Open Access Article
Open Access ArticleCharacterization of uranium in the extracellular polymeric substances of anaerobic granular sludge used to treat uranium-contaminated groundwater
Hailing Zhanga,
Mengxi Chenga,
Weidong Liua,
Fengyu Huangb,
Huanhuan Dinga,
Shicheng Lia,
Wei Guoa,
Yongpeng Wang *a and
Hexiang Huang*ab
*a and
Hexiang Huang*ab
aInstitute of Materials, China Academy of Engineering Physics, Jiangyou, Mianyang, Sichuan 621907, China. E-mail: wangyongpeng@caep.cn
bMianyang Yijing Anti Radiation Technology Co., LTD, Jiangyou, Mianyang, Sichuan 621907, China. E-mail: weiwei@caep.cn
First published on 24th November 2017
Abstract
Anaerobic granular sludge (AnGS) has been proven to be long-term effective for U(VI) removal and can be used as an inoculum for permeable reactive barriers, which is an innovative technology for remediation of uranium-contaminated groundwater. Considering their great ability in biosorption and bioreduction to common metal ions, extracellular polymeric substances (EPS) should play an important role in U(VI) removal and also in maintaining bioactivity of the AnGS due to toxicity accompanied with uranium. However, the roles of the EPS of AnGS in the uranium immobilization process are not clear. In this study, batch experiments were carried out by treating synthetic uranium-contaminated groundwater with AnGS, and uranium in EPS was extracted using four different methods. Moreover, speciation of uranium in EPS by filtration and inductively coupled plasma with mass spectroscopy and the reaction between isolated EPS and uranyl sulphate solution in a NaHCO3 medium were investigated. The results showed that about 12–16% of the total uranium immobilized by AnGS (extracted by the cation exchange resin (CER) method at 600 rpm for 1 h) was found to be present in EPS in its soluble ionic and particulate forms. For EPS-associated uranium obtained by the CER method, particulate uranium was proven to be the main form with sizes ranging from 24.7 nm to 171.3 nm. In the process of uranium immobilization using EPS isolated from non-reacted AnGS, both biosorption and bioreduction were involved. The findings of this study imply the important roles of EPS in the immobilization of uranium in groundwater using AnGS.
1. Introduction
Uranium mining and mine tailings cause uranium contamination to adjacent groundwater, where uranium levels have been reported to be as high as 50 mg L−1.1 Significant concerns regarding uranium behavior and remediation have been raised due to the hazardous nature of uranium related to its synchronous chemical toxicity and radiotoxicity. Uranium is mainly present as soluble hexavalent uranium (U(VI)) and insoluble tetravalent uranium (U(IV)) in the environment. In groundwater, U(VI) migrates mostly in the form of uranyl ion (UO22+) or uranyl carbonate complexes (e.g. UO2(CO3)22−).2 The transformation of U(VI) can be accomplished by some microorganisms. A number of cases of the reduction of soluble U(VI) to insoluble U(IV) (e.g. UO2) catalyzed by microorganisms, such as sulfate- or metal-reducing bacteria, have been reported. Indeed, microbial reduction has been widely proven as a cost-effective and promising bioremediation strategy for the treatment of uranium-contaminated groundwater.3–5 Besides the abovementioned pure cultures, mixed cultures, such as anaerobic granular sludge (AnGS), have also been proven long-term effective for the reduction of U(VI), which have been inoculated to sand column reactors.6,7 Hence, a permeable reactive barrier inoculated with AnGS will be very beneficial for future in situ remediation of uranium-contaminated groundwater due to its convenient maintenance and cost. Moreover, AnGS will also act as an inhabitant in groundwater systems, affecting the U(VI) migration behavior.Extracellular polymeric substances (EPS), located in the interspaces of bacteria inside the anaerobic granular sludge, have been proven to be important for maintaining the structural and functional integrity of AnGS aggregates. EPS are considered to have a strong influence on the migration and fate of many substances. First, EPS possess abundant functional groups and can immobilize large substances through biosorption or chelation. Many kinds of substances, including phosphorus,8 sulfanilamide,9 carbon nanotubes,10 and some minerals,11,12 have been detected in EPS. Moreover, because of their excellent chelation ability, EPS can also act as a template for mineral nucleation and induce the formation of minerals such as calcite13 and struvite.14 Furthermore, EPS produced by some metal-reducing bacteria have been demonstrated to contribute to the reduction of ferrous iron,15 silver ions,16,17 and U(VI).18
As a typical pollutant, uranium has been found to be immobilized by EPS of some anaerobic microbes through biosorption, biomineralization or bioreduction. For example, the EPS of Citrobacter sp. is thought to be involved in the biomineralization process of U(VI).19 The high biosorption capacity of EPS extracted from the anaerobic-activated sludge towards U(VI) has been proven by Yuan et al.20 Cao et al. (2011)18 first reported the significant contribution of bioreduction of the EPS of Shewanella sp. in HRCR-1 to U(VI) immobilization, and it was considered that the extracellular U(VI) reduction was most probably caused by the redox active c-cytochromes present in the EPS.21 Microbe species in AnGS are abundant, and several U(VI)-reducing bacteria have been identified to be inhabited in it.22,23 U(VI)-reduction was accomplished through an extracellular electron transfer by these functional microbes. Hence, the EPS matrix contains some electrochemically active substances and probably U(VI)-reducing active enzymes released by cell autolysis. Then, in the remediation process of uranium-containing groundwater using AnGS, the contributory factors of EPS for uranium immobilization may be numerous and complicated, and biosorption, biomineralization, and bioreduction are likely to be involved. Hence, uranium present in EPS was predicted to be complex such as soluble U(VI) ions (e.g. UO22+, UO2(CO3)22− etc.), U(VI)-phosphate precipitates (the product of adsorbed phosphate and UO22+), or also U(IV) precipitates. However, the chemical forms of extracellular uranium and their contents in EPS are still unclear. Therefore, a deeper insight into the characteristics, such as actual forms and contents, of uranium in EPS is desirable.
Regarding the two most probable states of the extracellular uranium, the soluble and particulate forms, different characterization methods are needed. To quantify the extracellular uranium content, a traditional extraction method has to be used. While for the accurate content of extracellular uranium, besides the previously reported influencing factors (e.g. the extraction efficiency of extracellular uranium, the leakage of intracellular uranium, etc), it is also necessary to evaluate the re-dissolution of insoluble U(IV) during the extraction process. For the extracellular uranium in the particulate form, little information (such as the chemical composition of the minerals, the mineral fraction, and the valence states of uranium inside the minerals) is available.
The main objective of this study was to characterize uranium in the EPS of AnGS, which was applied for the immobilization of uranium in groundwater. The extracellular uranium content and mineral fraction and the re-dissolution of insoluble U(IV) during various EPS extraction processes were investigated. Considering the probable roles of EPS in uranium bioreduction, the reduction of U(VI) to U(IV) minerals by single EPS extracted from AnGS was also explored. The present study will provide great reference value for better understanding the mechanism of uranium removal from groundwater in microbial remediation systems and will be beneficial for guiding related uranium remediation practices.
2. Materials and methods
2.1. Batch experiments for remediation using AnGS
The remediation of uranium-contaminated groundwater using AnGS was carried out in the batch mode to investigate the immobilization of uranium by microbial cells and EPS. The experiments were conducted in 160 mL serum bottles using 100 mL of synthetic uranium-contaminated groundwater (minerals solution, NaHCO3, and U(VI)) and 60 mL headspace. The mineral solution contained (mg L−1)23 NH4HCO3, 5; K2HPO4, 2; MgCl2, 2.1; Ca(OH)2, 1; yeast extract, 0.33 and a trace element solution (μg L−1): H3BO3, 0.5; FeSO4·7H2O, 28; ZnSO4·7H2O, 1.1; CuSO4·5H2O, 1.6; MnSO4·H2O, 2.5; (NH4)6Mo7O24·4H2O, 2.0; KAl(SO4)2·12H2O, 1.75; CoSO4·7H2O, 23.6; NiSO4·6H2O, 1.13; Na2SeO3·5H2O, 1; Na2WO4·2H2O, 5.2; and EDTA, 10. The minerals solution was boiled for 10 min and sparged with N2 to remove the dissolved oxygen. After cooling down to room temperature, 1 g L−1 NaHCO3 was dosed to adjust the pH to 7.0. Then, a 10 mM stock solution of uranyl sulfate (99.99% UO2SO4·3H2O obtained from Hubei Chushengwei Chemical Co., Ltd, China) and AnGS were successively added to the medium to a final U(VI) mass concentration of 100 mg L−1 and VSS concentration of 2000 mg L−1 (VSS: volatile suspended solids). The bottles were sparged with N2 to further remove the remaining oxygen and then sealed with butyl rubber stoppers and aluminum tear-off seals. The temperature was controlled at 25 ± 1 °C. In the time course of 13 days, a 1 mL mixed solution was taken at certain intervals and centrifuged at 8000 rpm for 5 min to determine U(VI) in the supernatant. After the reactions, 10 mL of the mixed solution was used for EPS extraction and 2 mL for the sequential extraction of uranium in the sludge. The batch test was repeated three times.The AnGS was stored anaerobically at 4 °C prior to the experiments, which was obtained from a full-scale up-flow anaerobic sludge blanket (UASB) reactor (Hefei, Anhui, China) treating starch wastewater and with a VSS content of 65%, moisture content of 94.9%, and specific acetoclastic methanogenic activity of 350 mg COD per g VSS per day. Prior to its addition, the sludge granules were washed with de-ionized water.
2.2. EPS extraction
Herein, four methods (sonication, EDTA, heating, cation exchange resin (CER), and a control method (centrifugation)) were used to extract the EPS from AnGS with immobilized uranium. As all the EPS extraction processes were carried out in an anaerobic glove box (99.9% N2), the centrifugation, sonication, EDTA, and CER methods were conducted at room temperature. To adequately extract the EPS, the sludge granules were first crushed and ground to flocs and suspended with a 100 mM anaerobic NaCl solution (previously boiled and sparged with N2) to the original volume. After this, 10 mL of the sludge solution was harvested and centrifuged at 8000 rpm for 5 min. Then, the sludge pellets were washed twice with the 100 mM anaerobic NaCl solution to remove any residual soluble substances in the media. Subsequently, the pellets were re-suspended with 10 mL of a 100 mM anaerobic NaCl solution (or 2% anaerobic EDTA solution during EDTA extraction). The control method was performed using a two-step centrifugation process: at first, centrifugation was carried out at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and subsequently, the supernatant was centrifuged at 14
000 rpm for 10 min, and subsequently, the supernatant was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 rpm (equivalent to 20
510 rpm (equivalent to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g) for 20 min.11,12 Sonication extraction was performed for 5 min or 10 min at 150 W. For the EDTA extraction method, the sludge pellets re-suspended in an EDTA solution were kept for 2 h to extract the EPS. For the heating extraction process, the sludge mixture was heated at 60 °C for 20 min or at 80 °C for 10 min in a water bath. For the CER procedure, the extraction process was conducted in a 50 mL beaker with 70 g resin per g VSS of CER (DOWEX MARATHON C, Na+-form, 20–50 mesh, Sigma-Aldrich) with the extraction times of 1 h or 2 h at 600 rpm.24 Thereafter, the suspensions were centrifuged at 10
000 g) for 20 min.11,12 Sonication extraction was performed for 5 min or 10 min at 150 W. For the EDTA extraction method, the sludge pellets re-suspended in an EDTA solution were kept for 2 h to extract the EPS. For the heating extraction process, the sludge mixture was heated at 60 °C for 20 min or at 80 °C for 10 min in a water bath. For the CER procedure, the extraction process was conducted in a 50 mL beaker with 70 g resin per g VSS of CER (DOWEX MARATHON C, Na+-form, 20–50 mesh, Sigma-Aldrich) with the extraction times of 1 h or 2 h at 600 rpm.24 Thereafter, the suspensions were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and subsequently at 14
000 rpm for 10 min and subsequently at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 rpm for 20 min, and the supernatant was obtained without further treatment, withholding the uranium precipitates.
510 rpm for 20 min, and the supernatant was obtained without further treatment, withholding the uranium precipitates.
In addition, to explore the re-dissolution of insoluble U(IV) during the EPS extraction, some amount of biologically reduced uranium, instead of the sludge, was used to repeat the EPS extraction procedure. The biologically reduced uranium was prepared according to the literature with a slight modification.25 The washed and crushed AnGS (the VSS concentration of 2000 mg L−1) was suspended in 100 mL of the mineral solution (the same as used in the batch tests) in a 160 mL serum bottle, amended with 2 g L−1 NaHCO3, 400 mg L−1 uranyl sulfate, and 20 mM acetate, and incubated at 30 °C under anaerobic conditions. At day 4, the sludge flocs were obtained and washed twice with 100 mM NaHCO3 buffer to remove any loosely absorbed U(VI). Then, the pellet was incubated in 1 M NaOH for 1 h to dissolve the cell membranes and proteins. The suspension was then centrifuged and washed four times with 1 M NaHCO3 to remove the remaining NaOH and any complexed U(VI). Finally, the pellet was sufficiently rinsed with deionized water until no U(VI) could be detected; this resulted in the formation of a purified U(IV) solid. Then, the U(IV) solid was re-suspended with a 100 mM anaerobic NaCl solution and divided into several aliquots to repeat the EPS extraction procedure. After centrifugation, the uranium concentration in the extracts was detected. The re-dissolution extent of insoluble U(IV) was evaluated as the ratio of uranium in the extracts to total uranium in the solution.
2.3. Chemical analysis
All chemicals used in this study were of analytical grade. The concentrations of total uranium (TU) in the supernatant, EPS (TUEPS) or sludge (TUSludge) were analyzed by inductively coupled plasma with mass spectroscopy (NEXION 350, Perkin Elmer), and all the samples were acidified using 5% HNO3 as previously described. The VSS of the AnGS was measured according to a standard method.26 The extraction efficiency was analyzed by the total concentration of carbohydrates, proteins, humic substances, and nucleic acids in the EPS extracts, which was determined as previously described.24The fractionation of uranium in the AnGS before and after uranium immobilization reaction was performed according to the literature,6,7 with successive extractions of anaerobic MilliQ water (overnight), anaerobic NaHCO3 (1 M, overnight), and HNO3 (10%, 4 h), representing the water soluble U(VI), adsorbed/complexed U(VI), and insoluble U(IV), respectively.
2.4. Quantification of the mineral fraction of uranium in the EPS extracts
Herein, two methods were used to quantify the mineral fraction of uranium in the EPS extracts. For the relatively large EPS-associated uranium particles (micron-size), a microporous membrane with a pore size of 0.22 μm was used to differentiate the soluble and particulate uranium in the EPS extracts as this pore size was proven to be efficient to intercept minerals with sizes ranging from several microns to tens of microns.11,12,27 To accurately quantify the EPS-associated uranium in its particulate form (>0.22 μm), both sonication (10 min at 150 W) and CER (1 h at 600 rpm), which have been proven to cause a low extent of U(IV) re-dissolution in the subsequent manuscript, have been chosen to perform the EPS extraction as abovementioned. After the extraction, about 30 mL of the EPS samples were filtered through 0.22 μm cellulose nitrate membranes, and the initial filtrate was discarded. The membranes were previously saturated by uranyl ions by soaking in a sufficient 50 mg L−1 uranyl sulfate solution and then rinsed with large amounts of MilliQ water. The difference between the uranium content in the EPS solution before and after filtration was evaluated to be the mineral fraction of uranium in the EPS (>0.22 μm). Moreover, six experiments for sonication and CER extraction were performed.As some nano-sized colloidal uranium may not be intercepted by the membrane, the EPS extracts after 0.22 μm filtration were analyzed using ICP-MS (NEXION 350, Perkin Elmer) in the single particle mode. In the single particle mode, the signal of the soluble uranyl ions was obviously different from the signal of the uranium-containing particles. The mineral fraction of the EPS-associated uranium within the EPS extracts after 0.22 μm filtration was evaluated by the difference between the total uranium concentration in EPS (acidified in 5% HNO3) and the concentration of soluble uranium ions. Herein, six experiments for sonication and CER extraction were performed.
On the other hand, for the characterization of the size distribution of the nano-sized uranium particles, a series of silver nanoparticles with known diameters and particle concentrations (Citrate NanoXact™ Silver, nanoComposix Inc.) were used as the standard particles. Moreover, one colloidal particle could be ionized in a plasma torch to be a flash of ions and was displayed as a transient signal to be detected by MS. The signal intensity presents the particle size, and the flash frequency accounts for the particle concentration.
2.5. U(VI) immobilization using EPS in a NaHCO3 medium
The EPS, used to determine the U(VI) immobilization, were isolated from the original AnGS (without reaction with U(VI)) using CER extraction (600 rpm for 1 h in an ice bath). Then, the crude EPS filtered through the 0.22 μm membrane was purified by dialysis (1000 MWCO, 24 h, anaerobic water).16,18 After removing the ions and small molecules, the EPS was divided into two aliquots and separately placed in dialysis tubing (1000 MWCO) against a UO2SO4 solution (25 mg U L−1, 1 g L−1 NaHCO3) for 24 h. To estimate the respective contribution of adsorption and reduction to U(VI) immobilization, the reaction process was conducted under two different conditions: anaerobic and aerobic. The anaerobic reaction was carried out in an anaerobic glove box (99.9% N2), whereas the aerobic reaction was performed under feeble and intermittent aeration conditions. The U(VI) concentrations in the bulk in the beginning (the dialysis tubing was previously immerged in the bulk and saturated with uranyl ions, and then, the EPS was injected), at 12 h, and at 24 h were measured. The decrease in the aqueous U(VI) concentration in the anaerobic reaction was attributed to both U(VI) adsorption and U(VI) reduction by EPS, whereas the aqueous U(VI) removed after the whole aerobic reaction was mainly caused by adsorption. Under aerobic conditions, uranium reduction could also inevitably occur. However, the apparent reduction amount of uranium under aerobic conditions should be rather low. Subsequently, the U(VI) reduction was determined by the difference between the abovementioned two reactions. The U(VI) immobilization process was repeated in triplicate.3. Results and discussion
3.1. Uranium immobilization using AnGS
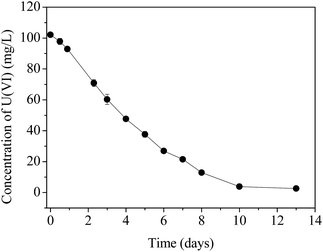
The time course of U(VI) immobilization by AnGS without external electron donors is shown in Fig. 1. The results indicated that the concentration of U(VI) in the supernatant decreased constantly and became stable after day 10, and a removal efficiency of above 97% was achieved.The uranium fraction in the AnGS, before and after the reaction, extracted by water, NaHCO3 and HNO3 are shown in Table 1. The high uranium recovery indicates the effectiveness of this method. Since natural uranium exists in starch wastewater, a little uranium is already present in the original AnGS, with a content of 0.06 ± 0.01 mg U per g SS. After the uranium immobilization reaction, the content of uranium in the sludge increased to 29.6 ± 2.8 mg U per g SS. At the start point of the reaction and at day 13, the absorbed U(VI) (e.g. U(VI) ions and U(VI)-phosphate minerals), extracted by both water and NaHCO3, accounted for about 62.5% and 44.6%, respectively. While HNO3 can extract about 37.5% and 55.4% uranium for the two reaction-point sludge, which represent the reduced uranium-U(IV) compounds.6 The results illustrate that the microorganisms capable of reducing U(VI), located in the AnGS, can effectively reduce U(VI) with an endogenous substrate. Furthermore, a higher U(IV) fraction implies that the activity of U(VI) reduction in the microorganisms was increased when they faced a high U(VI) content. In addition to biosorption, the bioreduction of U(VI) by AnGS occurred in the U(VI) removal process.
| Time | Anaerobic water | Anaerobic NaHCO3 | HNO3 | Recovery /% | |||
|---|---|---|---|---|---|---|---|
| Content /mg U per g SS | Fraction /% | Content /mg U per g SS | Fraction /% | Content /mg U per g SS | Fraction /% | ||
| 0 | 0.01 ± 0.001 | 14.3 ± 3.0 | 0.03 ± 0.00 | 48.2 ± 2.5 | 0.02 ± 0.004 | 37.5 ± 5.4 | 96.2 ± 5.4 |
| Day 13 | 0.80 ± 0.41 | 2.7 ± 1.4 | 12.4 ± 1.0 | 41.9 ± 3.5 | 16.4 ± 1.4 | 55.4 ± 4.8 | 92.3 ± 6.4 |
3.2. Uranium content in the EPS
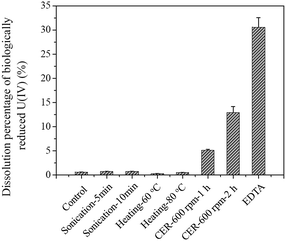
The total uranium contents of the EPS solutions extracted from AnGS using different methods were found to be largely dependent on the EPS extraction efficiency and the re-dissolution extent of insoluble U(IV) during the extraction process, as shown in Table 2 and Fig. 2 and 3. The TU contents in the EPS obtained from the control and sonication methods were low, which was consistent with their low EPS extraction efficiencies. Noticeably, the values of TUEPS/TUsludge (TUsludge = 29.5 mg U per g SS) obtained by both heating methods (60 °C for 20 min and 80 °C for 10 min) were low (2.06% and 1.20%, respectively) although a relatively high EPS content (Table 2) was extracted from the sludge. D'Abzac et al. (2010)11 and Bourven et al. (2011)12 have reported low mineral contents in heat-treated EPS extracted from AnGS and activated sludge. Therefore, the low TU content may also be attributed to the precipitation of the trapped inorganic ions (including uranyl ion) released from the disrupted EPS by heating. A significant amount of uranium was detected in the EPS solutions extracted using the EDTA method, which accounted for about 42.1% TU in the sludge (Fig. 2). This incredible uranium content should be caused by high re-dissolution extent of insoluble U(IV) during the EPS extraction process (Fig. 3). As a strong complexation agent, EDTA can dissolve the U(IV) minerals by forming U(IV)-EDTA complexes.28 For the CER extraction method, the TUEPS contents depend largely on the extraction time at the same stirring intensity. The ratio of TUEPS/TUsludge increased with the extraction time, but the re-dissolution of insoluble U(IV) also became severe when the extraction time was prolonged from 1 h to 2 h at 600 rpm. Comparatively, the extent of U(IV) re-dissolution was acceptable when the extraction time was 1 h at 600 rpm (Fig. 3).| Method | Carbohydrates | Proteins | Humic substances | Nucleic acids | Total EPS |
|---|---|---|---|---|---|
| Control | 2.27 ± 0.06 | 2.04 ± 0.09 | 0.59 ± 0.08 | 3.02 ± 0.05 | 7.92 ± 0.29 |
| Sonication-5 min | 2.26 ± 0.17 | 3.29 ± 0.11 | 1.59 ± 0.13 | 2.86 ± 0.05 | 10.0 ± 0.46 |
| Sonication-10 min | 2.38 ± 0.25 | 4.23 ± 0.23 | 0.93 ± 0.03 | 3.48 ± 0.06 | 11.0 ± 0.57 |
| Heating-60 °c | 7.27 ± 0.24 | 13.5 ± 0.43 | 13.2 ± 0.44 | 8.33 ± 0.08 | 42.3 ± 1.19 |
| Heating-80 °c | 10.9 ± 0.30 | 18.7 ± 0.21 | 10.6 ± 0.18 | 12.7 ± 0.25 | 52.9 ± 0.94 |
| CER-600 rpm-1 h | 8.5 ± 0.42 | 5.00 ± 0.40 | 14.4 ± 0.08 | 8.45 ± 0.46 | 36.4 ± 1.34 |
| CER-600 rpm-2 h | 13.5 ± 0.84 | 9.33 ± 0.19 | 9.12 ± 0.49 | 11.4 ± 0.66 | 43.4 ± 2.14 |
| EDTA | 18.8 ± 0.91 | 9.61 ± 1.10 | 10.4 ± 1.20 | 8.11 ± 0.62 | 46.9 ± 3.82 |
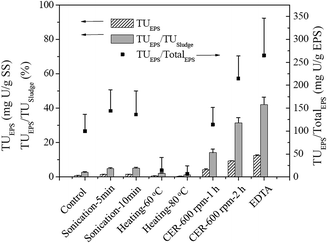 | ||
| Fig. 2 The TU content, TUEPS /TUSludge, and TUEPS/TotalEPS ratio of the EPS solution extracted from the AnGS. | ||
Fig. 1 shows the uranium contents in the EPS (expressed as TUEPS/TotalEPS) extracted using different methods. The results indicate that the control, sonication (5 min or 10 min at 150 W), and CER (1 h at 600 rpm) methods provided similar values for TUEPS/TotalEPS, which ranged from 100.1 to 144.0 mg U per g EPS. Contrary to other extraction methods, this value range may be closer to the real uranium content in EPS.
Based on the comprehensive consideration of the uranium and EPS extraction efficiency and U(IV) re-dissolution extent (Table 2 and Fig. 2 and 3), the sonication, heating, and EDTA methods were not suitable for EPS extraction to determine the species and contents of uranium in EPS because of their low uranium extraction efficiency or severe U(IV) re-dissolution. However, when the CER method was used for EPS extraction, the content of uranium in EPS (14.1 ± 2.0% TUsludge) obtained under the well controlled extraction conditions (70 g CER/g VSS, 600 rpm, 1 h) was more reasonable and reliable due to the low U(IV) re-dissolution extent, high uranium extraction efficiency, and low cell lysis extent, as proven previously.8,24 Moreover, the value of TUEPS/TotalEPS for CER (1 h at 600 rpm) (Fig. 2) confirmed the abovementioned conclusion. Then, for the 97% U(VI) removal at day 13 using AnGS (Fig. 1), the contributions were 13.7% and 83.3% for the EPS and AnGS's bulk, respectively. Actually, a higher fraction of uranium was found in the EPS of the original AnGS, extracted using the CER method of 1 h at 600 rpm, accounting for 62.0 ± 7.9% (data not shown). Comparatively, the EPS can reserve a higher fraction of uranium in the lower U(VI)-level system. The non-negligible uranium content in EPS indicated a strong uranium-accumulating ability of EPS when the AnGS immobilized uranium from groundwater.
3.3. Particulate uranium in the EPS
As shown in Table 3, some mineral uranium (>0.22 μm) in the EPS extracts was intercepted by the membrane filters, with a fraction of 2.4 ± 0.7% and 3.7 ± 0.9% for the sonication and CER methods, respectively. However, for the EPS extracts after 0.22 μm filtration, the particulate uranium was dominant as measured by ICP-MS, accounting for 80.9 ± 1.8% and 85.1 ± 2.2% for the sonication and CER methods, respectively. Upon combining the results of the two parts, it was observed that particulate uranium was the main form of uranium in the EPS. More particulate uranium in the tightly bound EPS was released using the CER method due to higher extraction efficiency of this method towards uranium.| Filtration (>0.22 μm) | ICP-MS (<0.22 μm) | Total uranium in EPS | ||||
|---|---|---|---|---|---|---|
| Soluble (%) | Particle (%) | Soluble (%) | Particle (%) | Soluble (%) | Particle (%) | |
| Sonication-10 min | 97.6 ± 0.74 | 2.4 ± 0.74 | 19.1 ± 1.8 | 80.9 ± 1.8 | 18.6 ± 2.6 | 81.4 ± 2.6 |
| CER-600 rpm-1 h | 96.3 ± 0.88 | 3.7 ± 0.88 | 14.9 ± 2.2 | 85.1 ± 2.2 | 14.3 ± 3.1 | 85.7 ± 3.1 |
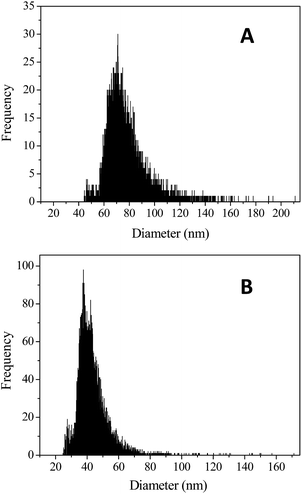
Single particle ICP-MS analysis has been successfully used in the size distribution tests of TiO2, ThO2, and UO2 colloids.29,30 As shown in Fig. 4, large amounts of nano-sized uranium particles were detected using ICP-MS. For the EPS extracts obtained using the sonication method, the size of the uranium particles was in the range from 44.3 to 211.1 nm (Fig. 4a), whereas for those obtained using the CER method, it ranged from 24.7 to 171.3 nm (Fig. 4b). The size of the uranium-containing particles in the EPS extracts obtained using the CER method (mean size of 46.0 nm) was much smaller than that obtained using the sonication method (mean size of 79.5 nm); this indicated that smaller uranium colloids occupied the tightly bound EPS. As reported by William et al. (2008), about 3.0 nm uraninite was formed by Shewanella oneidensis MR-1 and associated with EPS, as revealed by scanning electron microscopy (SEM).31 The much larger EPS-associated uranium colloids observed in this study may be due to the accumulation or growth of uranium nuclei with time. Moreover, even solids larger than 0.22 μm were present in the EPS.
It should be noted that the particle size obtained by ICP-MS was based on the detection of the signals of elemental uranium and not those of the real uranium-containing solids. While uranium is extremely larger than other element atoms (e.g. oxygen, phosphorus, etc.), its particle size could be very close to the real value.
The abovementioned results suggested that uranium particles were present in the EPS in the form of both micron-size and nano-size particles, which included U(VI) minerals or U(IV) minerals, or the both. Although difficult, analysis of the uranium valence states in minerals using SEM coupled with energy dispersive X-ray spectroscopy11,12 or other technologies in future investigations is necessary.
3.4. EPS-induced U(IV) mineral formation in a NaHCO3 medium
After 24 h reaction under anaerobic and aerobic conditions, the EPS extracted from the original AnGS can immobilize 21.9 ± 0.69% and 18.4 ± 0.26% U(VI) (Table 4), respectively. Hence, bioreduction also contributed to the U(VI) immobilization, accounting for 15.8 ± 1.49% of the U(VI) totally removed using the AnGS's EPS. The ratio of U(VI) removal via bioreduction in our study was lower than that observed for Shewanella sp. HRCR-1;18 this may be attributed to the difference in the active reducing enzyme contents in each EPS due to the excellent ability of dissimilatory metal reduction by Shewanella or the difference in the experimental operations (such as the pre-reduction of EPS by sodium dithionite in their tests). In addition, since bioreduction is inevitable even under aerobic conditions, bioreduction may be undervalued. Regardless of the different contributions from bioreduction in the uranium immobilization by EPS, two roles including biosorption and bioreduction were confirmed for microbial EPS in uranium remediation.| Time | Anaerobic removal (%) | Aerobic removal (%) | Adsorption contribution (%) | Reduction contribution (%) |
|---|---|---|---|---|
| 12 h | 8.12 ± 0.29 | 7.05 ± 0.28 | 86.8 ± 0.46 | 13.2 ± 0.46 |
| 24 h | 21.9 ± 0.69 | 18.4 ± 0.26 | 84.2 ± 1.49 | 15.8 ± 1.49 |
As indicated by previous studies, cytochrome C and flavins in EPS obtained from electroactive bacteria, such as Shewanella sp., have shown redox abilities towards metal ions.16,32,33 For the EPS of AnGS, UV/visible absorption spectroscopy was used to identify the presence of riboflavin or cytochromes C. The results indicated that cytochrome C was not detected in the EPS of AnGS. On the other hand, the two peaks at about 225 and 258 nm (data not shown) may be the characteristic peaks of riboflavin,32 implying the presence of extracellular riboflavin in the U(VI) reduction process. As indicated by Tapia-Rodriguez et al. (2010), Desulfovibrio and Clostridium spp., known as U(VI)-reducing bacteria, were identified in the AnGS cultured in brewery or related effluents. However, these bacteria could be rare in AnGS, even after stimulation by a high concentration of U(VI). Therefore, the extracellular cytochromes C may be too low to be detected or even not present. Interestingly, riboflavin may act as an electron mediator for U(VI) reduction by EPS. Moreover, humic substances may play roles in the U(VI) reduction process by the EPS of AnGS.33
The important roles of EPS in affecting the mobility of uranium in groundwater should deserve more attention. Notably, to simulate real groundwater environments, 1 g L−1 NaHCO3 was used as a buffer and complexed with uranyl ions to form uranyl–carbonate complexes. Therefore, as compared to the report on the reaction between EPS obtained from Shewanella sp. HRCR-1 and a pure uranyl solution,18 this study was closer to the reality occurring in uranium-contaminated groundwater.
3.5. The implications of this study
As a permeable hydrogel layer around cells, EPS are the inevitable pathway for mass transfer between microorganisms and the environment.34,35 Considering its great ability in uranium accumulation, in situ transformation of soluble U(VI) to insoluble U(IV) via bioreduction should be facilitated, which is favorable towards preventing the migration of uranium with groundwater and maintaining the bioactivity of AnGS.The results of uranium immobilization by AnGS without external electron donors proved the availability of the endogenous substrate, serving as electron donors for uranium bioreduction, which was in accordance with a previous study.23 Actually, a considerable part of the endogenous substrate was provided by EPS itself (especially the carbohydrates in EPS) or its degradation. Hence, EPS may also provide electron donors for the U(VI) bioreduction process; this has been observed in H2 production using AnGS when the substrate is in short supply.36 To clarify this, monitoring the variation of the contents of carbohydrates, proteins, and humic substances in the EPS during the entire process of uranium immobilization by AnGS is necessary.
Since a strong uranium-accumulating ability was found in the EPS of AnGS (this work) and Shewanella sp. HRCR-1,18 the interaction between the EPS and uranium needs further investigation.
4. Conclusions
Significant amount of uranium was found in the EPS of AnGS-treated uranium-containing groundwater, accounting for 12–16% TUsludge (extracted using the CER method with 70 g CER/g VSS at 600 rpm for 1 h). The results showed that uranium associated with EPS could be present in two forms: the soluble and particulate form. In addition to biosorption, bioreduction also contributed to uranium immobilization by the AnGS's EPS. The significant amount of uranium in the EPS matrix offers a new insight into the characteristics of extracellular uranium in the process of uranium immobilization for groundwater remediation using AnGS.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors wish to thank the NSFC (21407133, 21571163, and 51608498) and the Radiochemistry 909 Program in China Academy of Engineering Physics (CAEP) for providing partial support for this study.References
- W. M. Wu, J. Carley, T. Gentry, M. A. Ginder-Vogel, M. Fienen, T. Mehlhorn, H. Yan, S. Caroll, M. N. Pace, J. Nyman, J. Luo, M. E. Gentile, M. W. Fields, R. F. Hickey, B. Gu, D. Watson, O. A. Cirpka, J. Zhou, S. Fendorf, P. K. Kitanidis, P. M. Jardine and C. S. Criddle, Environ. Sci. Technol., 2006, 40, 3986–3995 CrossRef CAS PubMed.
- S. Sarri, P. Misaelides, D. Zamboulis, L. Papadopoulou and J. Warchoł, J. Radioanal. Nucl. Chem., 2013, 295, 1731–1736 CrossRef CAS.
- M. Martins, M. L. Faleiro, A. M. R. da Coasta, S. Chaves, R. Tenreiro, A. P. Matos and M. C. Costa, J. Hazard. Mater., 2010, 184, 89–96 CrossRef CAS PubMed.
- L. Newsome, K. Morris and J. R. Lloyd, Chem. Geol., 2014, 363, 164–184 CrossRef CAS.
- U. Suzuki, H. Mukai, T. Ishimura, T. D. Yokoyama, S. Sakata, T. Hirata, T. Iwatsuki and T. Mizuno, Sci. Rep., 2016, 6, 1–6 CrossRef PubMed.
- A. Tapia-Rodriguez, V. Tordable-Martinez, W. Sun, J. A. Field and R. Sierra-Alvarez, Biotechnol. Bioeng., 2011, 108, 2583–2591 CrossRef CAS PubMed.
- A. Luna-Velasco, R. Sierra-Alvarez, B. Castro and J. A. Field, Biotechnol. Bioeng., 2010, 107, 933–942 CrossRef CAS PubMed.
- H. L. Zhang, W. Fang, Y. P. Wang, G. P. Sheng, R. J. Zeng, W. W. Li and H. Q. Yu, Environ. Sci. Technol., 2013, 47, 11482–11489 CrossRef CAS PubMed.
- J. Xu, G. P. Sheng, Y. Ma, L. L. Wang and H. Q. Yu, Water Res., 2013, 47, 5298–5306 CrossRef CAS PubMed.
- L. L. Li, Z. H. Tong, C. Y. Fang, J. Chu and H. Q. Yu, Water Res., 2015, 70, 1–8 CrossRef PubMed.
- P. D'Abzac, F. Bordas, E. Joussein, E. van Hullebusch, P. N. L. Lens and G. Guibaud, Environ. Sci. Technol., 2010, 44, 412–418 CrossRef PubMed.
- I. Bourven, E. Joussein and G. Guibaud, Bioresour. Technol., 2011, 102, 7124–7130 CrossRef CAS PubMed.
- A. W. Decho, Ecol. Eng., 2010, 36, 137–144 CrossRef.
- Y. M. Lin, J. P. Bassin and M. C. M. van Loosdrecht, Water Res., 2012, 46, 986–992 CrossRef CAS PubMed.
- L. Castro, R. Y. Zhang, J. A. Munoz, F. Gonzalez, M. L. Blazquez, W. Sand and A. Ballester, Biofouling, 2014, 30, 501–511 CrossRef CAS PubMed.
- S. W. Li, X. Zhang and G. P. Sheng, Environ. Sci. Pollut. Res., 2016, 23, 8627–8633 CrossRef CAS PubMed.
- C. C. Li, Y. J. Wang, F. Dang and D. M. Zhou, J. Hazard. Mater., 2016, 308, 21–28 CrossRef CAS PubMed.
- B. Cao, B. Ahmed, D. W. Kennedy, Z. Wang, L. Shi, M. J. Marshall, J. K. Fredrickson, N. G. Isern, P. D. Majors and H. Beyenal, Environ. Sci. Technol., 2011, 45, 5483–5490 CrossRef CAS PubMed.
- L. Macaskie, K. Bonthrone, P. Yong and D. Goddard, Microbiol., 2000, 146, 1855–1867 CrossRef CAS PubMed.
- S. C. Yuan, S. B. Xie, Z. Yan and H. Ling, Municipal Engineering Technology, 2012, 30, 97–100 Search PubMed , in Chinese.
- B. Cao, L. Shi, R. Brown, Y. Xiong, J. Fredrickson, M. Romine, M. Marshall, M. Lipton and H. Beyenal, Environ. Microbiol., 2011, 13, 1018–1031 CrossRef CAS PubMed.
- N. Fernandez, E. E. Diaz, R. Amils and J. L. Sanz, Microb. Ecol., 2008, 56, 121–132 CrossRef CAS PubMed.
- A. Tapia-Rodriguez, A. Luna-Velasco, J. A. Field and R. Sierra-Alvarez, Water Res., 2010, 44, 2153–2162 CrossRef CAS PubMed.
- B. Frølund, R. Palmgren, K. Keiding and P. H. Nielsen, Water Res., 1996, 30, 1749–1758 CrossRef.
- D. A. Elias, J. M. Senko and L. R. Krumholz, J. Microbiol. Methods, 2003, 53, 343–353 CrossRef CAS PubMed.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association, Washington, DC, 1995 Search PubMed.
- J. H. Huang, E. J. Elzinga, Y. Brechbuehl, A. Voegelin and R. Kretzschmar, Environ. Sci. Technol., 2011, 45, 2804–2810 CrossRef CAS PubMed.
- Y. Suzuki, K. Tanaka, N. Kozai and T. Ohnki, Geomicrobiol. J., 2010, 27, 245–250 CrossRef CAS.
- C. Degueldre and P. Y. Favarger, Colloids Surf., A, 2003, 217, 137–142 CrossRef CAS.
- C. Degueldre, P. Y. Favarger, R. Rossé and S. Wold, Talanta, 2006, 68, 623–628 CrossRef CAS PubMed.
- D. B. William, J. T. McDonough, J. M. Senko, G. Zhang, A. C. Dohnalkova, S. D. Kelly, Y. Gorby and K. M. Kemner, Geochim. Cosmochim. Acta, 2008, 72, 4901–4915 CrossRef.
- Y. Xiao, E. Zhang, J. Zhang, Y. Dai, Z. Yang, H. E. M. Christensen, J. Ulstrup and F. Zhao, Sci. Adv., 2017, 3, e1700623 CrossRef PubMed.
- Y. Xiao and F. Zhao, Current Opinion in Electrochemistry DOI:10.1016/j.coelec.2017.09.016.
- S. Wang, M. Gao, Z. Wang, Z. She, C. Jin, Y. Zhao and Z. Li, RSC Adv., 2015, 5, 30737–30747 RSC.
- P. Xia, Q. Li, L. Tan, X. Sun, C. Song and S. Wang, RSC Adv., 2016, 6, 59438–59444 RSC.
- G. P. Sheng and H. Q. Yu, Appl. Microbiol. Biotechnol., 2007, 74, 208–214 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |