Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of different structured FePO4 for the enhanced conversion of methyl cellulose to 5-hydroxymethylfurfural
Yong Liua,
Zili Lia,
Yaohui Youa,
Xiaogang Zheng*a and
Jing Wen *b
*b
aCollege of Chemistry and Chemical Engineering, Neijiang Normal University, Neijiang, Sichuan 641100, China. E-mail: zhengxg123456@163.com; Fax: +86 0832 2341577; Fax: +86 0971 7762180; Tel: +86 0832 2341577 Tel: +86 0971 7762180
bKey Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China. E-mail: wj580420@163.com
First published on 3rd November 2017
Abstract
FePO4 catalysts with branch-like, flower-like, and spherical morphologies were synthesized for the conversion of methyl cellulose to 5-hydroxymethylfurfural (5-HMF) via a hydrothermal route. The molar ratio of Fe3+ and H2PO4− ions in the reaction system played a crucial role in the morphology of FePO4. Compared with flower-like, spherical and amorphous FePO4, branch-like FePO4 presented a better catalytic performance in the cellulose conversion and 5-HMF yield. The branch-like FePO4 retained a branch structure after recycling five times in the bi-phasic reaction process. The insolubility of low temperature and partial dissolution of elevated temperature were responsible for the excellent catalytic activity of the FePO4 phase-change catalyst. The combined effect of H+ ions and iron species generated from the hydrolysis of FePO4 can be favorable for the enhanced yield of 5-HMF.
1. Introduction
Due to the growing environmental awareness of greenhouse gas and the diminishing supply of petroleum resources, the catalytic conversion of renewable biomass feedstock into fuels, chemicals, and solvents is an alternative route to relieve the reliance on fossil resources.1,2 Many efforts focus on the development of innovative strategies for the transformation of biomass into chemicals to conquer the drawbacks including extensive, environmentally harmful, and high-cost pretreatment. Conversion of cellulosic biomass to 5-hydroxymethylfurfural (5-HMF) has triggered increasing attention.3–5 5-HMF, as a platform chemical, can be converted into valuable chemicals and fuels such as 2,5-furandicarboxylic acid, 2,5-dimethylfuran, and levulinic acid.6–10Compared with fructose and glucose, cellulose composed of linear glucose polymer chains is an ideal feedstock for the synthesis of 5-HMF due to its low cost and abundant resources.11,12 However, the poor solubility of cellulose and the formation of humins in aqueous media and organic solvents leads to the inferior conversion of cellulose and the low yield of 5-HMF in the catalytic system assisted with mineral acid.13–17 Ionic liquids combined with metal salts have been confirmed to break down the inferior conversion of cellulose into 5-HMF. Ionic liquids such as [Bmim]Br, [Bmim]Br, [EMIM]Cl, and imidazolium chloride ([C4C1im]Cl, [C2C1im]Cl, and [C4C1im]HSO4) present superior medium for the conversion of biomass feedstock due to their specific chemical and physical properties.18–25 Nevertheless, the hybrid reaction system of ionic liquids and metal salts is not suitable for the large-scale synthesis due to the high energy consumption, high cost, and environmental contamination.
Biphasic reaction systems26,27 and heterogeneous catalysis28–30 have been developed to address above challenges. These strategies are more suitable and cost effective for the large-scale transformation of cellulose into 5-HMF. However, these synthesis approaches usually suffer from the serious pollution and low 5-HMF yield if the cellulose and catalysts are not pretreated in biphasic system.31,32 This's ascribed to the insufficient contact and interaction between active sites of solid acid and insoluble cellulose in water and most organic solvents.33,34 Therefore, the phase transfer catalyst has been developed to achieve high conversion of cellulose into 5-HMF.35–38 The phase catalyst is characterized by the excellent activity of homogeneous acid catalyst and the effective separation and recovery of heterogeneous solid acid catalyst.39,40 Cellulose hydrogenation reaction assisted with H2WO4 and Ru/C presents the maximized yield of ethylene glycerol due to the effective contact between insoluble cellulose and soluble H2WO4 catalyst.29
Iron phosphate (FePO4) is a promising and inexpensive phase transfer catalyst for the efficient catalytic conversion of carbohydrates (such as glucose, fructose, and cellulose) into 5-HMF in biphasic system.36,37 FePO4 bulks are dissolved and transferred from solid state into the aqueous phase at high temperature (>140 °C), and re-precipitated to form solid bulks after cooling down to room temperature. Hence, this insolubility at room temperature and the partial dissolution at elevated temperatures of FePO4 can surmount the drawback of recycling and separation of homogeneous catalysts. The architecture of FePO4 may be crucial to the physical and chemical properties for the catalyzed synthesis of 5-HMF from cellulose during biphasic reaction process compared with CrPO4 and CrCl3 catalysts.20,28,38 This is attributed to the accelerated diffusion rate of molecules and the effective exposure of active sites to reactants. For example, the ordered mesoporous structure of Nb4W4 exhibits high amounts of Brønsted and Lewis acid sites, leading to the excellent catalytic performance in glucose conversion into 5-HMF.39 Due to the mesoporous structure and strong Brønsted acid sites, the mesoporous Nb2O5–WO3 and Ta2O5–WO3 catalysts also presented remarkable catalysis performance.40,41 However, the new insights into the effect of FePO4 microstructure on the chemical transformations of biomass feedstock remains elusive. The fundamental studies in the effect of FePO4 structure on the conversion of cellulose to 5-HMF in biphasic catalytic system is also scarce.
This work focused on the controllable synthesis of FePO4 for the catalytic conversion of methyl cellulose into 5-HMF. The branch-like, flower-like, and sphere FePO4 were prepared in a hydrothermal system with different molar ratio of FeCl3 and KH2PO4. The catalytic mechanism of FePO4 was also proposed for the conversion of methyl cellulose to 5-HMF.
2. Experimental
2.1. Reagents
Ferric chloride (FeCl3), potassium dihydrogen phosphate (KH2PO4), polyvinyl pyrrolidone (PVP, Mw = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), absolute ethanol (C2H5OH), methyl cellulose (1.0 × 105 mPa s, CAS: 9004-67-5), tetrahydrofuran (THF, C4H8O), and sodium chloride (NaCl) were purchased from Aladdin Industrial Corporation. These chemicals were of analytical grade without any further purification.
000), absolute ethanol (C2H5OH), methyl cellulose (1.0 × 105 mPa s, CAS: 9004-67-5), tetrahydrofuran (THF, C4H8O), and sodium chloride (NaCl) were purchased from Aladdin Industrial Corporation. These chemicals were of analytical grade without any further purification.
2.2. FePO4 preparation
FePO4 with branch-like, flower-like, and sphere structure were prepared by a hydrothermal route with different molar ratios of FeCl3 and KH2PO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2
3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Typically, 2.0 mmol FeCl3, 6.0 mmol KH2PO4, and 1.5 g PVP were dissolved in 60 mL ethanol solution (50 wt%) and stirred at room temperature for 3 h. Then, the above solution was transferred into a 100 mL Teflon-lined stainless autoclave and treated at 170 °C for 12 h. After cooling to room temperature, the obtained sample was centrifuged, washed with deionized water, dried at 60 °C for 10 h, and calcined at 500 °C for 3 h. Amorphous FePO4 was prepared by the precipitation route.
3). Typically, 2.0 mmol FeCl3, 6.0 mmol KH2PO4, and 1.5 g PVP were dissolved in 60 mL ethanol solution (50 wt%) and stirred at room temperature for 3 h. Then, the above solution was transferred into a 100 mL Teflon-lined stainless autoclave and treated at 170 °C for 12 h. After cooling to room temperature, the obtained sample was centrifuged, washed with deionized water, dried at 60 °C for 10 h, and calcined at 500 °C for 3 h. Amorphous FePO4 was prepared by the precipitation route.
2.3. Methyl cellulose conversion
The conversion of methyl cellulose into 5-HMF over FePO4 catalysts were performed in a 200 mL stainless steel autoclave under the N2 atmosphere of 5 bar and the given reaction temperature. Typically, 3.0 g NaCl and the given content of FePO4 and methyl cellulose were added to the mixed solution of 45 mL deionized water and 135 mL THF (TMF solution of 75%), and then stirred at room temperature for 2.0 h. The above mixture was placed into a 200 mL autoclave under the given test conditions. After cooled down to room temperature, the products were centrifuged, washed, and separated into solid residue, aqueous phase, and organic phase. The liquid fraction was analyzed by the high-performance liquid chromatography. The catalytic stability of FePO4 for the methyl cellulose converted into 5-HMF at 160 °C for 80 min was performed for five cycles under similar conditions. Before adding to the next reaction process, the used FePO4 bulks were centrifuged, washed with water three times, dried at 60 °C for 12 h, and calcined at 500 °C for 3 h.2.4. Characterization and analysis
XRD patterns of as-obtained FePO4 were measured by a Bruker D8 Advance X-ray Powder Diffractometer. FI-IR spectra were obtained by a Shimadzu® FTIR spectrometer, IRPrestige-21 model. SEM images were observed using a Hitachi S-3400 field emission electron microscope. XPS spectra were recorded on a Thermo Fisher Scientific Escalab 250 spectrometer. Fe3+ species in aqueous solution was analyzed via the Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) on Varian 710-ES equipped for axial viewing with a 1.12 megapixel CCD detector. The residue on the used FePO4 was conducted in a TA Q5000 instrument with a He flow rate of 50 mL min−1. Products were analyzed using an Agilent 1200 high-performance liquid chromatography equipped with an UV detector (280 nm) and a column (Zorbax SB-C18). 5-HMF and furfural were the main product and byproduct of methyl cellulose conversion, respectively. 5-HMF yield (Y) was defined as: Y (5-HMF) % = (moles of 5-HMF generated)/(glucose unit moles of methyl cellulose), where the mass of 5-HMF was the total content of 5-HMF in the water and organic phases.3. Results and discussion
3.1. Characterization of FePO4
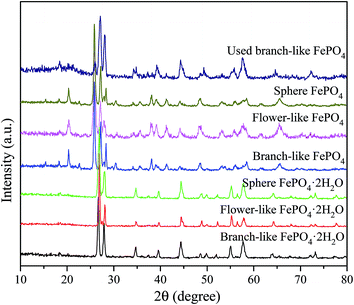
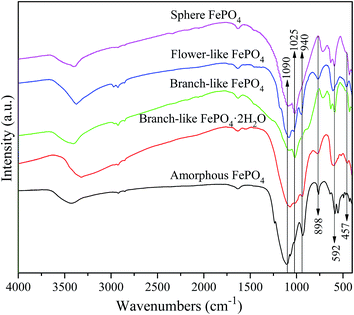
The XRD patterns of FePO4·2H2O generated via a hydrothermal route and FePO4 with different morphologies (including branch-like, flower-like, and sphere structure) were shown in Fig. 1. The typical peaks of FePO4·2H2O with different morphologies appeared at 2θ = 26.7°, 27.6°, 28.9°, 34.7°, 39.6°, 44.5°, 48.6°, 52.1°, 55.2°, 57.6°, 64.0°, and 73.3°, which were matched well with crystalline FePO4·2H2O phases (JCPDS no. 002-0250).42,43 The peaks of FePO4 samples at 2θ = 20.2°, 25.8°, 26.7°, 28.9°, 35.5°, 38.6°, 41.4°, 48.5°, 58.2°, and 65.7° were ascribed to the (100), (011), (012), (111), (110), (112), (200), (114), (212), and (124) planes of hexagonal FePO4 samples (JCPDS no. 29-0715), respectively.42–44 The (011) plane of FePO4 at 25.8° showed stronger peak intensity compared with other peaks in XRD pattern (Fig. 1), indicating that the FePO4 samples prepared via the hydrothermal route favored the growth along the (011) plane. The minor peak around 30.4° of fresh FePO4 was ascribed to the typical peak of Fe4(P2O7)3 phase (JCPDS no. 24-0526).42,45 Additional diffraction peaks of impurity were not detected in XRD patterns of FePO4, meaning that the as-prepared sample is pure FePO4. The typical peaks of FePO4 phases and FePO4·2H2O phases were detected in used branch-like FePO4 bulks for the conversion of methyl cellulose into 5-HMF after five cycled times. It's suggested that the new crystalline bulks were generated via the dissolution and recrystallization of branch-like FePO4 in the hydrothermal system.As shown in Fig. 2, the bands around 1630 cm−1 as well as the bands approximately 3400 cm−1 of all FePO4 samples were ascribed to the O–H vibration of adsorbed water molecules, while these typical bands of branch-like FePO4·2H2O exhibited at 1635 and 3328 cm−1. Compared with amorphous FePO4 bulks, the branch-like, flower-like, and sphere FePO4 samples presented slight difference in FT-IR spectrum. The asymmetric stretching vibration of PO4 group was detected at around 1090 cm−1. The bands at 1020 and 940 cm−1 arisen from the symmetric PO4-stretching mode associated with the Q0 PO43− tetrahedral.38 The peaks at 898 cm−1 of amorphous and flower-like FePO4 were also assigned to the symmetric stretching mode of PO4 group. Due to the difference in morphology, the peaks below 600 cm−1 of all FePO4 samples were related with the different Fe–O and P–O bending and stretching modes, leading to the different catalytic activity.
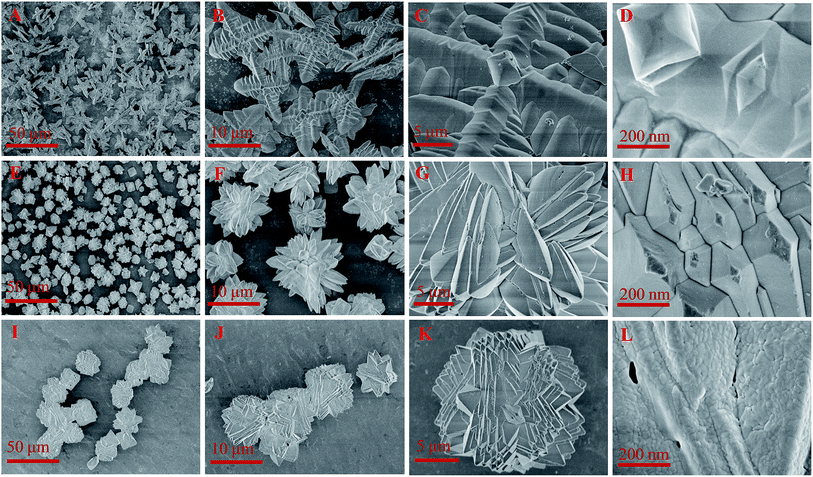
Fig. 3 presents the SEM images of FePO4 with different morphologies. The branch-like, flower-like, and sphere FePO4 were successfully achieved with the FeCl3/KH2PO4 molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2
3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. The branch-like FePO4 (Fig. 3A–D) was consisted of four main branch, of which the length and the width were 10 μm and 2 μm, respectively. The flower-like FePO4 with the particle diameter of around 10 μm (Fig. 3E–H) was regularly formed from small branch structure of 200 nm. Sphere FePO4 (Fig. 3I–L) with a diameter of 15 μm was consisted of small nanoparticles (<50 nm). The branch-like and flower-like FePO4 exhibited smooth surface structure (Fig. 3D and H), while the sphere FePO4 was rough surface structure (Fig. 3L).
3, respectively. The branch-like FePO4 (Fig. 3A–D) was consisted of four main branch, of which the length and the width were 10 μm and 2 μm, respectively. The flower-like FePO4 with the particle diameter of around 10 μm (Fig. 3E–H) was regularly formed from small branch structure of 200 nm. Sphere FePO4 (Fig. 3I–L) with a diameter of 15 μm was consisted of small nanoparticles (<50 nm). The branch-like and flower-like FePO4 exhibited smooth surface structure (Fig. 3D and H), while the sphere FePO4 was rough surface structure (Fig. 3L).
The formation of different structured FePO4 was related with the molar ratio of Fe3+ and H2PO4− ions in hydrothermal system. Surfactant PVP serving as the structure-directing molecule also played a crucial role in the synthesis of FePO4 with desired morphologies.46–48 FePO4 nanocrystallites generated from Fe3+ and PO43− ions were adsorbed by PVP and then aggregated in a certain direction. Due to the reduced surface free energies of the special crystal facets, the well-matched FePO4 particles with similar surface energy were assembled into oriented structure with the increase temperature.46,49 The increasing H+ ions released from H2PO4− could destroy the intrinsic crystal structure such as branch-like structure and change the surface energy of FePO4 nanocrystallites. These escaping nanocrystallites went into solution of excess H+ ions, nucleated and grew in a preferential direction with the assistance of surfactant, leading to the formation of flower-like and sphere FePO4.49 The molar ratio of Fe3+ and H2PO4− ions thus affected the morphology of FePO4 in PVP-assisted hydrothermal system.
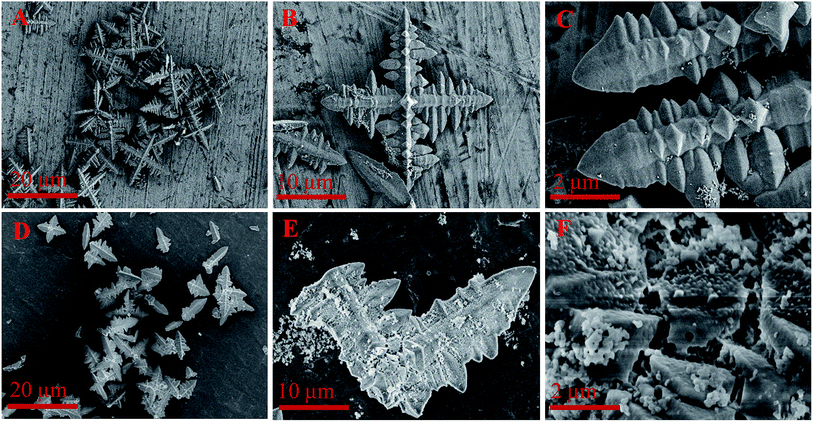
As shown in Fig. 4A–C, the used branch-like FePO4 after first cycle time retained four main branch structure with less nanosheets due to the partial dissolution in reaction system at high temperature. The branch structure of FePO4 was destroyed after the fifth cycle time under same conditions, and small particles randomly and gradually precipitated on the branch surface from the aqueous phase (Fig. 4D–F). It's indicated that FePO4 was partially dissolved at elevated temperature and randomly precipitated again in cooling process.
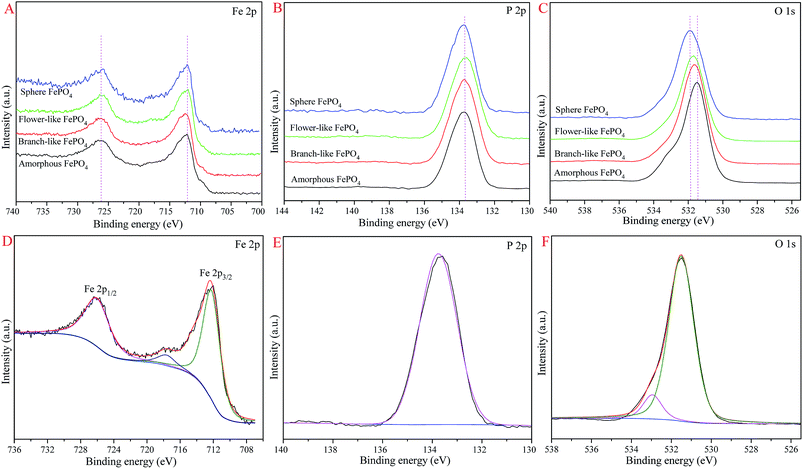
The XPS spectra of different structured FePO4 were exhibited in Fig. 5. The binding energies (BE) of Fe 2p and P 2p of these FePO4 samples were not shifted, while the BE of O 1s were slightly shifted, as shown in Fig. 5A–C. For branch-like FePO4 (Fig. 5D), the peaks at 726.1, 712.4, and 717.9 eV were assigned to the Fe 2p1/2, Fe 2p3/2, and satellite signal peaks, respectively.43,46 It's suggested that the Fe(III) element presented in fresh FePO4. The P 2p peak (Fig. 5E) was located at 133.7 eV, which was in agreement with previous work.43 Two oxygen signals of branch-like FePO4 generated from lattice oxygen and hydroxyl oxygen were observed at 531.5 and 533.0 eV in O 1s spectrum (Fig. 5F). As shown in Fig. 5C, the O 1s peaks (lattice oxygen) of sphere, flower-like, and amorphous FePO4 were located at 531.9, 531.7, and 531.4 eV, respectively. This slight BE variation of O 1s peaks was attributed to the difference in atomic ratio of lattice oxygen and hydroxyl oxygen of FePO4 with different morphologies.
3.2. Conversion of methyl cellulose into 5-HMF
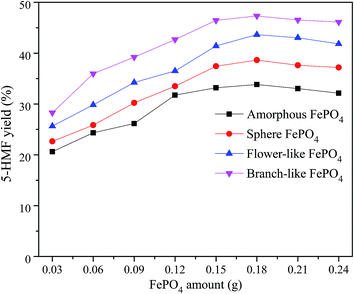
The phase-transfer catalysts such as H2WO4 and FePO4 are effective for the cellulose converted into 5-HMF in biphasic system composed of organic solvents and water.35,37 In the NaCl-assisted biphasic system, the cellulose contacted with soluble catalyst is converted into 5-HMF in the water phase, and then the obtained 5-HMF is rapidly extracted into organic phase to avoid side reaction.28,47,48 The optimal conversion conditions of methyl cellulose into 5-HMF were investigated in a biphasic reaction system combined with different structured FePO4.The 5-HMF yield increased with the increase of FePO4 content ranged from 0.03 to 0.18 g, and slightly decreased when the FePO4 dosage increased from 0.18 to 0.24 g, as shown in Fig. 6. In contrast with amorphous, sphere, and flower-like FePO4, the branch-like FePO4 was much more suitable for the methyl cellulose converted into 5-HMF. The highest 5-HMF yield was observed in the presence of 0.18 g branch-like FePO4. It could be ascribed to the effective contact between insoluble methyl cellulose and soluble Fe3+ ions in the biphasic system.36,37 The inferior 5-HMF yield for the conversion of methyl cellulose was obtained by the solid catalyst at low reaction temperature. With the increasing FePO4 amount, the excess Lewis acid sites formed upon elevating temperature (160 °C) was likely to catalyze 5-HMF to undesired products such as furfural, formic acid and levulinic acid, leading to a lower 5-HMF yield.6,20 Due to the sealed chamber and specific reaction conditions in hydrothermal system, it's difficult to detect the pH values of catalytic reaction process in this work. After cooling to room temperature, the pH values of these systems (Table 1) were slightly changed from 5.13 to 6.03 due to the poor insolubility of FePO4.
| Samples | FePO4 amount (g) | Fe3+ amountb (mg) | Furfural yield/% | pH valuec |
|---|---|---|---|---|
| a Conditions: methyl cellulose concentration of 2.5 g L−1, reaction temperature of 160 °C, reaction time of 80 min, THF solution volume of 180 mL, and NaCl dosage of 3.0 g.b Fe3+ amount in water phase after cooling to room temperature was detected by ICP-OES.c pH value of solution system after cooling to room temperature. | ||||
| Amorphous | 0.18 | 1.56 | 3.89 | 5.92 |
| Flower-like | 0.18 | 0.95 | 3.64 | 5.37 |
| Sphere | 0.18 | 1.38 | 3.52 | 5.67 |
| Branch-like | 0.24 | 1.14 | 4.35 | 5.04 |
| 0.21 | 0.97 | 4.27 | 5.13 | |
| 0.18 | 0.83 | 3.34 | 5.26 | |
| 0.15 | 0.72 | 3.56 | 5.85 | |
| 0.12 | 0.61 | 3.73 | 6.03 | |
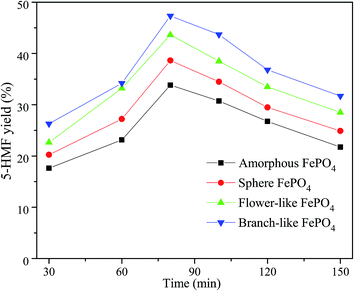
The 5-HMF yield increased with the reaction time varied from 30 min to 80 min, while decreased in a reaction time range of 80–150 min, as shown in Fig. 7. Among these different structured FePO4, the branch-like FePO4 exhibited the best catalytic activity for the conversion of methyl cellulose. This could be related with the contact efficiency between dissoluble Fe3+ ions and insoluble methyl cellulose molecules. The release rate of Fe3+ ions in water phase was greatly affected by the morphology of FePO4 at high temperature. FePO4 bulks were gradually dissolved in water phase with increasing reaction time at specified temperature (such as 160 °C), leading to an increasing Lewis acid sites (Fe3+ ions). It's reported that cellulose could be converted into furfural by Lewis acid catalysts such as Fe3+, Zn2+, Ca2+, and Cr3+ ions.26,32,51,52 These strong Lewis acid sites facilitated the formation of xylose from the retro-aldol reaction of fructose, and further converted xylose into furfural, leading to a lower 5-HMF yield.53,54
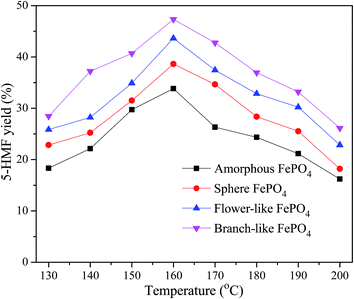
The effect of temperature on the 5-HMF yield over different morphological FePO4 was shown in Fig. 8. 5-HMF yield increased with the reaction temperature ranged from 130 to 160 °C, while decreased with the increasing reaction temperature from 160 to 200 °C. Compared with amorphous FePO4, regular structured FePO4 presented excellent catalytic activity, especially the branch-like morphology. It's suggested that FePO4 microstructure was responsible for the soluble rate of FePO4 to release soluble Fe ions and H+ ions. The soluble rate of branch-like FePO4 consisted of nanosheets (Fig. 3A–D) was higher than that of other structures at elevated temperature, leading to the increase of Fe3+ irons contacting with methyl cellulose. High level of reaction temperature favored the hydrolysis of FePO4 to form Fe3+ irons, which were likely to catalyze 5-HMF into formic acid and levulinic acid in the water phase.
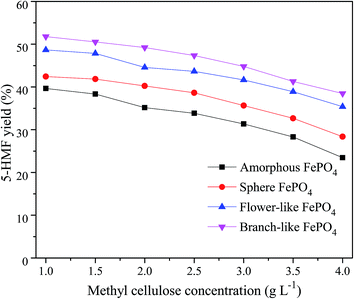
The methyl cellulose concentration as well as FePO4 amounts was important for the 5-HMF yield in aqueous solvent. As shown in Fig. 9, 5-HMF yield decreased with the increasing concentration of methyl cellulose under same conditions, which was agreed with previous works.36,37 Compared with the amorphous, flower-like, and sphere FePO4, the branch-like FePO4 exhibited better catalytic activity for methyl cellulose due to its higher soluble ability at 160 °C in water phase. The controllable branch-like structure could effectively restrain the dissolution rate of FePO4, and further affect the H+ ions released from the hydrolysis of Fe3+ ions. In addition, 5-HMF molecules were likely to react with and cross-polymerize glucose/fructose molecules to form humins during the dehydration process of methyl cellulose.37,53,54 As listed in Table 2, higher concentration of methyl cellulose induced to the degradation of 5-HMF to humins, thereby lowering the 5-HMF yield.
| Samples | Methyl cellulose concentration (g L−1) | Residue amountb (mg gcat−1) | Furfural yield/% |
|---|---|---|---|
| a Conditions: FePO4 amount of 0.18 g, reaction temperature of 160 °C, reaction time of 80 min, THF solution volume of 180 mL, and NaCl dosage of 3.0 g.b Residue amount deposited on catalyst surface was detected by TGA/DTA. | |||
| Amorphous | 2.5 | 96.5 | 3.89 |
| Flower-like | 2.5 | 75.2 | 3.64 |
| Sphere | 2.5 | 83.5 | 3.52 |
| Branch-like | 3.5 | 98.4 | 7.35 |
| 3.0 | 65.7 | 5.76 | |
| 2.5 | 43.8 | 3.34 | |
| 2.0 | 36.9 | 4.58 | |
| 1.5 | 32.6 | 4.20 | |
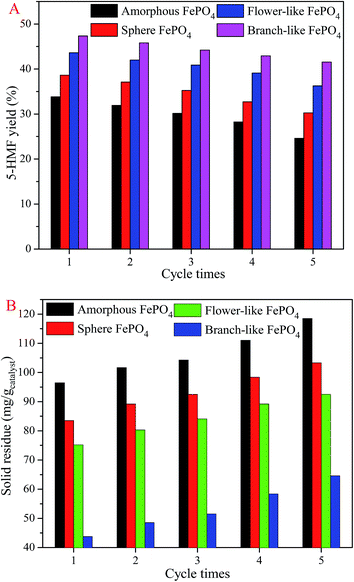
The catalyst stability of FePO4 for the conversion of methyl cellulose was performed five cycles at 160 °C for 80 min. The 5-HMF yield (Fig. 10A) decreased and the solid residue (Fig. 10B) increased after five cycle times. It's ascribed to the dissolved FePO4 could not completely transfer into the solid phase, leading to the FePO4 mass lose after cooling to room temperature.36,37 The residue deposited on the branch-like FePO4 surface was less than that of amorphous, flower-like, and sphere FePO4, indicating that the branch-like FePO4 retained superior catalytic activity compared with other structured FePO4. As shown in Fig. 4, the solid residue was from the unreacted methyl cellulose and the humins generated from 5-HMF.36,37 It's clearly illustrated that the FePO4 was partially dissolved at the evaluated temperature, and then randomly redeposited on the FePO4 surface in the temperature-fall period. The new diffraction peaks of FePO4·2H2O (Fig. 1) was detected in XRD pattern of used branch-like FePO4, which arisen from the recrystallization of dissolved FePO4.
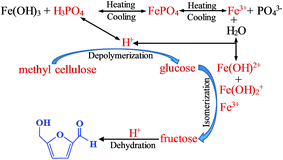
There were three steps for the conversion of methyl cellulose into 5-HMF over FePO4 catalyst in the biphasic system, as shown in Fig. 11. The methyl cellulose was depolymerized into glucose monomers by acid catalysts, then the glucose was isomerized into fructose by Lewis acid sites such as Fe3+ ions, and finally the fructose was dehydrated into 5-HMF over H+ ions.37,55 The Lewis acid sites (Fe3+ ions) and Brønsted acid sites (H+ ions) generated from the hydrolysis of FePO4 are thus suitable for the methyl cellulose converted into 5-HMF.36,37 It's reported that the ion strength and temperature were crucial for the H+ ions and soluble ions (Fe(OH)2+ and Fe(OH)2+) released from the hydrolysis of Fe3+ ions.55,56 With the elevating temperature, FePO4 can partially dissolve into Fe3+ ions, and then hydrolyzed to soluble iron species and H+ ions. These soluble iron species can isomerize glucose into fructose, and H+ ions can facilitate methyl cellulose to generate glucose and catalyze fructose to 5-HMF. This indicated that the dissolved FePO4 catalyst served as a “homogeneous” catalyst for the conversion of methyl cellulose. In addition, the low contact efficiency between insoluble methyl cellulose and solid FePO4 active sites was also responsible for the heterogeneous catalysis reaction of methyl cellulose into 5-HMF.36,37,50 It's noticed that the FePO4 mass loss leaded to the decreasing 5-HMF yield in the recycling process (Fig. 10). It's reasonable believed that the homogeneous catalysis of FePO4 played a crucial role in the conversion of methyl cellulose.
4. Conclusion
The flower-like, branch-like, and sphere FePO4 catalysts were successfully prepared via a hydrothermal route. Compared with amorphous, sphere, and flower-like FePO4, the branch-like FePO4 exhibited excellent catalytic activity and stability for the conversion of methyl cellulose into 5-HMF in the biphasic system. It's attributed to the Lewis acid sites (soluble iron species) and Brønsted acid sites (H+ ions) generated from the dissolved FePO4 at elevated temperature. The synergistic effect between iron species and H+ ions was favorable for the excellent catalytic activity for the methyl cellulose converted into 5-HMF. This dissolved FePO4 induced to the formation of FePO4·2H2O phase and the deterioration of branch-like structure during the phase-transfer catalytic process. The insolubility at room temperature and the dissolubility at high temperature were suitable for the potential application of FePO4 in large-scale conversion of methyl cellulose into 5-HMF.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of this work by the National Natural Science Foundation of China (Grant No.: 21506103 and 51608512), the Science and Technology Support Program of Sichuan Province (Grant No.: 2015GZ0170), the Major Training Program of the Education Department of Sichuan Province (Grant No.: 15CZ0026 and 17CZ0019), and Key Laboratory of Fruit Waste Treatment and Resource Recycling of the Sichuan Province College (Grant No.: KF17003).References
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499 CrossRef CAS PubMed.
- C. S. Maldonado, J. R. De la Rosaa, C. J. Lucio-Ortiz, J. S. Valente and M. J. Castaldid, Fuel, 2017, 198, 134 CrossRef.
- S. Xiao, B. Liu, Y. Wang, Z. Fang and Z. Zhang, Bioresour. Technol., 2014, 151, 361 CrossRef CAS PubMed.
- L. K. Ren, L. F. Zhu, T. Qi, J. Q. Tang, H. Q. Yang and C. W. Hu, ACS Catal., 2017, 7, 2199 CrossRef CAS.
- Q. Yang and T. Runge, ACS Sustainable Chem. Eng., 2016, 4, 6951 CrossRef CAS.
- V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997 CrossRef CAS PubMed.
- L. K. Ren, L. F. Zhu, T. Qi, J. Q. Tang, H. Q. Yang and C. W. Hu, ACS Catal., 2017, 7, 2199 CrossRef CAS.
- S. Jia, X. He and Z. Xu, RSC Adv., 2017, 7, 39221 RSC.
- J. Jae, W. Zheng, R. F. Lobo and D. G. Vlachos, ChemSusChem, 2013, 6, 1158 CrossRef CAS PubMed.
- H. Xin, T. Zhang, W. Li, M. Su, S. Li, Q. Shao and L. Ma, RSC Adv., 2017, 7, 41546 RSC.
- L. Hu, G. Zhao, X. Tang, Z. Wu, J. Xu, L. Lin and S. Liu, Bioresour. Technol., 2013, 148, 501 CrossRef CAS PubMed.
- H. Tang, N. Li, F. Chen, G. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Green Chem., 2017, 19, 1855 RSC.
- R. Weingarten, A. Rodriguez-Beuerman, F. Cao, J. S. Luterbacher, D. M. Alonso, J. A. Dumesic and G. W. Huber, ChemCatChem, 2014, 6, 2229 CrossRef CAS.
- R. Weingarten, J. Cho, R. Xing, W. C. Conner Jr and G. W. Huber, ChemSusChem, 2012, 5, 1280 CrossRef CAS PubMed.
- Z. Hu, Y. Peng, Y. Gao, Y. Qian, S. Ying, D. Yuan, S. Horike, N. Ogiwara, R. Babarao, Y. Wang, N. Yan and D. Zhao, Chem. Mater., 2016, 28, 2659 CrossRef CAS.
- M. I. Alam, S. De, B. Singh, B. Saha and M. M. Abu-Omar, Appl. Catal., A, 2014, 486, 42 CrossRef CAS.
- S. K. R. Patil, J. Heltzel and C. R. F. Lund, Energy Fuels, 2012, 26, 5281 CrossRef CAS.
- E. A. Pidko, V. Degirmenci and E. J. M. Hensen, ChemCatChem, 2012, 4, 1263 CrossRef CAS.
- H. Liu, H. Wang, Y. Li, W. Yang, C. Song, H. Li, W. Zhu and W. Jiang, RSC Adv., 2015, 5, 9290 RSC.
- S. Siankevich, Z. Fei, R. Scopelliti, P. G. Jessop, J. Zhang, N. Yan and P. J. Dyson, ChemCatChem, 2016, 9, 2089 CAS.
- H. Guo, A. Duereh, Y. Hiraga, T. M. Aida, X. Qi and R. L. Smith Jr, Chem. Eng. J., 2017, 323, 287 CrossRef CAS.
- S. Siankevich, Z. Fei, R. Scopelliti, G. Laurenczy, S. Katsyuba, N. Yan and P. J. Dyson, ChemCatChem, 2014, 7, 1647 CAS.
- M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397 CrossRef CAS PubMed.
- Q. Wang, K. Su and Z. Li, Mol. Catal., 2017, 438, 197 CrossRef CAS.
- Y. Shen, J. Sun, Y. Yi, M. Li, B. Wang, F. Xu and R. Sun, Bioresour. Technol., 2014, 172, 457 CrossRef CAS PubMed.
- B. Kassanov, J. Wang, Y. Fu and J. Chang, RSC Adv., 2017, 7, 30755 RSC.
- X. Guo, J. Tang, B. Xiang, L. Zhu, H. Yang and C. Hu, ChemCatChem, 2017, 9, 3218 CrossRef CAS.
- S. Q. Xu, X. P. Yan, Q. Bu and H. A. Xia, RSC Adv., 2016, 6, 8048 RSC.
- Z. Tai, J. Zhang, A. Wang, J. Pang, M. Zheng and T. Zhang, ChemSusChem, 2013, 6, 652 CrossRef CAS PubMed.
- A. Wang and T. Zhang, Acc. Chem. Res., 2015, 46, 1377 CrossRef PubMed.
- L. Y. Y. Jiang, C. M. Bohn, G. Li, D. Han, N. S. Mosier, J. T. Miller, H. I. Kenttamaa and M. M. Abu-Omar, Org. Chem. Front., 2015, 2, 1388 RSC.
- R. Sun, T. Wang, M. Zheng, W. Deng, J. Pang, A. Wang, X. Wang and T. Zhang, ACS Catal., 2015, 5, 874 CrossRef CAS.
- M. P. Pasternak, G. K. Rozenberg, A. P. Milner, M. Amanowicz, T. Zhou, U. Schwarz, K. Syassen, R. D. Taylor, M. Hanfland and K. Brister, Phys. Rev. Lett., 1997, 79, 4409 CrossRef CAS.
- Y. Zhang, E. A. Pidko and E. J. Hensen, Chem.–Eur. J., 2011, 17, 5281 CrossRef CAS PubMed.
- Z. W. Xi, N. Zhou, Y. Sun and K. L. Li, Science, 2001, 292, 1139 CrossRef CAS PubMed.
- L. Yang, X. P. Yan, S. Q. Xu, H. Chen, H. A. Xia and S. L. Zuo, RSC Adv., 2015, 5, 19900 RSC.
- H. Xia, S. Xu, X. Yan and S. Zuo, Fuel Process. Technol., 2016, 152, 140 CrossRef CAS.
- L. Zhang and R. K. Brow, J. Am. Ceram. Soc., 2011, 94, 3123 CrossRef CAS.
- J. Guo, S. Zhu, Y. Cen, Z. Qin, J. Wang and W. Fan, Appl. Catal., B, 2017, 200, 611 CrossRef CAS.
- C. Tagusagawa, A. Takagaki, A. Iguchi, K. Takanabe, J. N. Kondo, K. Ebitani, S. Hayashi, T. Tatsumi and K. Domen, Angew. Chem., Int. Ed., 2010, 49, 1128 CrossRef CAS PubMed.
- C. Tagusagawa, A. Takagaki, A. Iguchi, K. Takanabe, J. N. Kondo, K. Ebitani, T. Tatsumi and K. Domen, Chem. Mater., 2010, 22, 3072 CrossRef CAS.
- Y. Lu, T. Zhang, Y. Liu and G. Luo, Chem. Eng. J., 2012, 210, 18 CrossRef CAS.
- X. F. Guo and W. P. Ding, J. Fuel Chem. Technol., 2000, 28, 385 CAS.
- Y. Yin, Y. Hu, P. Wu, H. Zhang and C. Cai, Chem. Commun., 2012, 48, 2137 RSC.
- Y. D. Yu, Y. J. Zhu and J. Wu, Mater. Lett., 2017, 205, 158 CrossRef CAS.
- S. Guo, G. Zhang and J. C. Yu, J. Colloid Interface Sci., 2015, 448, 460 CrossRef CAS PubMed.
- P. Zhao, H. Liu, H. Zheng, Q. Tang and Y. Guo, Mater. Lett., 2014, 123, 128 CrossRef CAS.
- X. Zheng, S. Huang, D. Yang, H. Zhai, Y. You, X. Fu, J. Yuan, X. Zhou, J. Wen and Y. Liu, J. Alloys Compd., 2017, 705, 131 CrossRef CAS.
- P. Yang, B. Song, R. Wu, Y. Zheng, Y. Sun and J. K. Jian, J. Alloys Compd., 2009, 481, 450 CrossRef CAS.
- C. García-Sancho, I. Fúnez-Núñez, R. Moreno-Tost, J. Santamaría-González, E. Pérez-Inestrosa, J. L. G. Fierro and P. Maireles-Torres, Appl. Catal., B, 2017, 206, 617 CrossRef.
- J. M. J. M. Ravasco, J. A. S. Coelho, S. P. Simeonov and C. A. M. Afonso, RSC Adv., 2017, 7, 7555 RSC.
- H. Han, H. Zhao, Y. Liu, Z. Li, J. Song, W. Chu and Z. Sun, RSC Adv., 2017, 7, 3790 RSC.
- Q. Liu, F. Yang, H. Yin and Y. Du, RSC Adv., 2016, 6, 49760 RSC.
- G. Raveendra, M. Surenda and P. S. Sai Prasad, New J. Chem., 2017, 41, 8520 RSC.
- G. Li, E. A. Pidko and E. J. M. Hensen, Catal. Sci. Technol., 2014, 4, 2241 CAS.
- R. M. Milburn, J. Am. Chem. Soc., 1957, 79, 537 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |