of Ag+ (5 equiv.) in MeOH solution at room temperature, λex = 460 nm. | ||
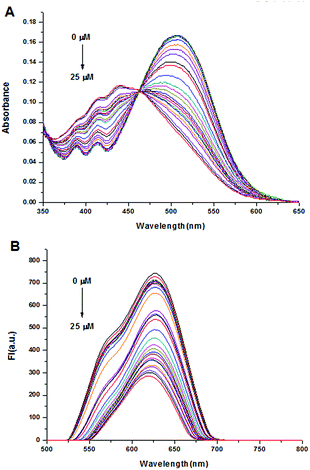
We further performed UV-vis and fluorescence titration experiments to research the sensing ability of probe DCPS-1 to Ag+ (0–5 equiv.). As shown in Fig. 4A, the subdued absorption at 510 nm and enhancement at 441 nm could be observed with the increasing Ag+ concentrations concomitantly. Moreover, the decreased fluorescence intensity at 620–628 nm can be easily found upon addition of increasing amounts of Ag+ (Fig. 4B). As shown in Fig. S9,† the plots of fluorescence intensity versus Ag+ concentration showed linear correlation in the range of 0–10 μM and the detection limit for Ag+ was deduced to as low as 21.2 nM (3σ/k), it is shown that our probe was highly sensitive to Ag+. In order to study the applicability of DCPS-1 in a real sample, DCPS-1 was used in the detection of Ag+ in water samples such as tap water, Pearl River water and waste water, the results shown in Table S1.† In a word, the visual readouts and far-red emission of probe DCPS-1 makes it a powerful tool for sensing in both environment and biology samples.
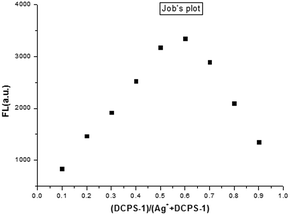
To further research the mechanism of the sensing process, NMR experiments were conducted. As shown in Fig. S8,† In the 1H NMR spectra of probe DCPS-1 and its complex, the chemical shifts of Ha, Hb, Hc and Hd shifted downfield from 2.61, 2.72, 3.61 and 6.77 ppm to 2.76, 2.87, 3.70 and 6.81 ppm upon adding Ag+ cations, respectively. The electronic withdrawing effect of silver ions can made chemical shifts of hydrogen from high to low field. Reversible experiment was carried out, as shown in Fig. S11.† when added chloride ions to eliminate Ag+, the UV-vis and fluorescence spectral of probe DCPS-1 basic recovery, this result confirmed that DCPS-1 bind to Ag+ rather than react with it. In order to research the binding stoichiometry between DCPS-1 and Ag+, a plot of fluorescence intensity versus the molecular fraction of [DCPS-1]/[Ag+ + DCPS-1] was provided in Fig. 5. It assured that the maximum fluorescence was about 0.5 indicating the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between DCPS-1 and Ag+ in the complexes. From the above results, the binding model was proposed for DCPS-1 and Ag+: two S atom and N was coordination with Ag+.35,36 Base on the 1H NMR experiment and HRMS spectrum (Fig. S7†), and the binding model was proposed as illustrated in Scheme 1. We calculated the quantum yields of DCPS-1 and [Ag+ + DCPS-1] (ØDCPS-1 = 0.56, ØDCPS-1+Ag+ = 0.34), this results confirmed that the fluorescence of DCPS-1 quenching occurs when Ag+ was added.
1 stoichiometry between DCPS-1 and Ag+ in the complexes. From the above results, the binding model was proposed for DCPS-1 and Ag+: two S atom and N was coordination with Ag+.35,36 Base on the 1H NMR experiment and HRMS spectrum (Fig. S7†), and the binding model was proposed as illustrated in Scheme 1. We calculated the quantum yields of DCPS-1 and [Ag+ + DCPS-1] (ØDCPS-1 = 0.56, ØDCPS-1+Ag+ = 0.34), this results confirmed that the fluorescence of DCPS-1 quenching occurs when Ag+ was added.
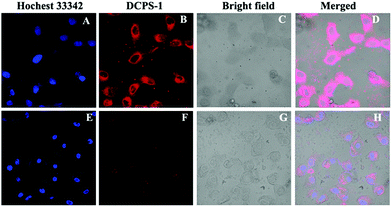
Encouraged by the outstanding properties of this probe for the detection of Ag+ in solution, the potential intracellular applications of probe DCPS-1 were investigated using confocal laser scanning microscopy. The cytotoxicity of probe DCPS-1 to cells was evaluated using a standard MTT assay. The results indicated that probe DCPS-1 exhibited very low cytotoxicity to living HeLa and 293T cells for 48 h (Fig. S10†). Living cell imaging assay was performed using HeLa cells. As shown in Fig. 6A–D, co-staining living HeLa cells with Hochest 33342 revealed that DCPS-1 alone exhibited strong fluorescence emission. While, the fluorescence intensity significantly decreased after incubation with Ag+ for 30 min (Fig. 6E–H). These results demonstrated that DCPS-1 is a useful tool for the detection of Ag+ in both environmental and in living cells.
Conclusions
In summary, a colorimetric and far-red fluorescent probe DCPS-1 for Ag+ was successfully developed. The vivid color change and turn-off emission make it simple and visual indicator for Ag+. Besides, probe DCPS-1 also exhibited high specificity and sensitivity, with the detection limit of 21.1 nM. Moreover, DCPS-1 was applied in the detecting and imaging of Ag+ in living cells successfully, which indicated that probe DCPS-1 is suitable for tracking intracellular Ag+ species. All these characteristics make it a useful tool for Ag+ detection in both environment and biomedical research field.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21502056), the Natural Science Foundation of Guangdong Province, China (2016A030310463, 2015A030310490), and the Fundamental Research Funds for the Central Universities (2017MS079).Notes and references
- W. C. Lee, C.-h. Wang, Y. H. Lin, W. C. Shih and T. G. Ong, Org. Lett., 2013, 15, 5358–5361 CrossRef CAS PubMed.
- D. Sarkar, RSC Adv., 2013, 3, 24389–24399 RSC.
- Y.-h. Huang, R. I. Dass, Z.-l. Xing and J. B. Goodenough, Science, 2006, 312, 254–257 CrossRef CAS PubMed.
- S. Biswas, Z. Huang, Y. Choliy, D.-y. Wang, M. Brookhart, K. K. Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2012, 134, 13276–13295 CrossRef CAS PubMed.
- K.-f. Chen, Y. D. Noh, K.-y. Li, S. Komarneni and D.-f. Xue, J. Phys. Chem. C, 2013, 117, 10770–10779 CAS.
- G. I. Hutchings, M. Brustb and H. Schmidbaur, Chem. Soc. Rev., 2008, 37, 1759 RSC.
- K. M. C. Wong and V. W. W. Yam, Coord. Chem. Rev., 2007, 251, 2477 CrossRef CAS.
- J. H. Schiller and G. Bittner, Clin. Cancer Res., 1999, 5, 4287 CAS.
- H.-y. Tan, J.-g. Liu, L.-f. Zhou, Y.-k. Li, J.-w. Yan and L. Zhang, RSC Adv., 2017, 7, 6583 RSC.
- C. Zhang, J.-f. Guo, A.-m. Ren and D. Wang, RSC Adv., 2017, 7, 49505 RSC.
- J. L. Barriada, A. D. Tappin, E. H. Evans and E. P. Achterberg, TrAC, Trends Anal. Chem., 2007, 26, 809–817 CrossRef CAS.
- M. N. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2011, 45, 6600–6607 CrossRef CAS PubMed.
- S.-s. Huang, S. He, Y. Lu, F.-f. Wei, X.-s. Zeng and L.-c. Zhao, Chem. Commun., 2011, 47, 2408–2410 RSC.
- M. Yamanaka, K. Hara and J. Kudo, Appl. Environ. Microbiol., 2005, 71, 7589 CrossRef CAS PubMed.
- G.-q. Wen, C.-y. Lin, M.-l. Tang, G.-s. Liu, A.-h. Liang and Z.-l. Jiang, RSC Adv., 2013, 3, 1941–1946 RSC.
- Z. Zhen, X.-y. Yang, M.-h. Shen, J.-f. Liu and Y.-g. Yin, J. Environ. Sci., 2015, 35, 62–68 CrossRef PubMed.
- Y. Yang, R. Behra, L. Sigg, P. Fernandez Freire, S. Pillai and K. Schirmer, Nanotoxicology, 2015, 9, 1–10 Search PubMed.
- J.-f. Zhang, Y. Zhou, J. Y. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- C.-b. Huang, X.-j. Peng, Z.-y. Lin, J.-l. Fan, A.-x. Ren and D.-x. Sun, Sens. Actuators, B, 2008, 133, 113–117 CrossRef CAS.
- S. Ma, J. He, M.-z. Guo, X.-h. Sun and M.-d. Zheng, RSC Adv., 2016, 6, 106608–106614 RSC.
- K. Van Meel, A. Smekens, M. Behets, P. Kazandjian and R. Van Grieken, Anal. Chem., 2007, 79, 6383–6389 CrossRef CAS PubMed.
- K. M. K. Swamy, H. N. Kim, J. H. Sob, Y. Kim, S. j. Kim and J. Yoon, Chem. Commun., 2009, 1234–1236 RSC.
- C. Huang, X. Peng, Z. Lin, J. Fan, A. Ren and D. Sun, Sens. Actuators, B, 2008, 133, 113 CrossRef CAS.
- A. Chatteriee, M. Santra, N. Won, S. Kim, J. K. Kim, S. B. Kim and K. H. Ahn, J. Am. Chem. Soc., 2009, 131, 2040 CrossRef PubMed.
- E. U. Akkaya and A. Coskun, J. Am. Chem. Soc., 2005, 127, 10464 CrossRef PubMed.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- T. Zhu, J. Du, W. Cao, J. Fan and X. Peng, Ind. Eng. Chem. Res., 2016, 55, 527 CrossRef CAS.
- G. Dey, P. Gaur, R. Giri and S. Ghosh, Chem. Commun., 2016, 52, 1887 RSC.
- M. Hu, J. Fan, J. Cao, K. Song, H. Zhang, S. Sun and X. Peng, Analyst, 2012, 137, 2107–2111 RSC.
- H.-h. Wang, L. Xue, Y.-y. Qian and H. Jiang, Org. Lett., 2010, 12, 292–295 CrossRef CAS PubMed.
- C. S. Park, J. Y. Lee, E.-j. Kang, J. E. Lee and S. S. Lee, Tetrahedron Lett., 2009, 50, 671–675 CrossRef CAS.
- L. Liu, D. Zhang, G. Zhang, J. Xiang and D. Zhu, Org. Lett., 2008, 10, 2271–2274 CrossRef CAS PubMed.
- R.-h. Yang, W.-h. Chan, A. W. M. Lee, P.-f. Xia and H.-k. Zhang, J. Am. Chem. Soc., 2003, 125, 2884–2885 CrossRef CAS PubMed.
- L.-q. Li and L.-j. Gao, Spectrochim. Acta, Part A, 2016, 152, 426–430 CrossRef CAS PubMed.
- C. Huang, A. Ren, C. Feng and N. Yang, Sens. Actuators, B, 2010, 151, 236–242 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of DCPS-1, experimental procedures, and supplemental spectra and graphs. See DOI: 10.1039/c7ra11933j |
| This journal is © The Royal Society of Chemistry 2017 |