Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anti-inflammatory flavonol acylglycosides from the aerial part of Lindera akoensis Hayata†
Hui-Chi Huang a,
Chung-Ping Yang‡
a,
Sheng-Yang Wangbc,
Chi-I Chang‡d,
Ping-Jyun Sung‡ef,
Guan-Jhong Huang‡
a,
Chung-Ping Yang‡
a,
Sheng-Yang Wangbc,
Chi-I Chang‡d,
Ping-Jyun Sung‡ef,
Guan-Jhong Huang‡ a,
Shih-Chang Chien*g and
Yueh-Hsiung Kuo*ah
a,
Shih-Chang Chien*g and
Yueh-Hsiung Kuo*ah
aDepartment of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University, Taichung 404, Taiwan
bDepartment of Forestry, National Chung-Hsing University, Taichung 402, Taiwan
cAgricultural Biotechnology Research Center, Academia Sinica, Taipei, 115, Taiwan
dDepartment of Biological Science and Technology, National Pingtung University of Science and Technology, Pingtung 912, Taiwan
eNational Museum of Marine Biology and Aquarium, Pingtung 944, Taiwan
fGraduate Institute of Marine Biotechnology, National Dong Hwa University, Pingtung 944, Taiwan
gExperimental Forest Management Office, National Chung-Hsing University, Taichung 402, Taiwan. E-mail: scchien@nchu.edu.tw; Tel: +886-4-2284-0397 ext. 600
hDepartment of Biotechnology, Asia University, Taichung 413, Taiwan. E-mail: kuoyh@mail.cmu.edu.tw; Tel: +886-4-2205-3366 ext. 5709
First published on 1st November 2017
Abstract
Five new flavonol acylglycosides, linderakosides A–E (1–5), together with 30 known compounds were isolated from the aerial part of Lindera akoensis Hayata. The structures were established using extensive spectroscopic analysis and comparison of NMR data with those of known compounds. The flavonol acylglycosides 1, 2, and 5 showed in vitro anti-inflammatory activity, which decreased LPS-stimulated nitrite production in RAW 264.7 cells. The structure activity relationships (SAR) of the flavonol acylglycoside compounds were also established to research for potential lead compounds as anti-inflammatory drugs.
Introduction
Lindera akoensis Hayata (Lauraceae) is an endemic large evergreen shrub that is widely distributed in Taiwan broad-leaved forests. This plant is a synonym of Benzoin akoense (Hayata) Kamik.1 L. akoensis has been used as a folk medicine for treatment of inflammation and trauma.2 Previous phytochemical studies revealed that the genus Lindera contains alkaloids,3 anthraquinone,4 aporphines, butanolides,5 essential oil,6 flavonoids, furanoids, chalconoids,5 phenolic compounds,7,8 and sesquiterpenoids.9,10 Earlier chemical and pharmacological studies on this endemic species of L. akoensis suggested that its butanolides account for its anti-inflammatory activities and antimycobacterial activities against Mycobacterium tuberculosis H37Rv.5,11,12 Previous studies on the aerial part of L. akoensis resulted in isolation of 10 butanolides, five lignans, and five flavonols.5,11 In the present study, further detailed chemical investigation of the same 95% ethanol extract of L. akoensis has resulted in isolation of five new flavonol acylglycosides (1–5) (Fig. 1), along with known compounds including three amides, eight apocarotenoids, 10 phenolic compounds, and nine porphyrinoids, which were isolated from this plant for the first time. Potential anti-inflammatory activity of isolates 1, 2, 4 and 5 was investigated via examining the inhibitory activity toward nitric oxide (NO) production induced by lipopolysaccharide in mouse macrophage RAW 264.7 cells tested in vitro (Table 1).| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| a The chemical shifts are expressed in δ ppm. The coupling constants (J) are expressed in Hz. | |||||
| 6 | 6.20, d (2.0) | 6.21, d (2.1) | 6.21, d (2.1) | 6.21, d (2.0) | 6.22, d (2.0) |
| 8 | 6.38, d (2.0) | 6.38, d (2.1) | 6.38, d (2.1) | 6.39, d (2.0) | 6.39, d (2.0) |
| 2′ | 7.88, d (8.8) | 7.88, d (8.8) | 7.81, d (8.5) | 7.37, br s | 7.90, d (8.9) |
| 3′ | 7.09, d (8.8) | 7.10, d (8.8) | 7.06, d (8.5) | 7.18, d (8.9) | |
| 5′ | 7.09, d (8.8) | 7.10, d (8.8) | 7.06, d (8.5) | 7.12, d (8.2) | 7.18, d (8.9) |
| 6′ | 7.87, d (8.8) | 7.88, d (8.8) | 7.81, d (8.5) | 7.38, d (8.2) | 7.90, d (8.9) |
| 1′′ | 5.48, d (1.4) | 5.40, d (1.0) | 5.51, d (1.0) | 5.60, br s | 5.72, d (1.4) |
| 2′′ | 5.52, dd (3.0, 1.4) | 5.49, dd (3.5, 1.0) | 4.23, dd (3.0, 1.0) | 4.23, br s | 5.54, dd (2.5, 1.4) |
| 3′′ | 3.93, dd (9.5, 3.0) | 3.94, dd (9.5, 3.5) | 3.89, dd (9.7, 3.0) | 3.94, dd (9.7, 3.2) | 4.17, dd (9.8, 2.5) |
| 4′′ | 3.39, dd (9.5, 6.7) | 3.28, t (9.5) | 4.90, t (9.7) | 4.91, t (9.7) | 4.97, t (9.8) |
| 5′′ | 3.39, qd (5.5, 6.7) | 3.49, qd (6.2, 9.5) | 3.28, qd (6.3, 9.7) | 3.23, qd (6.3, 9.7) | 3.31, qd (6.2, 9.8) |
| 6′′ | 0.99, d (5.5) | 0.96, d (6.2) | 0.76, d (6.3) | 0.78, d (6.3) | 0.85, d (6.2) |
| 2′′′ | 7.45, d (8.7) | 7.61, d (8.6) | 7.66, d (8.6) | 7.53, d (8.6) | 7.50, d (8.6) |
| 3′′′ | 6.79, d (8.7) | 6.75, d (8.6) | 6.74, d (8.6) | 6.83, d (8.6) | 6.82, d (8.6) |
| 5′′′ | 6.79, d (8.7) | 6.75, d (8.6) | 6.74, d (8.6) | 6.83, d (8.6) | 6.82, d (8.6) |
| 6′′′ | 7.45, d (8.7) | 7.61, d (8.6) | 7.66, d (8.6) | 7.53, d (8.6) | 7.50, d (8.6) |
| 7′′′ | 7.53, d (15.8) | 6.85, d (12.8) | 6.87, d (12.8) | 7.59, d (16.0) | 7.55, d (16.0) |
| 8′′′ | 6.33, d (15.8) | 5.78, d (12.8) | 5.72, d (12.8) | 6.29, d (16.0) | 6.27, d (16.0) |
| OCH3 | 3.89, s | 3.88, s | 3.89, s | 3.89, s | 3.87, s |
| 2′′′′ | 7.50, d (8.6) | ||||
| 3′′′′ | 6.85, d (8.6) | ||||
| 5′′′′ | 6.85, d (8.6) | ||||
| 6′′′′ | 7.50, d (8.6) | ||||
| 7′′′′ | 7.68, d (16.0) | ||||
| 8′′′′ | 6.42, d (16.0) | ||||
Results and discussion
The aerial part of L. akoensis was collected in Taiwan and extracted with 95% ethanol. The extract was partitioned into EtOAc and H2O layers. The EtOAc layer was purified using conventional chromatographic techniques yielding 35 compounds. Structures of the compounds were elucidated using spectroscopic techniques and these structures were compared with data from the literature.Compound 1 was isolated as a pale yellow solid. The molecular formula C31H28O12 was determined on the basis of its HR-ESI-MS (calcd for C31H28O12Na, 615.1473), which showed a pseudo-molecular ion peak at m/z 615.1479, corresponding to 18 degrees of unsaturation. The IR spectrum indicated the existence of a hydroxyl group (3426 cm−1), conjugated carbonyl group (1651 cm−1), and aromatic ring (1605 and 1513 cm−1). The UV absorption bands indicated λmax at 267 nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε 4.56) and 313 (log
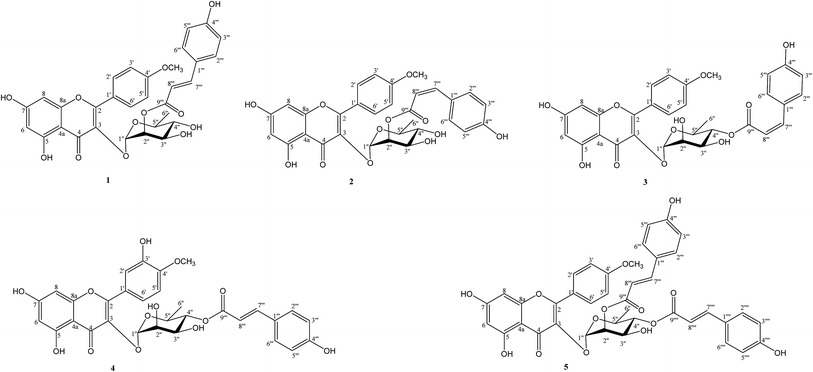
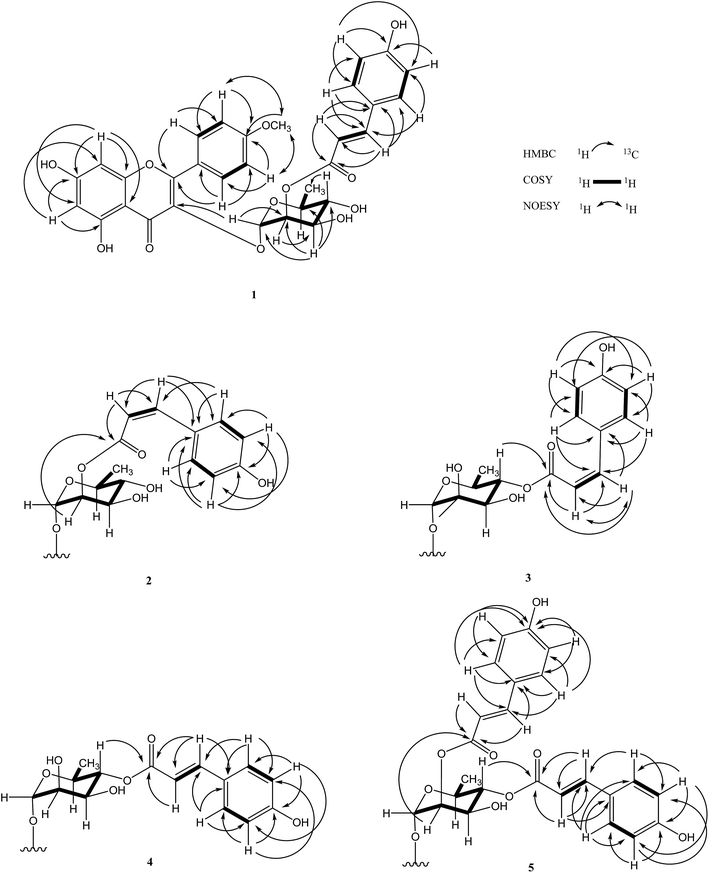
ε 4.56) and 313 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε 4.69) nm. The NMR spectra showed that 1 has a structure to similar to that of 4′-O-methylkaempferol-3-O-α-L-(4′′-E-p-coumaroyl)rhamnoside, which has been isolated from this plant previously.5 This evidence suggests that 1 is a flavonoid glycoside derivative from its characteristic yellow color and spectral properties. The 1H, DEPT, and HSQC spectra of 1 indicated the presence of a 5,7-dihydroxy A ring system [δH 6.20 (d, J = 2.0 Hz, H-6) and 6.38 (d, J = 2.0 Hz, H-8)] and a 1,4-disubstituted B ring [δH 7.09 (d, J = 8.8 Hz, H-3′,5′) and 7.88 (d, J = 8.8 Hz, H-2′,6′)] structure in flavonol, one sugar resonance [δH 5.48 (d, J = 1.4 Hz, H-1′′)/δC 100.8], and a methoxy group [δH 3.89/δC 56.2]. These data suggest that 1 contains a flavonol glycoside derivative.13 In the 13C NMR spectrum of 1, significant flavonol signals were observed at δC 159.0 (C-2), 136.0 (C-3), and 176.5 (C-4). The NOESY correlations between the methoxy singlet resonance with δH 7.09 (H-3′, 5′) suggested location of a methoxy at C-4′. HMBC correlations from δH 7.88 (2H, H-2′ and H-6′) to δC 159.0 (C-2), as well as δH 3.89 (OCH3) to 163.7 (C-4′), assumed that the aglycone of 1 was a kaempferide skeleton.14 For the glycone moiety, the carbon signal at δC 100.8 showed correlation with the anomeric proton at δH 5.48 in the HSQC. The distinct methyl proton of Rha H-6 (δH 0.99, 3H, d, J = 5.5 Hz) and small coupling constant (J = 1.4 Hz) of the anomeric proton were assigned as a α-L-rhamnopyranoside moiety using the characteristic 1H NMR signals. The α-L-rhamnopyranoside moiety was linked at C-3 of the flavone, from a cross-peak between H-1′′ (δH 5.48) of rhamnose and C-3 (δC 136.0) of the aglycon. In addition, a 1,4-disubstituted aromatic ring [δH 6.79 (d, J = 8.7 Hz, H-3′′′,5′′′) and 7.45 (d, J = 8.7 Hz, H-2′′′,6′′′)] as well as trans-olefinic signals [δH 7.53 and 6.33 (each 1H, d, J = 15.8 Hz)] were observed in the presence of an (E)-p-coumaroyl moiety. A detailed comparison of the 13C-NMR data between 1 and the afzelin literature data,15 the downfield shifts for C-2′′ of Rha (Δδ + 1.6 ppm) and upfield shifts for C-1′′ (Δδ − 2.7 ppm) and C-3′′ (Δδ − 1.9 ppm) of Rha, suggested that 1 was esterified at C-2′′. Furthermore, the HMBC correlation between the H-2′′ and C-9′′′ indicated that an (E)-p-coumaroyl moiety was located at the C-2′′ position. Accordingly, the structure of 1 was elucidated as 4′-O-methyl-2′′-(E)-p-coumaroylafzelin, and named linderakoside A.
ε 4.69) nm. The NMR spectra showed that 1 has a structure to similar to that of 4′-O-methylkaempferol-3-O-α-L-(4′′-E-p-coumaroyl)rhamnoside, which has been isolated from this plant previously.5 This evidence suggests that 1 is a flavonoid glycoside derivative from its characteristic yellow color and spectral properties. The 1H, DEPT, and HSQC spectra of 1 indicated the presence of a 5,7-dihydroxy A ring system [δH 6.20 (d, J = 2.0 Hz, H-6) and 6.38 (d, J = 2.0 Hz, H-8)] and a 1,4-disubstituted B ring [δH 7.09 (d, J = 8.8 Hz, H-3′,5′) and 7.88 (d, J = 8.8 Hz, H-2′,6′)] structure in flavonol, one sugar resonance [δH 5.48 (d, J = 1.4 Hz, H-1′′)/δC 100.8], and a methoxy group [δH 3.89/δC 56.2]. These data suggest that 1 contains a flavonol glycoside derivative.13 In the 13C NMR spectrum of 1, significant flavonol signals were observed at δC 159.0 (C-2), 136.0 (C-3), and 176.5 (C-4). The NOESY correlations between the methoxy singlet resonance with δH 7.09 (H-3′, 5′) suggested location of a methoxy at C-4′. HMBC correlations from δH 7.88 (2H, H-2′ and H-6′) to δC 159.0 (C-2), as well as δH 3.89 (OCH3) to 163.7 (C-4′), assumed that the aglycone of 1 was a kaempferide skeleton.14 For the glycone moiety, the carbon signal at δC 100.8 showed correlation with the anomeric proton at δH 5.48 in the HSQC. The distinct methyl proton of Rha H-6 (δH 0.99, 3H, d, J = 5.5 Hz) and small coupling constant (J = 1.4 Hz) of the anomeric proton were assigned as a α-L-rhamnopyranoside moiety using the characteristic 1H NMR signals. The α-L-rhamnopyranoside moiety was linked at C-3 of the flavone, from a cross-peak between H-1′′ (δH 5.48) of rhamnose and C-3 (δC 136.0) of the aglycon. In addition, a 1,4-disubstituted aromatic ring [δH 6.79 (d, J = 8.7 Hz, H-3′′′,5′′′) and 7.45 (d, J = 8.7 Hz, H-2′′′,6′′′)] as well as trans-olefinic signals [δH 7.53 and 6.33 (each 1H, d, J = 15.8 Hz)] were observed in the presence of an (E)-p-coumaroyl moiety. A detailed comparison of the 13C-NMR data between 1 and the afzelin literature data,15 the downfield shifts for C-2′′ of Rha (Δδ + 1.6 ppm) and upfield shifts for C-1′′ (Δδ − 2.7 ppm) and C-3′′ (Δδ − 1.9 ppm) of Rha, suggested that 1 was esterified at C-2′′. Furthermore, the HMBC correlation between the H-2′′ and C-9′′′ indicated that an (E)-p-coumaroyl moiety was located at the C-2′′ position. Accordingly, the structure of 1 was elucidated as 4′-O-methyl-2′′-(E)-p-coumaroylafzelin, and named linderakoside A.
Compound 2 was isolated as a pale yellow solid, with molecular formula obtained as C31H28O12 from HR-ESI-MS (m/z 615.1477 [M + Na]+, calcd 615.1473) analyses with 18 degrees of unsaturation. IR and UV spectra were nearly the same as those of 1. 1D and 2D NMR spectra analyses established a kaempferide glycoside skeleton, which was also closely related to that of 1. Compound 2 was identified as the Z-isomer of 1, according to cis-olefinic protons at δH 6.85 and 5.78 (each 1H, d, J = 12.8 Hz) in 1. Moreover, the (Z)-p-coumaroyl moiety position was determined to be C-2′′ using the HMBC correlations between H-2′′ (δH 5.47) and C-9′′′ (δC 167.3). Based on the obtained data, compound 2 was determined as 4′-O-methyl-2′′-(Z)-p-coumaroylafzelin, and named linderakoside B.
The molecular formula of compound 3 was given as C31H28O12 with 18 degrees of unsaturation from HR-ESI-MS at m/z 615.1476 [M + Na]+ (calcd 615.1473), and compound 3 exhibited the same molecular weight as 2. The NMR, UV, and IR data showed signal patterns similar to those of 2; however, the rhamnopyranose moiety substitution patterns differed. The 1H-NMR of rhamnose signals were easily assigned using the characteristic doublet signal of methyl. The 1H-NMR signal for Rha-6′′ (δH 0.76, d, J = 6.3 Hz) shifted upfield (Δδ − 0.20 ppm) compared with that of 2, which was shielded by the flavone C-ring, and the 1H-NMR signal of Rha-4′′ (δH 4.90, t, J = 9.7 Hz) appeared relatively downfield (Δδ + 1.51 ppm) using the esterified p-coumaroyl moiety.16 From 13C NMR spectra comparison of 3 and 2 in the L-rhamnose moiety, the downfield shifts for Rha C-1′′ (Δδ + 2.1 ppm) and Rha C-4′′ (Δδ + 2.4 ppm) and upfield shifts of Rha C-2′′ (−1.5) and C-5′′ (−3.3) in 3, implied that the (Z)-p-coumaroyl moiety was located at Rha C-4′′ in 3, instead of Rha C-2′′ as in 2. The HMBC correlation between δH 4.90 (H-4′′) and δC 167.9 (C-9′′′) of 3 indicated that the (Z)-p-coumaroyl moiety was located at the C-4 position. Thus, the structure of 3 was elucidated as 4′-O-methyl-4′′-(Z)-p-coumaroylafzelin, and named linderakoside C.
The molecular formula of compound 4 was obtained as C31H28O13 from HR-ESI-MS (m/z 631.1423 [M + Na]+, calcd 631.1422) with 18 degrees of unsaturation, and thus contains one more oxygen atom than 1. The spectroscopic features of 4 were closely related to the spectroscopic features of 1, except for the presence of aromatic ABX-coupling signals [δH 7.37 (1H, br s), 7.12 (1H, d, J = 8.2 Hz), and 7.38 (1H, d, J = 8.2 Hz)] rather than a 1,4-disubstituted B ring structure in flavonol, and the rhamnopyranoside unit substitution patterns differed. The 1H-NMR data for 4 showed that an aromatic ABX-coupling system was ascribed to the presence of hydroxyl and methoxy substituents. The 4′-OMe was deduced according to a NOESY correlation between a methoxy proton (δH 3.89) and H-5′ (δH 7.12) and HMBC correlation between δH 3.89 (OCH3) and δC 152.0 (C-4′), and thus the hydroxyl group was located at C-3′ (Fig. 2). From the HMBC spectrum, the correlation between δH 7.37 (H-2′) and δC 159.4 (C-2), 152.0 (C-4′), 148.0 (C-3′), and 124.5 (C-1′), δH 7.12 (H-5′) and δC 148.0 (C-3′), 152.0 (C-4′), and 124.5 (C-1′), and δH 7.39 (H-6′) and δC 159.4 (C-2), 112.7 (C-5′), 131.4 (C-2′), and 152.0 (C-4′), assumed that the flavone moiety of 4 was tamarixetin.17 The characteristic doublet methyl signal (CH3-6′′) of rhamnose upfield (δH 0.78, d, J = 6.3 Hz) and the triplet of H-4′′ downfield (δH 4.91, t, J = 9.7 Hz) in 4 was the same as in 3, which suggested that the (E)-p-coumaroyl moiety was located at Rha C-4′′ in 4. HMBC spectrum inspection showed correlations between δH 5.60 (Rha-1′′) and δC 135.7 (C-3) and between δH 4.91 (Rha-4′′) and δC 168.9 (C-9′′′), indicating Rha-C-1′′ linkage to C-3 of the flavone and of Rha-C-4′′ to (E)-p-coumaroyl-C-9′′′, respectively. The above evidence was used to identify 4 as a 2′′-(E)-p-coumaroyltamarixetin, and the compound was named linderakoside D.
With molecular formula calculated as C40H34O14 by HR-ESI-MS (m/z 761.1846 [M + Na]+ calcd 761.1841), further combined with the observation of 13C and DEPT spectra, compound 5 was suggested to have a similar kaempferide glycoside skeleton to 1. Comparing 5 with 1, there were similarities in both the UV and IR data and the 1H NMR spectra, but a difference appeared in the HR-ESI-MS analysis of one more (E)-p-coumaroyl moiety (C9H7O2). In the 1H NMR spectrum, ortho-coupled proton signals at δH 7.50, 6.85 (each 2H, d, J = 8.6 Hz, H-2′′′′, H-6′′′′) and trans-olefinic protons at δH 7.68, 6.42 (each 1H, d, J = 16.0 Hz, H-7′′′′, H-8′′′′) indicated that 5 possessed an E-olefinic functionality. NMR data for 5 compared with those of 1 revealed the downfield shifts for Rha C-4′′ (Δδ + 2.9 ppm) and upfield shifts of Rha C-3′′ (−1.9) and C-5′′ (−3.2) in 5, suggesting an additional p-coumaroyl moiety at C-4′′. This conclusion was supported by the HMBC correlation between H-4′′ (δH 4.97) of the L-rhamnose and C-9′′′′ (δC 168.4). Based on the above evidence, 5 was determined to be 4′-O-methyl-2′′,4′′-di-(E)-p-coumaroylafzelin, and named linderakoside E.
The 30 known compounds including three amides, moupinamide (6),18 N-p-coumaroyltyramine (7),19 and N-trans-sinapoyltyramine (8),19 eight apocarotenoids, 4,5-dihydroblumenol A (9),20 epiloliolide (10),21 (7E)-3β-hydroxy-5α,6α-epoxy-magastigmen-9-one (11),22 2α,4β-dihydroxy-2,6,6-trimethylcyclohexanone (12),23 (3S,4S,5S,6S,9R)-3,4-dihydroxy-5,6-dihydro-β-ionone (13),24 boscialin (14),25 grasshopper ketone (15),26 and loliolide (16),21 10 phenolic compounds, 2-hydroxymethyl-4-nitrophenol (17),27 4-hydroxy-3,5-dimethoxybenzaldehyde (18),28 isovanillin (19),28 p-hydroxybenzaldehyde (20),29 vanillin (21),30 p-hydroxybenzoic acid (22),31 4-hydroxy-3-methoxynitrobenzene (23),32 trans-ferulatic ester (24),33 2-methyl-4-nitrophenol (25),34 and 3-hydroxy-4-methoxybenzoic acid (26),35 and nine porphyrinoids, (132R)-132-hydroxypheophytin a (27),36 (132S)-132-hydroxypheophytin a (28),36 pheophytin a (29),37 (10S)-pheophytin a (30),38 pheophytin b (31),39 aristophyll-C (32),36 7′-oxoaristophyll-C (33),36 (132S)-132-hydroxypheophytin b (34),36 and methyl rel-(151R)-31,32-didehydro-151-hydroxy-71-oxo-173-O-phythylr-hodochlorin 15-acetate δ-lactone (35),40 were identified by comparison of their physical and reported spectroscopic data (Table 2).
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 159.0 | 159.1 | 158.8 | 159.4 | 159.1 |
| 3 | 136.0 | 136.1 | 136.0 | 135.7 | 135.2 |
| 4 | 179.5 | 179.7 | 179.6 | 179.7 | 179.4 |
| 4a | 106.1 | 106.1 | 106.1 | 106.1 | 106.1 |
| 5 | 158.7 | 158.7 | 158.8 | 158.8 | 158.8 |
| 6 | 100.1 | 100.1 | 100.1 | 100.2 | 100.2 |
| 7 | 166.1 | 166.1 | 166.3 | 166.3 | 166.2 |
| 8 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 |
| 8a | 163.6 | 163.4 | 163.4 | 163.4 | 163.4 |
| 1′ | 123.8 | 123.8 | 124.0 | 124.5 | 124.0 |
| 2′ | 131.8 | 131.8 | 132.1 | 131.4 | 132.0 |
| 3′ | 115.5 | 115.5 | 116.6 | 148.0 | 115.5 |
| 4′ | 163.3 | 163.7 | 163.4 | 152.0 | 163.7 |
| 5′ | 115.4 | 115.5 | 116.6 | 112.7 | 115.5 |
| 6′ | 131.9 | 131.8 | 132.0 | 131.4 | 132.0 |
| 1′′ | 100.8 | 100.8 | 102.9 | 102.5 | 99.5 |
| 2′′ | 73.5 | 73.5 | 72.0 | 71.9 | 73.3 |
| 3′′ | 70.8 | 70.7 | 70.2 | 70.3 | 68.6 |
| 4′′ | 72.3 | 72.3 | 74.7 | 75.0 | 74.9 |
| 5′′ | 73.7 | 73.2 | 69.9 | 69.9 | 69.9 |
| 6′′ | 17.9 | 17.9 | 17.8 | 17.8 | 17.9 |
| 1′′′ | 127.3 | 127.7 | 127.7 | 127.4 | 127.3 |
| 2′′′ | 131.4 | 133.9 | 134.0 | 131.5 | 131.4 |
| 3′′′ | 117.0 | 116.1 | 116.0 | 117.0 | 117.0 |
| 4′′′ | 161.4 | 160.2 | 160.3 | 161.5 | 161.6 |
| 5′′′ | 117.0 | 116.1 | 116.0 | 117.0 | 117.0 |
| 6′′′ | 131.4 | 133.9 | 134.0 | 131.5 | 131.4 |
| 7′′′ | 147.3 | 145.8 | 145.5 | 146.9 | 147.1 |
| 8′′′ | 115.0 | 116.3 | 116.0 | 115.4 | 114.8 |
| 9′′′ | 168.4 | 167.3 | 167.8 | 168.9 | 168.6 |
| OCH3 | 56.2 | 56.2 | 56.3 | 56.7 | 56.3 |
| 1′′′′ | 127.3 | ||||
| 2′′′′ | 131.4 | ||||
| 3′′′′ | 117.0 | ||||
| 4′′′′ | 161.6 | ||||
| 5′′′′ | 117.0 | ||||
| 6′′′′ | 131.4 | ||||
| 7′′′′ | 147.6 | ||||
| 8′′′′ | 115.1 | ||||
| 9′′′′ | 168.4 |
The potential anti-inflammatory activities of compounds 1, 2, 4, and 5 from L. akoensis were tested in vitro, by examining any decrease in LPS-stimulated nitrite production in RAW 264.7 cells (Table 3). Compounds 1, 2, and 5 exhibited significant inhibitory activities against nitric oxide production with IC50 values of 19.1, 25.1, and 9.4 μM, respectively. There was no significant change in cell viability among these active compounds (Table 3). These results are consistent with data from the literature on the kaempferide glycoside skeleton, in which the (E) or (Z)-p-coumaroyl moiety was located at Rha C-4′′ showing weak activity (IC50 > 50 μM).5 However, the (E) or (Z)-p-coumaroyl moiety was located at Rha C-2′′ in compounds 1 and 2 rather than at Rha C-4′′ in compound 4, and showed the highest inhibitory effects (IC50 < 25 μM). The activities of Rha C-2′′ with (E)-p-coumaroyl moiety (1) are better than Rha C-2′′ with (Z)-p-coumaroyl moiety (2). The disubstituted (E)-p-coumaroyl moieties of compound 5 were located at Rha C-4′′ and Rha C-2′′, and showed the strongest activity (IC50 < 10 μM). The monosubstituted (E)-p-coumaroyl group at Rha C-2′′ of compound 1 decreased activity. The observed structure–activity relationships (SAR) imply that the presence of the disubstituted (E)-p-coumaroyl groups at C-4 and C-2 of rhamnose moiety have an important role in enhancing the anti-inflammatory potential of kaempferide glycoside.
| Compound | Cytotoxicity IC50 (μM) | Inhibition of NO production IC50 (μM) |
|---|---|---|
| a Values are expressed as mean ± SD of three replicates. | ||
| 1 | 92.6 ± 0.51 | 19.1 |
| 2 | 92.2 ± 0.47 | 25.1 |
| 4 | 92.5 ± 0.13 | >50 |
| 5 | 90.9 ± 0.40 | 9.4 |
| Indomethacine | 182.9 ± 5.5 | |
Conclusions
This study investigated chemically the aerial part of L. akoensis and isolated five new flavonol acylglycosides, linderakosides A–E (1–5) along with 30 known compounds, including three amides (6–8), eight apocarotenoids (9–16), 10 phenolic compounds (17–26), and night porphyrinoids (27–35). These compounds were isolated from this plant for the first time. Compounds 1, 2, and 5 displayed potential anti-inflammatory activity with IC50 values of 9.4–25.1 μM, and exhibited no cytotoxic activity. These results provide a basis for evaluating the structure–activity relationships of flavonol acylglycosides, as well as for developing compound 5 as an anti-inflammatory drug.Experimental section
General experimental procedures
Optical rotations were obtained in MeOH using a JASCO P-1020 digital polarimeter. IR and UV spectra were recorded on a Shimadzu IR Prestige-21 Fourier transform infrared and a Shimadzu Pharmaspec-1700 UV-Visible spectrophotometer, respectively. 1D and 2D NMR spectra were measured in CDCl3 and referenced to δH 7.26 and δC 77.0, and were recorded on a Bruker AVANCE III-500 MHz spectrometer. The HRESIMS data were recorded on a Finnigan LCQ ion-trap mass spectrometer. Column chromatography was performed using silica gel (Merck, 30–65 μm), and TLC analysis was performed using aluminum pre-coated silica gel plates (Merck, Kieselgel 60 F254). HPLC was obtained with Shimadzu LC-6A apparatus equipped with an IOTA-2RI-detector. Phenomenex luna silica (Φ 250 × 10 column) were used for preparative purposes.Plant material
The aerial part of L. akoensis was collected in Taichung, Taiwan, in July, 2008. This material was identified by Prof. Yen-Hsueh Tseng, Department of Forestry, National Chung Hsing University, Taichung, Taiwan. A voucher specimen (CMU2008-06-LA) was deposited in the School of Pharmacy, China Medical University.Extraction and isolation
The dried aerial part of L. akoensis (5.9 kg) was extracted with 95% ethanol for 7 days (20 L, three times). The dried extract (337.8 g) was suspended in H2O and partitioned successively with ethyl acetate (EtOAc) and n-BuOH. The EtOAc layer was evaporated in vacuo to yield a residue (127.8 g) that was subjected to silica gel column chromatography (particle size 0.063–0.200 mm; Φ 250 × 15 column) and eluted with a gradient of increasing polarity with solvent of n-hexane/EtOAc solvent (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 0
1 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to give 21 fractions. Fraction 3 (10.0 g) was separated using semi-preparative HPLC (CH2Cl2/EtOAc, v/v 4
100) to give 21 fractions. Fraction 3 (10.0 g) was separated using semi-preparative HPLC (CH2Cl2/EtOAc, v/v 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford pure, 25 (14.1 mg), 26 (23.5 mg), 27 (16.2 mg), and 35 (4.5 mg). Fraction 11 (5.08 g) was fractioned after repeated chromatography over silica gel (n-hexane/acetone, v/v 50
1) to afford pure, 25 (14.1 mg), 26 (23.5 mg), 27 (16.2 mg), and 35 (4.5 mg). Fraction 11 (5.08 g) was fractioned after repeated chromatography over silica gel (n-hexane/acetone, v/v 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 0
1 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to afford Fr. 11-1–11-10. Fr. 11-6 (109.5 mg) was chromatographed on semi-preparative HPLC (CH2Cl2/EtOAc, v/v 7
100) to afford Fr. 11-1–11-10. Fr. 11-6 (109.5 mg) was chromatographed on semi-preparative HPLC (CH2Cl2/EtOAc, v/v 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford 10 (16.5 mg), 11 (2.1 mg), 12 (3.3 mg), 17 (6.2 mg), 18 (3.6 mg), and 21 (2.2 mg). Fr. 11-7 (98.7 mg) was further purified using semi-preparative HPLC (CH2Cl2/EtOAc, v/v 2
3) to afford 10 (16.5 mg), 11 (2.1 mg), 12 (3.3 mg), 17 (6.2 mg), 18 (3.6 mg), and 21 (2.2 mg). Fr. 11-7 (98.7 mg) was further purified using semi-preparative HPLC (CH2Cl2/EtOAc, v/v 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 24 (3.4 mg), 30 (2.9 mg), 31 (4.1 mg), 32 (4.4 mg), and 33 (2.3 mg). Fr. 11-8 (97.3 mg) was chromatographed on semi-preparative HPLC (CH2Cl2/EtOAc, v/v 2
1) to produce 24 (3.4 mg), 30 (2.9 mg), 31 (4.1 mg), 32 (4.4 mg), and 33 (2.3 mg). Fr. 11-8 (97.3 mg) was chromatographed on semi-preparative HPLC (CH2Cl2/EtOAc, v/v 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 19 (19.1 mg), 20 (12.3 mg), 22 (9.6 mg), 28 (8.3 mg), and 29 (12.4 mg). Fraction 15 (6.82 g) was re-separated by chromatography and semi-preparative HPLC (EtOAc/n-hexane, v/v 1
1) to afford 19 (19.1 mg), 20 (12.3 mg), 22 (9.6 mg), 28 (8.3 mg), and 29 (12.4 mg). Fraction 15 (6.82 g) was re-separated by chromatography and semi-preparative HPLC (EtOAc/n-hexane, v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford pure 6 (6.6 mg), 9 (9.8 mg), 23 (8.3 mg), and 34 (3.4 mg). Fraction 16 (7.15 g) was re-separated by chromatography and semi-preparative HPLC (EtOAc/n-hexane, v/v 2
1) to afford pure 6 (6.6 mg), 9 (9.8 mg), 23 (8.3 mg), and 34 (3.4 mg). Fraction 16 (7.15 g) was re-separated by chromatography and semi-preparative HPLC (EtOAc/n-hexane, v/v 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford 1 (6.5 mg), 2 (4.6 mg), 3 (0.8 mg), 4 (5.5 mg), 5 (6.5 mg), 7 (9.1 mg), and 8 (5.9 mg). Fraction 17 (1.25 g) was further passed over Sephadex LH-20 column and then purified by semi-preparative HPLC (EtOAc/n-hexane, v/v 2
3) to afford 1 (6.5 mg), 2 (4.6 mg), 3 (0.8 mg), 4 (5.5 mg), 5 (6.5 mg), 7 (9.1 mg), and 8 (5.9 mg). Fraction 17 (1.25 g) was further passed over Sephadex LH-20 column and then purified by semi-preparative HPLC (EtOAc/n-hexane, v/v 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to yield 13 (7.7 mg), 14 (6.5 mg), 15 (3.7 mg), and 16 (3.5 mg).
3) to yield 13 (7.7 mg), 14 (6.5 mg), 15 (3.7 mg), and 16 (3.5 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 313.0 (4.69), 277.0 (4.50), 267.0 (4.56), 247.0 (4.31), 211 (4.71) nm; IR νmax 3426, 1651, 1605, 1513, 1173 cm−1; HR-ESI-MS m/z: 615.1479 [M + Na]+ (calcd for C31H28O12Na, 615.1473).
ε) 313.0 (4.69), 277.0 (4.50), 267.0 (4.56), 247.0 (4.31), 211 (4.71) nm; IR νmax 3426, 1651, 1605, 1513, 1173 cm−1; HR-ESI-MS m/z: 615.1479 [M + Na]+ (calcd for C31H28O12Na, 615.1473).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 313.0 (4.60), 278.0 (4.48), 267.0 (4.57), 247.0 (4.34) nm, 211.0 (4.72); IR νmax 3426, 1651, 1605, 1512, 1173 cm−1; HR-ESI-MS m/z: 615.1477 [M + Na]+ (calcd for C31H28O12Na, 615.1473).
ε) 313.0 (4.60), 278.0 (4.48), 267.0 (4.57), 247.0 (4.34) nm, 211.0 (4.72); IR νmax 3426, 1651, 1605, 1512, 1173 cm−1; HR-ESI-MS m/z: 615.1477 [M + Na]+ (calcd for C31H28O12Na, 615.1473).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 312.0 (4.75), 247.0 (4.44), 211.0 (3.94) nm; IR νmax 3442, 2936, 1655, 1607, 1510, 1177 cm−1; HR-ESI-MS m/z: 615.1476 [M + Na]+ (calcd for C31H28O12, 615.1473).
ε) 312.0 (4.75), 247.0 (4.44), 211.0 (3.94) nm; IR νmax 3442, 2936, 1655, 1607, 1510, 1177 cm−1; HR-ESI-MS m/z: 615.1476 [M + Na]+ (calcd for C31H28O12, 615.1473).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 313.0 (4.42), 276.0 (4.27), 267.0 (4.33), 246.0 (4.24), 208.0 (4.61) nm; IR νmax 3426, 2932, 1651, 1604, 1512, 1173 cm−1; HR-ESI-MS m/z: 631.1423 [M + Na]+ (calcd for C31H28O13Na, 631.1422).
ε) 313.0 (4.42), 276.0 (4.27), 267.0 (4.33), 246.0 (4.24), 208.0 (4.61) nm; IR νmax 3426, 2932, 1651, 1604, 1512, 1173 cm−1; HR-ESI-MS m/z: 631.1423 [M + Na]+ (calcd for C31H28O13Na, 631.1422).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 314.0 (4.97), 248.0 (4.44) nm, 212 (4.85) nm; IR νmax 3402, 2936, 1651, 1605, 1512, 1173 cm−1; HR-ESI-MS m/z: 761.1846 [M + Na]+ (calcd for C40H34O14Na, 761.1841).
ε) 314.0 (4.97), 248.0 (4.44) nm, 212 (4.85) nm; IR νmax 3402, 2936, 1651, 1605, 1512, 1173 cm−1; HR-ESI-MS m/z: 761.1846 [M + Na]+ (calcd for C40H34O14Na, 761.1841).Cell culture
RAW264.7 (BCRC no. 60001) was purchased from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were maintained in plastic dishes containing Dulbecco's Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA, USA) with 5% CO2 incubator at 37 °C and subcultured every 3 days at a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 using 0.05% trypsin–0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
5 using 0.05% trypsin–0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
Nitric oxide (NO) production assay41
RAW 264.7 cells were incubated in a 96-well plate for 24 h and then pretreated with LPS (100 ng mL−1) with compounds (0, 3.125, 6.25, 12.5, 25 and 50 μg mL−1). Then, the supernatant (100 μL) was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthyl ethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room temperature for 10 min; the absorbance was measured at 540 nm with a Micro-Reader (Molecular Devices Orleans Drive, Sunnyvale, CA, USA). Using sodium nitrite to generate a standard curve, the concentration of nitrite was measured from absorbance at 540 nm.Cell viability assay11
RAW 264.7 cells (2 × 105 cells per well) were seeded in a 96-well plate containing DMEM medium with 10% FBS for 24 h. Then cells were treated with various concentrations of compounds 1, 2, 4, and 5 in the presence of 100 ng mL−1 LPS (lipopolysaccharide) and incubated for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μL of 0.5 mg mL−1 MTT for 2 h at 37 °C testing for cell viability. The medium was then discarded, and 100 μL dimethyl sulfoxide (DMSO) was added. The absorbance with cell viability was determined using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 570 nm.Statistical analysis
IC50 values were estimated using a non-linear regression algorithm (SigmaPlot 8.0; SPSS Inc., Chicago, IL, USA, 2002). Statistical evaluation was carried out by one-way analysis of variance (ANOVA followed by Scheffe's multiple range tests).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported from China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan (CHM 106-5-2 and CHM 106-4), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004), and the Ministry of Science and Technology (MOST 103-2320-B-039-009-MY3).Notes and references
- J. C. Liao, Flora of Taiwan, 2nd edn, 1996, vol. 2, pp. 456–458 Search PubMed.
- Department of Health, Committee on Chinese Medicine and Pharmacy. The Catalogue of Medicinal Plant Resources in Taiwan, Taipei, Taiwan, 2003 Search PubMed.
- Y. C. Chang, C. Y. Chen, F. R. Chang and Y. C. Wu, J. Chin. Chem. Soc., 2001, 48, 811–815 CrossRef CAS.
- G. Wei, H. Chen, F. Nie, X. Ma and H. Jiang, Anti-Cancer Agents Med. Chem., 2015, 15, 1–4 CrossRef.
- C. P. Yang, G. J. Huang, H. C. Huang, Y. C. Chen, C. I. Chang, S. Y. Wang, H. S. Chang, Y. H. Tseng, S. C. Chien and Y. H. Kuo, Int. J. Mol. Sci., 2013, 26, 9168–9181 CrossRef PubMed.
- G. Wei, L. Kong, J. Zhang, C. Ma, X. Wu, X. Li and H. Jiang, Nat. Prod. Res., 2016, 6, 1–3 Search PubMed.
- J. P. Lei, G. Q. Wei, J. J. Yuan, K. Z. Tan, Q. Y. Chen, T. Zhang, C. Y. Ma and H. Z. Jiang, Nat. Prod. Res., 2017, 31, 896–901 CrossRef CAS PubMed.
- G. H. Ma, C. W. Lin, H. Y. Hung, S. Y. Wang, P. C. Shieh and T. S. Wu, Nat. Prod. Commun., 2015, 10, 2131–2133 Search PubMed.
- Q. Liu, Y. H. Jo, S. B. Kim, Q. Jin, B. Y. Hwang and M. K. Lee, Bioorg. Med. Chem. Lett., 2016, 26, 4950–4954 CrossRef CAS PubMed.
- J. S. Yu, J. Baek, H. B. Park, E. Moon, S. Y. Kim, S. U. Choi and K. H. Kim, Arch. Pharmacal Res., 2016, 39, 1628–1634 CrossRef CAS PubMed.
- C. P. Yang, G. J. Huang, H. C. Huang, Y. C. Chen, C. I. Chang, S. Y. Wang, I. S. Chen, Y. H. Tseng, S. C. Chien and Y. H. Kuo, Molecules, 2012, 17, 6585–6592 CrossRef CAS PubMed.
- S. Y. Chang, M. J. Cheng, C. F. Peng, H. S. Chang and I. S. Chen, Chem. Biodiversity, 2008, 5, 2690–2698 CAS.
- T. Yahagi, A. Daikonya and S. Kitanaka, Chem. Pharm. Bull., 2012, 60, 129–136 CrossRef CAS PubMed.
- P. Curir, M. Dolci, V. Lanzotti and O. Taglialatela-Scafati, Phytochemistry, 2001, 56, 717–721 CrossRef CAS PubMed.
- L. A. Godínez, Rev. Soc. Quim. Mex., 1999, 43, 219–229 Search PubMed.
- S. G. Walmir, Y. Massayoshi and R. G. Otto, Phytochemistry, 1995, 39, 815–816 CrossRef.
- W. J. Peng, Y. W. Li, C. S. Zhu, X. W. Han and B. Yu, Carbohydr. Res., 2005, 340, 1682–1688 CrossRef CAS PubMed.
- D. D. Moreira and G. G. Leitao, Phytochem. Anal., 2001, 12, 223–225 CrossRef CAS PubMed.
- Y. C. Chang, C. Y. Chen, F. R. Chang and Y. C. Wu, J. Chin. Chem. Soc., 2001, 48, 811–815 CrossRef CAS.
- Y. H. Kuo and Y. C. Li, J. Chin. Chem. Soc., 1997, 44, 321–325 CrossRef CAS.
- Y. H. Kuo, J. M. Lo and Y. F. Chen, J. Chin. Chem. Soc., 2002, 49, 427–431 CrossRef CAS.
- R. S. Burden and H. F. Taylor, Tetrahedron Lett., 1970, 11, 4071–4074 CrossRef.
- N. Okada, K. Shirata, M. Niwano, H. Koshino and M. Uramoto, Phytochemistry, 1994, 37, 281–282 CrossRef CAS.
- C. Pérez, J. Trujillo, L. N. Almonacid, J. Trujillo, E. Navarro and S. J. Alonso, J. Nat. Prod., 1996, 59, 69–72 CrossRef.
- J. Busch, Y. Grether, D. Ochs and U. Sequin, J. Nat. Prod., 1998, 61, 591–597 CrossRef CAS PubMed.
- B. Y. Hwang, B. N. Su, H. Chai, Q. Mi, L. B. Kardono, J. J. Afriastini, S. Riswan, B. D. Santarsiero, A. D. Mesecar, R. Wild, C. R. Fairchild, G. D. Vite, W. C. Ros, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, S. M. Swanson and A. D. Kinghorn, J. Org. Chem., 2004, 69, 3350–3358 CrossRef CAS PubMed.
- Y. Zhou, G. Gao, H. Li and J. Qu, Tetrahedron Lett., 2008, 49, 3260–3263 CrossRef CAS.
- C. Y. Chen, F. R. Chang, C. M. Teng and Y. C. Wu, J. Chin. Chem. Soc., 1999, 46, 77–86 CrossRef CAS.
- K. Machida and M. Kikuchi, Phytochemistry, 1996, 41, 1333–1336 CrossRef CAS.
- S. Kobayashi, M. Kihara and Y. Yamahara, Chem. Pharm. Bull., 1978, 26, 3113–3116 CrossRef CAS.
- W. L. Lo, F. R. Chang, T. J. Hsieh and Y. C. Wu, J. Chin. Chem. Soc., 2002, 49, 421–426 CrossRef CAS.
- T. Ritter, K. Stanek, I. Larrosa and E. M. Carreira, Org. Lett., 2004, 6, 1513–1514 CrossRef CAS PubMed.
- K. Kawanishi and Y. Hashimoto, Phytochemistry, 1987, 26, 749–752 CrossRef CAS.
- W. B. Pan, L. M. Wei, L. L. Wei, C. C. Wu, F. R. Chang and Y. C. Wu, J. Chin. Chem. Soc., 2005, 52, 581–588 CrossRef CAS.
- H. Y. Ding, H. C. Lin, C. M. Teng and Y. C. Wu, J. Chin. Chem. Soc., 2000, 47, 381–388 CrossRef CAS.
- T. H. Lee, C. K. Lu, Y. H. Kuo, J. M. Lo and C. K. Lee, Helv. Chim. Acta, 2008, 91, 79–84 CrossRef CAS.
- M. Kobayashi, K. Ishida, S. Terabayashi and H. Mitsuhashi, Chem. Pharm. Bull., 1991, 39, 3348–3349 CrossRef CAS.
- S. Lötjönen and P. H. Hynninen, Synthesis, 1983, 9, 708–710 CrossRef.
- Y. Nakatani, G. Ourisson and J. P. Beck, Chem. Pharm. Bull., 1981, 29, 2261–2269 CrossRef CAS PubMed.
- H. Y. Lin, H. L. Chiu, Y. H. Lan, C. Y. Tzeng, T. H. Lee, C. K. Lee, Y. Y. Shao, C. R. Chen, C. I. Chang and Y. H. Kuo, Chem. Biodiversity, 2011, 8, 1701–1708 CAS.
- H. H. H. W. Schmidt and M. Kelm, in Methods in Nitric Oxide Research, John Wiley & Sons Ltd., New York, 1996, ch. 33, pp. 491–497 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for new compounds 1–5. See DOI: 10.1039/c7ra09063c |
| ‡ Equal contribution to this article. |
| This journal is © The Royal Society of Chemistry 2017 |