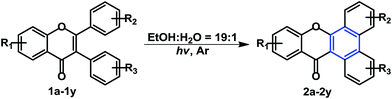DOI:
10.1039/C7RA09770K
(Paper)
RSC Adv., 2017,
7, 44333-44339
Annulation of 2,3-diphenyl-4H-chromen-4-ones via photo-induced hydrogen evolution†
Received
2nd September 2017
, Accepted 8th September 2017
First published on 14th September 2017
Abstract
An efficient photo-induced transition-metal-free direct hydrogen evolution and annulation of 2,3-diphenyl-4H-chromen-4-ones in EtOH–H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature was described. The reported methodology provided a quick and easy access to the synthesis of dibenzo[a,c]xanthen derivatives, which eliminates the use of any catalysts, oxidants and additives.
1, v/v) at room temperature was described. The reported methodology provided a quick and easy access to the synthesis of dibenzo[a,c]xanthen derivatives, which eliminates the use of any catalysts, oxidants and additives.
Introduction
In recent years, photochemical reactions have drawn great attention due to their environmentally friendly properties, sustainability and high atom efficiency compared to conventional transition-metal catalyzed reactions.1,2 Furthermore, photons have been recognized as an ideal clean reagent for organic reactions.3,4 Photochemical reactions have been successfully applied to the synthesis of phenanthrene derivatives and polycyclic aromatic hydrocarbons (PAHs),5,6 which are difficult to achieve via traditional chemical conditions.
Chromones are the core structures of flavonoids and isoflavonoids and have been extensively studied as biologically active substrates as well as drug molecules.7,8 Owing to their tremendous significance in biological and pharmaceutical activities,9 great effort has been made in the development of various chromone derivatives. Recently, transition-metal catalyzed C–H activation and annulation have been successfully applied for the synthesis of polycyclic derivatives of chromones. Yoshikai et al. reported Pd(dba)2 catalyzed cyclization of 3-iodoflavones with 2-(trimethylsilyl)phenyl triflate in the presence of CsF and P(o-tolyl)3 at 110 °C for 24 h to give dibenzo[a,c]xanthen derivative 2a in 79% (Scheme 1a).10
 |
| | Scheme 1 Synthetic approaches to dibenzo[a,c]xanthenes. | |
Alternatively, the irradiation of 2,3-diphenyl-4H-chromen-4-one 1a in acetone with a medium pressure mercury lamp under the air atmosphere for 1 h yielded trace amount of 2a (5%, Scheme 1b).11 It was proposed that 2a was generated via the well-known stilbene-phenanthrene type photocyclization of 2,3-diphenyl-4H-chromen-4-one 1a. Generally speaking, photocyclization of stilbene analogues always require the presence of oxidant (O2 or I2, KI, CuCl2, TCNE et al.) for the reaction to proceed smoothly.12–14 Luckily, we were able to obtain the cyclization products of 2,3-di(hetero)aryl-4H-chromen-4-ones without the requirement of any oxidant or additives.15 However, the annulation product for less reactive 2,3-diphenyl-4H-chromen-4-one substrate was not obtained. Following our investigation in the development of photo-induced transition metal-free cross-coupling reaction16 as well as the direct oxidative annulation,15,17,18 we would like to extend an efficient photo-induced intramolecular hydrogen evolution and annulation to 2,3-diphenyl-4H-chromen-4-ones substrate, which provides access to dibenzo[a,c]xanthen analogues (Scheme 1c).
Results and discussion
Initially, the photo-induced oxidative annulation of 2,3-diphenyl-4H-chromen-4-one (1a) was optimized and the corresponding data were presented in Table 1. Thus, irradiation of 1a in acetonitrile (100 mL) with a 500 W high-pressure mercury lamp at ambient temperature under argon atmosphere for 3 h gave 2a in the yield of 49% (entry 1). Annulation of 1a in acetone or dichloromethane did not improve the yield of 2a (38–43%, entries 2–3). While, similar yield of 2a was obtained in ethanol (56%, entry 4). It was interesting to find out that the yields of 2a were significantly affected with the presence of H2O (entries 5–8). The amount of additional water played a critical role towards the cyclization, since 2a was obtained in lower yields with either too much or too little water. The optimal yield was obtained when EtOH/H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was chosen as solvent (63%, entry 7). Meanwhile, the concentration of 1a was also explored (entries 7, 9–10) and 2a was obtained in highest yield when the reaction was performed in 5 mM scale (63%, entry 7). Finally, screening of irradiation time was conducted and lower yield of 2a was obtained (entries 11–12). When the cyclization was performed under open air, 2a was obtained in 32% yield (entry 13). Thus, the irradiation of 5 mM 1a in EtOH–H2O (19
1, v/v) was chosen as solvent (63%, entry 7). Meanwhile, the concentration of 1a was also explored (entries 7, 9–10) and 2a was obtained in highest yield when the reaction was performed in 5 mM scale (63%, entry 7). Finally, screening of irradiation time was conducted and lower yield of 2a was obtained (entries 11–12). When the cyclization was performed under open air, 2a was obtained in 32% yield (entry 13). Thus, the irradiation of 5 mM 1a in EtOH–H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at ambient temperature for 3 h under the argon atmosphere was determined to be the optimal condition.
1, v/v) at ambient temperature for 3 h under the argon atmosphere was determined to be the optimal condition.
Table 1 Optimization for the annulation of 1aa
With the optimized conditions in hand, the tolerance of various functional groups (methyl, tert-butyl, fluoro, chloro, hydroxyl and methoxyl) has been explored and the yields were summarized in Table 2. Generally speaking, the substrates bearing electron-donating groups (e.g., Me, OMe, t-Bu) gave the corresponding products 2a–2p in higher good yields (59–81%) comparing to those (2q–2x, 54–69%) bearing electron-withdrawing groups (e.g., F, Cl). Notably, the substrates bearing free hydroxyl group also yielded the corresponding annulation products 2y and 2z in high yields, which are better than the yields of 2l and 2e, respectively. There is a significant difference in the irradiation time as well. Substrates containing EWG usually require longer reaction time than those with EDG. The structures of 2 were characterized by 1H NMR, 13C NMR, HRMS and IR.
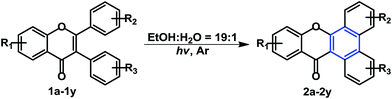
|
| All reactions were carried out on the 0.5 mmol scale with the irradiation of 500 W high-pressure mercury lamp under the argon atmosphere at room temperature until substrate was completely consumed (TLC monitor). The yields of 2q–2x was 39%, 45%, 49%, 31%, 46%, 40%, 43%, and 55%, for 3 h, respectively. |
 |
Based on our experimental data and a literature report,19 a plausible mechanism for the formation of 2a is depicted in Scheme 2. Initially, the irritation of 2,3-diphenyl-4H-chromen-4-one (1a) with a high-pressure mercury lamp generate intermediate A, followed by a thermal suprafacial [1,5]-H shift20 to give intermediate B. Spontaneous rearomatization of benzene ring is believed to be the force driving for [1,5]-sigmatropic shift. Subsequently, keto–enol isomerization of B led to the formation of a more stable syn-isomer C. Similar transformations have been reported for a number of structurally related compounds.15,21–24 It is worth mentioning that polar protic solvent EtOH–H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) plays an important role in the process of keto–enol isomerization, which accounts for higher yields of 2a comparing to MeCN, CHCl2 and Me2CO. In the end, the annulation product 2a was obtained along with the elimination of hydrogen molecule via syn-elimination from intermediate C, which could be easily explained by the restoration of aromaticity for the benzene ring and the entire conjugated system. To our delight, we have successfully detected the hydrogen via GC and the detail experiment was shown in ESI-Fig. 2.† The annulation product 2a was also produced under the open air (oxygen, Table 1, entry 13), and it indicated that the photochemical cyclization proceed through the S1 state.
1, v/v) plays an important role in the process of keto–enol isomerization, which accounts for higher yields of 2a comparing to MeCN, CHCl2 and Me2CO. In the end, the annulation product 2a was obtained along with the elimination of hydrogen molecule via syn-elimination from intermediate C, which could be easily explained by the restoration of aromaticity for the benzene ring and the entire conjugated system. To our delight, we have successfully detected the hydrogen via GC and the detail experiment was shown in ESI-Fig. 2.† The annulation product 2a was also produced under the open air (oxygen, Table 1, entry 13), and it indicated that the photochemical cyclization proceed through the S1 state.
 |
| | Scheme 2 Proposed reaction mechanism for the formation of 2a. | |
Encouraged by the further certified the proposed mechanism, we turned our attention to the reductive carbonyl. 1a was reduced to 3 under the presence of LiAlH4 AlCl3 in THF at 0 °C for 30 min to gave 3 (yield 44%, Scheme 3).25 As expected, no annulation product 4 was detected at the optimal reaction conditions, which could be explained to the lack of keto–enol tautomerization.
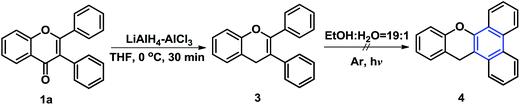 |
| | Scheme 3 Reductive carbonyl and attempt annulation of 3. | |
Conclusions
In summary, we have demonstrated a simple photo-induced direct hydrogen evolution and annulation for the synthesis of 14H-dibenzo[a,c]xanthen-14-one derivatives in 95% EtOH under room temperature. Comparing to conventional transition-metal-catalyzed annulation and oxidative photo-cyclization reaction, the reported methodology offers several notable advantages, including simple operation, mild reaction condition, no catalysts, oxidants and additives. Meanwhile, the failure of the cyclization of 3 and detection the H2 generated by the annulation of 1n sufficiently proved the rationality of the proposed mechanism.
Experimental sections
Unless otherwise noted, commercial reagents were purchased from Energy Chemical. All experiments were determined by thin layer chromatography (TLC). TLC used silica gel 60 GF254 plate. Column chromatography (200–300 mesh) was performed on silica gel. 1H and 13C NMR spectra were recorded on Bruker 400 or 600 MHzspectrometer. 1H and 13C NMR spectra were reported in parts per million (ppm) and referenced to the residual solvent peaks [CDCl3 (7.26 ppm) or DMSO-d6 (2.50)] for 1H NMR and the CDCl3 (77.16 ppm) or DMSO-d6 (39.52) for 13C NMR spectra. High-resolution mass spectra (HRMS) were obtained using the electron-spray ionization (ESI) technique. Melting points were measured with a X-5 micro-melting point apparatus and were uncorrected. IR spectra were recorded with a Nicollet 170SX FT-IR spectrophotometer with KBr pellets. According to the literature reported for the synthesis of 3-iodo-2-phenyl-4H-chromen-4-one, which using 2-phenyl-4H-chromen-4-ones (1 mmol), I2 (304 mg, 1.2 mmol) and Ce(NH4)2(NO3)6 (603 mg, 1.1 mmol) as starting materials and pre-dried MeCN (10 mL) as solvent a series of 3-iodoflavonoids compounds was obtained.26
General procedure for the synthesis of compounds 2a–2z
Substrate 2,3-diphenyl-4H-chromen-4-one 1a (149 mg, 0.5 mmol) was dissolved in EtOH–H2O (100 mL, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at ambient temperature in a quartz tube, and was degassed for 5 min via ultrasound, deaerated for 20 min by bubbling argon and irradiated with a high pressure mercury lamp (500 W) at room temperature for 3 h. Then the reaction mixture were removed under reduced pressure and purified by column chromatography (ethyl acetate/petroleum ether, 1
1, v/v) at ambient temperature in a quartz tube, and was degassed for 5 min via ultrasound, deaerated for 20 min by bubbling argon and irradiated with a high pressure mercury lamp (500 W) at room temperature for 3 h. Then the reaction mixture were removed under reduced pressure and purified by column chromatography (ethyl acetate/petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to give 2a (63%).
50) to give 2a (63%).
14H-dibenzo[a,c]xanthen-14-one (2a)10. 1H NMR (400 MHz, CDCl3) δ 10.19 (d, J = 8.4 Hz, 1H), 8.77 (d, J = 8.2 Hz, 1H), 8.68 (t, J = 8.5 Hz, 2H), 8.46 (d, J = 7.9 Hz, 1H), 7.87–7.68 (m, 6H), 7.51–7.47 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 178.33, 155.21, 154.42, 133.92, 133.80, 130.62, 128.99, 128.57, 127.75, 127.50, 127.32, 126.71, 126.68, 124.71, 124.10, 123.99, 123.97, 122.90, 122.32, 117.50, 112.70.
6-methoxy-12-methyl-14H-dibenzo[a,c]xanthen-14-one (2b). White solid. Yield: 72%, Mp: 229.3–231.9 °C. 1H NMR (400 MHz, CDCl3) δ 10.11 (d, J = 8.4 Hz, 1H), 8.42 (dd, J = 8.4, 5.7 Hz, 2H), 8.14 (s, 1H), 7.82 (d, J = 2.3 Hz, 1H), 7.69 (dd, J = 11.3, 4.1 Hz, 1H), 7.63–7.53 (m, 1H), 7.48 (dd, J = 8.5, 2.0 Hz, 1H), 7.41 (d, J = 8.4 Hz, 1H), 7.17 (dd, J = 9.0, 2.4 Hz, 1H), 3.97 (s, 3H), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 178.10, 161.54, 155.33, 152.54, 135.81, 134.79, 134.36, 129.75, 128.62, 127.84, 126.85, 126.22, 125.96, 123.59, 122.27, 117.97, 117.18, 116.91, 110.89, 104.35, 55.57, 21.18; IR (KBr), ν (cm−1) 2927, 1610, 1446, 1247, 1029, 925, 821, 628, 530; HRMS (ESI): calc. for C23H16O3 [M + H]+ 341.1178, found 341.1177.
6-Methoxy-2,12-dimethyl-14H-dibenzo[a,c]xanthen-14-one (2c). White solid. Yield: 78%, Mp: 222.4–224.2 °C. 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.45 (d, J = 8.4 Hz, 1H), 8.31 (d, J = 8.4 Hz, 1H), 8.15 (s, 1H), 7.81 (s, 1H), 7.50–7.38 (m, 3H), 7.17 (d, J = 9.0 Hz, 1H), 3.99 (s, 3H), 2.60 (s, 3H), 2.50 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 178.20, 161.59, 155.54, 152.61, 138.68, 135.97, 134.74, 134.32, 129.82, 127.75, 127.63, 125.98, 124.71, 123.67, 122.20, 117.65, 117.20, 116.57, 110.83, 104.14, 55.57, 22.22, 21.21; IR (KBr), ν (cm−1) 3101, 2914, 1612, 1442, 1238, 1130, 1029, 827, 622, 495; HRMS (ESI): calc. for C24H18O3 [M + H]+ 355.1334, found 355.1336.
3-(tert-Butyl)-6-methoxy-12-methyl-14H-dibenzo[a,c]xanthen-14-one (2d). Yellow solid. Yield: 81%, Mp: 255.6–258.1 °C. 1H NMR (400 MHz, CDCl3) δ 10.08 (d, J = 8.9 Hz, 1H), 8.60 (d, J = 8.9 Hz, 1H), 8.51 (d, J = 2.3 Hz, 1H), 8.20 (s, 1H), 8.01 (d, J = 2.3 Hz, 1H), 7.83 (dd, J = 8.9, 2.0 Hz, 1H), 7.51 (d, J = 2.8 Hz, 2H), 7.28 (dd, J = 9.1, 2.4 Hz, 1H), 4.05 (s, 3H), 2.50 (s, 3H), 1.51 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 178.21, 161.58, 155.19, 152.77, 149.03, 136.20, 134.80, 134.37, 127.69, 127.53, 126.93, 126.74, 126.22, 126.09, 123.73, 118.36, 118.16, 117.27, 116.16, 111.13, 105.19, 77.48, 77.16, 76.84, 55.68, 35.15, 31.60, 21.18; IR (KBr), ν (cm−1) 2941, 1623, 1396, 1259, 1168, 1047, 854, 800, 576; HRMS (ESI): calc. for C27H24O3 [M + H]+ 397.1804, found 397.1804.
12-Methyl-14H-dibenzo[a,c]xanthen-14-one (2e). White solid. Yield: 62%, Mp: 251.9–254.3 °C. 1H NMR (600 MHz, CDCl3) δ 10.20 (d, J = 8.3 Hz, 1H), 8.76 (d, J = 8.3 Hz, 1H), 8.68 (dd, J = 12.8, 8.3 Hz, 2H), 8.23 (s, 1H), 7.84 (dd, J = 11.2, 4.0 Hz, 1H), 7.78–7.72 (m, 2H), 7.69 (dd, J = 11.2, 4.0 Hz, 1H), 7.60–7.55 (m, 2H), 2.53 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 178.51, 155.25, 152.78, 135.17, 134.68, 133.96, 130.63, 129.24, 128.64, 127.88, 127.56, 127.39, 126.70, 126.12, 124.22, 124.18, 123.75, 122.98, 122.42, 117.38, 112.70, 21.21; IR (KBr), ν (cm−1) 3045, 2960, 1739, 1629, 1487, 1392, 1271, 1037, 804, 719, 518; HRMS (ESI): calc. for C22H14O2 [M + H]+ 311.1072, found 311.1071.
3-(tert-Butyl)-12-methyl-14-H-dibenzo[a,c]xanthen-14-one (2f). White solid. Yield: 71%, Mp: 208.3–211.1 °C. 1H NMR (400 MHz, CDCl3) δ 10.00 (d, J = 8.9 Hz, 1H), 8.56 (d, J = 8.3 Hz, 2H), 8.49 (d, J = 7.7 Hz, 1H), 8.07 (s, 1H), 7.79 (dd, J = 8.9, 1.9 Hz, 1H), 7.72–7.65 (m, 1H), 7.56 (t, J = 7.5 Hz, 1H), 7.40–7.33 (m, 2H), 2.42 (s, 3H), 1.51 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 178.20, 154.60, 152.56, 149.11, 134.82, 134.29, 133.91, 130.21, 127.52, 127.14, 126.96, 126.77, 126.58, 125.88, 124.05, 123.50, 122.63, 118.10, 117.23, 112.46, 35.14, 31.58, 21.10; IR (KBr), ν (cm−1) 3060, 2952, 1635, 1488, 1398, 1284, 1124, 1033, 923, 806, 763, 636, 522; HRMS (ESI): calc. for C26H22O2 [M + Na]+ 389.1517, found 389.1514.
3,12-Dimethyl-14-H-dibenzo[a,c]xanthen-14-one (2g). White solid. Yield: 68%, Mp: 231.8–233.5 °C. 1H NMR (400 MHz, CDCl3) δ 10.07 (d, J = 8.6 Hz, 1H), 8.75 (dd, J = 8.2, 1.0 Hz, 1H), 8.69 (d, J = 8.3 Hz, 1H), 8.46 (s, 1H), 8.23 (s, 1H), 7.86–7.80 (m, 1H), 7.76–7.70 (m, 1H), 7.62–7.55 (m, 3H), 2.63 (s, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 178.57, 154.69, 152.87, 136.41, 135.13, 134.59, 133.80, 130.49, 130.22, 127.75, 127.72, 127.28, 126.89, 126.13, 124.33, 124.23, 123.76, 123.01, 122.41, 117.41, 112.73, 21.99, 21.22; IR (KBr), ν (cm−1) 2910, 1930, 1610, 1431, 1398, 1286, 1139, 1043, 923, 819, 723, 584, 520; HRMS (ESI): calc. for C23H16O2 [M + Na]+ 347.1048, found 347.1052.
6,12-Dimethyl-14-H-dibenzo[a,c]xanthen-14-one (2h). White solid. Yield: 74%, Mp: 223.7–226.2 °C. 1H NMR (400 MHz, CDCl3) δ 10.12 (d, J = 8.3 Hz, 1H), 8.54 (d, J = 8.1 Hz, 1H), 8.45 (d, J = 8.1 Hz, 1H), 8.32 (s, 1H), 8.16 (s, 1H), 7.71 (t, J = 7.5 Hz, 1H), 7.63–7.59 (m, 1H), 7.52–7.40 (m, 3H), 2.58 (s, 3H), 2.50 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 178.32, 155.35, 152.63, 140.95, 134.92, 134.44, 133.93, 129.39, 128.94, 128.40, 127.79, 127.25, 126.41, 126.01, 124.01, 123.68, 122.74, 122.29, 121.83, 117.29, 111.94, 22.42, 21.18; IR (KBr), ν (cm−1) 3028, 2914, 1720, 1610, 1429, 1394, 1282, 1033, 919, 806, 524; HRMS (ESI): calc. for C23H16O2 [M + H]+ 325.1229, found 325.1224.
3,6,12-Trimethyl-14H-dibenzo[a,c]xanthen-14-one (2i). White solid. Yield: 72%, Mp: 254.3–256.8 °C. 1H NMR (400 MHz, CDCl3) δ 9.97 (d, J = 8.6 Hz, 1H), 8.41 (d, J = 8.3 Hz, 1H), 8.29 (s, 2H), 8.16 (s, 1H), 7.50 (dd, J = 11.1, 4.7 Hz, 2H), 7.45 (d, J = 8.4 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 2.58 (d, J = 3.9 Hz, 6H), 2.50 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 178.26, 154.83, 152.65, 140.68, 135.97, 134.79, 134.28, 133.69, 129.89, 128.74, 127.62, 127.31, 126.98, 125.97, 123.95, 123.64, 122.67, 122.20, 121.89, 117.28, 111.99, 22.38, 21.92, 21.17; IR (KBr), ν (cm−1) 2914, 1631, 1433, 1402, 1284, 1043, 869, 817, 669, 530; HRMS (ESI): calc. for C24H18O2 [M + H]+ 339.1385, found 339.1375.
3-(tert-Butyl)-6,12-dimethyl-14H-dibenzo[a,c]xanthen-14-one (2j). Yellow solid. Yield: 78%, Mp: 260.2–262.8 °C. 1H NMR (400 MHz, CDCl3) δ 10.02 (d, J = 8.9 Hz, 1H), 8.53 (d, J = 1.6 Hz, 1H), 8.35 (d, J = 8.3 Hz, 1H), 8.29 (s, 1H), 8.11 (s, 1H), 7.80 (dd, J = 8.9, 1.9 Hz, 1H), 7.43 (dd, J = 8.5, 1.9 Hz, 1H), 7.36 (dd, J = 8.0, 4.2 Hz, 2H), 2.57 (s, 3H), 2.46 (s, 3H), 1.54 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 178.14, 154.87, 152.56, 148.92, 140.63, 134.69, 134.19, 134.04, 128.67, 127.51, 127.02, 126.93, 126.45, 125.88, 123.96, 123.55, 122.43, 121.86, 118.04, 117.22, 111.84, 35.15, 31.64, 22.42, 21.11; IR (KBr), ν (cm−1) 3030, 2952, 1629, 1431, 1402, 1284, 1193, 1039, 850, 808, 659, 536; HRMS (ESI): calc. for C27H24O2 [M + H]+381.1855, found 381.1855.
6-Methyl-14H-dibenzo[a,c]xanthen-14-one (2k). Yellow solid. Yield: 70%, Mp: 185.7–187.6 °C. 1H NMR (600 MHz, CDCl3) δ 10.01 (d, J = 8.3 Hz, 1H), 8.38 (d, J = 8.1 Hz, 1H), 8.34 (dd, J = 7.8, 1.3 Hz, 1H), 8.23 (d, J = 8.2 Hz, 1H), 8.13 (s, 1H), 7.66–7.61 (m, 2H), 7.52 (dd, J = 11.0, 3.9 Hz, 1H), 7.45 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H), 7.26 (d, J = 8.2 Hz, 1H), 2.47 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 178.05, 155.16, 154.22, 140.90, 133.77, 133.53, 129.11, 128.80, 128.27, 127.66, 127.11, 126.58, 126.34, 124.50, 123.93, 123.78, 122.55, 122.17, 121.47, 117.46, 111.82, 22.34; IR (KBr), ν (cm−1) 3055, 2918, 1633, 1407, 1294, 1112, 1024, 873, 757, 520; HRMS (ESI): calc. for C22H14O2 [M + Na]+ 333.0891, found 333.0893.
3-(tert-Butyl)-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2l). Yellow solid. Yield: 73%, Mp: 212.2–214.4 °C. 1H NMR (600 MHz, CDCl3) δ 10.06 (d, J = 8.8 Hz, 1H), 8.60 (d, J = 1.8 Hz, 1H), 8.53 (d, J = 8.3 Hz, 1H), 8.45–8.38 (m, 2H), 7.82 (dd, J = 8.8, 2.0 Hz, 1H), 7.74–7.69 (m, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.48 (d, J = 8.2 Hz, 1H), 7.46–7.41 (m, 1H), 2.65 (s, 3H), 1.53 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 178.27, 155.14, 154.51, 149.22, 140.94, 134.31, 133.62, 128.88, 127.57, 127.10, 126.97, 126.73, 126.64, 124.57, 124.11, 124.07, 122.66, 121.93, 118.17, 117.58, 112.12, 35.20, 31.64, 22.51; IR (KBr), ν (cm−1) 3099, 2956, 1635, 1463, 1411, 1296, 1122, 1012, 873, 761, 644, 559, 470; HRMS (ESI): calc. for C26H22O2 [M + Na]+ 389.1517, found 389.1516.
2,6-Dimethyl-14H-dibenzo[a,c]xanthen-14-one (2m). Yellow solid. Yield: 75%, Mp: 218.5–221.1 °C. 1H NMR (600 MHz, CDCl3) δ 9.79 (s, 1H), 8.33 (d, J = 7.8 Hz, 1H), 8.23 (t, J = 8.1 Hz, 2H), 8.08 (s, 1H), 7.65 (dd, J = 11.1, 4.0 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H), 7.31 (d, J = 8.1 Hz, 1H), 7.24 (d, J = 9.2 Hz, 1H), 2.54 (s, 3H), 2.46 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 178.11, 155.29, 154.21, 140.76, 138.21, 133.86, 133.44, 129.12, 128.30, 127.81, 127.39, 126.52, 124.87, 124.44, 123.93, 123.75, 122.32, 122.04, 121.05, 117.44, 111.66, 22.34, 22.16; IR (KBr), ν (cm−1) 3028, 2914, 1637, 1415, 1307, 1172, 1033, 873, 761, 667, 576; HRMS (ESI): calc. for C23H16O2 [M + Na]+ 347.1048, found 347.1048.
11-Methoxy-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2n). White solid. Yield: 59%, Mp: 190.2–191.8 °C. 1H NMR (600 MHz, CDCl3) δ 10.15 (d, J = 8.2 Hz, 1H), 8.54 (d, J = 8.2 Hz, 1H), 8.40 (d, J = 8.8 Hz, 1H), 8.32 (s, 1H), 8.27 (d, J = 8.8 Hz, 1H), 7.70 (t, J = 7.4 Hz, 1H), 7.62–7.60 (m, 1H), 7.41 (d, J = 8.1 Hz, 1H), 6.96 (d, J = 7.4 Hz, 1H), 6.90 (s, 1H), 3.92 (s, 3H), 2.58 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 177.70, 164.23, 156.09, 155.24, 140.79, 133.78, 129.43, 128.92, 128.33, 128.09, 127.89, 127.31, 126.38, 123.80, 122.72, 122.27, 121.72, 117.99, 114.04, 111.91, 99.62, 55.95, 22.42; IR (KBr), ν (cm−1) 3016, 2918, 1622, 1444, 1249, 1031, 923, 783, 736, 630, 540; HRMS (ESI): calc. for C23H16O3 [M + Na]+ 341.1178, found 341.1178.
11-Methoxy-2,6-dimethyl-14H-dibenzo[a,c]xanthen-14-one (2o). Yellow solid. Yield: 70%, Mp: 221.4–223.8 °C. 1H NMR (600 MHz, CDCl3) δ 9.99 (s, 1H), 8.46 (d, J = 8.3 Hz, 2H), 8.33 (s, 1H), 8.29 (d, J = 8.8 Hz, 1H), 7.44 (dd, J = 11.3, 8.8 Hz, 2H), 7.01–6.95 (m, 2H), 3.95 (s, 3H), 2.61 (d, J = 8.9 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 177.87, 164.25, 156.16, 155.48, 140.77, 138.40, 133.96, 129.50, 128.52, 128.09, 127.96, 127.63, 125.14, 123.85, 122.58, 122.21, 121.37, 118.05, 114.06, 111.83, 99.67, 55.98, 22.45, 22.23; IR (KBr), ν (cm−1) 2912, 1627, 1444, 1257, 1172, 1022, 825, 690, 576, 466; HRMS (ESI): calc. for C24H18O3 [M + Na]+ 377.1154, found 377.1157.
3-(tert-Butyl)-11-methoxy-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2p). Yellow solid. Yield: 71%, Mp: 205.6–208.1 °C. 1H NMR (600 MHz, CDCl3) δ 10.07 (d, J = 8.8 Hz, 1H), 8.57 (d, J = 1.6 Hz, 1H), 8.40 (d, J = 8.3 Hz, 1H), 8.36 (s, 1H), 8.25 (d, J = 8.8 Hz, 1H), 7.80 (dd, J = 8.8, 1.9 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 6.92 (dd, J = 8.8, 2.3 Hz, 1H), 6.87 (d, J = 2.3 Hz, 1H), 3.89 (s, 3H), 2.61 (s, 3H), 1.53 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 177.66, 164.11, 156.10, 154.87, 148.98, 140.56, 133.98, 128.73, 128.00, 127.60, 127.09, 127.05, 126.47, 123.84, 122.51, 121.84, 118.04, 117.92, 113.92, 111.88, 99.57, 55.90, 35.17, 31.63; IR (KBr), ν (cm−1) 3095, 2952, 1627, 1409, 1255, 1170, 1033, 921, 825, 688, 557, 474; HRMS (ESI): calc. for C27H24O3 [M + Na]+ 419.1623, found 419.1623.
3-Fluoro-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2q). Yellow solid. Yield: 55%, Mp: 267.4–269.8 °C. 1H NMR (400 MHz, CDCl3) δ 10.23–10.14 (m, 1H), 8.58 (d, J = 8.3 Hz, 1H), 8.43 (d, J = 7.9 Hz, 1H), 8.28 (s, 1H), 8.20 (d, J = 10.7 Hz, 1H), 7.78–7.75 (m, 1H), 7.66 (d, J = 8.3 Hz, 1H), 7.55 (d, J = 8.3 Hz, 1H), 7.46 (dd, J = 17.2, 9.5 Hz, 2H), 2.65 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 178.16, 161.56 (d, 1J = 244.92 Hz), 155.08, 154.56, 141.44, 133.95, 133.34 (d, 4J = 3.87 Hz), 130.38 (d, 3J = 8.18 Hz), 129.79, 129.58 (d, 3J = 7.58 Hz), 126.82, 125.77, 124.85, 124.29, 124.04, 123.16, 122.37, 117.65, 116.78 (d, 2J = 21.72 Hz), 112.00, 107.86 (d, 2J = 22.17 Hz), 22.46; IR (KBr), ν (cm−1) 3060, 2918, 1934, 1635, 1469, 1411, 1226, 1116, 813, 750, 638, 536; HRMS (ESI): calc. for C22H13FO2 [M + H]+ 329.0978, found 329.0968.
3-Chloro-11-methoxy-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2r). White solid. Yield: 58%, Mp: 237.2–239.1 °C. 1H NMR (600 MHz, CDCl3) δ 10.09 (d, J = 9.0 Hz, 1H), 8.45 (d, J = 2.0 Hz, 1H), 8.42 (d, J = 8.8 Hz, 1H), 8.26 (d, J = 8.8 Hz, 1H), 8.22 (s, 1H), 7.61 (dd, J = 9.0, 2.1 Hz, 1H), 7.47 (d, J = 8.2 Hz, 1H), 7.00 (dd, J = 8.8, 2.3 Hz, 1H), 6.95 (d, J = 2.2 Hz, 1H), 3.97 (s, 3H), 2.61 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 177.41, 164.46, 156.14, 155.15, 141.23, 132.64, 132.62, 129.61, 129.53, 128.80, 128.50, 128.15, 127.72, 123.92, 122.81, 122.13, 121.94, 117.86, 114.25, 111.54, 99.72, 56.03, 22.41; IR (KBr), ν (cm−1) 2920, 1627, 1444, 1407, 1253, 1164, 1037, 906, 821, 759, 686, 538; HRMS (ESI): calc. for C23H15ClO3 [M + H]+ 375.0788, found 375.0783.
3-Fluoro-6-methoxy-12-methyl-14H-dibenzo[a,c]xanthen-14-one (2s). White solid. Yield: 64%, Mp: 253.4–256.1 °C. 1H NMR (600 MHz, CDCl3) δ 10.16 (s, 1H), 8.52 (d, J = 8.3 Hz, 1H), 8.16 (s, 1H), 8.04 (d, J = 8.3 Hz, 1H), 7.75 (s, 1H), 7.46 (dd, J = 31.1, 24.0 Hz, 3H), 7.25 (s, 1H), 4.02 (s, 3H), 2.50 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 177.90, 161.41 (d, 1J = 244.71 Hz), 161.86, 154.94, 152.79, 135.03, 134.96, 134.58, 130.52 (d, 3J = 7.88 Hz), 129.10 (d, 4J = 3.74 Hz), 126.33, 126.16 (d, 4J = 4.86 Hz), 123.71, 118.62, 117.69, 117.26, 116.85 (d, 2J = 21.81 Hz), 110.92, 107.72 (d, 2J = 21.99 Hz), 104.89, 55.73, 21.15; IR (KBr), ν (cm−1) 3105, 2964, 1917, 1610, 1458, 1234, 1026, 813, 636, 536; HRMS (ESI): calc. for C23H15FO3 [M + H]+ 359.1083, found 359.1089.
3-Fluoro-11-methoxy-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2t). White solid. Yield: 55%, Mp: 220.3–222.1 °C. 1H NMR (400 MHz, CDCl3) δ 10.21 (dd, J = 9.4, 6.3 Hz, 1H), 8.52 (d, J = 8.4 Hz, 1H), 8.31 (d, J = 8.5 Hz, 1H), 8.26 (s, 1H), 8.18 (dd, J = 10.9, 2.6 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.47–7.40 (m, 1H), 7.04–6.99 (m, 2H), 3.98 (s, 3H), 2.63 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 177.57, 164.46, 161.86, 161.46 (d, 1J = 224.65 Hz), 156.24, 141.07, 133.03 (d, 4J = 3.92 Hz), 130.44 (d, 3J = 8.09 Hz), 129.64, 129.50 (d, 3J = 8.03 Hz), 128.21, 125.89 (d, 4J = 1.74 Hz), 124.02, 123.04, 122.29, 117.96, 116.62 (d, 2J = 21.78 Hz), 114.22, 111.81, 107.71 (d, 2J = 22.50 Hz), 99.76, 56.03, 22.41; IR (KBr), ν (cm−1) 2911, 1627, 1448, 1409, 1255, 1109, 950, 819, 538; HRMS (ESI): calc. for C23H15FO3 [M + H]+ 359.1083, found 359.1083.
12-Fluoro-2,6-dimethyl-14H-dibenzo[a,c]xanthen-14-one (2u). White solid. Yield: 54%, Mp: 222.4–224.1 °C. 1H NMR (400 MHz, CDCl3) δ 9.85 (s, 1H), 8.42 (d, J = 8.4 Hz, 2H), 8.30 (s, 1H), 8.00 (dd, J = 8.5, 3.1 Hz, 1H), 7.57 (dd, J = 9.1, 4.1 Hz, 1H), 7.45–7.40 (m, 3H), 2.60 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 177.42, 159.45 (d, 1J = 243.59 Hz), 155.72, 150.60, 141.28, 138.64, 134.25, 129.02, 128.64, 128.21, 127.49, 125.15, 125.05 (d, 3J = 7.18 Hz), 123.98, 122.63, 122.26, 121.79 (d, 2J = 25.51 Hz), 121.14, 119.53 (d, 3J = 7.89 Hz), 111.46 (d, 2J = 23.85 Hz), 111.26, 22.46, 22.21; IR (KBr), ν (cm−1) 3080, 2916, 1895, 1635, 1485, 1272, 817, 655, 541; HRMS (ESI): calc. for C23H15FO2 [M + H]+ 343.1134, found 343.1134.
3-(tert-Butyl)-12-fluoro-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2v). Yellow solid. Yield: 63%, Mp: 240.2–241.8 °C. 1H NMR (400 MHz, CDCl3) δ 9.95 (d, J = 8.9 Hz, 1H), 8.55 (d, J = 1.5 Hz, 1H), 8.40 (d, J = 8.3 Hz, 1H), 8.35 (s, 1H), 7.97 (dd, J = 8.5, 3.0 Hz, 1H), 7.80 (dd, J = 8.9, 1.8 Hz, 1H), 7.50 (dd, J = 9.0, 4.1 Hz, 1H), 7.45–7.35 (m, 2H), 2.63 (s, 3H), 1.53 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.25, 159.35 (d, 1J = 243.61 Hz), 155.15, 150.57, 149.38, 141.13, 134.31, 128.89, 127.48, 127.10, 126.69, 126.61, 124.94 (d, 3J = 7.19 Hz), 124.01, 122.63, 121.68 (d, 2J = 25.36 Hz), 121.64, 119.49 (d, 3J = 7.87 Hz), 118.17, 111.43 (d, 2J = 23.58 Hz), 111.35, 35.21, 31.63, 22.51; IR (KBr), ν (cm−1) 3045, 2954, 1633, 1450, 1263, 1122, 1033, 813, 777, 543; HRMS (ESI): calc. for C26H21FO2 [M + Na]+ 407.1423, found 407.1418.
6-Fluoro-11-methoxy-2-methyl-14H-dibenzo[a,c]xanthen-14-one (2w). Yellow solid. Yield: 65%, Mp: 254.4–257.2 °C. 1H NMR (400 MHz, CDCl3) δ 9.97 (s, 1H), 8.61 (dd, J = 9.0, 5.9 Hz, 1H), 8.31 (dd, J = 14.5, 8.6 Hz, 2H), 8.16 (dd, J = 11.0, 2.0 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.39–7.33 (m, 1H), 7.01 (dd, J = 13.4, 4.6 Hz, 2H), 3.97 (s, 3H), 2.62 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 177.68, 164.43, 164.26 (d, 1J = 248.98 Hz), 156.14, 154.84, 139.41, 136.11 (d, 3J = 9.15 Hz), 128.25, 128.19, 127.78, 126.78 (d, 3J = 9.37 Hz), 124.64 (d, 4J = 3.72 Hz), 122.47, 120.21, 117.98, 115.81 (d, 2J = 23.56 Hz), 114.23, 111.9537, 111.9448, 108.21 (d, 2J = 22.61 Hz), 99.72, 56.02, 22.25; IR (KBr), ν (cm−1) 2974, 1897, 1623, 1417, 1271, 1166, 1051, 821, 607, 430; HRMS (ESI): calc. for C23H15FO3 [M + H]+ 359.1083, found 359.1083.
3-(tert-Butyl)-6-fluoro-11-methoxy-14H-dibenzo[a,c]xanthen-14-one (2x). White solid. Yield: 69%, Mp: 254.2–256.7 °C. 1H NMR (400 MHz, CDCl3) δ 10.01 (d, J = 8.9 Hz, 1H), 8.47 (dd, J = 8.9, 6.0 Hz, 1H), 8.38 (s, 1H), 8.22 (d, J = 8.9 Hz, 1H), 8.18–8.11 (m, 1H), 7.85–7.77 (m, 1H), 7.26 (d, J = 6.6 Hz, 1H), 6.92 (dd, J = 8.9, 2.0 Hz, 1H), 6.83 (s, 1H), 3.90 (s, 3H), 1.51 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 177.43, 164.26, 164.03 (d, 1J = 248.80 Hz), 156.04, 154.14, 149.38, 136.03 (d, 3J = 8.78 Hz), 128.04, 127.72, 127.31, 126.66 (d, 3J = 9.28 Hz), 126.48 (d, 4J = 3.55 Hz), 120.56, 118.26, 117.79, 115.89 (d, 2J = 23.63 Hz), 114.11, 111.95, 108.18 (d, 2J = 22.75 Hz), 99.56, 55.94, 35.18, 31.56; IR (KBr), ν (cm−1) 3099, 2960, 1625, 1442, 1255, 1166, 1035, 941, 827, 734, 578, 478; HRMS (ESI): calc. for C26H21FO3 [M + H]+ 401.1553, found 401.1562.
3-(tert-Butyl)-11-hydroxy-6-methyl-14H-dibenzo[a,c]xanthen-14-one (2y). Red solid. Yield: 78%, Mp: 291.6–293.4 °C. 1H NMR (600 MHz, DMSO) δ 10.75 (s, 1H), 9.83 (d, J = 8.1 Hz, 1H), 8.56 (s, 2H), 8.43 (d, J = 8.2 Hz, 1H), 7.98 (d, J = 8.6 Hz, 1H), 7.66 (d, J = 8.8 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 6.97 (s, 1H), 6.85 (d, J = 8.6 Hz, 1H), 2.37 (s, 3H), 1.34 (s, 9H); 13C NMR (150 MHz, DMSO) δ 176.37, 162.97, 155.74, 154.08, 148.71, 140.91, 133.12, 129.11, 127.64, 126.69, 126.56, 126.34, 125.96, 123.59, 122.80, 121.10, 118.47, 116.05, 114.70, 110.60, 101.83, 34.78, 31.22, 21.67; IR (KBr), ν (cm−1) 3189, 2958, 1620, 1415, 1269, 1174, 923, 821, 559, 468; HRMS (ESI): calc. for C26H22O3 [M + Na]+ 405.1467, found 405.1463.
6-Hydroxy-12-methyl-14H-dibenzo[a,c]xanthen-14-one (2z). White solid. Yield: 70%, Mp: 299.4–301.2 °C. 1H NMR (600 MHz, DMSO) δ 10.62 (s, 1H), 10.04 (d, J = 8.3 Hz, 1H), 8.63–8.59 (m, 2H), 8.07 (d, J = 1.8 Hz, 1H), 8.01 (s, 1H), 7.74–7.70 (m, 2H), 7.68–7.63 (m, 2H), 7.33 (dd, J = 8.9, 2.1 Hz, 1H), 2.46 (s, 3H); 13C NMR (150 MHz, DMSO) δ 176.84, 160.51, 155.20, 152.07, 135.61, 135.20, 134.28, 128.92, 128.36, 126.81, 126.44, 126.40, 126.20, 125.03, 122.80, 122.79, 118.15, 117.55, 116.00, 109.31, 107.12, 20.55; IR (KBr), ν (cm−1) 3145, 2912, 2314, 1608, 1436, 1244, 806, 619, 526; HRMS (ESI): calc. for C22H14O3 [M + Na]+ 349.0841, found 349.0839.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (No. 21672132, 21502110).
References
- M. Fagnoni, D. Dondi, D. Ravelli and A. Albini, Chem. Rev., 2007, 107, 2725–2756 CrossRef CAS PubMed.
- V. Dichiarante, M. Fagnoni and A. Albini, Green Chem., 2009, 11, 942–945 RSC.
- M. Fagnoni and A. Albini, Acc. Chem. Res., 2005, 38, 713–721 CrossRef CAS PubMed.
- Y. Inoue, Chem. Rev., 1992, 92, 741–770 CrossRef CAS.
- K. B. Jørgensen, Molecules, 2010, 15, 4334–4358 CrossRef PubMed.
- F. B. Mallory, K. E. Butler, A. Bérubé, E. D. Luzik Jr, C. W. Mallory, E. J. Brondyke, R. Hiremath, P. Ngo and P. J. Carroll, Tetrahedron, 2001, 57, 3715–3724 CrossRef CAS.
- A. Gaspar, M. J. Matos, J. Garrido, E. Uriarte and F. Borges, Chem. Rev., 2014, 114, 4960–4992 CrossRef CAS PubMed.
- S. Quideau, Angew. Chem., Int. Ed., 2006, 45, 6786–6787 CrossRef CAS.
- R. S. Keri, S. Budagumpi, R. K. Pai and R. G. Balakrishna, Eur. J. Med. Chem., 2014, 78, 340–374 CrossRef CAS PubMed.
- J. Yang and N. Yoshikai, Angew. Chem., Int. Ed., 2016, 55, 2870–2874 CrossRef CAS PubMed.
- M. C. Jiménez, M. A. Miranda and R. Tormos, Heterocycl. Chem., 1996, 43, 339–347 Search PubMed.
- F. B. Mallory, C. S. Wood and J. T. Gordon, J. Am. Chem. Soc., 1964, 86, 3094–3102 CrossRef CAS.
- C. S. Wood and F. B. Mallory, J. Org. Chem., 1964, 29, 3373–3377 CrossRef CAS.
- A. G. Neo, C. López, V. Romero, B. Antelo, J. Delamano, A. Pérez, D. Fernández, J. F. Almeida, L. Castedo and G. Tojo, J. Org. Chem., 2010, 75, 6764–6770 CrossRef CAS PubMed.
- J. Han, T. Wang, Y. Liang, Y. Li, C. Li, R. Wang, S. Feng and Z. Zhang, Org. Lett., 2017, 19, 3552–3555 CrossRef CAS PubMed.
- Q. Yang, T. Wei, Y. He, Y. Liang and Z.-T. Zhang, Helv. Chim. Acta, 2015, 98, 953–960 CrossRef CAS.
- Q. Wang, Z. Zhang, Z. Du, H. Hua and S. Chen, Green Chem., 2013, 15, 1048–1054 RSC.
- W. Wei, C. Li, T. Wang, D. Liu and Z. Zhang, Tetrahedron, 2016, 72, 5037–5046 CrossRef CAS.
- A. G. Lvov, V. Z. Shirinian, A. V. Zakharov, M. M. Krayushkin, V. V. Kachala and I. V. Zavarzin, J. Org. Chem., 2015, 80, 11491–11500 CrossRef CAS PubMed.
- R. Hoffmann and R. B. Woodward, Acc. Chem. Res., 1968, 1, 17–22 CrossRef CAS.
- A. G. Schultz and I. C. Chiu, J. Chem. Soc., Chem. Commun., 1978, 29 RSC.
- A. G. Schultz, W. Y. Fu, R. D. Lucci, B. G. Kurr, K. M. Lo and M. Boxer, J. Am. Chem. Soc., 1978, 100, 2140–2149 CrossRef CAS.
- A. G. Schultz, W. Y. Fu, R. D. Lucci, B. G. Kurr, K. M. Lo and M. Boxer, J. Am. Chem. Soc., 1978, 100, 2150–2162 CrossRef CAS.
- R. Sekiya and R. Kuroda, Chem. Commun., 2011, 47, 10097–10099 RSC.
- C. Valla, A. Baeza, F. Menges and A. Pfaltz, Synlett, 2008, 3167–3171 CAS.
- F. J. Zhang and Y. L. Li, Synthesis, 1993, 565–567 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra09770k |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Tao Wang
a,
Tao Wang a,
Yong Liangb,
Yangyang Zhanga,
Rui Wanga and
Zunting Zhang
a,
Yong Liangb,
Yangyang Zhanga,
Rui Wanga and
Zunting Zhang *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature was described. The reported methodology provided a quick and easy access to the synthesis of dibenzo[a,c]xanthen derivatives, which eliminates the use of any catalysts, oxidants and additives.
1, v/v) at room temperature was described. The reported methodology provided a quick and easy access to the synthesis of dibenzo[a,c]xanthen derivatives, which eliminates the use of any catalysts, oxidants and additives.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was chosen as solvent (63%, entry 7). Meanwhile, the concentration of 1a was also explored (entries 7, 9–10) and 2a was obtained in highest yield when the reaction was performed in 5 mM scale (63%, entry 7). Finally, screening of irradiation time was conducted and lower yield of 2a was obtained (entries 11–12). When the cyclization was performed under open air, 2a was obtained in 32% yield (entry 13). Thus, the irradiation of 5 mM 1a in EtOH–H2O (19
1, v/v) was chosen as solvent (63%, entry 7). Meanwhile, the concentration of 1a was also explored (entries 7, 9–10) and 2a was obtained in highest yield when the reaction was performed in 5 mM scale (63%, entry 7). Finally, screening of irradiation time was conducted and lower yield of 2a was obtained (entries 11–12). When the cyclization was performed under open air, 2a was obtained in 32% yield (entry 13). Thus, the irradiation of 5 mM 1a in EtOH–H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at ambient temperature for 3 h under the argon atmosphere was determined to be the optimal condition.
1, v/v) at ambient temperature for 3 h under the argon atmosphere was determined to be the optimal condition.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) plays an important role in the process of keto–enol isomerization, which accounts for higher yields of 2a comparing to MeCN, CHCl2 and Me2CO. In the end, the annulation product 2a was obtained along with the elimination of hydrogen molecule via syn-elimination from intermediate C, which could be easily explained by the restoration of aromaticity for the benzene ring and the entire conjugated system. To our delight, we have successfully detected the hydrogen via GC and the detail experiment was shown in ESI-Fig. 2.† The annulation product 2a was also produced under the open air (oxygen, Table 1, entry 13), and it indicated that the photochemical cyclization proceed through the S1 state.
1, v/v) plays an important role in the process of keto–enol isomerization, which accounts for higher yields of 2a comparing to MeCN, CHCl2 and Me2CO. In the end, the annulation product 2a was obtained along with the elimination of hydrogen molecule via syn-elimination from intermediate C, which could be easily explained by the restoration of aromaticity for the benzene ring and the entire conjugated system. To our delight, we have successfully detected the hydrogen via GC and the detail experiment was shown in ESI-Fig. 2.† The annulation product 2a was also produced under the open air (oxygen, Table 1, entry 13), and it indicated that the photochemical cyclization proceed through the S1 state.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at ambient temperature in a quartz tube, and was degassed for 5 min via ultrasound, deaerated for 20 min by bubbling argon and irradiated with a high pressure mercury lamp (500 W) at room temperature for 3 h. Then the reaction mixture were removed under reduced pressure and purified by column chromatography (ethyl acetate/petroleum ether, 1
1, v/v) at ambient temperature in a quartz tube, and was degassed for 5 min via ultrasound, deaerated for 20 min by bubbling argon and irradiated with a high pressure mercury lamp (500 W) at room temperature for 3 h. Then the reaction mixture were removed under reduced pressure and purified by column chromatography (ethyl acetate/petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to give 2a (63%).
50) to give 2a (63%).