Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
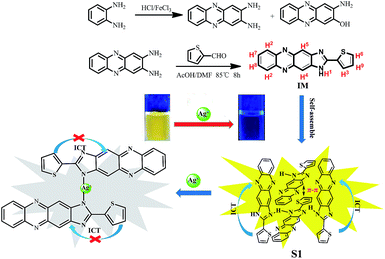
A novel self-assembled supramolecular sensor based on thiophene-functionalized imidazophenazine for dual-channel detection of Ag+ in an aqueous solution†
Hai-Xiong
Shi
,
Wen-Ting
Li
,
Qiao
Li
,
Hai-Li
Zhang
,
You-Ming
Zhang
 ,
Tai-Bao
Wei
,
Tai-Bao
Wei
 *,
Qi
Lin
*,
Qi
Lin
 and
Hong
Yao
and
Hong
Yao
Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: weitaibao@126.com
First published on 21st November 2017
Abstract
Herein, a novel self-assembled supramolecular sensor (S1) based on thiophene-functionalized imidazophenazine for Ag+ was designed and synthesized. It showed dual-channel detection properties for Ag+ based on the competitive mechanism of supramolecular self-assembly with high sensitivity and selectivity even in the presence of other metal ions. Its detection limit for Ag+ is 8.18 × 10−9 M. Upon the addition of Ag+, the solution changes from yellow to light purple and the fluorescence is quenched. Furthermore, the sensing mechanism between Ag+ and S1 is investigated via IR and 1H NMR spectroscopy, mass spectrometry, and density functional theory calculations.
Introduction
With the development of modern industry, increasing industrial emissions containing metal ions have caused serious water pollution, which affects food and agriculture and further threatens human health and the environment.1 Among the important metal ions, silver has attracted extensive attention due to its wide applications in the fields of electricity, photography, imaging, and pharmacy.2 In fact, trance amounts of silver ions can control bacteria breeding or rehabilitate and reconstruct essential human tissue.3 However, as is well known, excess silver ions can inactivate sulfhydryl enzymes; on the other hand, silver ions can combine with amine, imidazole, and carboxyl groups of various metabolites, such as high molecular weight protein and metallothionein, in the tissue of the cytosol fraction, resulting in harmful effects to humans.4 Thus, the maximum bearable concentration of Ag(I) issued by the World Health Organization (WHO) is 0.5 μM in drinking water.5Considering the importance of Ag(I) in industrial production and in daily life, the development of techniques for sensing and monitoring Ag(I) is in great demand. There are various available methods to monitor Ag+, such as electrothermal atomic absorption spectrometry (ETAAS), voltammetry, inductively coupled plasma atomic emission spectrometry (ICP-AES), inductively coupled plasma mass spectroscopy (ICP-MS), and potentiometry.6 However, most of them are expensive, time-consuming, or unsuitable for on-site and real-time monitoring. Therefore, the development of fast, efficient, sensitive, and selective methods for the detection of Ag+ is necessary for both the environment and human health.7 In comparison, fluorescence techniques exhibit attractive advantages, such as high selectivity and sensitivity, real-time detection, practical-simple sample preparation, and great potential in high throughput detection of Ag+ on-site. However, some of these probes suffer from serious drawbacks such as complicated synthesis procedures and poor solubility, sensitivity, and selectivity in aqueous media.8 Due to the efforts of researchers, numerous Ag+ sensors with good properties have been reported. However, most of them exhibit ultraviolet or fluorescent single channel recognition or are less selective.9 Moreover, it is worthwhile to note that sensors for the detection of Ag+ in aqueous systems are relatively scarce although Ag+ usually exists in water or sewerage. Consequently, the design and synthesis of colorimetric and fluorescent chemosensors with high sensitivity and selectivity for the quantification of Ag+ in aqueous media via a simple reaction is still an intriguing area of research.10
In view of this and based on our previous research in host–guest chemistry,11 we designed and synthesized a sensor molecule IM (Scheme 1) based on thiophene-functionalized imidazophenazine via a simple reaction; this sensor could self-assemble into a supramolecular system and form a supramolecular chemosensor S1. This sensor combines the phenazine fluorescent signaling subunit, which has attracted extensive attention due to its excellent photophysical properties. In addition, among the different fluorogens, it is very sensitive to conformational changes.12 Consequently, phenazines are ideal platforms for the development of cation, anion, and neutral molecule recognition platforms. Moreover, there are only a few reports on ion recognition in host–guest chemistry. Compared to some Ag+ sensors reported in previous studies, the Ag+ sensor reported herein is a self-assembled supramolecular sensor (S1). The Ag+ sensing mechanism is based on a competition between self-assembly of the sensor molecules and Ag+ coordination with the sensor molecules. This novel competitive mechanism provides the S1 sensor with better Ag+ detection sensitivity. The detection limit of S1 for Ag+ is 8.18 × 10−9 M, which is lower than that reported in some previous studies.8 Therefore, S1 is utilized as a supramolecular self-assembled sensor to study the recognition performance of Ag+.
Results and discussion
To investigate the Ag+ recognition abilities of the S1 sensor in an aqueous solution, we performed a series of host–guest recognition experiments. The recognition profiles of the chemosensor S1 towards various metal ions, including Fe3+, Hg2+, Ag+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, and Mg2+, were primarily investigated using UV-vis spectroscopy and fluorescence spectroscopy in a DMSO/water (v:v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, buffered with HEPES pH = 7.2) solution.
1, buffered with HEPES pH = 7.2) solution.
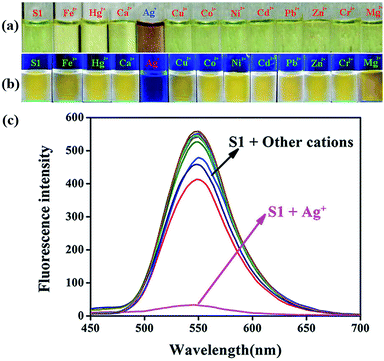
As shown in Fig. 1, the sensor immediately responded with obvious color changes (from yellow to light purple) when Ag+ was added to the S1 solution at room temperature. In the corresponding UV-vis spectra, the strength of the absorption peak at 413 nm decreased, and the peak red-shifted to 376 nm (Fig. S1†). However, yellow fluorescence with one emission band centered at 547 nm appeared when the S1 sensor solution was excited at 400 nm. The fluorescence intensity of the peak at 547 nm sharply decreased with the addition of the Ag+ solution. No significant UV-vis or fluorescence spectra changes were observed when solutions of other cations (such as Fe3+, Hg2+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, and Mg2+) were added to the S1 sensor solution.
Subsequently, we further investigated the supramolecular sensor S1 recognition for Pd2+, as shown in Fig. S2 and S3;† however, S1 could not sense Pd2+. These results confirmed that the self-assembly supramolecular sensor S1 could sense Ag+ with high sensitivity and selectivity.
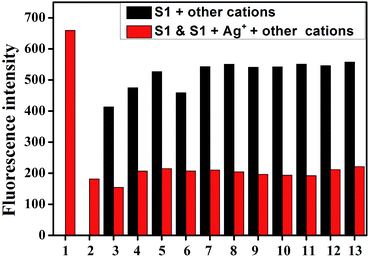
The specific selectivity of a sensor towards an analyte in the presence of other competitive species is important. To explore the utility of the S1 sensor as an ion-selective chemosensor for Ag+, competitive experiments were carried out in the presence of 10 equiv. of Ag+ and 10 equiv. of Fe3+, Hg2+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, and Mg2+ metal ions in DMSO/water (v:v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, buffered with HEPES pH = 7.2) binary solutions of the S1 sensor (Fig. 2 and S4†). From the bar diagram, it was observed that none of these ions induced any significant change in the fluorescence and UV-vis absorbance spectrum of the sensor. Therefore, it is clear that the interference of other ions is negligible during the detection of Ag+. These results further suggested that S1 could be used as a sensor for Ag+ over a wide range of cations. Thus, S1 could selectively and instantly (dual-channel) detect Ag+ in DMSO/water (v:v = 1
1, buffered with HEPES pH = 7.2) binary solutions of the S1 sensor (Fig. 2 and S4†). From the bar diagram, it was observed that none of these ions induced any significant change in the fluorescence and UV-vis absorbance spectrum of the sensor. Therefore, it is clear that the interference of other ions is negligible during the detection of Ag+. These results further suggested that S1 could be used as a sensor for Ag+ over a wide range of cations. Thus, S1 could selectively and instantly (dual-channel) detect Ag+ in DMSO/water (v:v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, buffered with HEPES pH = 7.2).
1, buffered with HEPES pH = 7.2).
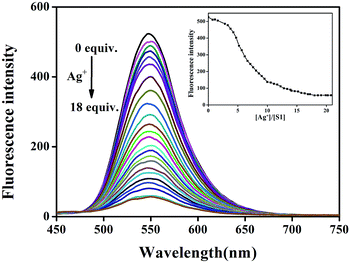
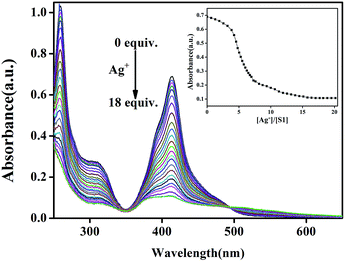
To evaluate the Ag+-responsive nature of S1, fluorescence and UV-vis absorbance titrations with Ag+ at varying concentrations were conducted. As shown in Fig. 3, upon the addition of Ag+ to the S1 sensor, the emission peak at 547 nm gradually diminished, and the fluorescence of S1 was almost quenched by 18.0 equiv. of Ag+ ions. In the UV-vis spectra (Fig. 4), the initial absorption peak at 413 nm gradually decreased, and simultaneously, a broad shoulder peak appeared at 530 nm and an isosbestic point at 495 nm was clearly observed. This should be attributed to the damage of intramolecular hydrogen-bonding interactions in the sensor and confirms the coordination of the sensor and Ag+. Based on the equation LOD = K × δ/S,13 the detection limits obtained from the UV-vis and the fluorescence spectra were 8.75 × 10−7 M and 8.18 × 10−9 M for silver ions, respectively.14 This data indicates that the sensor can detect Ag+ at very low concentrations in the environment with high selectivity and sensitivity. This result also shows that S1 has higher sensitivity for silver ions as compared to other reported Ag+ sensors (Table S1†). Therefore, S1 can be used as a potential colorimetric and fluorescent sensor for Ag+ recognition.
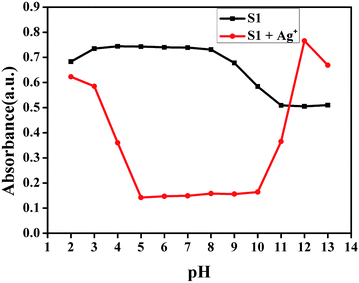
Since the pH value of the environment around a chemosensor usually influences its detection toward target metal ions due to the protonation or deprotonation reaction of the sensor, the influence of pH value on the absorbance and emission bands of S1 in a DMSO/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v:v, buffered with HEPES pH = 7.2) solution has been investigated. As shown in Fig. 5 and S7,† when the pH increased from 5 to 10, the absorbance and emission values remained almost unchanged. These results indicated that Ag+ could be clearly detected via UV-vis and fluorescence spectral measurement using S1 over a wide pH range.
1, v:v, buffered with HEPES pH = 7.2) solution has been investigated. As shown in Fig. 5 and S7,† when the pH increased from 5 to 10, the absorbance and emission values remained almost unchanged. These results indicated that Ag+ could be clearly detected via UV-vis and fluorescence spectral measurement using S1 over a wide pH range.
The self-assembly and Ag+ recognition mechanism of the S1 sensor were investigated using concentration titration, ESI-MS, 1H NMR titration, and DFT methods. To establish the supramolecular self-assembly mechanism, we performed the concentration titration in the absence and presence of different concentrations of S1. As shown in Fig. 6, the fluorescence intensity of the peak at 547 nm sharply increased with the addition of S1; this suggested that with the gradual increase in the concentration of S1, the self-assembled system was formed by π–π stacking and H-bonding in the aqueous solution. Moreover, the XRD spectra (Fig. S8†) support the abovementioned competitive mechanism. The free sensor S1 exhibits a peak at 2θ = 23.5° corresponding to the d spacing of 3.82 Å, which suggests that π–π stacking exists. In addition, compared to S1–Ag, this peak disappeared; this is attributed to the coordination of Ag+ with the S1 sensor. Based on all the abovementioned tests, we confirmed that IM self-assembly formed S1via π–π stacking and H-bonding in the aqueous solution.
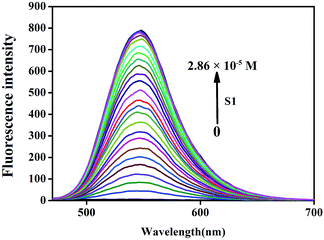 | ||
Fig. 6 Fluorescence spectra in the presence of different concentrations of S1 (0–2.86 × 10−5 M) in the DMSO/H2O (v:v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, buffered with HEPES pH = 7.2) solution. 1, buffered with HEPES pH = 7.2) solution. | ||
The reversibility of the sensor function was tested by the addition of I− to the S1–Ag complex. With the alternative addition of Ag+ and I− to the aqueous solution of the self-assembled S1 sensor, the fluorescence emission of the tested solution exhibited alternating reviving and quenching processes (Fig. S9†). This off–on–off switching process was repeated several times. Therefore, the S1 sensor can be considered a good ON–OFF fluorescence switch.
The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the S1 and Ag+ complex was further confirmed by the results of the ESI-MS studies. The free probe shows a main peak at m/z = 301.09, corresponding to [IM − H+] (ESI-MS negative ion mode) (Fig. S14†), and the complex is confirmed by the appearance of a peak at m/z = 731.12, assignable to [2IM + Ag+ + H2O + H+] (ESI-MS positive ion mode) (Fig. S15†). The Job plot between S1 and Ag+ was implemented, demonstrating a 2
1 stoichiometry of the S1 and Ag+ complex was further confirmed by the results of the ESI-MS studies. The free probe shows a main peak at m/z = 301.09, corresponding to [IM − H+] (ESI-MS negative ion mode) (Fig. S14†), and the complex is confirmed by the appearance of a peak at m/z = 731.12, assignable to [2IM + Ag+ + H2O + H+] (ESI-MS positive ion mode) (Fig. S15†). The Job plot between S1 and Ag+ was implemented, demonstrating a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for S1 and Ag+, as shown in Fig. S16.†
1 stoichiometry for S1 and Ag+, as shown in Fig. S16.†
The FTIR spectra of the receptor IM and its complex with Ag+ were obtained to examine the binding sites (Fig. S10†). In the FTIR spectrum of the free probe IM, the characteristic absorption band of S1–Ag+ for N–H (3428 cm−1) of the imidazole group was absent, and an obvious enhancement was observed for the C–N absorption bands from 1322 to 1421 cm−1; this confirmed the binding of S1 with silver.
To gain more information about the complexation between S1 and Ag+, we performed an 1H NMR titration in the absence and presence of different concentrations of Ag+. As shown in Fig. S11,† the 1H NMR titration of S1 in DMSO-d6 (10 mmol) with Ag+ in D2O (1 mol) established the stoichiometry and binding site towards the sensor response. Most interestingly, the N–H peak (H1) of the imidazole group in IM disappeared after the addition of 2.0 equiv. of Ag+ to D2O;15 this proved that S1 was strongly bound with Ag+. Moreover, the signal of the hydrogen atoms in the phenazine (H2, H4, H5, H7, and H8) and thiazole (H3, H6, and H9) rings showed a significant downfield shift; this suggested that the intermolecular π–π stacking was destroyed after the addition of Ag+, and it further caused the deshielding for the hydrogen protons and resulted in downfield shifts.
To further investigate the binding behavior of S1 with Ag+ ions, we optimized the structure of IM and IM–Ag+ complex by applying the B3LYP/6-31G(d, p) method in the gas phase using the Gaussian-09 computational code.16 The optimized structures of IM and IM–Ag+ are shown in Fig. S12 and S13,† respectively. The short N34–H49⋯N23 distance of 2.041 Å indicates the presence of an intramolecular hydrogen bond in IM. Additionally, some of the relevant optimized geometric parameters are presented in Tables S2–S5.† The relevant occupied molecular orbital HOMO (−0.295 a.u.) of IM was localized mainly to the thiophene groups, whereas the unoccupied orbital LUMO (−0.227 a.u.) was localized over the phenazine and imidazole groups. The HOMO–LUMO energy gap (DE) for IM was found to be 0.068 a.u. (Fig. 7). On the other hand, upon interaction with Ag+, the HOMO (−0.291 a.u.) and LUMO (−0.279 a.u.) were almost localized on the entire molecule, where the electron density was polarized toward the whole molecule. The HOMO–LUMO energy (DE) for IM with Ag+ was found to be 0.012 a.u. These results suggested that the fluorescence quenching was most probably attributed to the prevention of IM intramolecular charge transfer (ICT) by Ag+ (Scheme 1).
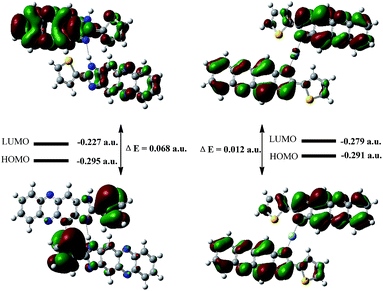 | ||
| Fig. 7 DFT-optimized Frontier molecular orbitals and HOMO–LUMO energy gaps for S1 (left) and S1–Ag+ (right). | ||
To investigate the practical application of the chemosensor S1, test strips were prepared by immersing filter paper in a DMSO/water (v:v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, buffered with HEPES pH = 7.2) solution of S1 and then drying them in air. The test strips containing S1 were utilized to sense Ag+ and other metal ions. As shown in Fig. 8, when Ag+ and other ions were added to the test kits, an obvious color change was only observed for the Ag+ solution under a 365 nm UV-lamp. The potential competitive ions did not influence the detection of Ag+ by the test strips. Therefore, the test strips could conveniently detect Ag+ in the solution.
1, buffered with HEPES pH = 7.2) solution of S1 and then drying them in air. The test strips containing S1 were utilized to sense Ag+ and other metal ions. As shown in Fig. 8, when Ag+ and other ions were added to the test kits, an obvious color change was only observed for the Ag+ solution under a 365 nm UV-lamp. The potential competitive ions did not influence the detection of Ag+ by the test strips. Therefore, the test strips could conveniently detect Ag+ in the solution.
Conclusions
In summary, we developed a novel self-assembled supramolecular sensor S1 that could recognize Ag+ over other metal ions with significant UV-vis absorption and fluorescence quenching changes with highly selective and sensitive properties based on the competitive mechanism of supramolecular self-assembly. The obvious color changes and pronounced ON–OFF-type fluorescence signaling behavior can be seen by the naked eyes. The limit of detection for Ag+ was as low as 8.18 × 10−9 M in an aqueous solution, which was lower than that in some previously reported research. We believe that the sensor will be regarded as an environmentally friendly material that can sense Ag+ in an aqueous solution and make a great contribution to the development of silver ion sensors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (21661028; 21574104; 21262032), the Natural Science Foundation of Gansu Province (1506RJZA273), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- (a) T. Anand, D. Sivaraman, P. Anandh, D. Chellappa and S. Govindarajan, Tetrahedron Lett., 2014, 55, 671–675 CrossRef CAS; (b) L. J. Bian, X. Ji and W. J. Hu, J. Agric. Food Chem., 2014, 62, 4870–4877 CrossRef CAS.
- H.-T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- (a) C. M. Niemietz and S. D. Tyerman, FEBS Lett., 2002, 531, 443–447 CrossRef CAS; (b) M. S. Pedroso, J. G. Bersano and A. Bianchini, Environ. Toxicol. Chem., 2007, 26, 2158–2165 CrossRef CAS.
- X. B. Zhang, Z. X. Han, Z. H. Fang, G. L. Shen and R. Q. Yu, Anal. Chim. Acta, 2006, 562, 210–215 CrossRef CAS.
- M. Hu, J. Fan, J. Cao, K. Song, H. Zhang, S. Sun and X. Peng, Analyst, 2012, 137, 2107–2111 RSC.
- (a) M. Hadioui, S. Leclerc and K. J. Wilkinson, Talanta, 2013, 105, 15–19 CrossRef CAS; (b) H. Yang, X. Liu, R. Fei and Y. Hu, Talanta, 2013, 116, 548–553 CrossRef CAS; (c) Z. Szigeti, A. Malon, T. Vigassy, V. Csokai, A. Grun, K. Wygladacz, N. Ye, C. Xu, V. J. Chebny, I. Bitter, R. Rathore, E. Bakker and E. Pretsch, Anal. Chim. Acta, 2006, 572, 1–10 CrossRef CAS; (d) G. Yan, Y. Wang, X. He, K. Wang, J. Su, Z. Chen and Z. Qing, Talanta, 2012, 94, 178–183 CrossRef CAS.
- (a) B. Zhang, J. Sun, C. Bi, G. Yin, L. Pu, Y. Shi and L. Sheng, New J. Chem., 2011, 35, 849–853 RSC; (b) Z. Xu, S. Zheng, J. Yoon and D. R. Spring, Analyst, 2010, 135, 2554–2559 RSC; (c) H.-H. Wang, L. Xue, Y.-Y. Qian and H. Jiang, Org. Lett., 2010, 12, 292–295 CrossRef CAS; (d) C. Huang, A. Ren, C. Feng and N. Yang, Sens. Actuators, B, 2010, 151, 236–242 CrossRef CAS; (e) T. Chen, W. Zhu, Y. Xu, S. Zhang, X. Zhang and X. Qian, Dalton Trans., 2010, 39, 1316–1320 RSC; (f) C. S. Park, J. Y. Lee, E.-J. Kang, J.-E. Lee and S. S. Lee, Tetrahedron Lett., 2009, 50, 671–675 CrossRef CAS; (g) L. Liu, D. Zhang, G. Zhang, J. Xiang and D. Zhu, Org. Lett., 2008, 10, 2271–2274 CrossRef CAS; (h) S. Iyoshi, M. Taki and Y. Yamamoto, Inorg. Chem., 2008, 47, 3946–3948 CrossRef CAS; (i) C. Huang, X. Peng, Z. Lin, J. Fan, A. Ren and D. Sun, Sens. Actuators, B, 2008, 133, 113–117 CrossRef CAS; (j) R.-H. Yang, W.-H. Chan, A. W. M. Lee, P.-F. Xia and H.-K. Zhang, J. Am. Chem. Soc., 2003, 125, 2884–2885 CrossRef CAS; (k) C. Liu, S. Huang, H. Yao, S. He, Y. Lu, L. Zhao and X. Zeng, RSC Adv., 2014, 4, 16109–16114 RSC; (l) A. Affrose, S. D. S. Parveen, B. S. Kumar and K. Pitchumani, Sens. Actuators, B, 2015, 206, 170–175 CrossRef CAS; (m) Q. Lin, Q. P. Yang, B. Sun, T.-B. Wei and Y.-M. Zhang, Chin. J. Chem., 2014, 32, 1255–1258 CrossRef CAS; (n) Y. Li, H. Yu, G. Shao and F. Gan, J. Photochem. Photobiol., A, 2015, 301, 14–19 CrossRef CAS.
- (a) C. Liu, S. Huang, H. Yao, S. He, Y. Lu, L. Zhao and X. Zeng, RSC Adv., 2014, 4, 16109–16114 RSC; (b) Y.-T. Wu, J.-L. Zhao, L. Mu, X. Zeng, G. Wei, C. Redshaw and Z. Jin, Sens. Actuators, B, 2017, 252, 1089–1097 CrossRef CAS; (c) C. Chen, H. Liu, B. Zhang, Y. Wang, K. Cai, Y. Tan, C. Gao, H. Liu, C. Tan and Y. Jiang, Tetrahedron, 2016, 72, 3980–3985 CrossRef CAS; (d) Y. Jiang, W. Kong, Y. Shen and B. Wang, Tetrahedron, 2015, 71, 5584–5588 CrossRef CAS; (e) T.-B. Wei, H. L. Zhang, W. T. Li, W. J. Qu, J. X. Su, Q. Lin, Y. M. Zhang and H. Yao, Chin. J. Chem., 2017, 35, 1311–1316 CrossRef CAS.
- (a) F. Qu, J. Liu, H. Yan, L. Peng and H. Li, Tetrahedron Lett., 2008, 49, 7438–7441 CrossRef CAS; (b) X. Liu, X. Yang, Y. Fu, C. Zhu and Y. Cheng, Tetrahedron, 2011, 67, 3181–3186 CrossRef CAS; (c) F. Li, F. Meng, Y. Wang, C. Zhu and Y. Cheng, Tetrahedron, 2015, 71, 1700–1704 CrossRef CAS; (d) Y. Wang, H. Q. Chang, W.-N. Wu, X.-L. Zhao, Y. Yang, Z. Q. Xu, Z. H. Xu and L. Jia, Sens. Actuators, B, 2017, 239, 60–68 CrossRef CAS.
- (a) X. H. Li, X. H. Gao, W. Shi and H. M. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS; (b) Z. Q. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC; (c) K. Zscharnack, T. Kreisig, A. A. Prasse and T. Zuchner, Anal. Chim. Acta, 2014, 834, 51–57 CrossRef CAS; (d) S. Goswami, A. K. Das, A. Manna, A. K. Maity, P. Saha, C. K. Quah, H. Fun and H. A. Abdel-Aziz, Anal. Chem., 2014, 86, 6315–6322 CrossRef CAS; (e) R. Kramer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef CAS; (f) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 1995, 24, 197–202 RSC.
- (a) B. B. Shi, P. Zhang, T.-B. Wei, H. Yao, Q. Lin and Y.-M. Zhang, Chem. Commun., 2013, 49, 7812–7814 RSC; (b) Q. Lin, T. T. Lu, J. C. Lou, G. Y. Wu, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 12224–12227 RSC; (c) Q. Lin, F. Zheng, L. Liu, P. P. Mao, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2016, 6, 111928–111933 RSC; (d) Q. Lin, T. T. Lu, X. Zhu, T. B. Wei, H. Li and Y. M. Zhang, Chem. Sci., 2016, 7, 5341–5346 RSC; (e) Q. Lin, T. T. Lu, X. Zhu, B. Sun, Q. P. Yang, T. B. Wei and Y. M. Zhang, Chem. Commun., 2015, 51, 1635–1638 RSC; (f) Q. Lin, P. P. Mao, L. Liu, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2017, 7, 11206–11210 RSC; (g) Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Chem.–Eur. J., 2014, 20, 11457–11462 CrossRef CAS; (h) X. B. Cheng, H. Li, F. Zheng, Q. Lin, H. Yao, Y. M. Zhang and T. B. Wei, RSC Adv., 2016, 6, 20987–20993 RSC.
- (a) Y. X. Zhao, Y. Y. Liu, Z. Y. Wang, W. H. Xu, B. Liu, J. H. Su, B. Wu and X. J. Yang, Chem. Commun., 2015, 51, 1237–1239 RSC; (b) C. G. Wang, G. Li and Q. C. Zhang, Tetrahedron Lett., 2013, 54, 2633–2636 CrossRef CAS; (c) Q. L. Xu, C. H. Heo, G. G. Kim, H. W. Lee, H. M. Kim and J. Y. Yoon, Angew. Chem., Int. Ed., 2015, 54, 4890–4894 CrossRef CAS.
- M. H. Yang, P. Thirupathi and K. H. Lee, Org. Lett., 2011, 13, 5028–5031 CrossRef CAS PubMed.
- (a) L. Yang, X. Li, J. Yang, Y. Qu and J. Hua, ACS Appl. Mater. Interfaces, 2013, 5, 1317–1326 CrossRef CAS; (b) F. Zheng, F. Zeng, C. Yu, X. Hou and S. Wu, Chem.–Eur. J., 2013, 19, 936–942 CrossRef CAS.
- M. Shellaiah, M. V. R. Raju, A. Singh, H.-C. Lin, K.-H. Wei and H.-C. Lin, J. Mater. Chem. A, 2014, 2, 17463–17476 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheese-man, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Strat-mann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Ste-fanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. John-son, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 09W, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis of S1, NMR spectra, and other materials. See DOI: 10.1039/c7ra09597j |
| This journal is © The Royal Society of Chemistry 2017 |