Open Access Article
Open Access ArticleEffects of optical-inert ions on upconversion luminescence and temperature sensing properties of ScVO4:10%Yb3+/2%Er3+ nano/micro-particles†
Peng Lia,
Linna Guo *a,
Chenxi Lianga,
Tiesheng Li*a,
Penglei Chen
*a,
Chenxi Lianga,
Tiesheng Li*a,
Penglei Chen b,
Minghua Liu
b,
Minghua Liu *b and
Yangjie Wu
*b and
Yangjie Wu *a
*a
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, The Key Lab of Chemical Biology and Organic Chemistry of Henan Province, The Key Lab of Nano-information Materials of Zhengzhou, Zhengzhou, 450001, P. R. China. E-mail: guolinna@zzu.edu.cn; lts34@zzu.edu.cn
bBeijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 3rd November 2017
Abstract
In this paper, ScVO4:10%Yb3+/2%Er3+ nano/micro-particles doped with optical-inert metal ions including the alkali metal ions (Li+/Na+/K+), alkaline-earth metal ions (Mg2+/Ca2+/Sr2+/Ba2+) and lanthanide ions (Y3+/Gd3+/Lu3+) were synthesized by a conventional solid-state method. X-ray diffraction studies show that the prepared ScVO4:10%Yb3+/2%Er3+ whether single-doping, codoping or tridoping optical-inert metal ions are highly crystalline in nature with tetragonal phase structure when the doping concentration ≤ 10%. Under a 980 nm laser diode excitation, the upconversion luminescence was enhanced significantly by single doping of Li+, Na+, K+, Mg2+, Ca2+, Sr2+, Ba2+, Y3+, Gd3+ or Lu3+, showing the strongest green emission with 5 mol% Li+ dopant. For codoping optical-inert metal ions system, it is found that Li+/Gd3+ couple is the most effective codopant, leading to an drastic increase of the UC luminescence centered at 554 nm by a factor of 15.3 compared to optical-inert metal ions free sample, while the factor of Li+/Ca2+/Gd3+ tridoping system is only 4.6. This work aims to investigate the origin of UC luminescence enhancement for ScVO4:10%Yb3+/2%Er3+ after codoping optical-inert ions based on the systematical analyses of the structures, morphologies, chemical states of elements, oxygen defects, optical absorption properties, etc. Furthermore, temperature-sensing performance was also investigated using the fluorescence intensity ratio technique. This opens a new window for studying the cooperation of the optical-inert ions doping effect on improving UC luminescence and temperature sensitivity properties.
1 Introduction
Infrared-to-visible upconversion (UC) of rare-earth (RE) ion-doped phosphors (UCPs) has been studied extensively in the past few years, due to their huge potential applications in the fields of solid-state lasers,1,2 3D display,3,4 fluorescence labeling,5,6 bioimaging,7,8 therapy9,10 and temperature sensor,11–13 etc. Despite the renewed interests and rapid progress being made, the UC efficiency of the currently available materials is not high enough for truly wide spread applications. It is therefore imperative to find ways to significantly improve the efficiency. Generally, the UC intensity and quantum efficiency under excitation at 980 nm is governed by the following three factors: (1) high electronic transition probabilities of the dopants, (2) the energy transfer rate from sensitizer (Yb3+) to emission centers (Tm3+, Er3+, Ho3+ etc.) and (3) the last but the most important, the intensity of Ln3+-doped UC materials strongly depends on intra 4f–4f transition probabilities and is also influenced by the crystal field symmetry and crystallinity of the samples.14 In general, there were some strategies that have been employed to achieve high efficiency in UC materials: (1) selection of novel host materials; (2) tailoring local crystal field; (3) plasmonic enhancement; (4) engineering energy transfers; (5) suppression of surface-related deactivations;9 and (6) the effective Bragg reflection of photonic crystals.15,16 Among them, the more simple and commonly used method is tailoring local crystal field. Therefore, it is possible to modulate UC emissions by tailoring the local crystal field of the luminescent centers in an inorganic host matrix. As is well known that beyond these sensitizer (Yb3+) and activators (Tm3+, Er3+, Ho3+ etc.), the periodic table also provides a rich diversity of optical-inert elements that can be used to modulate emission, or to alter the symmetry around activators, thus influencing UC luminescence (UCL) properties by altering electronic transition probabilities and enhance the UC efficiency. Meanwhile, it is more cheap and economic to enhance UCL intensity through doping the majority of optical-inert ions. By this means, common optical-inert ions include alkali metal ions (Li+, Na+, K+), alkaline-earth metal ions (Ca2+, Mg2+, Sr2+, Ba2+), transition metal ions (Mn2+, Fe3+) and some other inactive lanthanide ions (Ln3+ = Gd3+, Lu3+) have been doped into various UCPs to modulate their luminescence properties.17–22For example, Chen et al.23 discovered a 10 times increase in blue emissions (1D2 → 3F4, 1G4 → 3H6) in Gd2O3:Yb3+,Tm3+ nanoparticles by doping with 6% Li+. Song's group24 demonstrated 10 and 4 times increases in red (5F4, 5S2 → 5I8) and green (5F5 → 5I8) emissions in Y2O3:3%Li+,4%Yb3+,1%Ho3+ nanoparticles. Hom et al.25 reported that the NIR to NIR UC emission intensity of 10 mol% K+ substituted ZnMoO4:Tm3+,Yb3+ nanocrystals increased by 21-fold compared with K+ free sample. Our group26 found that white UC emission was achieved in the Lu6O5F8:6%Yb3+,0.3%Er3+,0.4%Tm3+, 5%Li+ compared to Li+ free sample with the same activator concentration, besides, the integrated UC emission intensity of Lu6O5F8:20%Yb3+,1%Er3+, 3%Li+ is 5.5 times as strong as that of commercial UC phosphor (NaYF4:20%Yb3+,2%Er3+). Aside from alkali metal ions, alkaline-earth metal ions can also be used to tailor the local crystal field. Chen and Wang27 explored that UC luminescence intensities of NaGdF4:Yb3+/Er3+ were enhanced by about 200 times upon introducing Ca2+ dopants into the phosphors, probably due to a modification of the crystal structure of NaGdF4 and an improvement in the crystallinity. Haase and co-workers28 prepared CaAl12O19:Mg2+,Yb3+,Er3+ UCPs, in which the green and red emissions of the Er3+ ions was improved 4 and 1.5 times, respectively, compared with the counterparts without Mg2+ ions. On the other hand, not only the local site symmetry, but also phase transition may be induced with a higher concentration of host-ion substitution by non-active Ln3+. Liu and co-workers29 investigated a NaYF4:Er3+,Yb3+ system in which host ion substitution influences the nanocrystal growth process to give simultaneous control over the crystallographic phase, size and optical emission properties of the resulting nanocrystals. It was demonstrated that NaYF4 nanocrystals can be rationally tuned in size (down to ten nanometres), phase (cubic or hexagonal) and UC emission color (green to blue) as well as intensity by replacing Y3+ with Gd3+. Zhang et al.30 discovered that the UC enhancement with size decrease has been realized in β-NaLuF4:Yb3+/Er3+ nanocrystals (NCs) through doping with Y3+ ions. Compared with β-NaLu0.78Yb0.2Er0.02F4 and β-NaY0.78Yb0.2Er0.02F4 prepared under the same condition, the green UC emission is enhanced by a factor of 1.8 and 16, respectively, for β-NaLu0.48Y0.3Yb0.2Er0.02F4. UC luminescence of NaY0.95−xYb0.03Er0.002ScxF4 was enhanced obviously by tridoping Sc3+ ions, contrasted to the untridoped one, especially for higher energy emission.21 Comprehensively, it shows a rising trend, indicating that optical-inert ions are becoming a more and more important and hot topic in the field of UCL enhancement. It can be concluded from above reports, most researchers have studied the effect of co-doping with alkali metal ions, alkaline-earth metal ions and inactive Ln3+ ions individually on the structural and UCL properties of phosphors.31–33 Comparatively, there is rare research about the effect of combination these optical inert metal ions doping and lack of knowledge on the intercommunication between codopants on the UC luminescence in controlling the properties of UCPs to the best of our knowledge.
In addition, reasons for the UC emission intensity enhancement through optical-inert co-dopants is yet to be determined with complete certainty, and this investigation is still an important and challenging research field. Most reports described the experiment results to the following fact. Some optical inert ions such as Li+ may directly act as a flux or sensitizer, and the others could enter into the host lattice, creating oxygen vacancies or altering the crystal field surrounding the activator, then affecting the luminescence performances of the phosphors.34–39 It is very important to inquire into the characteristics of codopants so as to further understand the mechanism of enhanced luminescence, and also help us to look for some more effective codoping ions. Such studies would be further helpful to understand the mechanisms involved in enhanced luminescence of optical-inert ions and RE co-doped materials.
In our previous work,40 it is demonstrated that ScVO4:Yb3+/Er3+ is a green-emitting phosphor with good monochromaticity, while its emission efficiency still needs for the further improvement. So in this work, we focused on the UCL enhancement of ScVO4:Yb3+/Er3+ through different kinds of optical-inert ions (Li+/Na+/K+, Mg2+/Ca2+/Sr2+/Ba2+ and Y3+/Gd3+/Lu3+) single doping as well as combination doping. In order for horizontal comparison, the concentrations of Li+/Na+/K+, Mg2+/Ca2+/Sr2+/Ba2+ and Y3+/Gd3+/Lu3+ are chosen to be with the same value, respectively. Meanwhile, combination these optical inert metal ions is a wishful and challengeable task since their radius and valance are different. Overall, the present work provides a comparative study including some powerful evidences and a deep understanding on the origin of UCL enhancement for the ScVO4:Yb3+/Er3+ phosphors after optical-inert ions doping in either way.
On the other hand, during the operation of electronic and photonic devices, temperature is needed to be monitored for the best performance. Therefore, accurate sensing and mapping of temperature in a non-invasive way is a challenging field of research.41–44 Hence, this research-need motivated us to tailor the structural and UCL properties of ScVO4:Yb3+/Er3+ nano/microcrystals with optical-inert ions incorporation and to study the temperature sensing performance. Based on the above points, in this work, we employed a modified molten salt method to prepare Yb3+/Er3+-codoped ScVO4 UCPs, adjusting UC luminescence and temperature-sensing performance by codoping optical-inert ions (Li+/Na+/K+, Mg2+/Ca2+/Sr2+/Ba2+ and Y3+/Gd3+/Lu3+), to achieve the purpose of kill two birds with one stone.
Comprehensively, it shows a rising trend, indicating that optical-inert ions are becoming a more and more important and hot topic in the field of UCL enhancement. It can be concluded from above reports, most researchers have studied the effect of co-doping with alkali metal ions, alkaline-earth metal ions and inactive Ln3+ ions individually on the structural and UCL properties of phosphors. Comparatively, there is rare research about the effect of combination these optical inert metal ions doping and lack of knowledge on the intercommunication between codopants on the UC luminescence in controlling the properties of UCPs to the best of our knowledge.
2 Experimental
2.1 Synthesis
The raw materials Sc2O3 (99.99%), Yb2O3 (99.99%), Er2O3 (99.99%), and NH4VO3 (A. R.) were used directly without any further purification. The stoichiometric raw materials were ground in an agate mortar and heated to 800 °C for 4 h in air. Then the as-synthesized samples were slowly cooled to room temperature.2.2 Characterization
The phase purity was determined using a Rigaku D/MAX-2400 powder X-ray diffractometer (XRD) with Cu Kα radiation (λ = 1.54178 Å) operating at 40 kV and 60 mA. The size, shape and structure of the as-prepared samples were characterized by SEM (S-4800). The room temperature Raman spectra were measured at a backscattering geometry using an Ar-ion laser (Coherent Innova 70) operating at 488 nm as the excitation source. Spectra were recorded by a double grating spectrometer (Spex 1403) equipped with standard photon counting equipment. XPS In the measurements of UC emission spectra, a continuous 980 nm laser diode (LD) with maximum power of 1.0 W was used as an excitation source, and the emission was collected using a HORIBA JOBIN YVON Fluorolog-3 spectrofluorometer system. The chemical components were analyzed by Fourier transform infrared spectroscopy (FT-IR, Nicolet NEXUS 670). All the spectral measurements were performed at room temperature.3 Results and discussion
3.1 Effects of optical-inert ions on phase and morphology of ScVO4:10%Yb3+/2%Er3+
Fig. 1a shows the XRD patterns of optically inert ions (Li+, Ca2+, Gd3+) single-doped, co-doped or tri-doped ScVO4:10%Yb3+/2%Er3+ phosphors. The diffraction peaks of ScVO4:Yb3+/Er3+ (optically inert ions free) can be well indexed, following a pure zircon-type structure. The main diffraction peaks shift toward smaller angles corresponding to the standard pattern of ScVO4 (JCPDS No. 06-0260) referenced below, indicating that the substitution of Sc3+ ions by Yb3+/Er3+ ions cause the host lattice to expand because the ionic radii of Yb3+ (0.86 Å), Er3+ (0.88 Å) is larger than that of Sc3+ (0.75 Å). Besides, it was noticed that the samples were still well crystalline and no other impurity phase was generated upon optically inert ions doping. To further evaluate the effect of optically inert ions on crystal structure, the main diffraction peaks (220) are compared for ScVO4:10%Yb3+/2%Er3+ with different kinds of optically inert ions doped or not, as show in Fig. 1b and c. Although the main diffraction peak moves irregular with doping one, two or three kinds of optically inert ions, it is clear that the peak shift towards smaller angles on the whole. In the tetragonal ScVO4 host lattice, the optically inert ions (Li+, Ca2+ or Gd3+) can enter ScVO4 crystal site through substituting for the Sc3+ ions or interstitial sites or coexist in the two ways. Especially, it is more complicated that two or three optically inert ions co-doped into the system of ScVO4:Yb/Er. According to the Bragg's law 2dsinθ = nλ, where d is the interplanar distance, θ is the diffraction angle, and λ is the diffraction wavelength. Regardless optically inert ions (Li+ (0.76 Å), Ca2+ (1.00 Å) or Gd3+ (0.94 Å)) with larger radius substituted the Sc3+ (0.75 Å) or occupy the interstitial sites, the interplanar distance increased, resulting in the (220) position shifting toward the lower 2θ angle. Our experimental result is in accordance with this theoretical analysis, while different optical inert ions doping or doping method would cause the XRD peak of (220) to produce different degrees of shift to the left. | ||
| Fig. 1 (a) XRD patterns, (b) shifting of main diffraction peaks, (c) changes in main diffraction peaks position of Li+, Ca2+, Gd3+ doped ScVO4:10%Yb3+/2%Er3+ (“No” represents ScVO4:10%Yb3+/2%Er3+). | ||
3.2 SEM studies
The surface morphology and crystallinity of solid host materials are important parameters which determine the emission characteristics of phosphors. The microstructural analysis of ScVO4:10%Yb3+/2%Er3+ and optically inert ions doped ScVO4:10%Yb3+/2%Er3+ samples was performed with the help of SEM examination (Fig. 2). From Fig. 2, it is clear from the SEM image that particles with the variation sizes are not uniformly distributed throughout the surface, which is mainly caused by the inhomogeneous distribution of temperature during synthesis of the material by the solid-state technique. On the whole, the synthesized particles without optically inert ions doping appear chips-like shape, whereas cobblestone-like shape and agglomerated irregular polyhedron morphology particles were obtained when Ca2+ or Li+ ions were doped, while chips-like shape was basically remained when Gd3+ doped. From the local point of view, Fig. 2a shows that agglomeration chips-like nanoparticles with diameter of about 140–400 nm and length of about 750–1770 nm were obtained in the ScVO4:10%Yb3+/2%Er3+. As shown in Fig. 2b, when Gd3+ was further doped into the ScVO4:10%Yb3+/2%Er3+ sample, the morphology did not change greatly, remained the chips-like shape except the size were enlarged of diameter ranging from 344 nm to 707 nm and length 1134–1879 nm, due to the radius of Gd3+ is similar with that of Sc3+. In sharp contrast, when the smallest metal ions Li+ doped into the lattice, the SEM image (Fig. 2c) display an irregular stone-like with corner angle appearance, and the surface just looks completely smooth and integrated. If Ca2+ was introduced into the ScVO4:10%Yb3+/2%Er3+ system, the resulting nanoparticles appear nearly smooth and much more uniform cobblestone in shape. Whereas, as shown in Fig. 2e–g, if doping combination two kinds of Li+, Ca2+ or Gd3+ into ScVO4:10%Yb3+/2%Er3+, it is worth noting that the surface just looks coarse and imperfect, and the size are not uniform, ranging from 200–1000 nm. Especially for Li+/Ca2+/Gd3+ tri-doped into ScVO4:10%Yb3+/2%Er3+, as displayed in Fig. 2h, both diameter and length decreased more quickly, resulting in small agglomerated nanoparticles with size of 200–500 nm. These results obviously indicate that the optically-inert ions doping indeed alters the particle growth process. To express the changeable morphology and size with different optical-inert ions clearly, the detailed size and shape of all these samples were listed in Table 1.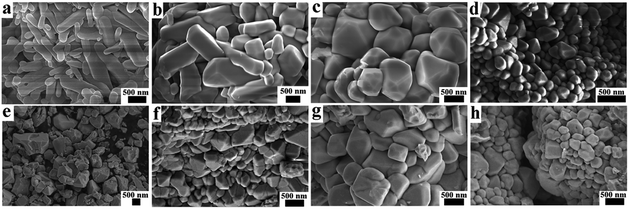 | ||
| Fig. 2 SEM of (a) optically-inert ions free, (b) Gd3+, (c) Li+, (d) Ca2+, (e) Li+/Gd3+, (f) Ca2+/Gd3+, (g) Li+/Ca2+, (h) Li+/Ca2+/Gd3+ co-doped ScVO4:10%Yb3+/2%Er3+. | ||
| Optically-inert ions | Morphology | Size (nm) |
|---|---|---|
| No | Chips-like | Length: 750–1770; diameter: 140–440 |
| Li+ | Smooth stone | 971–1208 |
| Ca2+ | Cobblestone | 176–317 |
| Gd3+ | Chips-like | Length: 1134–1879; diameter: 344–707 |
| Li+/Ca2+ | Smooth stone | 400–800 |
| Li+/Gd3+ | Stone fragments | 400–1400 |
| Ca2+/Gd3+ | Smooth stone | 200–600 |
| Li+/Ca2+/Gd3+ | Rough stone | 200–500 |
3.3 UC photoluminescence properties
 | ||
| Fig. 3 XRD patterns (a), UC emission spectra (b) of Li+ (1–10 mol%) doped ScVO4:10%Yb3+/2%Er3+ samples. | ||
As well as Li+, other alkali metal ions like Na+ or K+ might possibly influence the final crystal structure and UC luminescence, so Li+, Na+ or K+ with the same doping concentration 5 mol% were studied and compared in a similar way. The XRD patterns of different alkali metal ions doped ScVO4:10%Yb3+/2%Er3+ are presented in supporting Fig. S1a.† All of the XRD patterns could clearly be indexed to the pure tetragonal phase of ScVO4 (JCPDS No. 06-0260), and no trace of other phases or impurities were observed, indicating all the optical-inert ions and Yb3+/Er3+ ions are incorporated into the ScVO4 host matrix and formed a solid solution structure. The UC luminescent performance of alkali metal ions doping ScVO4:10%Yb3+/2%Er3+ was studied, as exhibited in Fig. S1b.† Obviously, addition of alkali metal ions significantly intensified the UC emission intensities of all the two emission bands and the trend of increment is the same for the two emission bands as: Li+ > Na+ > K+ > No alkali metal ions. It is also observed that all the UC bands are splitted into several Stark components. The UC luminescence splitting from 4S3/2 → 4I15/2 and 2H11/2 → 4I15/2 transitions of Er3+ was observed results from the coordination field effect of host matrices. Similar phenomenon were also observed in previous reports.45
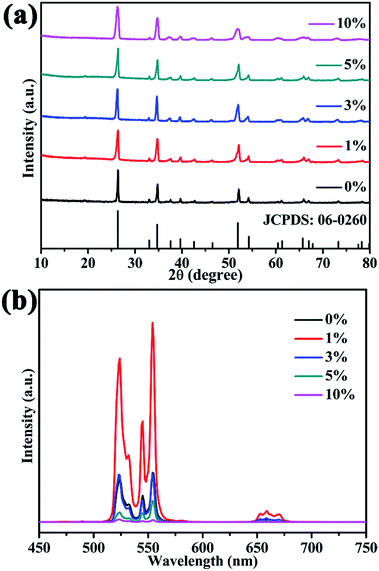 | ||
| Fig. 4 XRD patterns (a), UC emission spectra (b) of Ca2+ (1–10 mol%) doped ScVO4:10%Yb3+/2%Er3+ samples. | ||
Similarly, other alkaline earth metal ions (Mg2+, Sr2+ or Ba2+) with doping concentration 1 mol% were also studied to investigate their influence on the final crystal structure and UCL properties. Fig. S2a† depicts XRD patterns of the ScVO4:10%Yb3+/2%Er3+ doped with alkaline earth metal ions (Mg2+, Sr2+ or Ba2+), all the diffraction peaks of samples still correspond to the tetragonal structure (JCPDS No. 06-0260) and no other impurity phase was detected. The normalized UC emission spectra of calcined ScVO4:10%Yb3+/2%Er3+ doped with alkaline earth metal ions (Mg2+, Ca2+, Sr2+ or Ba2+) are shown in Fig. S2b.† Obviously, addition of alkaline earth metal ions enhanced the UC emission intensities and the trend of increment is as: Sr2+ > Ca2+ > Mg2+ > Ba2+ > No alkaline earth metal ions. Doping with alkaline earth metal ions (Mg2+, Ca2+, Sr2+ or Ba2+) intensified the UC emission by almost 2.5, 3.7, 4.7 and 2.3 fold to that of optically inert ions-absent sample, respectively.
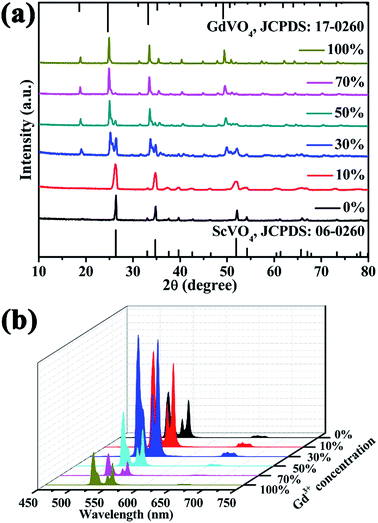
In order to reveal the concentration-dependent UCL properties and obtain the optimum concentration of Gd3+ ions doping in host lattice, the Gd3+ concentration dependent UCL spectra of the ScVO4:10%Yb3+/2%Er3+ samples are shown in Fig. 5b. As can be evidently seen that the doping of Gd3+ ion (even Gd3+ ion concentration reached 100 mol%) cannot change the position of typical emission of Er3+. The UC emission intensity enhanced with rising Gd3+ doping content from 0 to 30 mol%, and then started to weaken when the Gd3+ concentration exceeds 30 mol%. The UC emission intensities at 554 nm in ScVO4:10%Yb3+/2%Er3+ nanocrystals doped with 30 mol% Gd3+ are about 3.2 times than that of Gd3+-absent sample. The red emission intensity has a little change after Gd3+ introducing. In stark contrast, the UC luminescence intensity of GdVO4:10%Yb3+/2%Er3+ (Gd3+ content reaches up to 100 mol%) is 1.2 times lower than that of ScVO4:10%Yb3+/2%Er3+ (Gd3+ concentration 0 mol%), indicating that ScVO4 is more suitable as UCL host than GdVO4.
From the analysis above, when the Gd3+ content is less than or equal to 10 mol%, all the X-ray diffraction peaks of the sample can be well indexed as pure ScVO4 phase. Therefore, the 10 mol% doping content of Y3+, Lu3+ was chosen for the following investigations. Firstly, XRD patterns of these samples are shown in Fig. S3.† All the diffraction peaks can be indexed to those of the tetragonal phase ScVO4 (JCPDS card No. 06-0260). Influences of non-luminescent Y3+, Gd3+ or Lu3+ dopant on the UCL properties of ScVO4:10%Yb3+/2%Er3+ phosphors were compared and studied, as shown in Fig. S3b.† Obviously, addition of Y3+/Gd3+/Lu3+ significantly intensified the UC emission at the green as well as red region and the order of increment was Lu3+ > Gd3+ > Y3+ > inactive ions free. It is worthwhile pointing out that substitution of 10 mol% Lu3+ intensified the UC emission by almost 3.4 fold than that of non-active RE ions free-sample.
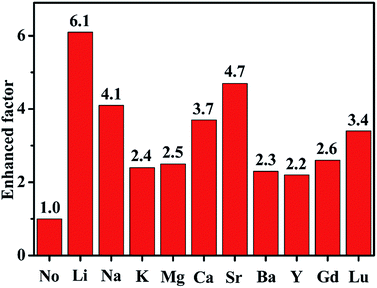
Fig. 6 summaries the UCL intensity of ScVO4:10%Yb3+/2%Er3+ with various optical-inert ions single substitution. It can be seen clearly that optical-inert ions doping enhanced the UCL intensity in different degree. Among them, 5 mol% Li+ doping shows the biggest enhancement, by almost 6.1-fold compared to that of ScVO4:10%Yb3+/2%Er3+
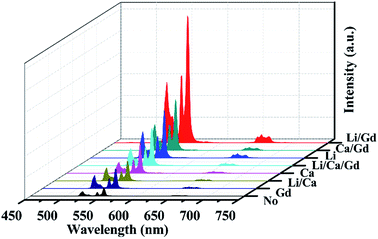
No matter in which kind of doping way, the UC emission peak positions remain unaltered, while the UCL intensity varied differently. Among these samples, the Li+/Gd3+ codoped ScVO4:10%Yb3+/2%Er3+ phosphor has the highest emission intensity, and the intensity of UC luminescence enhanced by a factor of 15.3 compared to the optical-inert ions free sample, and the codoping of Ca2+/Gd3+ ions followed. That is to say the combination of Li+/Gd3+ or Ca2+/Gd3+ present more excellent UCL intensity than that of corresponding optical-inert ions single doped or free samples. While, the combination of Li+/Ca2+/Gd3+ or Li+/Ca2+ weakened the UCL intensity compared to that of the Li+ or Ca2+ single doped samples, respectively.
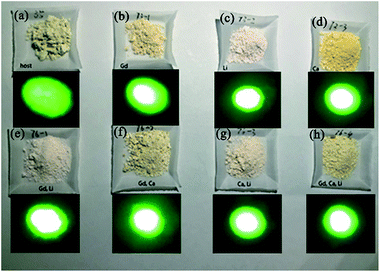
Fig. 8 shows the emission colors of optical-inert ions doped ScVO4:10%Yb3+/2%Er3+ samples under excitation at 980 nm. The apparent color difference in the digital images (collected using a Canon Power Shot G7) also show that optical-inert ions doped ScVO4:10%Yb3+/2%Er3+ samples has bright green emission under NIR laser excitation. Furthermore, the green light spot is biggest in the Li+/Gd3+ codoped system, and smallest in ScVO4:10%Yb3+/2%Er3+ samples, which is agree well with the measured spectra results.
3.4 Reasons for UC emission intensity enhanced by non-optical ions doping
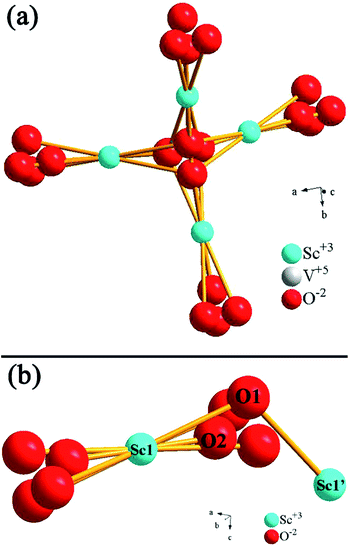
To reveal the reasons for UC emission intensity enhancing trend in our system as follows: 5%Li+ > 1%Ca2+ > 10%Gd3+ > optical-inert ions free sample, XRD Rietveld refinement, SEM, XPS etc. were employed for the crystal structure analysis and luminescence performance investigation.Generally, the factors affecting luminescence intensity are various and complex, including its crystal structure, shape, size, phonon modes, etc.1,14 In our work, it can be seen above that phase of all prepared samples is the same with the standard pattern of ScVO4 (JCPDS Card, File No. 06-0260), indicating that those optical-inert ions get incorporated into the ScVO4 matrix without phase separation, so the benefit of phase effect on the luminescence augmentation can be excluded. However, doping those optical-inert ions leads to the change of lattice cell. From the enlarged spectra which are dominated by the shifting of main diffraction peaks (220) of tetragonal phase ScVO4 as shown in Fig. 1b and c, we can see that the peak shifts changed as the different optical-inert ions doping. To see how crystal structure quantitatively evolves along with the addition of optical-inert ions (Li+, Ca2+ or Gd3+), these raw data of XRD were analyzed by Rietveld refinement method.46–49 Fig. 9 shows the Rietveld analysis pattern of ScVO4:Yb3+/Er3+ sample and Li+, Ca2+ or Gd3+ single doped ScVO4:Yb3+/Er3+ samples, in which the black crosses, red solid lines, green solid lines, blue lines and magenta bars characterized experimental patterns, calculated patterns, background patterns, differences and Bragg position, respectively. The deviation between the calculated and experimental results are expressed by blue lines, which are shown between the background line and the Bragg reflection line. The refinement results and the main refinement parameters are shown in Table 2 and Table 3. Profile factor Rp lies between 8.24% to 9.91%, weighted profile factor Rwp is 6.79–8.03%.
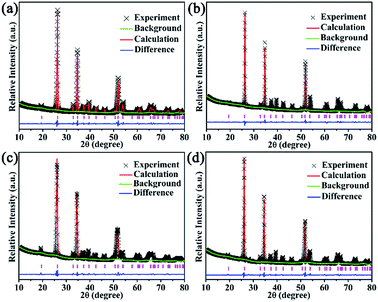 | ||
| Fig. 9 The Rietveld analysis pattern of (a) ScVO4:10%Yb3+/2%Er3+, (b) Li+, (c) Ca2+, (d) Gd3+ doped ScVO4:10%Yb3+/2%Er3+. | ||
| Bond lengths and angles | No | Li+ | Ca2+ | Gd3+ |
| Crystal system | Tetragonal | Tetragonal | Tetragonal | Tetragonal |
| Space group | I41/amd | I41/amd | I41/amd | I41/amd |
| Z | 4 | 4 | 4 | 4 |
| Cell parameters/Å | a = b = 6.8289, c = 6.1709 | a = b = 6.8375, c = 6.1705 | a = b = 6.8454, c = 6.1728 | a = b = 6.8148, c = 6.1597 |
| Cell volume/Å3 | 287.77 | 288.48 | 289.25 | 286.07 |
| Profile factor Rp | 9.89% | 8.24% | 9.91% | 9.77% |
| Weighted profile factor Rwp | 7.97% | 6.79% | 7.77% | 8.03% |
| Bond lengths and angles | No | Li+ | Ca2+ | Gd3+ |
| Sc1–O1 | 2.377(8) Å | 2.408(4) Å | 2.376(16) Å | 2.365(5) Å |
| Sc1–O2 | 2.129(11) Å | 2.116(6) Å | 2.134(21) Å | 2.139(7) Å |
| Sc1–O–Sc1′ | 112.4(5)° | 111.83(29)° | 112.2(7)° | 112.16(33)° |
These results indicate that the crystal structure data of these samples by Rietveld structural refinement are well matched with experimental data. Due to similarity between refined patterns, we list the refined profile of the sample of ScVO4:Yb3+/Er3+ as an example. For ScVO4:Yb3+/Er3+, Rp = 9.89%, Rwp = 7.97%. The refinement results indicate that ScVO4:Yb3+/Er3+ and Li+, Ca2+ or Gd3+ single doped ScVO4:Yb3+/Er3+ phosphors belong to tetragonal phase, and its space group is I41/amd with Z = 4. The refined unit cell parameters are a = b = 6.8289 Å, c = 6.1709 Å and cell volume V = 287.77 Å3 for ScVO4:Yb3+/Er3+ sample. For Li+, Ca2+ or Gd3+ single doped ScVO4:Yb3+/Er3+ samples, the unit cell parameters are a = b = 6.8375 Å, c = 6.1705 Å, cell volume V = 288.48 Å3; a = b = 6.8454 Å, c = 6.1728 Å, cell volume V = 289.25 Å3 and a = b = 6.8148 Å, c = 6.1597 Å, cell volume V = 286.07 Å3, respectively. Obviously, the trend of increment for the cell volume V as: Ca2+ > Li+ > No optical-inert ions > Gd3+. The emergence of the result may be caused by that these optical-inert ions can be doped into the host lattice through the substitution or occupation of the interstitial. As shown in Fig. 10, for tetragonal phase ScVO4, each Sc3+ ion is eight-coordinated by O atoms. In 10%Yb3+/2%Er3+-doped ScVO4 sample, the two kinds of Sc–O bond lengths are 2.377(8) and 2.129(11) Å, respectively. The angles of Sc–O–Sc is 112.4(5)°. The corresponding bond lengths and bond angles of Li+, Ca2+ or Gd3+ single doped ScVO4:Yb3+/Er3+ samples are deposited in Table 2. Compared with the ScVO4:Yb3+/Er3+ sample, the decreased Sc–O average bond length will change the surrounding environment of Yb3+ and Er3+ and break the local crystal field symmetry around the Er3+ ions, leading to a low symmetric site of the Er3+ ions, which can make an enhancement in UC efficiency. It is worthwhile pointing out that Sc–O average bond length of Li+ doping sample changed the most, in accordance with the UCL intensity of this sample is the highest.
Besides, it is considered that the larger size and more regular morphology has higher UCL intensity, while it is noticed from the Table 1 that the size are not consistant with the UC luminescence intensity perfectly, thus, the benefit of the crystalline size effect on the luminescence enhancement is not an important factor. Thus, the enhancement mechanisms of optical-inert ions should be searched from other directions.
On one hand, to investigate chemical composition of the material surface, a well-known, extensively used X-ray photon spectroscopy (XPS) technique was used.50–52 Fig. 11a and b shows the XPS spectra of Sc(2p), V 2p3/2 and V 2p1/2 regions (between 455–467 eV) for ScVO4:Yb3+/Er3+ sample and Li+, Ca2+, Gd3+ single doped ScVO4:Yb3+/Er3+ samples. These results confirm the +3 oxidation state of Sc and V in its +5 oxidation state.51,53 Moreover, the XPS spectra of O 1s are used as a probe for investigating the presence of oxygen ion vacancies on the surface of sample. The peaks were de-convoluted using Lorentzian function. In the case of ScVO4:Yb3+/Er3+ sample, the two peaks well fitted to BE ∼529.57 (P1) and 531.97 eV (P2) with FWHM ∼1.08 and 1.32 eV, respectively. Upon optical-inert ions doping, all the peaks showed asymmetric behaviour towards higher BE. Moreover, the decrease extent of the P2/P1 ratio of optical-inert ions doped samples is as follows: 1%Ca2+ < 5%Li+ < 10%Gd3+, as shown in Fig. 11c. This is probably because of the creation of oxygen ion vacancies and/or surface defects through the sample surface with introduction of optical-inert ions into the host matrix. It can be concluded from the analysis of XPS spectra that the creation of appropriate amounts of oxygen ion vacancies and/or surface defects through the sample surface with the introduction of optical-inert ions into the host matrix, which are beneficial to the stronger UCL. This is because of a lower or optimal proportion of optical-inert ions incorporation into the host lattice, thus inducing a fast ET from the host to the Er3+ ion. This may create the vacancies that act as the sensitizer, mixing the charge-transfer states. Optical-inert ions addition increased the UCL intensity by increasing the radiative transition probability. However, an increase in the optical-inert ions concentration or type over a certain limit (such as 10%Gd3+ in this work) generates a significant amount of oxygen ion vacancies in the lattice. Consequently, the crystal lattice collapses, and the luminescence intensity decreases. Therefore, the brightness increases with oxygen ion vacancies concentration to certain extent, if above this point, the luminescence begins to decrease, then quenching behavior appears as a result.
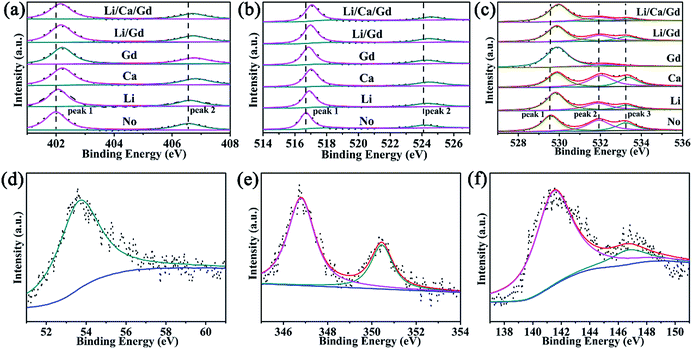 | ||
| Fig. 11 XPS and their peak fitting curves of (a) Sc 2p, (b) V 2p, (c) O 1s, (d) Li 1s, (e) Ca 2p, and (f) Gd 4d of Gd3+, Li+, Ca2+ doped ScVO4:10%Yb3+/2%Er3+, respectively. | ||
Based on the experiment results described above, in ScVO4 phosphor, it can be concluded that optical-inert ions doping induced change of local symmetry and oxygen vacancy generated should be the main reason that is responsible for UC emission enhancement. In addition, morphology and size of the obtained samples also contributes to the enhanced UC emission. In order to interpret the effects of these optical-inert ions doping on the UCL process, the dependence of emission intensity on the pump power for the red, green emission was measured, in order to better verify the role of the optical-inert ions played in the energy transfer of UC emissions. As a commonly used method, the relationship between integrated emission intensity I and excitation power P, Iem = Pn is often used to provide the information of n photons involved in the UC process.14 For comparison, the power dependence of S0–S4 samples are shown in Fig. 12. Considering the energy transfer UC process, the population of emissive levels will be greatly influenced by the energy transfer process showing a changeable n value. It is demonstrated whether in ScVO4:10%Yb3+/2%Er3+ (S0) or optical-inert ions doped ScVO4:10%Yb3+/2%Er3+ (S1–S4), the slop value n are all approximate to 2 both for the green and red. As the slope denotes the number of NIR photons absorbed to generate one frequency upconverted photon under unsaturated conditions, the green and red emissions are two-photon processes both in the UCPs doping optical-inert ions or not. The UC mechanism of this system is similar to our previous work.40
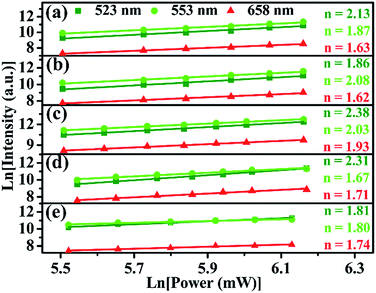 | ||
| Fig. 12 Pump power dependence of the fluorescent bands centered at 523 nm, 554 nm and 659 nm in (a) Gd3+/Ca2+, (b) Li+, (c) Gd3+, (d) Ca2+, (e) without co-doped ScVO4:10%Yb3+/2%Er3+. | ||
3.5 Temperature sensing properties ScVO4:10%Yb3+/2%Er3+ and 5%Li+/10%Gd3+ codoped ScVO4:10%Yb3+/2%Er3+
It is also interesting to find that ScVO4:10%Yb3+/2%Er3+ micro-particles have optical-thermal sensing performance. To investigate influences of optical-inert ions on the temperature-sensing behaviour of ScVO4:10%Yb3+/2%Er3+, 5%Li+/10%Gd3+ codoped ScVO4:10%Yb3+/2%Er3+ with the highest UC luminescence intensity was chosen as a representative. It is well known that the 2H11/2 and 4S3/2 levels of the Er3+ ion are thermally coupled, leading to the change of the transitions of 2H11/2 → 4I15/2 (522 nm) and 4S3/2 → 4I15/2 (554 nm) of Er3+ at different temperatures.54–57 According to Boltzmann distribution theory, the emission intensity ratio (R) of the 522 nm and 554 nm transitions can be written as follows:43
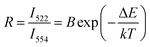 | (1) |
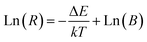 | (2) |
The UC emission spectra at various temperatures and curves of the emission intensity ratio (R) versus temperature (T/K) for ScVO4:10%Yb3+/2%Er3+ and ScVO4:10%Yb3+/2%Er3+/5%Li+/10%Gd3+ are presented in Fig. 13a and b, respectively. The intensity of the UC emission bands around 522 nm and 554 nm seemed to drastically vary with increasing temperature of the sample. The plots of Ln(R) versus 1/T are exhibited in Fig. 13c and d, the slope (−ΔE/k) is a very important parameter to judge the optical temperature sensing ability of Er3+ doped materials, and the linear fitting of the experimental data gave slope and intercept equal to −655.15 ± 16.22 and −511.12 ± 38.57 for both of these two samples, respectively. Besides, the sensor sensitivity is another important coefficient of a sensing material. The sensor sensitivity (S) can be defined as
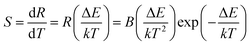 | (3) |
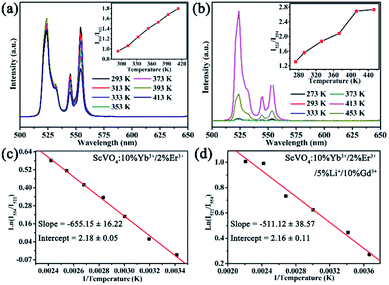 | ||
| Fig. 13 Green UC emission spectra of ScVO4:10%Yb3+/2%Er3+ (a) and ScVO4:10%Yb3+/2%Er3+/5%Li+/10%Gd3+ (b) phosphors on increasing temperatures and the variation of emission intensity ratio (R) as function of absolute temperature (inset). (c, d) Monolog plots of R as a function of inverse absolute temperature for these two samples, fitted by eqn (2). | ||
Actually, with increasing temperature, the absolute sensitivity for both two samples first increases, then reach a certain temperature it starts decreasing (Fig. 14). It is noteworthy that the sensitivity increases dramatically in the ScVO4:10%Yb3+/2%Er3+/5%Li+/10%Gd3+ sample compared to the Li+/Gd3+ free sample. At the temperature of 260 K, the sensitivity of ScVO4:10%Yb3+/2%Er3+/5%Li+/10%Gd3+ reached its maximum value of about 0.0092 K−1, while the maximum sensitivity of ScVO4:10%Yb3+/2%Er3+ only 0.0073 K−1 at 330 K. The results indicated that the multifunctional optical-inert ions Li+/Gd3+ could be used not only to enhance the temperature sensor sensitivity but also the UCL intensity.
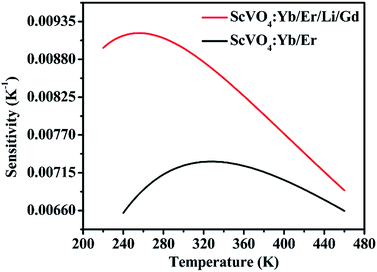 | ||
| Fig. 14 Relative sensitivity of the as-prepared ScVO4:10%Yb3+/2%Er3+ and 5%Li+/10%Gd3+ codoped ScVO4:10%Yb3+/2%Er3+ samples for optical thermometry as a function of temperature, fitted by eqn (3). | ||
4. Conclusions
To sum up, this study reveals that the influences of different optical-inert metal ions including alkali metal, alkaline earth metal as well as inactive Ln3+ ions were discussed by varying the doping content and the combination way. Successful incorporation of optical inert ions into ScVO4 lattices was supported by XRD and XPS. Under a 980 nm laser diode excitation, for optical-inert metal ions single doping system, 5% Li+ is demonstrated as the most effective dopant to enhance the UCL intensity as high as 6.1 times among Li+/Na+/K+/Mg2+/Ca2+/Sr2+/Ba2+/Y3+/Gd3+/Lu3+. For codoping or tridoping optical-inert metal ions system, it is found that Li+/Gd3+ couple is the most effective codopant, leading to an drastic increase by a factor of 15.3 compared to optical-inert metal ions free sample. In addition, the color coordinates of all samples are located in the green region. The UC luminescence enhancement after codoping optical-inert ions were ascribed to the interaction of a variety of factors, such as larger size, oxygen vacancy, crystal environment distortation. Furthermore, temperature-sensing performance was also investigated using the fluorescence intensity ratio technique. For ScVO4:10%Yb3+/2%Er3+, the maximum sensitivity was found to be 0.0073 K−1 at 330 K. Surprisingly, 5%Li+/10%Gd3+ codoped ScVO4:10%Yb3+/2%Er3+ with the highest UC luminescence intensity was as a temperature sensor with maximum sensitivity of 0.0092 K−1 at 260 K. The investigation results establish the understanding of optical-inert metal ions as dopants for adjusting UCL performance and may be helpful for researchers to develop quick responsive UCL materials. This opens a new window for studying the cooperation of the optically inert ions doping effect on improving UC luminescence and temperature sensitivity properties of ScVO4:10%Yb3+/2%Er3+ phosphors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge China Postdoctoral Science Foundation (2016M592308), Chinese Academy of Sciences (Grants XDA09030203), the NSFH (142300410223), and Student Innovation and Entrepreneur ship Program of Zhengzhou University (SIEP, 2015xjxm180) for their financial support.References
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Y. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- H. H. Fang, J. Yang, J. Feng, T. Tamao, S. Hotta and H. B. Sun, Laser Photonics Rev., 2014, 8, 687–715 CrossRef.
- F. Wang and X. G. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- L. L. Han, Y. H. Wang, L. N. Guo, L. Zhao and Y. Tao, Nanoscale, 2014, 6, 5907–5917 RSC.
- H. R. Yang, C. M. Han, X. J. Zhu, Y. Liu, K. Y. Zhang, S. J. Liu, Q. Zhao, F. Y. Li and W. Huang, Adv. Funct. Mater., 2016, 26, 1945–1953 CrossRef CAS.
- Y. Liu, Q. Q. Su, M. Chen, Y. Dong, Y. B. Shi, W. Feng, Z. Y. Wu and F. Y. Li, Adv. Mater., 2016, 28, 6625–6630 CrossRef CAS PubMed.
- W. B. Hu, X. M. Lu, R. C. Jiang, Q. L. Fan, H. Zhao, W. X. Deng, L. Zhang, L. Huang and W. Huang, Chem. Commun., 2013, 49, 9012–9014 RSC.
- S. H. C. Askes, W. Pomp, S. L. Hopkins, A. Kros, S. Wu, T. Schmidt and S. Bonnet, Small, 2016, 12, 5579–5590 CrossRef CAS PubMed.
- G. Y. Chen, H. L. Qiu, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- H. Dong, S. R. Du, X. Y. Zheng, G. M. Lyu, L. D. Sun, L. D. Li, P. Z. Zhang, C. Zhang and C. H. Yan, Chem. Rev., 2015, 115, 10725–10815 CrossRef CAS PubMed.
- W. Yu, W. Xu, H. W. Song and S. Zhang, Dalton Trans., 2014, 43, 6139–6147 RSC.
- Y. Tian, B. N. Tian, C. E. Cui, P. Huang, L. Wang and B. J. Chen, RSC Adv., 2015, 5, 14123–14128 RSC.
- Y. Y. Tian, Y. Tian, P. Huang, L. Wang, Q. F. Shi and C. E. Cui, Chem. Eng. J., 2016, 297, 26–34 CrossRef CAS.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS PubMed.
- J. Y. Liao, Z. W. Yang, H. J. Wu, D. Y. Yan, J. B. Qiu, Z. G. Song, Y. Yang, D. C. Zhou and Z. Y. Yin, J. Mater. Chem. C, 2013, 1, 6541–6546 RSC.
- B. Shao, Z. W. Yang, Y. D. Wang, J. Li, J. Z. Yang, J. B. Qiu and Z. G. Song, ACS Appl. Mater. Interfaces, 2015, 7, 25211–25218 CAS.
- G. Y. Chen, H. C. Liu, H. J. Liang, G. Somesfalean and Z. G. Zhang, J. Phys. Chem. C, 2008, 112, 12030–12036 CAS.
- Q. Cheng, J. H. Sui and W. Cai, Nanoscale, 2012, 4, 779–784 RSC.
- H. Lin, D. K. Xu, D. D. Teng, S. H. Yang and Y. L. Zhang, New J. Chem., 2015, 39, 2565–2572 RSC.
- M. K. Mahata, T. Koppe, T. Mondal, C. Brüsewitz, K. Kumar, V. K. Rai, H. Hofsäss and U. Vetter, Phys. Chem. Chem. Phys., 2015, 17, 20741–20753 RSC.
- Q. M. Huang, J. C. Yu, E. Ma and K. M. Lin, J. Phys. Chem. C, 2010, 114, 4719–4724 CAS.
- J. Tang, L. Chen, J. Li, Z. Wang, J. H. Zhang, J. G. Zhang, Y. S. Luo and X. J. Wang, Nanoscale, 2015, 7, 14752–14759 RSC.
- Q. Sun, H. Zhao, X. Q. Chen, F. P. Wang, W. Cai and Z. H. Jiang, Mater. Chem. Phys., 2010, 123, 806–810 CrossRef CAS.
- Y. F. Bai, Y. X. Wang, G. Y. Peng, K. Yang, X. R. Zhang and Y. L. Song, J. Alloys Compd., 2009, 478, 676–678 CrossRef CAS.
- H. N. Luitel, R. Chand, H. Hamajima, Y. R. Gaihre, T. Shingae, T. Yanagita and T. J. Watari, J. Mater. Chem. B, 2016, 4, 6192–6199 RSC.
- L. N. Guo, Y. H. Wang, Y. Z. Wang, J. Zhang, P. Y. Dong and W. Zeng, Nanoscale, 2013, 5, 2491–2504 RSC.
- L. Lei, D. Q. Chen, J. Xu, R. Zhang and Y. S. Wang, Chem.–Asian J., 2014, 9, 728–733 CrossRef CAS PubMed.
- V. Singh, V. K. Rai, I. J. Lee, I. Ledoux-Rak, K. Al-Shamery, J. Nordmann and M. Haase, Appl. Phys. B, 2012, 106, 223–228 CrossRef CAS.
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, nature, 2010, 463, 1061 CrossRef CAS PubMed.
- G. T. Xiang, J. H. Zhang, Z. D. Hao, X. Zhang, G. H. Pan, Y. S. Luo and H. F. Zhao, CrystEngComm, 2015, 17, 3103–3109 RSC.
- H. J. Liang, Y. D. Zheng, G. Y. Chen, L. Wu, Z. G. Zhang and W. W. Cao, J. Alloys Compd., 2011, 509, 409–413 CrossRef CAS.
- D. G. Li, W. P. Qin, P. Zhang, L. L. Wang, M. Lan and P. B. Shi, Opt. Mater. Express, 2017, 7, 329–340 CrossRef.
- L. Mukhopadhyay, V. K. Rai, R. Bokolia and K. Sreenivas, J. Lumin., 2017, 187, 368–377 CrossRef CAS.
- Y. F. Bai, Y. X. Wang, K. Yang, X. R. Zhang, Y. L. Song and C. H. Wang, Opt. Commun., 2008, 281, 5448–5452 CrossRef CAS.
- Q. Cheng, J. H. Sui and W. Cai, Nanoscale, 2012, 4, 779–784 RSC.
- V. Mahalingam, R. Naccache, F. Vetrone and J. A. Capobianco, Opt. Express, 2010, 20, 111–119 CrossRef PubMed.
- J. H. Chung, S. Y. Lee, K. B. Shim, S. Y. Kweon, S. C. Ur and J. H. Ryu, Appl. Phys. A, 2012, 108, 369–373 CrossRef CAS.
- X. W. Wang, X. Zhang, Y. B. Wang, H. Y. Li, J. Xi, T. Wei, Q. W. Huang, X. J. Xie, L. Huang and W. Huang, Dalton Trans., 2017, 46, 8968–8974 RSC.
- S. S. Du, D. Y. Wang, Q. P. Qiang, X. L. Ma, Z. B. Tang and Y. H. Wang, J. Mater. Chem. C, 2016, 4, 7148–7155 RSC.
- C. X. Liang, L. N. Guo, P. Li, T. S. Li, P. L. Chen, M. H. Liu and Y. J. Wu, J. Alloys Compd., 2017, 715, 37–42 CrossRef CAS.
- S. S. Zhou, K. M. Deng, X. T. Wei, G. C. Jiang, C. K. Duan, Y. H. Chen and M. Yin, Opt. Commun., 2013, 291, 138–142 CrossRef CAS.
- F. Huang, Y. Gao, J. C. Zhou, J. Xu and Y. S. Wang, J. Alloys Compd., 2015, 639, 325–329 CrossRef CAS.
- M. Xu, D. Q. Chen, P. Huang, Z. Y. Wan, Y. Zhou and Z. G. Ji, J. Mater. Chem. C, 2016, 4, 6516–6524 RSC.
- I. Hyppänen, N. Perälä, R. Arppe, M. Schäferling and T. Soukka, ChemPhysChem, 2017, 18, 692–701 CrossRef PubMed.
- A. J. Huang, Z. W. Yang, C. Y. Yu, J. B. Qiu and Z. G. Song, J. Am. Ceram. Soc., 2017, 100, 4994–4998 CrossRef CAS.
- I. Sosnowska, W. Schäfer, W. Kockelmann, K. H. Andersen and I. O. Troyanchuk, Appl. Phys. A, 2002, 74(Suppl. 1), S1040–S1042 CrossRef CAS.
- T. Katsufuji, M. Masaki, A. Machida, M. Moritomo, K. Kato, E. Nishibori, M. Takata, M. Sakata, K. Ohoyama, K. Kitazawa and H. Takagi, Phys. Rev. B, 2002, 66, 134434 CrossRef.
- F. W. Kang, L. J. Li, J. Han, D. Y. Lei and M. Y. Peng, J. Mater. Chem. C, 2017, 5, 390–398 RSC.
- J. G. Cheng, P. L. Li, Z. J. Wang, Y. S. Sun, Q. Y. Bai, Z. L. Li, M. M. Tian, C. Wang and Z. P. Yang, J. Mater. Chem. C, 2017, 5, 127–133 RSC.
- B. J. Tan, K. J. Klabunde and P. M. A. Sherwood, J. Am. Chem. Soc., 1991, 113, 855–861 CrossRef CAS.
- G. Silversmit, D. Depla, H. Poelman, G. B. Marin and R. D. Gryse, J. Electron Spectrosc. Relat. Phenom., 2004, 135, 167–175 CrossRef CAS.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- B. P. Singh, A. K. Parchur, R. S. Ningthoujam, P. V. Ramakrishna, S. Singh, P. Singh, S. B. Rai and R. Maalej, Phys. Chem. Chem. Phys., 2014, 16, 22665–22676 RSC.
- G. Paez and M. Strojnik, Appl. Opt., 2003, 42, 3251–3258 CrossRef PubMed.
- S. F. Leon-Luis, U. R. Rodriguez-Mendoza, I. R. Martin, E. Lalla and V. Lavin, Sens. Actuators, B, 2013, 176, 1167–1175 CrossRef CAS.
- J. S. Liao, L. L. Nie, Q. Wang, S. J. Liu, H. R. Wen and J. P. Wu, RSC Adv., 2016, 6, 35152–35159 RSC.
- S. B. Liu, S. F. Liu, M. Zhou, X. Y. Ye, D. J. Hou and W. X. You, RSC Adv., 2017, 7, 36935–36948 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10035c |
| This journal is © The Royal Society of Chemistry 2017 |