 Open Access Article
Open Access ArticleIn vitro and in vivo characterization of strontium-containing calcium sulfate/poly(amino acid) composite as a novel bioactive graft for bone regeneration
Wu Juna,
Wang Pengb,
Jiang Dianmingc,
Li Hongb,
Luo Conga,
Liu Xinga,
Qu Xiangyanga,
Cao Yujianga and
Li Ming *a
*a
aDepartment of Orthopaedics, Children's Hospital of Chongqing Medical University, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, China International Science and Technology Cooperation base of Child development and Critical Disorders, Chongqing 400014, People's Republic of China. E-mail: liming3180@126.com; Fax: +86 23 63634105; Tel: +86 23 63634105
bSichuan Guona Science and Technology Co., Ltd, Chengdu 610041, People's Republic of China
cCenter of Bone and Trauma, The Third Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
First published on 27th November 2017
Abstract
Herein, strontium (Sr) was successfully doped into strontium-containing calcium sulfate/poly(amino acid) (CS/PAA) composites to develop a novel bioactive bone graft, whose degradability, biocompatibility, and bioactivity were investigated in vitro and in vivo. Compared to CS/PAA composites, Sr-CS/PAA composites showed a slower degradation rate, such that, approximately, 20% less mass was lost at the end of the in vitro degradation tests. Moreover, strontium was gradually released into the degradation media along with the degradation of Sr-CS/PAA composites. In vitro, MC3T3-E1 cells adhered well on the Sr-CS/PAA composites, and the optical density values showed no significant difference between Sr-CS/PAA groups and CS/PAA groups on days 4 and 7. However, the alkaline phosphatase activity of the Sr-CS/PAA groups was significantly higher than that of the CS/PAA groups. The in vivo osteogenesis of Sr-CS/PAA composites was evaluated in rabbit femoral condyle critical bone defects. The results confirmed that Sr-CS/PAA composites exhibited superior capacity of repair for bone defects than CS/PAA composites. Taken together, Sr-CS/PAA composites were demonstrated as promising candidates for bone regeneration, showing appropriate degradation rate, superior biocompatibility and biological activity.
1. Introduction
Repairing large bone defects secondary to severe trauma, infectious focal cleaning, and tumor resection are yet significant clinical challenges.1 Several materials, exhibiting advantages and disadvantages, are used for bone grafting. Autologous bone grafting is a gold standard of bone grafting owing to its excellent histocompatibility. However, its clinical application is not widespread due to (i) limitations of bone availability and (ii) additional morbidity associated with bone harvesting.2,3 Allografts have great availability; however, their use involves the potential risk of immune responses, graft failure, and disease transmission.4 Therefore, a variety of synthetic bone graft substitutes has been explored to repair large bone defects effectively.5Calcium sulfate (CS) has been used as a bone graft material for more than one century.6,7 Although CS is osteoconductive, biocompatible, and nontoxic, it has been confirmed by several studies;8,9 the rapid degradation might not be similar to the new bone tissue formation.10 CS is completely reabsorbed over a period of approximately 5–8 weeks by dissolution.11 However, large bone defects caused by severe trauma, infectious focal cleaning, and tumor resection could not completely repair over such a short duration. Therefore, CS is not recommended as a bone graft separately in these cases.
To overcome this limitation, our group developed a calcium sulfate/poly(amino acids) (CS/PAA) composite material, which not only retained the desirable properties of CS, such as favorable biocompatibility and osteoconductivity but also underwent a slow rate of degradation.12 However, this kind of CS-based material does not have osteoinductivity, which is a crucial characteristic in the process of bone formation. Thus, we hypothesized that a novel CS/PAA composite possessed osteoinductive capacity that might exhibit better repairing performance of the bone defects.
Strontium (Sr) is a trace element in the bone and vital for the bone growth. It can inhibit bone resorption by inhibiting the activity of osteoclasts as well as, promote the proliferation and differentiation of osteoblasts to promote bone formation.13–16 Sr-containing materials have aroused great interest and have been widely studied in the field of bone tissue engineering. The Sr-containing hydroxyapatite was shown to accelerate bone growth and osteointegration in normal and osteoporotic bone significantly.17–19 Sr-substituted bioactive glass exhibited better ability to promote bone forming than the bioactive glass without strontium.20,21 Li et al. prepared a novel resorbable Sr-containing α-CS hemihydrate bone substitute and evaluated the bone repair ability in the bone defects of rat tibia. These results suggested that the bone repair performance of Sr–CaS was superior to CaS alone.22 Based the satisfactory effects achieved by the Sr-containing bone substitute, we further hypothesized that the Sr-containing CS/PAA composites would theoretically enhance the curative effects of bone defects repairing.
In this report, Sr was incorporated with CS/PAA composite to develop a novel bioactive bone graft substitute. The degradability and biocompatibility were characterized in vitro, and the bone repair performance was investigated in vivo.
2. Materials and methods
2.1 Preparation of Sr-CS/PAA composites
The Sr-containing CS/PAA(Sr-CS/PAA) composites were synthesized using melt polycondensation method. Briefly, the aqueous solution of 10 mg mL−1 strontium sulfate (Kelong Chemistry Co., Ltd, China) was mixed with CS dihydrate (Kelong Chemistry) at a solid to the liquid loading of 2 g mL−1. The pastes were mixed and completely hardened at room temperature. The specimens were washed several times with distilled to remove the residual soluble salts. After drying at room temperature for 72 h, the Sr-containing CS was prepared.23 2 g L-lysine, 6 g L-proline, 6 g L-alanine, 7 g L-phenylalanine, 12.5 g gamma-aminobutyric acid, and 108 g 6-aminocaproic acid (Shanghai Experimental Reagents Co., Ltd., Shanghai, China) were added to a 250 mL reaction flask. Subsequently, 50 mL purified water was slowly added and stirred continuously until all the amino acids were solubilized. The reaction flask was heated and maintained at 200 °C until complete evaporation of the water. After the temperature of the system had increased to 220 °C, 187 g of Sr–CS powder was added and mixed in the flask. Subsequently, the system was maintained at 220 °C, followed by 230 °C for 2 h, respectively. The Sr-CS/PAA was obtained as the vapor evaporated from the CS dihydrate. To avoid unexpected oxidation reactions, the whole procedure was continuously protected using nitrogen gas. The composites were shifted into a Teflon mould (5 mm diameter and 1 mm thickness) to ensure a standardized shape of the specimens for in vitro experiments. In addition, after cooling down to room temperature, some composites were shattered into granules with the dimension of 1–3 mm for in vivo experiments. Then, the specimens were sterilized with ethylene oxide prior to evaluation. The CS/PAA composites were synthesized using the same method.2.2 In vitro degradation
The in vitro degradation tests were carried out in the simulated body fluid (SBF, pH 7.4), which was prepared according to the method described by Su et al.24 The ion concentrations of SBF were 142 mmol L−1 of Na+, 5 mmol L−1 of K+, 2.75 mmol L−1 of Ca2+, 1.5 mmol L−1 of Mg2+, 148.3 mmol L−1 of Cl−, 4.2 mmol L−1 of HCO3−, 1.1 mmol L−1 of HPO42−, and 0.5 mmol L−1 of SO42−. The solution was adjusted to pH 7.4 using tris-hydroxymethylaminomethane and hydrochloric acid. The initial mass (M0) of the Sr-CS/PAA specimens was weighed accurately. The specimens were soaked in SBF solution with a solid/liquid mass ratio of 1 g/30 mL in polyethylene tubes that were incubated at 37 °C in a water bath shaking at 70 rpm. Every week, the SBF was replaced with fresh solution. At the predetermined time intervals, the samples were dried and weighed accurately (Mt). The mass loss was calculated as the percent change in the weight as shown in the equation: T = (M0 − Mt)/M0 × 100%. The pH values of the SBF solutions were assayed by a pH meter at room temperature condition. In addition, the mass and pH values of the CS/PAA groups (controls) were detected. The Sr ions in the supernatant of the Sr-CS/PAA groups were measured by inductively coupled plasma-atomic emission spectroscopy. All samples were assayed in quintuplicate.2.3 In vitro biocompatibility
MC3T3-E1 cells (clonal osteoblast-like mouse calvarial cells) were selected for evaluating the biocompatibility of the Sr-CS/PAA and CS/PAA composites. The cells were cultured in 24-well plate containing alpha-modified Eagle's Minimum Essential Medium (α-MEM; HyClone; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone; Thermo Fisher Scientific) and 1% penicillin/streptomycin (Beyotime Institute of Biotechnology, Shanghai, China) at 37 °C in a humidified 5% CO2 atmosphere. The medium was replaced every two days. The Sr-CS/PAA and CS/PAA samples were immersed into the culture medium for 24 h prior to seeding. The cells (5 × 104 cells per mL) were seeded onto the surface of each sample.The adhesion and morphology of the MC3T3-E1 cells on the surface of each sample were detected by scanning electron microscope (SEM, JSM-6500LV, JEOL, Japan) at a voltage of 20 kV. MTT (3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide) assay was used to determine the cell viability of MC3T3-E1 cells. Briefly, after 1, 4 and 7 days of culture, the original medium was replaced by fresh medium, MTT solution was added to each well, and then cell culture was continued for 4 h. Subsequently, the culture medium was removed, and dimethylsulfoxide was added to dissolve the formazan crystals. Finally, the optical density (OD) was detected at 570 nm with a microplate reader. The alkaline phosphatase (ALP) activity was measured using an Alkaline Phosphatase Colorimetric Assay Kit (ImmunoWay Biotechnology Co.; Newark, USA). The absorbance was recorded at 405 nm, following which, the protein content was calculated from a standard curve after normalizing to the total protein content that was determined by the bicinchoninic acid (BCA) method using the Beyotime protein assay kit (Beyotime Institute of Biotechnology). The results were expressed in U mg−1 protein. The assays were conducted in triplicate.
2.4 In vivo evaluation
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Chongqing Medical University and approved by the Animal Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Twenty-seven healthy New Zealand white rabbits of either gender, weighing 2.5–3.5 kg, were enrolled in this study.The animals were anesthetized via intravenous injection (1.0 mL kg−1) using pentobarbital sodium solution (3 wt%). A longitudinal lateral incision was made, and the lateral femoral condyle was exposed layer-by-layer. The bone defects, 6.5 mm diameter and 10 mm depth, were created by an electric drill. Intermittent drilling and continuous saline irrigation minimized the thermal damage of the adjacent bones. After copious physiological saline irrigation, the defects were filled with Sr-CSr/PAA granules, or CS/PAA granules, or left unfilled. The incisions were aligned and sutured precisely. The animals were raised in individual cages under standard conditions and allowed complete activity post-surgery.25
The animals were euthanized by administering an overdose of sodium pentobarbital at 4, 8, and 12 weeks post-surgery. The femoral condyles were excised and immediately immersed in 4% paraformaldehyde. The specimens were observed by micro-computed tomography (CT) scanning (Viva CT40; SCANCO Medical AG, Brüttisellen, Switzerland) at 70 kV and 114 mA. The specimens were reconstructed, and the newly formed bone was analyzed by calculating the trabecular number (Tb.N) and bone volume percent (BV/TV%). The BV/TV% was the percentage of newly formed bone volume in the total bone defect volume.
The femoral condyles were dehydrated by an ethanol gradient and embedded in methyl methacrylate without decalcification. Subsequently, the specimens were sliced and ground to a thickness of 50 μm, followed by staining with Van Gieson stain to observe the bone regeneration microscopically (BX51; Olympus Co., Tokyo, Japan).
2.5 Statistics
Quantitative data were presented as mean ± standard deviation. The statistical analysis was performed using the SPSS 17.0 statistical software package (SPSS Inc, Chicago, IL, USA). One-way analysis of variance test was used to analyze the statistical difference between the different groups. p ≤ 0.05 was considered as statistically significant.3. Results
3.1 In vitro degradation
As shown in Fig. 1A, the CS/PAA composites exhibited a rapid degradation during the first week with 22.34 ± 0.95% mass loss. Subsequently, the degradation rate was decelerated gradually. After 12 weeks, the mass loss of Sr-CS/PAA composites and CS/PAA composites was 31.30 ± 1.17% and 50.72 ± 1.46%, respectively (p < 0.05). The pH values of the SBF solution in the CS/PAA groups dropped sharply from 7.4 to 6.37 ± 0.08 at the first week, and then gradually rose to neutrality at the fourth week. However, the pH values declined from 7.4 to 6.73 ± 0.07 at the first week, followed by a rapid increase at 7.0 at the second week in the Sr-CS/PAA groups (Fig. 1B). Significant differences were observed between the pH values of CS/PAA and Sr-CS/PAA groups during the first four weeks (p < 0.05). The cumulative Sr ions released from Sr-CS/PAA composites were detected and shown in Fig. 2. A large volume of Sr ions was released from the Sr-CS/PAA composites during the first week, followed by a gradual increase. The cumulative concentration of Sr ions was 121.9 ± 4.3 μmol L−1 at the end of 12 weeks. However, there were no Sr ions in the CS/PAA groups.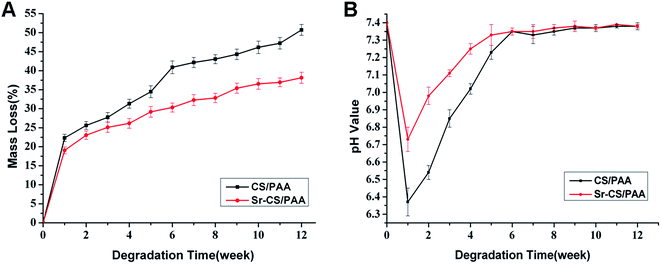 | ||
| Fig. 1 In vitro degradation of Sr-CS/PAA and CS/PAA composites. (A) Mass loss; (B) changes in pH values. | ||
3.2 In vitro biocompatibility
The in vitro biocompatibility of Sr-CS/PAA composites was investigated by culturing the composites with MC3T3-E1 cells. The MC3T3-E1 cells adhered tightly (with pseudopodia) on the surfaces of the Sr-CS/PAA composites (Fig. 3A), showing excellent cell viability on the composite surfaces.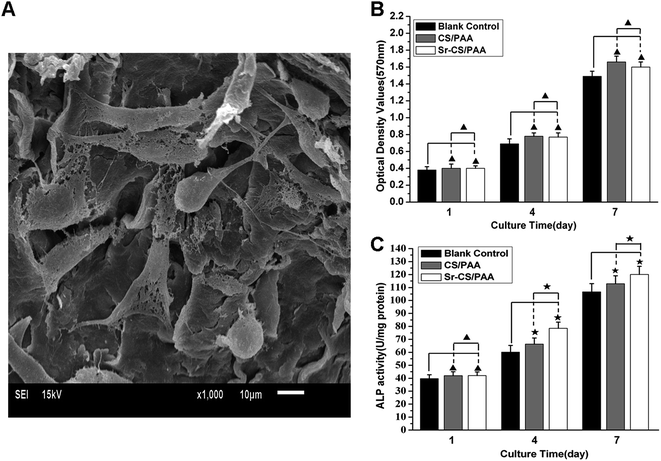 | ||
| Fig. 3 MC3T3-E1 cells cultured with Sr-CS/PAA composites. (A) SEM images; (B) MTT assays and (C) ALP activity (▲, p > 0.05; ★, p < 0.05). | ||
The results of MTT assays were shown in Fig. 3B. The OD values increased steadily in both CS/PAA and Sr-CS/PAA groups (p < 0.05), indicating that both materials did not exert negative effects on the proliferation of MC3T3-E1 cells. The ODs showed no significant difference between Sr-CS/PAA and CS/PAA groups on days 4 and 7 (p > 0.05).
The ALP activity during the 7 day culture with CS/PAA or Sr-CS/PAA was presented in Fig. 3C. The ALP activity was significantly increased by prolonging the duration of the culture (p < 0.05). Moreover, higher ALP activity was obtained in Sr-CS/PAA groups than the CS/PAA groups (p < 0.05).
3.3 In vivo evaluation
The osteogenesis of Sr-CS/PAA composites was evaluated in vivo. Two rabbits succumbed to mortality due to diarrhea at 26 days after surgery and were replaced by two new rabbits. All the incisions healed without dehiscence and infectious signs such as swelling, febrility, and effusions. Neither abscess nor inflammation was not observed in the bone or muscle tissues around the implantation sites.The micro CT images were shown in Fig. 4. The defect was evident in the untreated group at the end of the experiment. In the CS/PAA and Sr-CS/PAA groups, the defects were completely repaired at 12 weeks post-surgery. Moreover, the trabecular density as determined by visual inspection was higher in the Sr-CS/PAA than the CS/PAA groups. In order to measure the amount and quantity of new bone formation, the Tb.N and BV/TV% in each group were determined by micro CT analysis (Fig. 4C and D). At 12 weeks post-implantation, the Tb.N values were 0.33 ± 0.04 in the untreated groups, 0.93 ± 0.05 in the CS/PAA groups, and 1.25 ± 0.07 in the Sr-CS/PAA groups. The BV/TV% values of the untreated, CS/PAA, and Sr-CS/PAA groups were 8.1 ± 0.7, 23.5 ± 0.9, and 30.3 ± 1.4%, respectively. The Tb.N and BV/TV% in the Sr-CS/PAA and CS/PAA groups were significantly higher than the untreated group (p < 0.05). Moreover, the Sr-CS/PAA groups revealed significantly higher values of Tb.N and BV/TV% at 12 weeks after implantation as compared to the CS/PAA groups (p < 0.05).
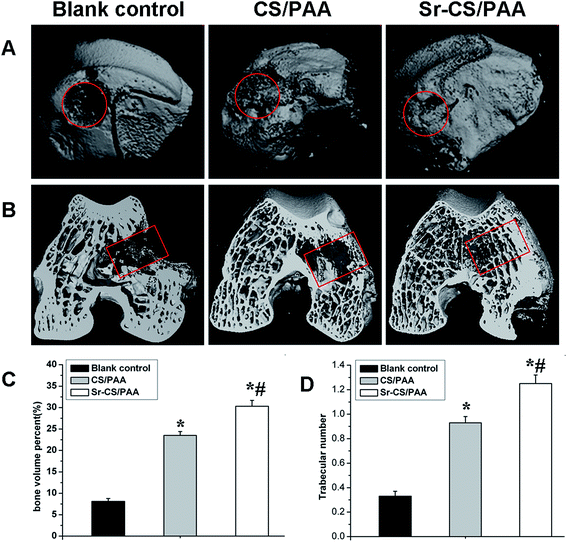 | ||
| Fig. 4 Micro CT images of femoral condyle defects at 12 weeks after the operation. (A) The gross images; (B) the cross-section images; (C) BV/TV% and (D) Tb.N. (*p < 0.05; #p < 0.05). | ||
The in vivo osteogenesis of Sr-CS/PAA composites was further detected by Van Gieson staining (Fig. 5). The unfilled bone defects showed the limited presence of the newly formed bone at the twelfth week post-surgery. The newly formed osseous tissues were not formed into bone trabeculas to repair the bone defects (Fig. 5A). However, in both CS/PAA (Fig. 5B) and Sr-CS/PAA groups (Fig. 5C), a large amount of mature and regularly arranged new bone trabeculas were formed that were connected to the host bone. Moreover, the number and density of trabecular bone in the Sr-CS/PAA groups were markedly high, and the defects were almost repaired.
4. Discussion
In the area of bone tissue engineering, CS grafts have been proved to show adequate biocompatibility and osteoconductivity with the drawback of rapid degradation.6–10 To overcome this shortage, our research group has previously developed a PAA copolymer, which is a partial analogy to the polypeptide architecture of natural collagen protein in the normal bone.26,27 The copolymer is combined with CS for the development of a CS/PAA composite material. The CS/PAA composite retains the desirable properties of CS, such as favorable biocompatibility and osteoconductivity.12 However, CS/PAA composite does not have osteoinductivity, which is a critical factor in the process of bone defects healing. Sr exerts a certain osteoinductive effect by inhibiting the activity of osteoclast and promoting the proliferation and differentiation of osteoblasts. Thus, in this study, the bone defects healing is accelerated by Sr doping into the CS/PAA composite to develop a novel composite, and its in vitro and in vivo characters are investigated in this study.The degradability of a bone graft plays a major role in bone repair.11,28 As an ideal bone graft, the degradation rate is preferably matched with that of osteogenesis such that the bone replacement occurred after the absorption of the implants.29 Rapid degradation not only leads to a destruction of mechanically efficient support structure but also alters the bone defect micro-environment. Since the pH values of the degradation media after soaking the PAA composites were maintained at approximately 7.4, the changes are induced by the degradation of inorganic substances.6 The calcium and sulfate ions are released into the degradation media, and the calcium ions trapped the hydroxyl ions in water molecules, leading to the formation of hydrogen ions. The increased hydrogen ions concentration reduces the pH value of the degradation media.30 Previous studies have revealed that the stable pH environment was vital for the metabolic activity, proliferation, and differentiation of osteoblasts.31 After 12 week soaking in the SBF solution, the mass loss of Sr-CS/PAA was 31.30 ± 1.17%, which was significantly lower than that of CS/PAA 50.72 ± 1.46% (p < 0.05). The incorporation of Sr into CS/PAA composites reduced the degradation rate of the composite. The media soaking the Sr-CS/PAA composites exhibited a rather stable pH environment. In the in vivo study, the Sr-CS/PAA granules were packed into the defects; however, still, some amount of porosity was retained to facilitate the tissue ingrowth. Next, the voids formed by the degradation of granules were gradually filled with the regenerated osseous tissues. A small amount of residual Sr-CS/PAA granules were observed in the defects at the twelfth week when the bone defect was almost entirely repaired, suggesting that the degradation rate was approximately equivalent to that of the osteogenesis. These results suggested that the Sr-containing CS/PAA composites had been improved in the degradability for a perfect bone graft.
The biocompatibility and biological properties are crucial factors in bone tissue regeneration.32 Thus, the biocompatibility of the Sr-CS/PAA composites is investigated by cell morphologies and proliferation of MC3T3-E1 cells. In the present study, the SEM analysis determined that the MC3T3-E1 cells spread well and adhere tightly to the surface of all the Sr-CS/PAA composites, indicating that the composites do not induce any cytotoxicity. Moreover, the OD values of the Sr-CS/PAA groups are significantly higher than that of the CS/PAA groups on days 4 and 7. According to Zhang et al., the enhanced effects of Sr-containing scaffolds on the proliferation and differentiation of MC3T3-E1 cells are caused by Sr release and stable pH environment.21 The Sr ions are released from the Sr-CS/PAA composites into the media as shown in Fig. 2. Since strontium can promote the proliferation and differentiation of osteoblasts for bone formation,33,34 its incorporation into CS/PAA matrix can improve the biological properties of the composite material and exert positive effects on cell proliferation and viability. The stimulatory effect on the proliferation and cell viability of osteoblasts are demonstrated by the ALP activity in MC3T3-E1 cells. ALP is an early marker of differentiation of osteoblasts, and it participates in the process of mineralization.35 In addition, the ALP activity in the Sr-CS/PAA composite group is significantly higher than that in the CS/PAA group or blank plates at days 4 and 7 of culture, indicating a promotion of osteogenesis by the Sr-CS/PAA composite. The results are consistent with that of the previous studies.21,36
A critical size defect is defined as the smallest tissue defect that would not completely heal during the lifetime of an animal.37,38 According to Hollinger et al., a 6 mm bone defect is regarded as the critical size defect in rabbits.39 However, Zhao et al. constructed a drilling hole in the femoral condyle, with 6.5 mm diameter and 6 mm depth, and the defect implanted without anything is naturally healed after 12 weeks. The results suggested that the bone defect was not a critical parameter.12 Qi et al. found a cavitary defect in the femoral condyle with the same diameter, while 0.4 mm excess depth would not completely heal during the lifetime of an animal.25 These results were in agreement with our study. Therefore, we used the cavitary defect of 6.5 mm diameter and 10 mm depth as the critical size defect in rabbit femoral condyle, which is used as the animal model for the in vivo evaluation of Sr-CS/PAA composites in this study.
The biocompatibility and biological activity of the Sr-CS/PAA composites are further verified in the animal bone defect model, which is crucial in evaluating a biomaterial. All the Sr-CS/PAA-treated femoral condyle and surrounding muscle tissues neither show a significant foreign body reaction nor chronic inflammation, indicating excellent biocompatibility of the composite in vivo. Furthermore, quantitative analysis of the CT images suggests that the number and density of trabecular bone in the Sr-CS/PAA composites-treated groups are markedly higher than that in the CS/PAA-treated groups (Fig. 4C and D). The capacity of repair of the bone defects of Sr-CS/PAA composites is superior to the CS/PAA composites, which is caused by the incorporation of Sr into CS/PAA matrix. Taken together, the in vitro and in vivo tests results exhibits excellent biocompatibility and biological activity of the Sr-CS/PAA composites.
5. Conclusions
The Sr was successfully doped in the CS/PAA composites to develop a novel bioactive bone graft substitute. The in vitro degradation studies suggested that the degradation rate of Sr-CS/PAA composites is slow. The released Sr stimulated the proliferation and differentiation of MC3T3-E1 cells. Furthermore, the in vivo evaluation confirmed that the Sr-CS/PAA composites have excellent biocompatibility; their degradation rate matched with that of osteogenesis and exhibited a better capacity for the repair of critical bone defects than the CS/PAA composites. These findings indicated that the Sr-CS/PAA composite is a promising candidate for bone regeneration as a bone graft substitute, displaying an appropriate degradation rate, superior biocompatibility and biological activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The materials used in this study were provided by the Sichuan Guona Science and Technology Co., Ltd, China. The authors thank Zhao Zenghui, Qi Xiaotong, Qiao Bo, Wen Congyou, and Shen Honglin for the technical assistance.References
- P. Bernstein, M. Bornhäuser, K. P. Günther and M. Stiehler, Orthopä, 2009, 38, 1029–1037 CrossRef CAS PubMed.
- H. C. Pape, A. Evans and P. Kobbe, J. Orthop. Trauma, 2010, 24, S36–S40 CrossRef PubMed.
- G. M. Calori, E. Mazza, M. Colombo and C. Ripamonti, Injury, 2011, 42, S56–S63 CrossRef PubMed.
- D. L. Wheeler and W. F. Enneking, Clin. Orthop. Relat. Res., 2005, 435, 36–42 CrossRef.
- A. J. Salinas and M. Vallet-Regí, RSC Adv., 2013, 3, 11116–11131 RSC.
- A. S. Coetzee, Arch. Otolaryngol., 1980, 106, 405–409 CrossRef CAS PubMed.
- Y. He, J. Gao, X. Li, Z. Ma, Y. Zhang, M. Li, Y. Zhang, X. Wang, H. Qiu and Y. Liu, J. Biomater. Sci., Polym. Ed., 2010, 21, 1313–1330 CrossRef CAS PubMed.
- C. M. Kelly, R. M. Wilkins, S. Gitelis, C. Hartjen, J. T. Watson and P. T. Kim, Clin. Orthop. Relat. Res., 2001, 382, 42–50 CrossRef.
- S. Gitelis, P. Piasecki, T. Turner, W. Haggard, J. Charters and R. Urban, Orthopedics, 2001, 24, 162–166 CAS.
- K. N. Lewis, M. V. Thomas and D. A. Puleo, J. Mater. Sci.: Mater. Med., 2006, 17, 531–537 CrossRef CAS PubMed.
- M. V. Thomas and D. A. Puleo, J. Biomed. Mater. Res., Part B, 2009, 88, 597–610 CrossRef PubMed.
- Z. H. Zhao, Z. X. Quan, D. M. Jiang, H. Li, L. Guo and Y. G. Yan, J. Mater. Sci., 2013, 48, 2022–2029 CrossRef CAS.
- P. J. Marie, P. Ammann, G. Boivin and C. Rey, Calcif. Tissue Int., 2011, 69, 121–129 CrossRef.
- Z. Gu, H. Xie, C. Huang, H. Peng, H. Tan, L. Li and X. Yu, RSC Adv., 2014, 4, 2783–2792 RSC.
- S. Peng, X. S. Liu, G. Zhou, Z. Li, K. D. Luk, X. E. Guo and W. W. Lu, J. Bone Miner. Res., 2011, 26, 1272–1282 CrossRef CAS PubMed.
- Y. Li, J. Li, S. Zhu, E. Luo, G. Feng, Q. Chen and J. Hu, Biochem. Biophys. Res. Commun., 2012, 418, 725–730 CrossRef CAS PubMed.
- W. Zhang, Y. Shen, H. Pan, K. Lin, X. Liu, B. W. Darvell, W. W. Lu, J. Chang, L. Deng, D. Wang and W. Huang, Acta Biomater., 2011, 7, 800–808 CrossRef CAS PubMed.
- J. Yan, J. F. Sun, P. K. Chu, Y. Han and Y. M. Zhang, J. Biomed. Mater. Res., Part A, 2013, 101, 2465–2480 CrossRef PubMed.
- D. Guo, K. Xu, X. Zhao and Y. Han, Biomaterials, 2005, 26, 4073–4083 CrossRef CAS PubMed.
- E. Gentleman, Y. C. Fredholm, G. Jell, N. Lotfibakhshaiesh, M. D. O'Donnell, R. G. Hill and M. M. Stevens, Biomaterials, 2010, 31, 3949–3956 CrossRef CAS PubMed.
- J. H. Zhang, S. C. Zhao, Y. F. Zhu, Y. J. Huang, M. Zhu, C. L. Tao and C. Q. Zhang, Acta Biomater., 2014, 10, 2269–2281 CrossRef CAS PubMed.
- X. Li, C. P. Xu, Y. L. Hou, J. Q. Song, Z. Cui, S. N. Wang, L. Huang, C. R. Zhou and B. Yu, Biomed. Mater., 2014, 9, 045010 CrossRef PubMed.
- J. Wang, L. Zhang, X. Sun, X. Chen, K. Xie, M. Lin, G. Yang, S. Xu, W. Xia and Z. Gou, Biomed. Mater., 2014, 9, 045002 CrossRef PubMed.
- B. Su, X. H. Peng, D. M. Jiang, J. Wu, B. Qiao, W. C. Li and X. T. Qi, PLoS One, 2013, 8, e68342 CAS.
- X. T. Qi, H. Li, B. Qiao, W. C. Li, X. Y. Hao, J. Wu, B. Su and D. M. Jiang, Int. J. Nanomed., 2013, 8, 4441–4452 CrossRef PubMed.
- H. Li, Y. Yan, J. Wei, J. Ma, M. Gong, X. Luo and Y. Zhang, J. Mater. Sci.: Mater. Med., 2011, 22, 2555–2563 CrossRef CAS PubMed.
- H. Li, S. C. Tao, Y. G. Yan, G. Y. Lv, Y. F. Gu, X. M. Luo, L. L. Yang and J. Wei, J. Biomater. Sci., Polym. Ed., 2014, 25, 1194–1210 CrossRef CAS PubMed.
- C. G. Ambrose and T. O. Clanton, Ann. Biomed. Eng., 2004, 32, 171–177 CrossRef PubMed.
- I. Vroman, M. Dottori, E. Fortunati, S. Mattioli and J. M. Kenny, Polym. Degrad. Stab., 2010, 95, 2126–2146 CrossRef.
- J. T. Nan, H. Li, G. Y. Lv, Y. L. Liu, Y. F. Zhang and Y. G. Yan, J. Funct. Mater., 2010, 6, 1057–1060 Search PubMed.
- K. Qiu, X. J. Zhao, C. X. Wan, C. S. Zhao and Y. W. Chen, Biomaterials, 2006, 27, 1277–1286 CrossRef CAS PubMed.
- R. A. Péreza, J. E. Won, J. C. Knowles and H. W. Kim, Adv. Drug Delivery Rev., 2013, 65, 471–496 CrossRef PubMed.
- P. Yin, F. F. Feng, T. Lei, X. H. Zhong and X. C. Jian, J. Biomed. Mater. Res., Part A, 2014, 102, 621–627 CrossRef PubMed.
- Z. Gu, H. Wang, L. Li, Q. Wang and X. Yu, Biomed. Mater., 2012, 7, 065007 CrossRef PubMed.
- Y. Wang, S. Zhang, X. Zeng, L. L. Ma, W. Weng, W. Yan and M. Qian, Acta Biomater., 2007, 3, 191–197 CrossRef CAS PubMed.
- J. C. Wang, L. Zhang, X. L. Sun, X. Y. Chen, K. L. Xie, M. Lin, G. J. Yang, S. Z. Xu, W. Xia and Z. R. Gou, Biomed. Mater., 2014, 9, 045002 CrossRef PubMed.
- M. Azami, S. Tavakol, A. Samadikuchaksaraei, M. S. Hashjin, N. Baheiraei, M. Kamali and M. R. Nourani, J. Biomater. Sci., Polym. Ed., 2012, 23, 2353–2368 CAS.
- K. Khanna, A. Jaiswal, R. V. Dhumal, N. Selkar, P. Chaudhari, V. P. Soni, G. R. Vanage and J. Bellare, RSC Adv., 2017, 7, 37522–37533 RSC.
- J. O. Hollinger and J. C. Kleinschmidt, J. Craniofac. Surg., 1990, 1, 60–68 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |


