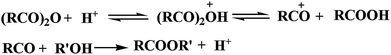Open Access Article
Open Access ArticleEsterification mechanism of lignin with different catalysts based on lignin model compounds by mechanical activation-assisted solid-phase synthesis†
Xiaohong Zhaoab,
Yanjuan Zhang*a,
Liping Weia,
Huayu Hua,
Zuqiang Huang *a,
Mei Yanga,
Aimin Huanga,
Juan Wua and
Zhenfei Fenga
*a,
Mei Yanga,
Aimin Huanga,
Juan Wua and
Zhenfei Fenga
aSchool of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China. E-mail: zhangyj@gxu.edu.cn; huangzq@gxu.edu.cn
bCollege of Materials and Environmental Engineering, Hezhou University, Hezhou 542899, China
First published on 10th November 2017
Abstract
In order to learn about the esterification mechanism of lignin by mechanical activation-assisted solid-phase synthesis (MASPS) technology, lignin model compounds, p-hydroxy benzaldehyde (H), vanillin and vanillyl alcohol (G), and syringaldehyde (S), were used in the reaction with acetic anhydride, with 4-dimethyl amino pyridine (DMAP), sodium acetate, and sulfuric acid as catalysts. FTIR, NMR, and UV/vis analyses of the products showed that all of the catalysts could enhance the esterification. Both the phenolic hydroxyl and aliphatic hydroxyl participated in the esterification and the reactivity of the basic structural units of lignin had a descending order of H, G, and S. Oxidations could happen in the presence of unsaturated groups such as aldehyde in the lignin model compounds. The catalytic mechanism of the three kinds of catalyst was different, and the catalytic activity had a descending order of DMAP, sodium acetate, and sulfuric acid. The reactivity of phenolic hydroxyl was higher than that of aliphatic hydroxyl with DAMP as the catalyst, but the reactivity of aliphatic hydroxyl was higher than that of phenolic hydroxyl with sodium acetate or sulfuric acid as the catalyst. With sulfuric acid as the catalyst, some side reactions took place and resulted in the ring cleavage or cross-linking of the benzene ring. Consistency verification indicated that the use of lignin model compounds for studying the esterification mechanism of lignin was reasonable and feasible.
1. Introduction
Lignocellulosic biomass, as a sustainable alternative for energy, fuel and chemical production, attracts a lot of attention.1,2 Lignin is one of the three main components of lignocellulosic biomass.3 It is estimated that more than 150 billion tons of lignin are produced annually by plant growth worldwide.4 More than 60 billion tons of industrial lignin was obtained as a byproduct of pulp in paper-making and biofuel industries, but only approximately 2% was used for producing lignin-based materials due to the deficiency in properties of lignin, including brittleness, low reactivity, poor compatibility with polymers, etc., limiting its application scope.5 The remainder mainly has been burned as an energy source or discarded as waste, leading to the waste of resources and growing environmental problems.Chemical modification of lignin can efficiently improve its performances and expand its applications. More superior lignin-based products can be gotten from modified lignin such as aminated lignin, hydroxymethylated lignin, methyl methacrylate (MMA) grafted lignin, and urea-formaldehyde modified lignin.6–8 Furthermore, more excellent characteristic(s) of modified lignin endow its more applications. For example, modified lignin can be used as a curing agent or flexibilizer of epoxy resins.9
Esterification of lignin is commonly used for three objectives: the analysis of hydroxyl content, the modification of lignin, and the graft of lignin with other polymer.10–12 As a method of modification, it can improve the photothermal stability, water resistance, oxidation resistance, compatibility with non-polar polymers, and dissolution in non-polar solvents of lignin.13–15 The esterification is usually carried out in liquid phase with acid, acid anhydride, or acyl chloride as esterifying agent, pyridine, 4-dimethyl amine pyridine (DMAP), or 1-methyl imidazole as catalyst, and acid anhydride, pyridine, THF, 1,4-dioxane, or N-methyl pyrrolidone as solvent.16,17 The reaction usually needs a long time and the recovery of modified lignin is time-consuming and tedious. So, some new preparation methods such as supercritical carbon dioxide as solvent, microwave, and reactive extrusion have been paid close attention.18–20
In order to strengthen the activity of lignin and avoid the solvent pollution in liquid synthesis and tedious collection of the product, an environmentally friendly and effective mechanical activation-assisted solid-phase synthesis (MASPS) technology was adopted to prepare acetylated lignin in our previous work,21 and the esterification mechanism of lignin was discussed. However, it is difficult to get comprehensive information of the mechanism due to its complicated structure, and more feasible ways are required to further investigate the esterification mechanism of lignin.
Lignin is composed of three phenyl propane units of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), which connected with C–O–C and C–C.22 Lignin model compounds, such as the monomers of these three phenyl propane units or their dimer, trimer, tetramer and hexamer, are usually used to study the correlated theory of lignin for their definite structure.23 For example, lignin model compounds have been used to study the pyrolysis chemistry, bond dissociation enthalpies, laccase promoted oxidation, peroxidative oxidation, aerobic oxidation, and reductive degradation of lignin.24–27 The degree of reaction and related chemical metrology data are easily monitored and the products are simple with the use of lignin model compounds. The physical and chemical properties of lignin can be speculated based on those of its model compounds, leading to further promote the research on application performances of lignin and broaden the application fields.
In this paper, four lignin model compounds, p-hydroxy benzaldehyde (H), vanillin and vanillyl alcohol (G), and syringaldehyde (S) (their structures are shown in Fig. S1†), were acetylated by MASPS with DMAP, sodium acetate, and sulfuric acid as catalysts. The resulting samples were analyzed by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) and Ultraviolet/visible (UV/vis) spectrometer. The reactivity of basic structure units (H, G, and S) was discussed by comparing the three kinds of aldehyde, and the reactivity of hydroxyl groups was discussed by comparing two representative compounds of G type. For systematic investigation on the acetylation of typical lignin model compounds, the comprehensive information of esterification mechanism of lignin by MASPS could be obtained to give more theoretical support for chemical modification of lignin.
2. Experimental
2.1. Materials
The p-hydroxy benzaldehyde (98%), vanillin (≥99%), vanillyl alcohol (98%), and syringaldehyde (98%) were purchased from Aladdin Industrial Corporation. Enzymatic hydrolysis lignin (95.92% of lignin (10.60% of acid soluble and 85.32% of klason lignin), 1.05% of ash, and 3.02% of polysaccharide, with pH of 7) and alkali lignin (90.45% of lignin (13.43% of acid soluble and 77.02% of klason lignin), 8.085% of ash, and 8.11% of polysaccharide, with pH of 7) were kindly supplied by Ji'nan Yang Hai Chemical Co., Ltd. (China). All other chemical reagents were of analytical grade (DMAP >99.5%, H2SO4 >98%, CH3COONa ≥ 99%) and were obtained commercially without further purification.2.2. Esterification of lignin model compounds
The esterification of lignin model compounds by MASPS was carried out in the same customized stirring ball mill (reactor) as the esterification of lignin.28 In order to learn about the catalytic action of DMAP, two feeding ways were used. In one way, the reactants and DMAP were added in the reactor at the same time. In the other way, one reactant and DMAP were pre-reacted for 5 min and then the other reactant was added to the reactor. When other two catalysts (sodium acetate and sulfuric acid) were used, the reactants and catalyst were simultaneously added to the reactor. A fixed amount of milling balls (300 mL, the ratio of milling balls to material was 6 mL g−1) was first added to a jacketed stainless steel tank (1200 mL), and then a mixture of lignin model compound, acetic anhydride (n(acetic anhydride)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(lignin model compounds) = 3), and catalyst (2 wt% of the reactants) was added to the tank and subjected to milling at the speed of 300 rpm with the temperature of 80 °C for 1.5 h. The crude products were refined by dissolving in alcohol and precipitated with water. Specifically, the milling balls were washed with 50 mL anhydrous ethanol, then 50 mL water was added into the wash solution and left to stand until the product were precipitated out. The suspension was filtered by vacuum filtration and the filter cake was washed with deionized water until the filtrate was neutral. In order to avoid being oxidized, the filter cake was vacuum dried at 35 °C for 24 h, and then the product was sealed with a sealing bag and stored in a silica-gel desiccator.
n(lignin model compounds) = 3), and catalyst (2 wt% of the reactants) was added to the tank and subjected to milling at the speed of 300 rpm with the temperature of 80 °C for 1.5 h. The crude products were refined by dissolving in alcohol and precipitated with water. Specifically, the milling balls were washed with 50 mL anhydrous ethanol, then 50 mL water was added into the wash solution and left to stand until the product were precipitated out. The suspension was filtered by vacuum filtration and the filter cake was washed with deionized water until the filtrate was neutral. In order to avoid being oxidized, the filter cake was vacuum dried at 35 °C for 24 h, and then the product was sealed with a sealing bag and stored in a silica-gel desiccator.
2.3. Characterization
Chemical structure and quantitative analysis of the esterified samples were carried out by FTIR, NMR and UV/vis spectrometer. The operating conditions of these analyses were same as the esterified lignin and provided in ESI.†13,283. Results and discussion
There are a lot of catalysts for biomass conversion, including metal catalysts, base catalysts, acid catalysts, and so on.1,2,29,30 Either acid catalyst or alkaline catalyst is effective for the esterification of lignin.31 In order to further learn about the esterification mechanism of lignin by MASPS, the esterification of lignin model compounds were systematically studied with DMAP (usual base) and sodium acetate (salt of strong alkali weak acid) as representatives of alkaline catalyst and sulfuric acid as a representative of acidic catalyst.3.1. FTIR analysis
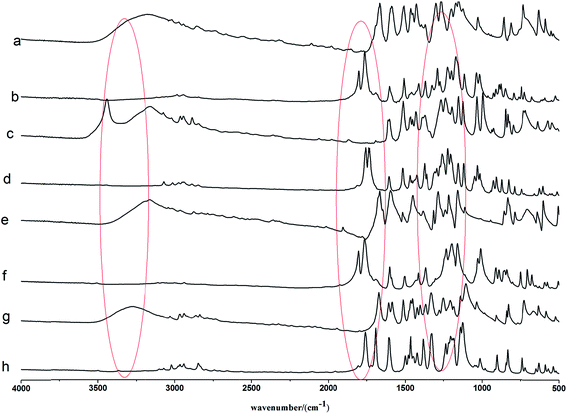
The FTIR spectra of lignin model compounds and their esters catalyzed by DMAP are shown in Fig. 1, and those of the esterified samples catalyzed by sodium acetate and sulphuric acid are shown in ESI (Fig. S2 and S3).† The main assignments are presented in Table 1.| Band position (cm−1) | Assignment |
|---|---|
| 3400 | O–H stretching of aromatic and aliphatic OH groups |
| 2939 | Alkyl group |
| 1760 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of phenolic ester O stretch of phenolic ester |
| 1740 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of aliphatic ester O stretch of aliphatic ester |
| 1800 | Characteristic band of anhydride |
| 1597 | Aromatic skeletal vibration |
| 1510 | Aromatic skeletal vibration |
| 1459 | O–CH3 deformation |
| 1222 | C–O–C of aromatic acetyl groups |
As shown in Fig. 1, three regions assigning to O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C had obvious changes for the products catalyzed by DMAP. The C
O and C–O–C had obvious changes for the products catalyzed by DMAP. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of phenolic ester appeared in all the esterified lignin model compounds for the esterification of phenolic hydroxyl, and a C
O stretch of phenolic ester appeared in all the esterified lignin model compounds for the esterification of phenolic hydroxyl, and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of aliphatic ester peak appeared in esterified vanillyl alcohol due to the reaction of aliphatic hydroxyl. Moreover, the esterified vanillin and p-hydroxy benzaldehyde exhibited the characteristic peak of anhydride. It should not be the residual acetic anhydride since it did not appear in other esters as the same process was carried out. This may due to that the oxidation of aldehyde generated COOH groups, which could form anhydride by dehydration during the following drying process. For all the esterified lignin model compounds, the intensity of hydroxyl decreased greatly for the esterification of hydroxyl groups. Esterification also led to the enhancement in peak intensity of C–O–C of aromatic acetyl groups.
O stretch of aliphatic ester peak appeared in esterified vanillyl alcohol due to the reaction of aliphatic hydroxyl. Moreover, the esterified vanillin and p-hydroxy benzaldehyde exhibited the characteristic peak of anhydride. It should not be the residual acetic anhydride since it did not appear in other esters as the same process was carried out. This may due to that the oxidation of aldehyde generated COOH groups, which could form anhydride by dehydration during the following drying process. For all the esterified lignin model compounds, the intensity of hydroxyl decreased greatly for the esterification of hydroxyl groups. Esterification also led to the enhancement in peak intensity of C–O–C of aromatic acetyl groups.
The peaks of the products catalyzed by sodium acetate were nearly the same as those catalyzed by DMAP (Fig. S2†), illustrating the same functional groups in these products. But some differences in the FTIR spectra of the products with different catalysts could be observed. Different extent of esterification and oxidation led to different intensity of the absorbance of ester groups and carboxyl groups, and the peak of hydroxyl in the products catalyzed by sodium acetate was still obvious. The absorption peaks of aliphatic ester link and phenolic ester link in acetylated vanillyl alcohol catalyzed by DMAP nearly showed the same height, but the absorbance of aliphatic ester link was stronger than that of phenolic ester link in the products catalyzed by sodium acetate. So the catalytic activity and selectivity of these two kinds of alkaline catalysts for esterification of lignin model compounds were different. DMAP possessed higher activity and selectivity than sodium acetate.
The difference between the products catalyzed by alkaline catalysts and acidic catalyst was also obvious. For the products catalyzed by sulphuric acid (Fig. S3†), there was nearly no absorption peak of anhydride in acetylated vanillin and p-hydroxy benzaldehyde, and the characteristic peaks of carbonyl became wide. The absorbance of aliphatic ester link was stronger than that of phenolic ester link in acetylated vanillyl alcohol. The characteristic absorption peaks of acetylated syringaldehyde catalyzed by sulphuric acid were similar with those catalyzed by alkaline catalysts. The peak intensity of hydroxyl was also very weak because most of the hydroxyl groups participated in the reaction. The characteristic peaks of alkyl became complex for the presence of part ring-opening or coupling reaction. New aldehyde or ketone might be generated and resulted in the shift of the characteristic peak of carbonyl to lower wavenumber. Based on the above comparative analysis of different FTIR spectra, it could be concluded that some side reactions happened during the esterification of lignin model compounds with sulphuric acid as catalyst.
3.2. NMR analysis
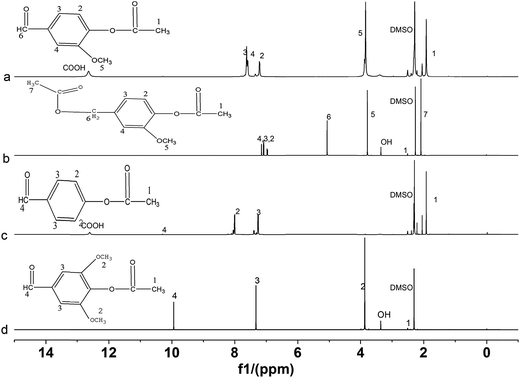
NMR spectra of acetylated lignin model compounds are shown in Fig. 2 and 3 and Fig. S4–S7.† As shown in Fig. 2, all the characteristic peaks were visible. The peaks at 1.89 ppm (CH3COO), 3.80 ppm (CH3O), 7.18 ppm (m- and p-aromatic hydrogen in benzene of CH3O), 7.60 ppm (o-aromatic hydrogen in benzene of CH3O), and 12.00 ppm (COOH) appeared in the acetylated vanillin. The peaks at 2.08 ppm (CH3COO in alcohol ester), 2.40 ppm (CH3COO in phenol ester), 3.80 ppm (CH3O), 7.15, 7.0 and 6.95 ppm (m-, p- and o-aromatic hydrogen of CH3O in benzene) appeared in the acetylated vanillyl alcohol. The peaks at 1.90 ppm (CH3COO), 8.00 and 7.20 ppm (m- and o-aromatic hydrogen of ester group in benzene), and 12.00 ppm (COOH) appeared in the acetylated p-hydroxy benzaldehyde. The peaks at 2.4 ppm (CH3COO), 3.80 ppm (CH3O), 7.3 ppm (aromatic hydrogen in benzene), and 10.00 ppm (CHO) appeared in acetylated syringaldehyde.The peaks of acetyl groups at 1.50–2.50 ppm appeared in all the products, implying that all the lignin model compounds were esterified with DMAP as catalyst. As the incomplete esterification resulted from the presence of steric hindrance between reactants, the acetylated vanillyl alcohol and syringaldehyde still exhibited the characteristic peak of OH at 3.50 ppm. For the vanillyl alcohol and syringaldehyde, the characteristic peak of aldehyde groups at 10.00 ppm decreased but that of carboxyl groups at 12.00 ppm increased obviously after esterification, which indicate that most of the aldehyde was oxidized to carboxyl during the esterification of lignin model compounds by MASPS. But the oxidation of syringaldehyde was unconspicuous.
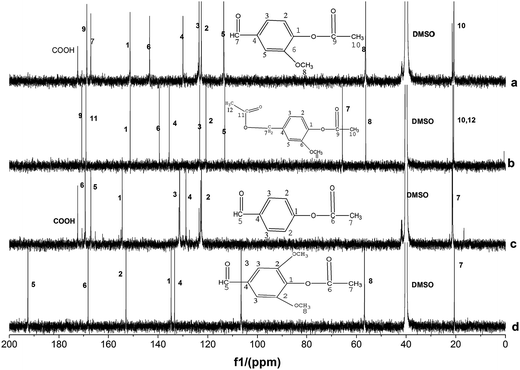
13C-NMR analysis of different samples showed similar results (Fig. 3). The characteristic peaks such as 20.9 ppm (CH3 in CH3COO), 169.0 ppm (CO in CH3COO), 56.0 ppm (CH3O), 167.0 ppm (CHO), 152.0 ppm (aromatic-carbon connected with CH3COO), 144.0 ppm (aromatic-carbon connected with CH3O), 130.0 ppm (aromatic-carbon connected with CHO), 114.0, 122.0 and 123.0 ppm (m-, p- and o-aromatic-carbon of CH3O), and 172 ppm (COOH) appeared in the acetylated vanillin. The peaks at 20.9 ppm (CH3 in CH3COO), 169.0 ppm (CO in alcohol ester), 171.0 ppm (CO in phenol ester), 56.0 ppm (CH3O), 66.0 ppm (benzyl group), 152.0 ppm (aromatic-carbon connected with CH3COO), 140.0 ppm (aromatic-carbon connected with CH3O), 136.0 ppm (aromatic-carbon connected with CH2), and 114.0, 120.0 and 123.0 ppm (m-, p- and o-aromatic-carbon of CH3O) appeared in the acetylated vanillyl alcohol. The peaks at 20.9 ppm (CH3 in CH3COO), 170.0 ppm (CO in CH3COO), 167.0 ppm (CHO), 154.0 ppm (aromatic-carbon connected with CH3COO), 129.0 ppm (aromatic-carbon connected with CHO), 122.5 and 131.0 ppm (m- and o-aromatic-carbon of CH3COO), and 173.00 ppm (COOH) appeared in the acetylated p-hydroxy benzaldehyde. The peaks at 20.9 ppm (CH3 in CH3COO), 192.0 ppm (CO in CH3COO), 56.0 ppm (CH3O), 134.0 ppm (aromatic-carbon connected with CH3COO), 144.0 ppm (aromatic-carbon connected with CH3O), 130.0 ppm (aromatic-carbon connected with CHO), 153 ppm (m-aromatic-carbon of CHO), and 169.0 ppm (CHO) appeared in acetylated syringaldehyde. The characteristic peaks of acetyl at 0.0–40.0 ppm and those of ester carbonyl at 165.0–175.0 ppm in all the products also confirmed the successful esterification of all the lignin model compounds. The characteristic peak of COOH at 173.0 ppm appeared in the 13C-NMR spectra of acetylated vanillin and acetylated p-hydroxy benzaldehyde, which is consistent with FTIR and 1H-NMR analyses.
The products catalyzed by sodium acetate showed similar NMR spectra (Fig. S4 and S5†) with those catalyzed by DMAP. Their difference mainly presented in the intensity of characteristic peaks of hydroxyl. All the products catalyzed by sodium acetate still had strong characteristic peaks of hydroxyl, while those of the products catalyzed by DMAP nearly disappeared, only acetylated vanillyl alcohol and syringaldehyde had weak characteristic peaks.
Compared with the esterification of lignin model compounds catalyzed by alkaline catalyst, the esterification catalyzed by sulphuric acid (Fig. S6 and S7†) had more side reactions. In 1H-NMR spectra, there was not only characteristic peak of aldehyde but also two new peaks around 10.00 ppm. The m-aromatic-hydrogen of CHO no longer overlapped, and more new peaks of hydrocarbon appeared below 4.00 ppm in acetylated vanillin. The characteristic peaks of aromatic hydrogen at 6.00–8.00 ppm and those of alkyl groups around 4.00 ppm became complex in the acetylated vanillyl alcohol, p-hydroxy benzaldehyde, and syringaldehyde. In 13C-NMR spectra, the characteristic peaks of aromatic-carbon at 100.0–150.0 ppm for all the samples also became complex.
In order to compare the reactivity of basic structure units, the content of ester were calculated from integral area of 1H-NMR spectra. With the region of 6.20–8.00 ppm assigned to aromatic hydrogen as reference, the area of CH3 in CH3COO at the region of 1.60–2.50 ppm, during which 1.60–2.10 ppm assigned to alcohol ester and 2.10–2.50 ppm assigned to phenol ester, was calculated and marked as A1, A2, A3, and A4 for acetylated vanillin, vanillyl alcohol, p-hydroxy benzaldehyde, and syringaldehyde, respectively. Then, the content of ester could be expressed as A1, A2, 4/3 A3, and 2/3 A4, respectively. The calculated results are shown in Table 2.
| Sample | DMAP | Sodium acetate | H2SO4 |
|---|---|---|---|
| a The calculation process is presented in ESI. | |||
| Acetylated vanillin | 1.55 | 0.65 | 0.47 |
| Acetylated vanillyl alcohol | 2.02 (alcohol 0.81, phenol 1.21) | 1.11 (alcohol 1.01, phenol 0.11) | 0.70 (alcohol 0.45, phenol 0.25) |
| Acetylated p-hydroxy benzaldehyde | 3.28 | 1.33 | 0.78 |
| Acetylated syringaldehyde | 0.99 | 0.11 | 0.13 |
Based on theoretical analysis and NMR analysis, eqn (1) was established to obtain the relationship between ester content and integral area.
n1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n2 = a1 n2 = a1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a2 a2
| (1) |
In theory, ester content of acetylated p-hydroxy benzaldehyde, vanillin and syringaldehyde was less than 1 mol mol−1 material, and that of acetylated vanillyl alcohol was less than 2 mol mol−1 material, but some of the calculated results were more than the theory values due to the interference of DMSO solvent whose characteristic peak was at 2.5 ppm. Since the operating conditions of NMR analysis were the same, the interference of DMSO solvent affected the absolute value but did not affect the change rule of esters. So ester contents of acetylated lignin model compounds calculated from integration of NMR spectra were still meaningful. As can be seen from Table 2, either the alkaline catalyst or acidic catalyst was used, the ester content of the product showed the following order: acetylated p-hydroxy benzaldehyde (H) > acetylated vanillyl alcohol (G) > acetylated vanillin (G) > acetylated syringaldehyde (S). The reactivity of the basic structure units could be inferred from the ester content of the products of three kinds of aldehyde which had only one phenolic hydroxyl, and the results showed the following order: H > G > S. For the G type of vanillin and vanillyl alcohol, the hydroxyl content of the latter is twice as the former, but for their esters, the ester content of the latter is less than the twice of the former. So the introduction of hydroxyl could affect the reactivity of the primary hydroxyl. For the acetylated vanillyl alcohol catalyzed by DMAP, the content of alcohol ester was more than that of phenol ester; while for that catalyzed by sodium acetate, the content of phenol ester was more than that of alcohol ester. So the reactivity of hydroxyl was different with the use of different catalysts.
3.3. UV/vis analysis
The esterification of lignin model compounds catalyzed by three catalysts was proved by the above FTIR and NMR analyses. In order to investigate the effects of lignin model compounds and catalyst on the efficiency of esterification, DE was analyzed by UV/vis spectrometer and calculated according to eqn (2),32 and the results are shown in Table 3. Although the oxidation of aldehyde to carboxylic acid might have certain effect, the effect was not obvious because most of the maximum absorption (except vanillyl alcohol whose maximum absorption was at 310 nm) appeared around 350 nm was separated from the peak of 300 nm.| DEwhole = 1 − C2/C1 | (2) |
| Sample | DE (%) | ||
|---|---|---|---|
| DMAP | Sodium acetate | Sulphuric acid | |
| Acetylated vanillin | 55.28 ± 0.17 | 41.88 ± 0.13 | 22.57 ± 0.07 |
| Acetylated vanillyl alcohol | 57.05 ± 0.18 | 51.05 ± 0.16 | 36.27 ± 0.11 |
| Acetylated p-hydroxy benzaldehyde | 61.38 ± 0.18 | 84.45 ± 0.23 | 58.54 ± 0.18 |
| Acetylated syringaldehyde | 33.43 ± 0.11 | 32.78 ± 0.09 | 12.78 ± 0.04 |
Under the catalytic action of these three catalysts, DE exhibited the following order: p-hydroxy benzaldehyde > vanillyl alcohol > vanillin > syringaldehyde. So the reactivity of the basic structure units of lignin showed the following order: H > G > S, which is consistent with the NMR analysis.
The reactivity of phenolic hydroxyl and aliphatic hydroxyl was preliminarily analyzed by comparing the vanillin and vanillyl alcohol. The phenolic hydroxyl contents of vanillin and vanillyl alcohol before esterification were 17/154 and 17/152, respectively. If the DEs of phenolic hydroxyl and alcohol hydroxyl was x and y, respectively, and the phenolic hydroxyl content of vanillin and vanillyl alcohol after esterification was 17(1 − x)/(154 + 43x+43y) and 17(1 − x)/(152 + 43x), respectively (the calculation process is provided in ESI†). Therefore, DEwhole−vanillin and DEwhole−vanillyl alcohol could be calculated as follows:
| DEwhole−vanillin = 1 − [(1 − x)/(152 + 43x)]/(1/152) | (3) |
| DEwhole−vanillyl alcohol = 1 − [(1 − x)/(154 + 43x + 43y)]/(1/154) | (4) |
The measured values were 55.28% and 57.05% for the acetylated vanillin and vanillyl alcohol catalyzed by DMAP, respectively (Table 3). When the DEwhole−vanillin and DEwhole−vanillyl alcohol were substituted by measured values, the results were x = 0.4908 and y = 0.1747. So the reactivity of aliphatic hydroxyl was higher than that of phenolic hydroxyl. Similarly, according to the measured values of the products catalyzed by sodium acetate and sulphuric acid, roots of the equation were x = 0.3597 and y = 0.7445 for the former, and x = 0.1851 and y = 0.8134 for the latter, indicating the reverse reactivity of aliphatic hydroxyl and phenolic hydroxyl.
3.4. Catalytic mechanism of DMAP
The processes of DMAP-catalyzed acylation of lignin with acid anhydride had been discussed in our previous works.21,33 The esters were obtained through two routes. In the first route, the nitrogen atom in pyridine ring of DMAP attacks the carbonyl of Ac2O to form an ion pair of acetate and acetylpyridinium. Then, the hydroxyl is added to the acetylpyridinium and forms an ester and deactivated (protonated) catalyst. In the second route, hydroxyl is added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond of Ac2O through a four-membered or six-membered-ring transition state with DMAP, and then an ester is generated. Using vanillin and vanillyl alcohol as examples, the processes were studied here by pre-reaction of DMAP with one of the reactants, and the results are shown in Table 4.
O double bond of Ac2O through a four-membered or six-membered-ring transition state with DMAP, and then an ester is generated. Using vanillin and vanillyl alcohol as examples, the processes were studied here by pre-reaction of DMAP with one of the reactants, and the results are shown in Table 4.
| Sample | Reaction condition | DE (%) |
|---|---|---|
| Acetylated vanillin | Vanillin were added after 5 min of pre-reaction of DMAP and Ac2O | 66.89± 0.21 |
| Ac2O were added after 5 min of pre-reaction of DMAP and vanillin | 58.91± 0.19 | |
| No catalyst | 20.95 ± 0.07 | |
| No pre-reaction | 55.28 ± 0.17 | |
| Acetylated vanillyl alcohol | Vanillyl alcohol were added after 5 min of pre-reaction of DMAP and Ac2O | 82.86 ± 0.22 |
| Ac2O were added after 5 min of pre-reaction of DMAP and vanillyl alcohol | 63.36 ± 0.18 | |
| No catalyst | 31.11 ± 0.09 | |
| No pre-reaction | 57.05 ± 0.17 |
The esterification efficiency was low for either vanillyl alcohol or vanillin without catalyst, and it increased significantly with the use of catalyst. So DMAP is a good catalyst for the esterification of lignin. Compared with the esterification that all the reactants and DMAP were added at the same time, the esterification that one reactant and DMAP were pre-reacted for 5 min and then the other reactant was added showed higher efficiency. So the DMAP-catalyzed acylation of alcohol or phenol with acid anhydride by MASPS was carried out through two routes, which was similar with that by liquid-phase synthesis.33 The pre-reaction of DMAP and Ac2O is in favor of the formation of acetylpyridinium, and the pre-reaction of DMAP and alcohol or phenolic hydroxyl group is in favor of the formation of four-membered or six-membered-ring transition state due to the formation of hydrogen bond between N and H. Therefore, pre-reaction could improve the esterification of lignin, and the first route was the main way since the product prepared by the first route had a higher DE than that by the second route.
3.5. Catalytic mechanism of sodium acetate
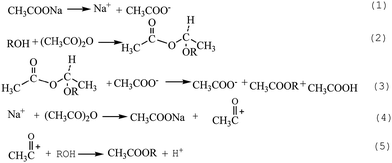
Sodium acetate is commonly considered to play a part of salt in the esterification by liquid-phase synthesis (Scheme 1).34 As a Lewis acid, the sodium ion attacks the anhydride and turns it to nucleophilic group CH3CO+, which then reacts with alcohol. And CH3COO−, as a Lewis base, captures the proton of the intermediate formed from alcohol and acid anhydride, leading to bond breaking of the intermediate to form the ester and carboxylate radical. In our previous studies about the solid-phase esterification of cellulose, cassava stillage residue, and starch, it was found that proper metal salts such as ZnCl2 and K2CO3 could improve the esterification of polysaccharide polymers by MASPS.35,36 So sodium acetate also plays the role of salt effect in the esterification of lignin by MASPS.3.6. Catalytic mechanism of sulphuric acid
The mechanism of esterification catalyzed by acid in liquid phase is shown in Scheme 2.37 Acid anhydride accepts protons from inorganic acid and forms acyl cation through decarboxylation. Then the acyl cation reacts with alcohol to generate the ester. Sulphuric acid can also provide proton during the esterification of lignin by MASPS. It can be seen from FTIR and NMR spectra that the absorption peaks of hydroxyl groups nearly disappeared, the characteristic peaks of benzene ring had some change, and the characteristic peaks of aldehydes and ketones enhanced. These indicate that the dehydration reaction of lignin model compounds could happen for the strong water absorption of concentrated sulfuric acid. Sulfuric acid can dissociate and generate proton under the water from dehydration and absorption, and thus enhance the esterification, as shown in Scheme 2. The dehydration can consume most of the sulfuric acid, and only a small part play the role of catalyst. Moreover, the water generated from the dehydration of lignin model compounds can easily cause the hydrolysis of anhydride, resulting in poor reactivity. Therefore, the products catalyzed by sulfuric acid had the lowest DE.3.7. Consistency verification
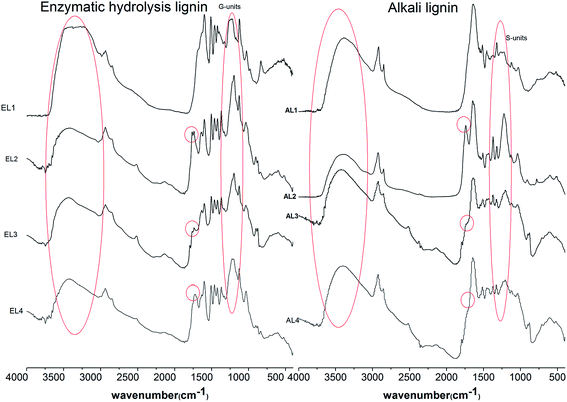
In order to verify the representativeness of lignin model compounds, the esterification of enzymatic hydrolysis lignin with DMAP as catalyst by different feeding way and that of different types of lignin (enzymatic hydrolysis lignin and alkali lignin) with different catalysts by the same process were studied, and the results are shown in Table 5 and Fig. 4. The DE of lignin was only 19.41% without catalyst, while it increased obviously with the addition of catalyst. The two pre-reaction further enhanced the esterification and the DEs were higher than that of no pre-reaction. The esterified lignin prepared by the process that lignin was added after 5 min of pre-reaction of DMAP and Ac2O had the highest DE. So the effect of feeding way on the esterification of lignin was similar with that of vanillin and vanillyl alcohol. For the FTIR spectra of lignin, the difference between different types of lignin mainly showed at 1400–1100 cm−1. The characteristics of G and S units can be seen at the peaks of 1260 cm−1 (C–H plane vibrations of G unit), 1220 cm−1 (C–O–C of G unit), and 1330 cm−1 (C–O and C–C of S unit). It can be easy to observe from Fig. 4 that the G unit was relatively large for enzymatic hydrolysis lignin, while the S unit was relatively large for alkali lignin. Difference between lignin and its ester mainly showed at 3200–3500 cm−1 and 1700–1760 cm−1. After esterification, the peaks at 1760 and 1740 cm−1 corresponding to ester increased, while the peak at 3400 cm−1 corresponding to hydroxyl decreased. For the esterification of either enzymatic hydrolysis lignin or alkali lignin, the relative strength of reactivity between different catalysts was the same. No matter which catalyst was used, the esterification of enzymatic hydrolysis lignin was more obvious than that of alkali lignin, so the reactivity of G unit was larger than that of S unit. It was also consistent with the above analysis results. Therefore, the lignin model compounds used here were reasonable and feasible for the study of esterification mechanism of lignin.| Sample | Reaction condition | DE (%) |
|---|---|---|
| Acetylated enzymatic hydrolysis lignin | Lignin were added after 5 min of pre-reaction of DMAP and Ac2O | 85.04 ± 0.26 |
| Ac2O were added after 5 min of pre-reaction of DMAP and lignin | 80.01 ± 0.24 | |
| No catalyst | 19.41 ± 0.06 | |
| No pre-reaction | 77.59 ± 0.24 | |
| Acetylated enzymatic hydrolysis lignin | No pre-reaction, catalyzed by DMAP | 77.59 ± 0.24 |
| No pre-reaction, catalyzed by CH3COONa | 43.64 ± 0.13 | |
| No pre-reaction, catalyzed by H2SO4 | 30.23 ± 0.11 | |
| Acetylated alkali lignin | No pre-reaction, catalyzed by DMAP | 51.92 ± 0.18 |
| No pre-reaction, catalyzed by CH3COONa | 20.03 ± 0.07 | |
| No pre-reaction, catalyzed by H2SO4 | 15.60 ± 0.05 |
4. Conclusions
DMAP, sodium acetate, and sulfuric acid could catalyze the esterification of lignin model compounds. Both the phenolic hydroxyl and aliphatic hydroxyl participated in the esterification, and the reactivity of the basic structure units of lignin had the descending order of H, G, and S. Oxidations could take place with the presence of unsaturated groups such as aldehyde in lignin model compounds. The reactivity of phenolic hydroxyl was higher than that of aliphatic hydroxyl with DMAP as catalyst, but the reactivity of aliphatic hydroxyl was higher than that of phenolic hydroxyl with sodium acetate or sulfuric acid as catalyst. The esterification catalyzed by DMAP had the highest efficiency, but that catalyzed by sulfuric acid had the lowest efficiency.DMAP enhanced the esterification through two routes, and sodium acetate played the role of salt effect during it catalyzed the acylation. Sulfuric acid could dissociate and generate proton under the water from dehydration of lignin model compounds and adsorption by concentrated sulfuric acid, thus enhanced the esterification. Some side reactions took place and resulted in the change of benzene ring with sulfuric acid as catalyst. The use of lignin model compounds for studying the esterification mechanism of lignin had been proved to be reasonable and feasible by consistency verification between lignin model compounds and real lignins.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Natural Science Foundation of China (No. 51463003 and 21666005), the Guangxi Science and Technology Plan Project of China (Grant No. AB16380305), Guangxi Distinguished Experts Special Foundation of China, and the Scientific Research Foundation of Guangxi University (Grant No. XJPZ160713).References
- X. Chen, H. Yang, Z. Zhong and N. Yan, Green Chem., 2017, 19, 2783–2792 RSC.
- Y. Wang, S. De and N. Yan, Chem. Commun., 2016, 52, 6210–6224 RSC.
- B. Zhang, C. Li, T. Dai, G. W. Huber, A. Wang and T. Zhang, RSC Adv., 2015, 5, 84967–84973 RSC.
- T. Q. Hu, Chemical Modification, Properties, and Usage of Lignin, Springer, US, 2002 Search PubMed.
- D. Z. Ye, L. Jiang, C. Ma, M. Zhang and X. Zhang, Int. J. Biol. Macromol., 2014, 63, 43–48 CrossRef CAS PubMed.
- X. Yue, F. Chen and X. Zhou, BioResources, 2011, 6, 2022–2034 CAS.
- H. Shiyan, G. Fang, S. Li, G. Liu and G. Jiang, BioResources, 2014, 9, 4971–4980 CrossRef.
- H. Li, Z. Pang, P. Gao and L. Wang, RSC Adv., 2015, 5, 54387–54394 RSC.
- J. Qin, M. Woloctt and J. Zhang, ACS Sustainable Chem. Eng., 2014, 2, 188–193 CrossRef CAS.
- P. Månsson, Holzforschung, 1983, 37, 143–146 CrossRef.
- K. A. Y. Koivu, H. Sadeghifar, P. A. Nousiainen, D. S. Argyropoulos and J. Sipilä, ACS Sustainable Chem. Eng., 2016, 4, 5238–5247 CrossRef CAS.
- L. Dehne, C. Vila Babarro, B. Saake and K. U. Schwarz, Ind. Crops Prod., 2016, 86, 320–328 CrossRef CAS.
- E. Hult, J. Ropponen, K. Poppius-Levlin, T. Ohra-Aho and T. Tamminen, Ind. Crops Prod., 2013, 50, 694–700 CrossRef CAS.
- S. Luo, J. Cao and A. G. McDonald, Ind. Crops Prod., 2017, 97, 281–291 CrossRef CAS.
- L. Dehne, C. Vila, B. Saake and K. U. Schwarz, J. Appl. Polym. Sci., 2016, 134, app.44582 Search PubMed.
- R. Fang, X. Cheng and W. Lin, BioResources, 2011, 2874–2884 CAS.
- Y. Teramoto, S. Lee and T. Endo, Polym. J., 2009, 41, 219–227 CrossRef CAS.
- N. Cachet, S. Camy, B. Benjelloun-Mlayah, J. Condoret and M. Delmas, Ind. Crops Prod., 2014, 58, 287–297 CrossRef CAS.
- F. Monteil-Rivera and L. Paquet, Ind. Crops Prod., 2015, 65, 446–453 CrossRef CAS.
- J. H. Bridson, D. J. van de Pas and A. Fernyhough, J. Appl. Polym. Sci., 2013, 128, 4355–4360 CrossRef CAS.
- X. Zhao, Z. Huang, Y. Zhang, M. Yang, D. Chen, K. Huang, H. Hu, A. Huang, X. Qin and Z. Feng, J. Appl. Polym. Sci., 2017, 134, 44276 Search PubMed.
- Y. Ma, Z. Du, F. Xia, J. Ma, J. Gao and J. Xu, RSC Adv., 2016, 6, 110229–110234 RSC.
- U. Weißbach, S. Dabral, L. Konnert, C. Bolm and J. G. Hernández, Beilstein J. Org. Chem., 2017, 13, 1788–1795 CrossRef PubMed.
- S. G. Yao, M. S. Meier, R. B. Pace III and M. Crocker, RSC Adv., 2016, 6, 104742–104753 RSC.
- T. Elder, Energy Fuels, 2014, 28, 1175–1182 CrossRef CAS.
- M. Chen, G. Zeng, C. Lai, J. Li, P. Xu and H. Wu, RSC Adv., 2015, 5, 52307–52313 RSC.
- J. K. Mobley, S. G. Yao, M. Crocker and M. Meier, RSC Adv., 2015, 5, 105136–105148 RSC.
- X. Zhao, Y. Zhang, H. Hu, Z. Huang, M. Yang, D. Chen, K. Huang, A. Huang, X. Qin and Z. Feng, Int. J. Biol. Macromol., 2016, 91, 1081–1089 CrossRef CAS PubMed.
- H. Konnerth, J. Zhang, D. Ma, M. H. G. Prechtl and N. Yan, Chem. Eng. Sci., 2015, 123, 155–163 CrossRef CAS.
- J. Zhang, J. Teo, X. Chen, H. Asakura, T. Tanaka, K. Teramura and N. Yan, ACS Catal., 2014, 4, 1574–1583 CrossRef CAS.
- W. A. W. I. Thielemans, Lignin and carbon nanotube utilization in bio-based composites, University of Delaware, 2004 Search PubMed.
- L. A. M. Nevárez, L. B. Casarrubias, A. Celzard, V. Fierro, V. T. Muñoz, A. C. Davila, J. R. T. Lubian and G. G. Sánchez, Sci. Technol. Adv. Mater., 2011, 12, 045006 CrossRef PubMed.
- S. Xu, I. Held, B. Kempf, H. Mayr, W. Steglich and H. Zipse, Chem.–Eur. J., 2005, 11, 4751–4757 CrossRef CAS PubMed.
- A. L. B. Tchoubar, Salt effects in organic and organometallic chemistry, New York, VCH Publisher, 1991 Search PubMed.
- Y. Zhang, T. Gan, H. Hu, Z. Huang, A. Huang, Y. Zhu, Z. Feng and M. Yang, Ind. Eng. Chem. Res., 2014, 53, 2114–2120 CrossRef.
- Z. Huang, Y. Tan, Y. Zhang, X. Liu, H. Hu, Y. Qin and H. Huang, Bioresour. Technol., 2012, 118, 624–627 CrossRef CAS PubMed.
- V. Casson, D. G. Lister, M. F. Milazzo and G. Maschio, J. Loss Prev. Process Ind., 2012, 25, 209–217 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10482k |
| This journal is © The Royal Society of Chemistry 2017 |