Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ytterbium-mediated synthesis of 2,4-diarylpyrroles from α-bromo oxime ethers†
Xinxin Zhangab,
Songlin Zhenga and
Songlin Zhang *a
*a
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, P. R. China. E-mail: zhangsl@suda.edu.cn; Fax: +86-512-65880352
bDepartment of Chemistry, Xi'an Jiaotong-Liverpool University, 111 Renai Road, SIP Suzhou, 215123, Jiangsu Province, P. R. China
First published on 27th November 2017
Abstract
A novel and efficient ytterbium promoted reductive cyclisation dimerization of α-bromo-oxime ethers affording 2,4-diarylpyrroles with high regioselectivity has been developed. Compared with the reported synthesis methods, the method has the following advantages: readily available and safe starting material, one-pot single step operation and mild and neutral reaction conditions.
Pyrroles are one of the most versatile and important classes of heterocycles as well as building blocks, being present in many natural products,1 synthetic medicinal agents2 and functionalized materials.3 As a consequence, various synthetic methods have been developed for the preparation of pyrroles.4 However, most of the existing methods lead to pyrroles with functional groups at various positions and therefore further synthetic operation is required to afford simple alkyl or aryl substituted pyrroles. Particularly, synthetic approaches to simple 2,4-diaryl substituted pyrroles are even more limited.5–8 Common limitations in the synthesis of 2,4-diaryl substituted pyrroles are: explosive substrates,5 multiple synthetic steps,6 harsh reaction conditions with high pressure7 and limited substrate scope.8 Therefore, the establishment of a simple and convenient synthetic method for preparation of 2,4-diaryl substituted pyrroles continues to be actively pursued.
The rapid development of lanthanides, especially Samarium reagents, in organic synthesis has been recently achieved.9,10c Ytterbium is one of the most important rare earth metals, due to its role as a reducing agent11 and its reactivity towards various electrophilic organic compounds.12 In addition, the reaction of an ytterbium-type Grignard reagent with electrophiles is another important application of ytterbium.13,14
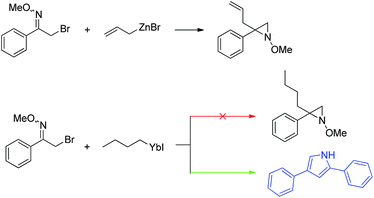
In the course of other studies in progress in this laboratory, we found that Barbier reaction of α-bromo-oxime ethers with butyl bromide promoted by ytterbium afforded unexpected product – 2,4-diarylpyrrole instead of aziridine (Scheme 1).15
Rational analysis of the result reveals that is a reductive cyclisation dimerization reaction of α-bromo-oxime promoted with ytterbium. In view of the importance of this class of compound and the limitation of previous synthesis methods, we believe it is necessary to further investigate and develop this method to afford 2,4-diarylpyrroles. Herein, we report a convenient one-pot protocol for the synthesis of 2,4-diarylpyrroles by the reductive cyclisation dimerization reaction of α-bromo-oxime promoted with ytterbium.
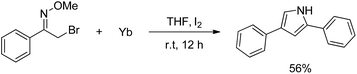
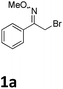
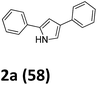
Our exploration of this transformation began with 2-bromo-1-phenylethanone O-methyl oxime (E/Z mixture) 1a (Table 1) as substrate. It was found that when 1a was treated with 1.5 equiv. of Yb, catalytic amount I2 in THF, 2-bromo-1-phenylethanone O-methyl oxime had been completely consumed and a new product had been formed, it was interesting to notice the formation of 2,4-diphenylpyrrole in 58% yield instead of the proposed products shown in Scheme 1.
| Entry | Reductant (yield)b | Entry | Reductant (yield)b |
|---|---|---|---|
| a Unless noted, the reaction was take under a nitrogen atmosphere at room temperature.b Isolated yield.c 12 h α-bromo oxime/Yb = 1/1.d 12 h α-bromo oxime/Yb = 1/1.5.e 12 h α-bromo oxime/Yb = 1/2.f 0 °C, 12 h, α-bromo oxime/Yb (1/1.5).g 65 °C, 12 h, α-bromo oxime/Yb (1/1.5). | |||
| 1 | Yb (51c/58d/48e/56f/37g) | 6 | Nd (48) |
| 2 | 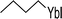 (33) (33) |
7 | Dy (44) |
| 3 | YbI2 (35) | 8 | Mg (28) |
| 4 | SmI2 (38) | 9 | Zn (0) |
| 5 | Sm (54) | 10 | In (0) |
Then other reductants were employed in the controlled experiments to select the suitable reductant. The results in Table 1 shown that 2,4-diphenyl-1H-pyrrole was obtained in 33%, 35% and 38% yields when butyl ytterbium(II) iodide, YbI2 and SmI2 were successively used as the reductants in dry THF at room temperature (Table 1, entries 2–4). Some other metals except of zinc and indium, such as samarium, neodymium, dysprosium and magnesium can also promote the reaction to get product 2a in different yields (Table 1, entry 5–10). As we can see, ytterbium is the most suitable reductant.
In order to investigate the effect of the configuration of substrates on yield, the reaction of (Z)-2-bromo-1-phenylethanone O-methyl oxime 1aa with ytterbium was performed, the yield did not change obviously compared with 1a (Scheme 2).
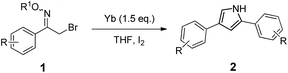
The scope of the above reductive process was further investigated by extending the substrate to other α-halo oxime ethers. As summarized in Table 2, an array of α-halo oxime ethers was suitable for this pyrrole formation process.
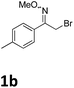
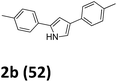
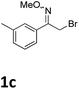
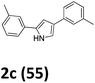
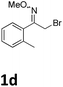
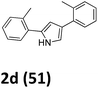
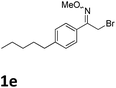
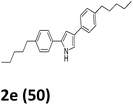
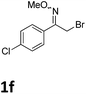
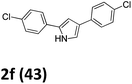
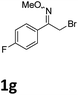
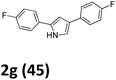
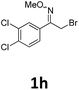
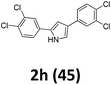
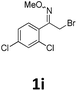
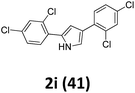
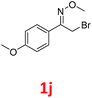
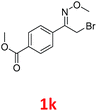
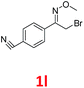
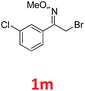
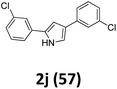
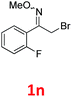
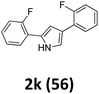
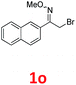
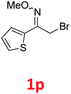
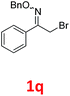
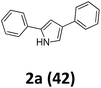
As shown in Table 2, moderate yields were obtained with α-bromo oxime ethers bearing electron-donating groups on the aryl ring, such as p-methyl (1b), m-methyl (1c), o-methyl (1d), p-pentyl (1e) (Table 2, entries 2–5). With α-bromo oxime ethers bearing electron-withdrawing groups on the aryl ring, such as 4-chloro (1f), 4-fluoro (1g), 3,4-dichloro (1h) and 2,4-dichloro (1i), the products were obtained in lower yields than electron-donating groups (Table 2, entries 6–9). However, other functional groups on the aryl ring 1j–l failed to afford the corresponding pyrrole products (Table 2, entries 10–12). Electron-withdrawing groups at meta- and ortho-position on the aryl ring (1m and 1n) were more reactive and provided the products 2,4-diarylpyrroles in higher yields than 1f–i. Fused ring compound such as 2-bromo-1-(naphthalen-2-yl)ethanone O-methyl oxime (1o) in this reaction leads to a complex mixtures and only few amounts of product was detected by LC-MS. Moreover, a heterocyclic substrate such as 2-bromo-1-(thiophen-2-yl) ethanone O-methyl oxime (1p) is unreactive and no corresponding pyrrole was achieved. Then, 2-bromo-1-phenylethanone O-benzyl oxime (1q) was used as the substrate to investigate the reaction, the corresponding 2,4-diphenylpyrrole 2a was afforded in 42% yield under the aforementioned conditions.
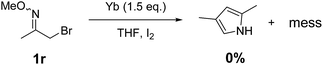
To further determine the reactivity of alkyl α-bromo oxime ethers, 1-bromopropan-2-one O-methyl oxime (1r) as substrate was used under the standard reaction conditions. Also, the reaction leads to a complex mixtures and no expected pyrrole was obtained (Scheme 3).
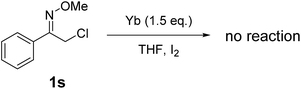
Finally, we investigated the reaction of (Z)-2-chloro-1-phenylethanone O-methyl oxime (1s) with ytterbium to examine the reactivity of α-chloro oxime ethers. Unfortunately, ytterbium cannot promote the reaction to generate 2,4-diphenylpyrrole (Scheme 4).
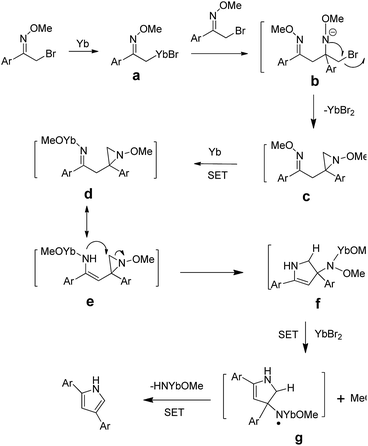
Although the detailed mechanism of the above reaction has not been clarified, a plausible mechanism is proposed in Scheme 5. The cleavage of the C–Br bond could be achieved through two single electron transfer (SET) process, in which Yb would donate two electrons to afford intermediate a. Another α-bromo oxime ether would then react with a through nucleophilic addition to afford b. Then intermediate c could be formed from b through intramolecular substitution. The cleavage of the O–N bond could be achieved through two single electron transfer (SET) process, in which Yb would donate two electrons to afford intermediate d, and whose tautomer e. Then intermediate f could be formed from e through intramolecular substitution. The one single electron transfer (SET) process takes place in which YbBr2 would donate one electron to afford intermediate g. After the intramolecular SET process, intermediate g could afford the final product.
Conclusions
In conclusion, a novel, one-pot method for the preparation of 2,4-diarylpyrroles from α-bromo oxime ethers has been developed, which is mediated by ytterbium. In this reaction, the starting materials are readily available and the reaction conditions are mild and neutral. This work may provide a useful method for the preparation of 2,4-diarylpyrroles. Researches to explore the mechanism of this process and to develop other new uses of rare earth metals are under investigation in our group.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) Pyrroles, Part II, ed. R. A. Jones, Wiley, New York, 1992 Search PubMed; (b) H. Fan, J. N. Peng, M. T. Hamann and J. F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (c) A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef PubMed; (d) A. Grube and M. Kock, Org. Lett., 2006, 8, 4675 CrossRef CAS PubMed; (e) M. Fujita, Y. Nakao, S. Matsunaga, M. Seiki, Y. Itoh, J. Yamashita, R. W. van Soest and N. Fusetani, J. Am. Chem. Soc., 2003, 125, 15700 CrossRef CAS PubMed; (f) D. L. Boger, C. W. Boyce, M. A. Labroli, C. A. Sehon and Q. Jin, J. Am. Chem. Soc., 1999, 121, 54 CrossRef CAS.
- (a) G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238 CrossRef CAS PubMed; (b) A. Andreani, A. Cavalli, M. Granaiola, M. Guardigli, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, M. Recanatini and A. Roda, J. Med. Chem., 2001, 44, 4011 CrossRef CAS PubMed; (c) P. G. Baraldi, M. C. Nunez, M. A. Tabrizi, E. D. Clercq, J. Balzarini, J. Bermejo, F. Estévez and R. Romagnoli, J. Med. Chem., 2004, 47, 2877 CrossRef CAS PubMed; (d) S. K. Srivastava, S. N. Miller, M. D. Aceto, J. R. Traynor, J. W. Lewis and S. M. Husbands, J. Med. Chem., 2004, 47, 6645 CrossRef CAS PubMed.
- (a) P. Novak, K. Muller, K. S. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207 CrossRef CAS PubMed; (b) P. Dydio, D. Lichosytab and J. Jurczak, Chem. Soc. Rev., 2011, 40, 2971 RSC; (c) S. K. Kim and J. L. Sessler, Chem. Soc. Rev., 2010, 39, 3784 RSC.
- (a) T. L. Gilchrist, J. Chem. Soc., Perkin Trans. 1, 1999, 1, 2849 RSC; (b) O. A. Tarasova, N. A. Nedolya, V. Y. Vvedensky, L. Brandsma and B. A. Trofimov, Tetrahedron Lett., 1997, 38, 7241 CrossRef CAS; (c) V. F. Ferreira, M. C. B. de Souza, A. C. Cunha, L. O. Pereira and M. L. Ferreira, Org. Prep. Proced. Int., 2001, 33, 411 CrossRef CAS.
- (a) X. Fan, X. Zhang and Y. Zhang, J. Chem. Res., 2005, 11, 750 CrossRef; (b) X. Fan and Y. Zhang, Tetrahedron Lett., 2002, 43, 1863 CrossRef CAS; (c) F. Chen, T. Shen, Y. Cui and N. Jiao, Org. Lett., 2012, 14, 4926 CrossRef CAS PubMed.
- (a) B. B. Thompson and J. Montgomery, Org. Lett., 2011, 13, 3289 CrossRef CAS PubMed; (b) M. J. Hall, S. O. McDonnell, J. Killoran and D. F. O'Shea, J. Org. Chem., 2005, 70, 5571 CrossRef CAS PubMed; (c) W. Zhao and E. M. Carreira, Chem.–Eur. J., 2006, 12, 7254 CrossRef CAS PubMed.
- (a) S. L. Buchwald, M. W. Wannamaker and B. T. Watson, J. Am. Chem. Soc., 1989, 111, 776 CrossRef CAS; (b) E. M. Campi, W. Jackson and Y. Nilsson, Tetrahedron Lett., 1991, 32, 1093 CrossRef CAS; (c) R. Umeda, T. Mashino and Y. Nishiyama, Tetrahedron, 2014, 70, 4395 CrossRef CAS.
- (a) M. Nitta and T. Kobayashi, Chem. Lett., 1983, 12, 1715 CrossRef; (b) L. W. Deady, Tetrahedron, 1967, 23, 3505 CrossRef; (c) A. Padwa, R. Gruber and D. Pashayan, J. Org. Chem., 1968, 33, 454 CrossRef CAS.
- (a) Y. Fujiwara, K. Takaki and Y. Taniguchi, J. Alloys Compd., 1993, 192, 200 CrossRef CAS; (b) W. Robert, Aldrichimica Acta, 1995, 28, 77 Search PubMed; (c) M. Yu, Y. Zhang and W. Bao, Chin. J. Chem., 1999, 17, 4 CrossRef CAS.
- (a) J. C. Di and S. L. Zhang, Synlett, 2008, 10, 1491 Search PubMed; (b) X. D. Liu, S. L. Zhang and J. C. Di, Synthesis, 2009, 16, 2749 Search PubMed; (c) Y. Y. Hu, T. Zhao and S. L. Zhang, Chem.–Eur. J., 2010, 16, 1697 CrossRef CAS PubMed; (d) Y. Li, Y. Y. Hu and S. L. Zhang, Chem. Commun., 2013, 49, 10635 RSC.
- (a) Z. Hou, Y. Fujiwara and H. Taniguchi, J. Org. Chem., 1988, 53, 3118 CrossRef CAS; (b) Z. Hou, H. Taniguchi and Y. Fujiwara, Chem. Lett., 1987, 16, 305 CrossRef; (c) K. Takaki, Y. Tsubaki, S. Tanaka, F. Beppu and Y. Fujiwara, Chem. Lett., 1990, 203 CrossRef CAS.
- (a) Z. Hou, H. Yamazaki, K. Kobayashi, Y. Fujiwara and H. Taniguchi, J. Chem. Soc., Chem. Commun., 1992, 722 RSC; (b) Z. Hou, H. Yamazaki, Y. Fujiwara and H. Taniguchi, Organomentallics, 1992, 11, 2711 CrossRef CAS; (c) Y. Makioka, S. Y. Uebori, T. M. Suno, Y. Taniguchi, K. Takaki and Y. Fujiwara, Chem. Lett., 1994, 611 CrossRef; (d) Z. Hou, K. Takamine, O. Aoki, H. Shiraishi, Y. Fujiwara and H. Taniguchi, J. Org. Chem., 1988, 53, 6077 CrossRef CAS; (e) Y. Makioka, M. Tsuno, K. Takaki, Y. Taniguchi and Y. Fujiwara, Chem. Lett., 1995, 851 Search PubMed; (f) K. Takaki, S. Tanaka and Y. Fujiwara, Chem. Lett., 1991, 493 CrossRef CAS; (g) Y. Makioka, S. Uebori, M. Tsuno, Y. Taniguchi, K. Takaki and Y. Fujiwara, J. Org. Chem., 1996, 61, 372 CrossRef CAS; (h) K. Takaki, U. Tsubaki, S. Tanaka, F. Beppu and Y. Fujiwara, Chem. Lett., 1990, 203 CrossRef CAS.
- (a) Z. Hou, N. Mine, Y. Fujiwara and H. Taniguchi, J. Chem. Soc., Chem. Commun., 1985, 1700 RSC; (b) T. Fukagawa, Y. Fujiwara, K. Yokoo and H. Taniguchi, Chem. Lett., 1981, 10, 1771 CrossRef; (c) T. Fukagawa, Y. Fujiwara and H. Taniguchi, Chem. Lett., 1982, 11, 601 CrossRef; (d) K. Yokoo, T. Fukagawa, Y. Yamanaka, H. Taniguchi and Y. Fujiwara, J. Org. Chem., 1984, 49, 3237 CrossRef CAS.
- (a) A. Y. Sukhorukov and S. L. Ioffe, Chem. Rev., 2011, 111, 5004 CrossRef CAS PubMed; (b) K. Narasaka and M. Kitamura, Eur. J. Org. Chem., 2005, 4505 CrossRef CAS.
- S. Zheng and S. Zhang, RSC Adv., 2016, 6, 26437 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10909a |
| This journal is © The Royal Society of Chemistry 2017 |