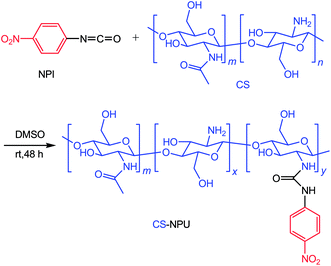
Open Access Article
Open Access ArticleA study on the synthesis and anion recognition of a chitosan-urea receptor
Kuerbanjiang Rouzi ab,
Abuderixiti Abulikemuc,
Jie Zhaoa and
Biao Wu*a
ab,
Abuderixiti Abulikemuc,
Jie Zhaoa and
Biao Wu*a
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, Shaanxi 710069, P. R. China. E-mail: wubiao@nwu.edu.cn; krbnjn2003@126.com; Fax: +86 29 81535020; Tel: +86 29 81535020
bInstitute of Chemistry and Chemical Engineering, Xinjiang University, Urumqi, 830046, P. R. China
cCollege of Chemistry and Chemical Engineering, Xinjiang Normal University, Urumqi, 830054, Xinjiang, P. R. China. E-mail: aarexit@mail.ustc.edu.cn
First published on 1st November 2017
Abstract
A new chitosan based colorimetric anion receptor bearing nitrophenyl as a chromogenic signal group, and urea moiety as recognition site was designed and synthesized. The receptor structure was characterized by FTIR and 1H NMR. The sensing abilities of the receptor for anions were investigated with UV-vis methods. During the addition of PO43− and F− anions, the sensor responded with a dramatic color change, and no change was observed in the presence of other anions. The UV-vis absorption titration data showed that the receptor shows selective recognition and sensing in DMSO-H2O (1%) for PO43− and F− anions. The Job's curve showed that 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry complexes were formed between the receptor and the anion.
2 stoichiometry complexes were formed between the receptor and the anion.
Introduction
Anions play important roles in biological, pharmaceutical, chemical, biochemical and environmental research.1–7 The detection, isolation and recognition of anions has attracted much interest. The design and synthesis of simple and selective colorimetric anion recognition tools via artificial receptors are hot topics in host guest anion recognition. Usually, anion receptors consist of recognition sites and signal reporter groups. These include general recognition sites by urea/thiourea, amine/amide, phenol, guanidine, and pyrrole-containing structural units with hydrogen bond donors.8–10 The reporting group is often the chromophore with a π conjugated system, such as a nitro phenyl, azophenyl, or anthraquinone group. These can construct an anionic chromophore carrier to realize naked eye detection of anion.11,12 Among many analysis methods, naked eye detection of anions is widely used because it is simple and easy to operate, without any spectroscopic instrumentation. Researchers have developed and applied many colorimetric anion receptors containing one or more (thio) urea subunits for anion sensing during the past decades.13 Moreover, selectivity is related to the binding affinity of the receptor–anion interaction; in this sense, results in quite stable strongly hydrogen-bonded complexes with different anions such as acetate, phosphate or fluoride.14It is well-known that urea/thiourea with a nitrophenyl group as a signaling unit showed an enhancement in both the hydrogen-bond donor tendency and acidity.15 For example, Aldrey16 and others17,18 have reported the colorimetric receptors where nitrophenyl was treated as a signaling unit and urea/thiourea moieties as binding sites.
The selectivity for special analyte of the host molecule could be rationalized on the basis of not only the guest basicity but also shape complementarity between the host and the anionic guests. We recently developed a series of ortho-phenylene bridged oligourea receptors that exhibit excellent binding affinity and selectivity toward tetrahedral anions (sulfate and phosphate) and have proven to be promising receptors for anion coordination and recognition.19
Chitosan (CS) is a linear β-(1,4)-linked polysaccharide that is composed of glucosamine and N-acetylglucosamine obtained by the partial deacetylation of chitin-the second most abundant biopolymer in nature.20–22 Chitosan is non-toxic, biocompatible and biodegradable. It is widely used in biotechnology, pharmaceuticals, cosmetics, agriculture, food science and textiles.23 However, due to strong intra- and intermolecular hydrogen bonding interactions, chitosan is only soluble in acids. It is insoluble in neutral or alkaline aqueous solutions and thus its utility is largely limited. Therefore, it is necessary to introduce functional groups to transform the chemical structure of chitosan as well as to improve its solubility, functionality, and applications.
In recent years, chitosan thiourea and its derivatives have been extensively studied in various applications such as antimicrobial and antifungal activity,24–28 chelating adsorbents for adsorption of heavy metal ions,29 inorganic anions (such as fluoride, sulfate, nitrate, and phosphate),30–32 and macromolecular organic compounds.33 In addition, chitosan and its derivatives have been used as stationary materials for chromatographic columns,34 as a colorimetric optical sensor for the detection of Ni2+, Pd2+, Cd2+, and Hg2+ ions,35–38 as a carrier in biosensors,39 as an electrochemical sensor for the detection of F−, NO3−, I− anions,40,41 or as a photochemical sensor for the detection of H2S, NH3, or CO gases.42–44
However, to the best of our knowledge, there is no report showing about chitosan (thio) urea as an anion recognition tool. Here we designed and synthesized chitosan based colorimetric anion receptors for three main reasons: (i) chitosan contains many free and active groups of amino (–NH2) and hydroxyl (–OH). Multiple urea, hydroxyl and amino groups are good hydrogen bond donors for the construction of the chitosan based anion receptors. It is known that stronger interactions in the host-guest systems are usually a consequence of a larger number of H-bonds in the complex.10 Therefore, the complex stability highly depends on the strength and the number of the H-bonds. (ii) The 4-nitrophenyl group was appended to the urea moiety, and the presence of –NO2– electron-withdrawing group enhanced the acidity, and colorimetric recognition ability of the receptor and (iii) the receptor synthesis is simple.
Here, a 4-nitrophenylurea chitosan (CS-NPU) receptor was prepared through the reaction of chitosan with 4-nitrophenyl isocyanate. The receptor structure was characterized by FTIR and 1H NMR and its anion sensing properties were studied by UV-vis analytical methods. In addition, sensing behaviors can be realized via the “naked eye” determination because it has a remarkable color response.
Experimental
Materials and chemicals
Chitosan (degree of deacetylation = 95.20%, average molecular weight Mη = 4.75 × 105, viscosity = 100–200 mPa s) was purchased from Shanghai Aladdin Industrial Corporation (China). This was dried in a vacuum drying at 50 °C for 48 h before use. The 4-nitrophenyl isocyanate (NPI) and tetrabutylammonium salts (TBA) of all anions (where [(Bu)4N]2SO4 is 50% water solution) were purchased from Alfa Aesar and used directly. The DMSO was dried prior to use with CaH2. It was distilled under reduced pressure. Other reagents were of analytical grade without further purification.Determination of degree of deacetylation (DDA) and molecular weight
The degree of deacetylation of chitosan was determined via a potentiometric titration according to the literature.45 0.2 g of chitosan was dissolved in 25 mL of 0.1 M HCl and excess HCl was back titrated with a solution of 0.1 M NaOH using a pHSJ-4F meter (INESA, Shanghai). The differential and integral titration curves were drawn between solution pH and volume of alkali added. This produced an integral curve with two inflexions. The DDA of chitosan has been calculated as follows:
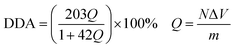 | (1) |
The molecular weight was determined by measuring relative viscosity with an Ubbelohde viscometer at 25 ± 0.5 °C. The solvent system was 0.1 M CH3COOH and 0.1 M CH3COONa. The molecular weight was calculated from the intrinsic viscosity based on the Mark–Houwink eqn (2):
| [η] = KMα | (2) |
Degradation of CS by hydrogen peroxide
The degradation process of chitosan was performed according to the literature.48 Chitosan (6 g) was dissolved in 200 mL acetic acid solution 5.0% (w/v) under room temperature for 1 h with stirring and heating to 65 °C. Next, 6 mL H2O2 was added to obtain the CS solution. The resulting solution was stirred at 65 °C for 6 h. The solution was added into a 10% sodium hydroxide solution. The product precipitated and was then filtered and washed with deionized water until the pH = 7. The solid was lyophilized for three days at −50 °C. The deacetylation degree of the degradation CS was 94.30%, and the Mη value was 2.37 × 104 g mol−1.Preparation of chitosan-nitrophenylurea (CS-NPU) receptor
The chitosan was modified via reaction between the amino group on the chitosan and the active isocyanate group of NPI as presented in Scheme 1. The treated CS (calculated by the glucosamine units: 0.180 g, 1 mmol) was added into a two-necked flask evacuated under vacuum and flushed with dry nitrogen three times. This was then added into 30 mL of dry DMSO in the flask. The mixture was stirred 30 h at 60 °C. Then NPI (0.246 g, 1.5 mmol) was added into the flask, and the resulting mixture was stirred at room temperature for 48 h. Finally, a pale yellow solid was filtered, washed three times with DMSO and acetone and then dried in vacuum.Characterizations
Fourier transform infrared spectroscopy
Fourier transform infrared spectra (FTIR) of CS-NPU were recorded in solid state using BRUKER EQUINOX-55 spectrometer (Germany). The spectra were collected in 4000–400 cm−1 region using KBr pellets. All samples were dried in a vacuum oven at 60 °C for 24 h before testing.Nuclear magnetic resonance spectroscopy
1H NMR spectroscopy was used to determine the chemical structures of the CS and CS-NPU. The 1H NMR spectra were obtained using a VARIAN INOVA-400 spectrometer (400 MHz, USA) with 5% acetic acid-d4/water-d2 as solvents.UV-vis titrations
The absorption spectra were recorded with a UV-178 UV-visible spectrophotometer (Shimadzu, Japan). The concentration of the receptor solution (1.0 × 10−5 M) in DMSO/H2O was calculated on the basis of the monomeric units and the guest anions (2.0 × 10−3 M) in DMSO. In each experiment a small amount (2−5 μL) of guest anion samples were added to the receptor L (2 mL) solution. The spectrum was then scanned. All measurements were performed at room temperature.Absorption titrations and Hill plots49
The sample solutions with varying amounts of anions were prepared similar to the above procedure. The UV-vis absorption was measured. The titration curve was obtained by plotting the absorbance at the proper wavelength versus the [anion]/[monomeric units of receptor] ratio. The binding data were further analyzed using the Hill equation (eqn (3)):
log[Y/(1 − Y)] = n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[anion] + n log[anion] + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka Ka
| (3) |
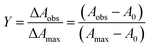 | (4) |
Solubility test
The solubility of the chitosan and the modified chitosan was tested in common organic solvents such as acetone, carbon tetrachloride, dichloromethane, trichloromethane, ethanol, methanol, tetrahydrofuran(THF), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) at 25 °C. The samples were soaked in each solvent at the concentration of 5 mg mL−1, the results are presented in Table 2.Results and discussion
Characterization of CS-NPU receptor
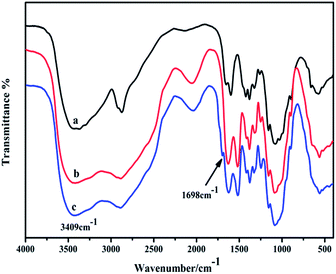
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), the amide II δ (N–H) at 1602 cm−1,50 and the ν (C–N) at 1592 cm−1. The δ (CH2) was at 1425 cm−1 and the δ (CH3) was at 1378 cm−1. There were pronounced bands at 1170 cm−1 and 1072 cm−1 for the C–O–C and C–O stretching vibrations, respectively. Degraded CS shows few new or enhanced absorption peaks. The intensity of the absorption peak is the main difference in the degraded material. This indicates that CS and its degradation products have the same molecular structure. The basic structural unit CS survives oxidative degradation. This further shows that the active functional groups –NH2 and –OH remain (Fig. 1(b)). According to the characteristic frequency of the infrared absorption spectrum, there are new peaks, at 1698 cm−1 urea ν (C
O)), the amide II δ (N–H) at 1602 cm−1,50 and the ν (C–N) at 1592 cm−1. The δ (CH2) was at 1425 cm−1 and the δ (CH3) was at 1378 cm−1. There were pronounced bands at 1170 cm−1 and 1072 cm−1 for the C–O–C and C–O stretching vibrations, respectively. Degraded CS shows few new or enhanced absorption peaks. The intensity of the absorption peak is the main difference in the degraded material. This indicates that CS and its degradation products have the same molecular structure. The basic structural unit CS survives oxidative degradation. This further shows that the active functional groups –NH2 and –OH remain (Fig. 1(b)). According to the characteristic frequency of the infrared absorption spectrum, there are new peaks, at 1698 cm−1 urea ν (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1327 cm−1 (–NO2), 1517 cm−1 (phenyl) and 709 cm−1 (phenyl) (Fig. 1(c)). The characteristic vibration peak of the C
O), 1327 cm−1 (–NO2), 1517 cm−1 (phenyl) and 709 cm−1 (phenyl) (Fig. 1(c)). The characteristic vibration peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the urea moiety of modified chitosan is consistent with the reported literature.51
O stretching of the urea moiety of modified chitosan is consistent with the reported literature.51
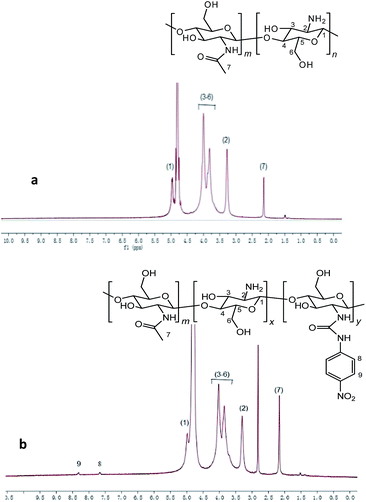
The 1H NMR spectrum of CS-NPU is shown in (Fig. 2(b)). The aromatic proton peaks from δ 7.50 to 8.50 ppm are characteristic for mono substituted benzene derivatives. Based on the assignment of the 1H NMR spectra of CS-NPU, we concluded that the phenyl urea group has been successfully introduced to the chitosan.29,48
Recognition performance
UV-vis spectra titrations
The anion sensing behavior of receptor (4.0 × 10−5 M) was determined by UV-vis spectrophotometric methods in DMSO/H2O solution. Absorption titrations were carried out with a receptor (4.0 × 10−5 M) in DMSO/H2O. The receptor was titrated upon incremental addition of tetrabutylammonium fluoride (1.0 × 10−5 M) and phosphate (1.0 × 10−5 M) in DMSO up to 2 equivalents. The titrations were carried out for all anions, and the resulting spectra are shown in Fig. 4. The UV-vis spectra of the receptor in DMSO/H2O were dominated by strong absorption peak at 351 nm.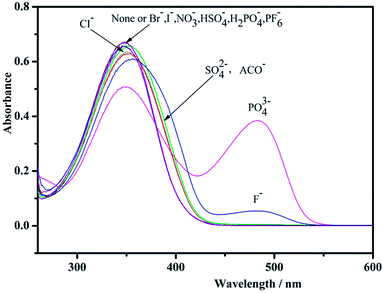 | ||
| Fig. 4 UV-vis absorbance of L in DMSO/H2O upon addition of different anions ([anion]/[L] = 10); [monomeric units of L] = 1 × 10−5 mol L−1. | ||
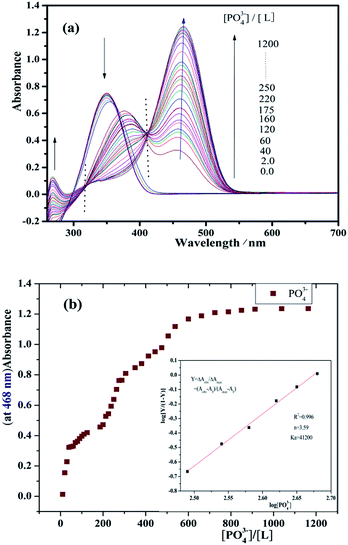
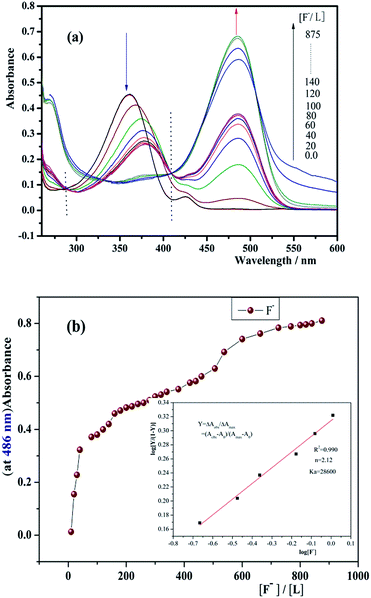
During the addition of PO43− to the solution of receptor, prominent changes were observed in UV-vis absorption spectra due to complexation between receptor and anion. The ICT band at 351 nm for the receptor is shown in Fig. 5(a). This disappears gradually upon formation of new peak at 468 nm (bathochromic shift). Clear isosbestic points were observed at 317 and 412 for L. The absorbance intensity of L was saturated with 1200 equiv. of PO43−. On the other hand, Fig. 6(a) shows that as the F− anion increased, the absorption peak at 351 nm gradually decrease, and a new peak appeared at 486 nm. Clear isosbestic points were observed at 286 and 409 nm. The absorbance intensity of L was saturated with 875 equiv. of F−. The addition of other anionic species as their tetrabutylammonium salts did not give any response. This shows the specificity of the receptor for selective binding interaction with PO43− and F− anions. The data confirm that the receptor has higher selectivity for phosphate and fluoride over other anions.
The Job's plot
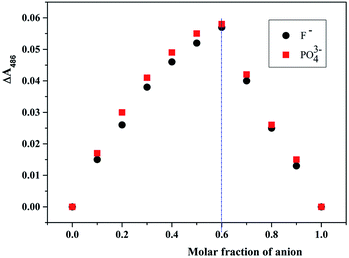
Next, we used a Job's plot (continuous variation) to establish the stoichiometric ratio between receptor and anionic guests by continuous variation in mole fraction of L ([host]/[host] + [guest]), and the total concentration of L and anionic guest was constant (1.0 × 10−4 M). Fig. 7 shows the Job plot for L and the anion complex. The receptor L/F− complex concentration approaches a maximum when the mole fraction of L is 0.6. This means that L and F− form 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes. We found similar stoichiometric ratios for L with PO43−.
2 complexes. We found similar stoichiometric ratios for L with PO43−.
Hill plot analysis
On the basis of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, the corresponding binding constants (Ka) of L for F− and PO43− anions were calculated in DMSO based on the UV-vis titration experiments by Hill plots the results are presented in Table 1. Hill plots of L for F− and PO43− are shown in Fig. 5(b) and 6(b). The slope and the intercept of Hill plots shows that Ka = 4.12 × 104 M−1 and n = 3.59 for L/PO43− (correlation coefficient: R2 = 0.996) and Ka = 2.86 × 104 M−1 and n = 2.12 for L/F− (R2 = 0.990). This shows that L has strong binding ability to PO43− and F−, and it forms a stable complex. The n value was greater than 1, and the titration curve of L was S shaped. This indicated that the effects are positive and cooperative between L and PO43− or F−.57
2 stoichiometry, the corresponding binding constants (Ka) of L for F− and PO43− anions were calculated in DMSO based on the UV-vis titration experiments by Hill plots the results are presented in Table 1. Hill plots of L for F− and PO43− are shown in Fig. 5(b) and 6(b). The slope and the intercept of Hill plots shows that Ka = 4.12 × 104 M−1 and n = 3.59 for L/PO43− (correlation coefficient: R2 = 0.996) and Ka = 2.86 × 104 M−1 and n = 2.12 for L/F− (R2 = 0.990). This shows that L has strong binding ability to PO43− and F−, and it forms a stable complex. The n value was greater than 1, and the titration curve of L was S shaped. This indicated that the effects are positive and cooperative between L and PO43− or F−.57
| Receptor | anion | Ka (M−1) |
|---|---|---|
| L | F− | 4.12 × 104 |
| L | PO43− | 2.86 × 104 |
Solubility of NPU-chitosan
The solubility of the CS and CS-NPU were studied in different solvents at 25 °C (Table 2). CS is insoluble in water or any selected organic solvent but soluble in dilute aqueous acids due to protonation of the amino groups. As shown in the table, CS-NPU is insoluble in carbon tetrachloride, dichloromethane, acetone, trichloromethane, ethanol, methanol, and THF, and it is swelled in strong polar solvents such as DMSO and DMF. NPU-CS is soluble in DMSO/H2O solution and acetic acid solution (5%, v/v).| Sample | Solubility | ||
|---|---|---|---|
| DMSO | DMF | Ethanol | |
a (+: Soluble; ±: partially soluble or swelled; −: insoluble), 25 °C, 48 hours, and chitosan: reagent = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. 1.5. |
|||
| CS | − | − | − |
| NPU-CS | ± | ± | − |
| Sample | Solubility | ||
|---|---|---|---|
| Trichloromethane | Carbon tetrachloride | Methanol | |
| CS | − | − | − |
| NPU-CS | − | − | − |
| Sample | Solubility | ||
|---|---|---|---|
| THF | Acetone | Dichloromethane | |
| CS | − | − | − |
| NPU-CS | − | − | − |
Possible recognition mechanism
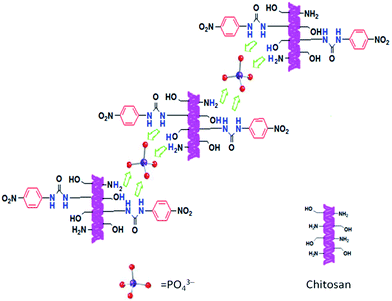
The solution is yellow when the receptor molecules coexist with phosphate anion. Upon addition of a small amount of proton solvent methanol, the solution gradually became colorless. The absorption peak at 351 nm increased gradually to the state without addition of anions. At the same time, the absorption peak of 468 nm gradually disappeared. This may be due to the competition of hydrogen bonding site of the methanol molecule to the anion with receptor molecule. These spectral data indicate the essence of the hydrogen bonding between the host and guest.Fabbrizzi and other research group's studies showed that (thio)urea is a good H-bonds donor and excellent receptor for tetrahedral anions and Y-shaped anions through the formation of multi topic H-bonds.14,58 Thus, for a given NH-containing receptor (the acid), selectivity should be mainly related to the basicity of the A− anion; the higher anion basicity, the stronger the hydrogen-bonding interaction.
Phenylurea–chitosan receptor was selective toward PO43−, forming stable complexes. The possible reasons are as follows: (i) the attachment of the strong electron-withdrawing group (–NO2) to the aromatic substituent in receptor L enhances the acidity of the urea NH proton, which could increase the stability of the PO43− complex. (ii) Many urea, hydroxyl and amino groups in phenylurea–chitosan receptor can form multiple hydrogen bond donors. The complex stability highly depends on the strength and the number of the H-bonds.10 The incorporation of additional H-bonding groups to the receptor, in principle, leads to the formation of more stable anion complexes. (iii) The basicity of phosphate anions also strongly contributes to the selectivity of CS-NPU to phosphate anions. (IV) Urea is an appropriate receptor for oxoanions because it can form two N–H O bonds with two consecutive oxygen atoms of the anion. The proposed anion-sensing mechanism of CS-NPU with anions in DMSO/H2O solution is depicted in Scheme 2.
Fluoride is a particular case as it has a size comparable to that of oxygen but holds an integral negative charge. Thus, among anions, F− establishes the strongest H-bond interaction with an NH fragment of the urea subunit. In particular, the interaction should correspond to an advanced stage of the proton transfer. In addition, the highest value with F− was observed and explained by its high charge density, small dimension and high basicity. In the presence of strong electron-withdrawing groups on the receptor, there is also a possibility for double deprotonation of the urea receptor with F−.
Conclusions
In summary, we reported the synthesis and anion recognition characteristics of a new chitosan compound. During the addition of PO43− and F− anions, the sensor responded and could be detected with naked-eyes via a change from colourless to yellow for PO43− and yellowish for F− at room temperature. Other anions such as Cl−, Br−, I−, NO3−, PF6−, ClO4−, AcO−, H2PO4−, HSO4−, and SO42− had no response. The mechanism of this anion receptor is likely hydrogen bonding interactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 21271149).References
- V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC
.
- M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC
.
- P. A. Gale and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3595–3596 RSC
.
- A. F. Li, J. H. Wang, F. Wang and Y. B. Jiang, Chem. Soc. Rev., 2010, 39, 3729–3745 RSC
.
- A. E. Hargrove, S. Nieto, T. Z. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS PubMed
.
- E. M. Boyle, S. Comby, J. K. Molloy and T. Gunnlaugsson, J. Org. Chem., 2013, 78, 8312–8319 CrossRef CAS PubMed
.
- T. Gunnlaugsson, P. E. Kruger, P. Jensen, J. Tierney, H. D. P. Ali and G. M. Hussey, J. Org. Chem., 2005, 70(26), 10875–10878 CrossRef CAS PubMed
.
- B. Wu, X. Huang, J. Liang, Y. Liu, X.-J. Yang and H. M. Hu, Inorg. Chem. Commun., 2007, 10, 563–566 CrossRef CAS
.
- K. Pandurangan, J. A. Kitchen and T. Gunnlaugsson, Tetrahedron Lett., 2013, 54, 2770–2775 CrossRef CAS
.
- V. B. Bregović, N. Basarić and K. M. Majerski, Coord. Chem. Rev., 2015, 295, 80–124 CrossRef
.
- M. Y. Wei, S. G. Li, C. D. Jia and B. Wu, Chem. J. Chin. Univ., 2011, 32, 1939–1949 CAS
.
- J. M. Kang, J. H. Lee, Y. H. Kim, S. K. Lee, E. Y. Kim, H. G. Lee and C. Ki, J. Inclusion Phenom. Macrocyclic Chem., 2012, 74, 177–182 CrossRef
.
- D. D. Su, H. T. Niu, Y. Wang, J. Q. He and J. P. Cheng, Chem. J. Chin. Univ., 2010, 31, 714–717 CAS
.
- M. Boiocchi, L. D. Boca, D. Esteban-Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed
.
- J. Kang, Y. J. Lee, S. K. Lee, J. H. Lee, J. J. Park, Y. Kim, S. J. Kim and C. Kim, Supramol. Chem., 2010, 22, 267–273 CrossRef CAS
.
- A. Aldrey, V. García, C. Lodeiro, A. Macías, P. Pérez-Lourido, L. Valencia, R. Bastida and C. Núñez, Tetrahedron, 2013, 69, 4578–4585 CrossRef CAS
.
- J. Shao, H. Lin and H. K. Lin, Talanta, 2008, 75, 1015–1020 CrossRef CAS PubMed
.
- Y. J. Kim, H. Kwak, S. J. Lee, J. S. Lee, H. J. Kwon, S. H. Nam, K. Lee and C. Kim, Tetrahedron, 2006, 62, 9635–9640 CrossRef CAS
.
- X. L. Wang, C. D. Jia, X. J. Huang and B. Wu, Inorg. Chem. Commun., 2011, 14, 1508–1510 CrossRef CAS
.
- R. A. A. Muzzarelli, Carbohydr. Polym., 2011, 84, 54–63 CrossRef CAS
.
- M. N. V. Ravi Kumar, R. A. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. J. Domb, Chem. Rev., 2004, 104, 6017–6084 CrossRef PubMed
.
- M. N. V. Ravi Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef
.
- H. P. Li, H. Li and Y. Liu, Iran. Polym. J., 2016, 25, 277–284 CrossRef CAS
.
- M. Eweis, S. S. Elkholy and M. Z. Elsabee, Int. J. Biol. Macromol., 2006, 38, 1–8 CrossRef CAS PubMed
.
- S. S. Elkholy, H. A. Salem, M. Eweis and M. Z. Elsabee, Int. J. Biol. Macromol., 2014, 70, 199–207 CrossRef CAS PubMed
.
- N. A. Mohamed and N. Y. Al-mehbad, Int. J. Biol. Macromol., 2013, 57, 111–117 CrossRef CAS PubMed
.
- Z. H. Li, F. Yang and R. D. Yang, Int. J. Biol. Macromol., 2015, 75, 378–387 CrossRef CAS PubMed
.
- Z. M. Zhong, R. E. Xing, S. Liu, L. Wang, S. B. Cai and P. C. Li, Carbohydr. Res., 2008, 343, 566–570 CrossRef CAS PubMed
.
- M. Monier and D. A. Abdel-Latif, J. Hazard. Mater., 2012, 209–210, 240–249 CrossRef CAS PubMed
.
- A. Rajeswari, A. Amalraj and A. Pius, J. Environ. Chem. Eng., 2015, 3, 2331–2341 CrossRef CAS
.
- X. Liu and L. F. Zhang, Powder Technol., 2015, 277, 112–119 CrossRef CAS
.
- N. Viswanathan, C. S. Sundaram and S. Meenakshi, J. Hazard. Mater., 2009, 161, 423–430 CrossRef CAS PubMed
.
- J. Q. Xue, Y. J. Guo, Q. Bi, W. B. Mao and J. X. Li, Mod. Phys. Lett. B, 2013, 27, 1341031 CrossRef
.
- S. Tang, J. D. Liu, Q. Bin, K. Q. Fu, X. C. Wang, Y. B. Luo, S. H. Huang and Z. W. Bai, J. Chromatogr. A, 2016, 1476, 53–62 CrossRef CAS PubMed
.
- R. Raghunandhan, L. H. Chen, H. Y. Long, L. L. Leam, P. L. So, X. Ning and C. C. Chan, Sens. Actuators, B, 2016, 233, 31–38 CrossRef CAS
.
- M. Monier, D. A. Abdel-Latif and Y. G. Abou El-Reash, J. Colloid Interface Sci., 2016, 469, 344–354 CrossRef CAS PubMed
.
- V. N. Mehta, H. Basu, R. K. Singhal and S. K. Kailasa, Sens. Actuators, B, 2015, 220, 850–858 CrossRef CAS
.
- K. Chauhan, P. Singh and R. K. Singhal, ACS Appl. Mater. Interfaces, 2015, 7, 26069–26078 CAS
.
- L. Wang, W. Wen, H. Y. Xiong, X. H Zhang, H. S. Gu and S. F. Wang, Anal. Chim. Acta, 2013, 758, 66–71 CrossRef CAS PubMed
.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Appl. Clay Sci., 2005, 28, 199–208 CrossRef CAS
.
- X. Yao, G. H. Lu, X. G. Wu and T. Zhan, Electroanalysis, 2001, 13, 923–926 CrossRef
.
- A. Yu. Mironenko, A. A. Sergeev, A. E. Nazirov, E. B. Modin, S. S. Voznesenskiy and S. Y. Bratskaya, Sens. Actuators, B, 2016, 225, 348–353 CrossRef CAS
.
- I. M. El-Sherbiny, A. Hefnawy and E. Salih, Int. J. Biol. Macromol., 2016, 86, 782–788 CrossRef CAS PubMed
.
- A. Bayram, C. Ozbek, M. Şenel and S. Okur, Sens. Actuators, B, 2017, 241, 308–313 CrossRef CAS
.
- K. C. Gupta and F. H. Jabrail, Carbohydr. Polym., 2006, 66, 43–54 CrossRef CAS
.
- X. Jiang, L. R. Chen and W. Zhong, Carbohydr. Polym., 2003, 54, 457–463 CrossRef CAS
.
- L. Y. Chen, Y. M. Du and H. Q. Wu, J. Appl. Polym. Sci., 2002, 82, 1233–1241 CrossRef
.
- Z. K. Wang, S. J. Chen, J. W. Y. Lam, W. Qin, R. T. K. Kwok, N. Xie, Q. L. Hu and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 8238–8245 CrossRef CAS PubMed
.
- M. J. Su, W. Wan, X. Yong, X. W. Lu, R. Y. Liu and J. Q. Qu, Chin. J. Polym. Sci., 2013, 31, 620–629 CrossRef CAS
.
- S. P. Chen, G. Z. Wu and H. Y. Zeng, Carbohydr. Polym., 2005, 60, 33–38 CrossRef CAS
.
- C. M. Fernandez, J. Heinamaki, M. Rasanen, S. L. Maunu, M. Karjalaien, O. M. NietoAcosta, A. Iraizoz Colarte and J. Yliruusi, Carbohydr. Polym., 2004, 58, 401–408 CrossRef
.
- M. Lavertu, A. Xia, A. N. Serreqi, M. Berrada, A. Rodrigues, D. Wang, M. D. Buschmann and A. Gupta, J. Pharm. Biomed. Anal., 2003, 32, 1149–1158 CrossRef CAS PubMed
.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS
.
- E. S. Abdou, K. S. A. Nagy and M. Z. Elsabee, Bioresour. Technol., 2008, 99, 1359–1367 CrossRef CAS PubMed
.
- M. Monier, D. A. Abdel-Latif and Y. G. Abou El-Reash, J. Colloid Interface Sci., 2016, 469, 344–354 CrossRef CAS PubMed
.
- O. Ahmet, S. Erdemir and O. Kocyigit, J. Mol. Struct., 2013, 1048, 392–398 CrossRef
.
- M. Takeuchi, M. Ikeda, A. Sugasaki and S. Shinkai, Acc. Chem. Res., 2001, 34, 865–873 CrossRef CAS PubMed
.
- D. H. Lee, K. H. Lee and J. I. Hong, Org. Lett., 2001, 3, 5–8 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2017 |