Open Access Article
Open Access ArticleHematite nanoparticle clusters with remarkably high magnetization synthesized from water-treatment waste by one-step “sharp high-temperature dehydration”†
L. Yu. Novoselova
Institute of Petroleum Chemistry, Siberian Branch of the Russian Academy of Sciences, 4 Academichesky Ave., 634055 Tomsk, Russia. E-mail: novoselova@ipc.tsc.ru; Tel: +7-3822-491-623
First published on 3rd November 2017
Abstract
Hematite (α-Fe2O3) nanoparticle clusters with an exceptionally high magnetization (51 emu g−1), comparable to that of nanoscale Fe3O4 and γ-Fe2O3, were synthesized for the first time. This material was prepared from water-treatment waste (iron removal precipitate) after single-step exposure to high temperature without any support (template, catalyst, surfactant, or others). The key factor was the using of a new approach, namely, “sharp high-temperature dehydration” of iron hydroxides containing adsorbed water.
Nanomaterials based on iron oxides have attracted a great deal of global research interest because of their ready availability, low cost, environmental compatibility, high performance, and wide range of uses.1 Iron oxide-based nanomaterials are highly promising materials that are used in diverse fields such as environmental protection and remediation,2 catalysis,3 sensing (e.g., of alcohols and gases such as CO, NO2, etc.),4 energy storage and conversion,5 biotechnology, and medicine.6 Such nanomaterials have been used in the development of pigments,7 electromagnetic-wave-absorbing materials,8 and other products. However, syntheses of iron oxide-based nanomaterials generally include iron salts (as Fex+ precursors), nonaqueous solvents, and surfactants (which contradicts the principles of “green chemistry”9); sometimes, catalysts, templates, oxidizers, fuels, and other substances are also necessary.10 These processes often suffer from one or more disadvantages, including their multistage character, complexity of hardware design, duration, pollution from toxic reagents, high cost due to the use of expensive reagents, and the use of nonrenewable sources of iron.
Magnetite (Fe3O4)- and maghemite (γ-Fe2O3)-based nanomaterials have drawn interest primarily because of their magnetic properties,11 which enable their manipulation using external magnetic fields. The magnetization values for bulk Fe3O4 and bulk γ-Fe2O3 are ∼90 and ∼74 emu g−1,12 respectively, and as their particle sizes decrease to the nanoscale range, their magnetizations noticeably decline.13 Hematite (α-Fe2O3) is conventionally considered non-magnetic iron oxide, and is not regarded as a material for applications that require external magnetic fields. According to prior reports, its magnetization value is typically less than 1 emu g−1.14
However, recent research has shown that materials may change their properties or even acquire completely new and unique properties upon nanonization.15 For example, hematite nanoparticles and nanorods, recently synthesized, demonstrated a magnetization of 21, 85, and 65 emu g−1.16 In these cases, a template-assisted synthesis was integrated with a high-temperature combustion stage;16a other procedures included pulse galvanostatic synthesis in the presence of external magnetic fields followed by calcination16b and microwave combustion synthesis.16c However, the reported procedures have some drawbacks, for example, a greater number of materials and reagents used (template, oxidizer, and fuel16a and nucleation starter16b) and rather cumbersome template synthesis and iron-impregnation stages.16a Also it should be noted here that in all cases, the source of Fe3+ was not a renewable material16 (Table S3 and Section 3.4, ESI†).
Herein, the synthesis of hematite nanoparticle clusters (HNPCs) with remarkably high magnetization of 51 emu g−1, comparable to those of nanoscale magnetite and maghemite, is reported. HNPCs were obtained in a single-step thermal process in which the precursor was treated with the burner without any support (Scheme 1) (for more details, see Section 2.2, ESI†).
 | ||
| Scheme 1 Schematic representation of the synthesis of HNPCs: (a) burner (schematic image), and (b) initial iron removal precipitate (IRP) material. | ||
The iron (Fe3+) precursor for the synthesis is known as iron removal precipitate (IRP), a waste product obtained from the treatment of natural groundwater for drinking, household, and industrial needs. This waste material is abundant worldwide, especially in regions where groundwater is characterized by an elevated iron content. The FTIR, XRF, XRD, and thermal analysis data collectively show that the IRP starting material is a powder based, primarily, on amorphous iron hydroxides containing a rather large amount of water (Section 3.2, ESI†).
Upon treatment of the IRP with the gas burner flame, a sharp, almost instantaneous evaporation of water takes place, which strips the water out of the material and practically disintegrates the particles of the initial powder at different hierarchical levels. At the same time, oxygen entering the open tube from the air contributes to the synthesis of the most thermodynamically stable form of iron oxide, i.e., hematite. We refer to this procedure as “sharp high-temperature dehydration (SHTDH)”.
The key factor of the SHTDH is the very sharp, short exposure to high temperature of the amorphous iron oxyhydroxide phases (the Fe3+ ion sources) that contain a considerable amount of adsorbed water in an oxidizing atmosphere. Here, the (i) sharp, high-temperature exposure involves no preheating of the initial material prior to the treatment. The material is exposed to a high temperature immediately. (ii) The short exposure means that there is no maintaining the product at an elevated temperature. Such a holding period could lead to the gradual recrystallization of the synthesized particles, accompanied by an increase in their size and a change in their properties. For more details see Section 4, ESI.†
The described synthesis of HNPCs by SHTDH can be viewed, on the one hand, as a version of the thermal decomposition of iron precursors. However, it most likely could serve as a starting point for developing a new approach for producing iron oxide-based nanomaterials. It should be noted that this approach agrees well with the principles of “green chemistry,” and, on the other hand, in our opinion, it could be extended to the synthesis of nanomaterials with other compositions.
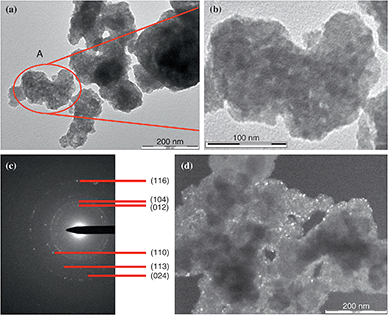
The results of transmission electron microscopy (TEM) of the powder obtained from the thermal treatment (Fig. 1a–d and S2, ESI†) indicate that hematite nanoparticle clusters were synthesized. These clusters display irregular, somewhat elongated shapes with dimensions of approximately 50–200 nm. Each cluster consists of multiple individual nanoparticles with a mean size of less than 10 nm. Furthermore, the clusters tend to aggregate slightly, rather than remain as isolated entities.13c,17 This tendency can be explained by the lack of any special techniques to prevent aggregation of the particles in the nanomaterial (e.g., application of various surfactants, polymers, etc.) during its preparation. The electron microdiffraction pattern (Fig. 1c), which can be used to clarify atomic crystal structures for phase identification,18 confirms that hematite was obtained.
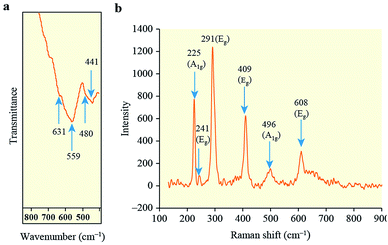
The obtained HNPCs were investigated using FTIR spectroscopy. In the region of 400–800 cm−1, two broad transmission bands centered at 441 and 559 cm−1 and two bands as bends (shoulders) at 480 and 631 cm−1 are observed (Fig. 2a). The peaks at 480 and 559 cm−1 can be associated with the perpendicular modes of the Fe–O stretching vibrations in α-Fe2O3, and the peaks at 441 and 631 cm−1 can be ascribed to α-Fe2O3 lattice modes that have polarization parallel to the c-axis.19 Thus, the FTIR spectroscopy data confirm that hematite was prepared in this work. Some broadening of the peaks may have been caused by particle aggregation,19c which was noted above in the discussion of the TEM results.
The prepared HNPCs were also characterized by Raman spectroscopy. By this method, iron oxide phases α-Fe2O3, Fe3O4 and γ-Fe2O3 can be identified nearly unambiguously. The XRD method, for instance, cannot explicitly differentiate between Fe3O4 and γ-Fe2O3.20 To avoid phase changes, which may be induced by the excitation source during collection of the Raman spectra, as previously observed for iron oxides,21 the spectrum in this study was collected using a low laser power of ∼0.8 mW.
At least six lines are clearly visible in the Raman spectrum, at ∼225, 241, 291, 409, 496, and 608 cm−1 (Fig. 2b). The peaks at 225 and 496 cm−1 are assigned to the A1g modes of hematite, and those at 241, 291, 409, and 608 cm−1 are attributed to the Eg modes of hematite.21,22 The obtained spectrum thus exhibits six of the seven spectral signatures deemed diagnostic for hematite, and does not contain any peaks associated with either maghemite or magnetite. There is also a shift of the characteristic lines towards lower wavenumbers, which is common for nanoparticles.22
Thus, the combined TEM, FTIR, and Raman data clearly demonstrate that hematite nanoparticle clusters were obtained in the present study.
The field dependence of the magnetization of the HNPCs is presented in Fig. 3. The data show an exceptionally high value of saturation magnetization for the HNPCs, reaching 51 emu g−1 at an applied field of H = 6.5 kOe and temperature T = 300 K. Although this magnetization value is somewhat lower than those obtained for recently synthesized hematite nanoparticles16c and nanorods,16b it substantially exceeds that of α-Fe2O3 nanoparticles, 21 emu g−1 (H = 10 kOe, T = 300 K)16a and, as far as we know, is currently one of the highest of all values obtained for hematite.
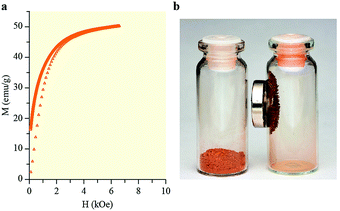 | ||
| Fig. 3 (a) Dependence of the HNPCs magnetization on the magnetic field. (b) Images of (left) initial IRP material and (right) synthesized HNPCs material with a magnet. | ||
Furthermore, the HNPCs magnetization is comparable to or even higher than those of magnetite and maghemite, which are conventionally considered as magnetic iron oxides. For example, for Fe3O4 nanoparticles prepared at 25, 40, and 60 °C, the saturation magnetization values were 45, 50, and 50 emu g−1, respectively (H = 20 kOe, T = 25 °C).23 In the case of 42, 30, and 19 nm Fe3O4 nanoflowers, saturation magnetization values of 33, 45, and 36 emu g−1 were observed, respectively (H = 30 kOe, T = 250 K),24 whereas, for porous γ-Fe2O3, this parameter was approximately 9 emu g−1 (H = 5 kOe, room temperature).20b
The high magnetization value of the HNPCs can be explained by specific effects of the multilevel hierarchical organization of their structure. On the one hand, the presence of the small individual hematite nanoparticles with dimensions of less than 10 nm can play a major role in this phenomenon; the high magnetization of such nanoparticles can be associated with the contribution of uncompensated surface spins, which tend to interact ferromagnetically.16a On the other hand, the clustering of these individual nanoparticles and the tendency of the resulting clusters to assemble, which we noted when discussing the TEM results, can contribute to the enhancement of magnetization. For example, Ge et al.13c mentioned that clusters with larger sizes exhibited higher values of magnetization (however, these results describe the behavior of magnetite clusters rather than hematite clusters, as in our case). The interaction of these clusters, in distinction from isolated ones, apparently results in the formation of structures of another hierarchical level (with larger sizes), which increases the value of the magnetization.
Based on these results, new applications of hematite nanomaterials can be anticipated, especially in fields in which a material with both high magnetization and thermodynamic stability is required. The former enables the efficient control of a material's movement in different environments using magnetic fields,2a,c,d and, if necessary, separation of the material from those environments.2b,e,3b The latter helps the material to maintain the required properties upon exposure to an extended temperature range. Hematite is considered the most thermodynamically stable phase of iron oxide,14a in contrast to magnetite and maghemite, which are usually oxidized to α-Fe2O3 at elevated temperature.20b,25
In summary, the hematite nanoparticle clusters with an exceptionally high magnetization of 51 emu g−1, comparable to the magnetization values of nanoscale magnetite and maghemite, were synthesized for the first time. They were prepared from IRP (a renewable, globally available waste material consisting of iron hydroxides with adsorbed water that is produced during the removal of iron from groundwater). The newly described synthesis has many advantages, including rapidity, environmental friendliness, and single-stage operational simplicity. It does not require modifiers such as templates, catalysts, or surfactants, and avoids the use of toxic and/or expensive reagents or nonrenewable sources of iron. Thus, the synthesis of HNPCs by SHTDH agrees well with the principles of “green chemistry”.
It is expected that these HNPCs will find many applications in the development of advanced materials. In addition, the data presented in this paper may (i) lead to a revision of ideas about the magnetic properties of iron oxides, particularly hematite; (ii) suggest the replacement of magnetite and/or maghemite nanomaterials in many applications by hematite nanomaterials as more thermodynamically stable; and (iii) lead to the development of a new approach for the synthesis of nanomaterials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author expresses gratitude to employees of Tomsk Regional Common Use Center for fruitful cooperation. The author is also grateful to the Joint-use Center of Tomsk Scientific Center of SB RAS for providing access to the Nicolet 5700 FTIR spectrometer.Notes and references
- (a) L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426 CrossRef CAS; (b) C. Yang, J. J. Wu and Y. L. Hou, Chem. Commun., 2011, 47, 5130 RSC; (c) T. Peik-See, A. Pandikumar, L. H. Ngee, H. N. Ming and C. C. Hua, Catal. Sci. Technol., 2014, 4, 4396 RSC; (d) S. Lee, A. Fursina, J. T. Mayo, C. T. Yavuz, V. L. Colvin, R. G. S. Sofin, I. V. Shvets and D. Natelson, Nat. Mater., 2008, 7, 130 CrossRef CAS PubMed; (e) S. C. Warren, K. Voitchovsky, H. Dotan, C. M. Leroy, M. Cornuz, F. Stellacci, C. Hebert, A. Rothschild and M. Graetzel, Nat. Mater., 2013, 12, 842 CrossRef CAS PubMed; (f) M. Hu and Y. Yamauchi, Chem.–Asian J., 2011, 6, 2282 CrossRef CAS PubMed.
- (a) Z. Liu, H. S. Wang, C. Liu, Y. J. Jiang, G. Yu, X. D. Mu and X. Y. Wang, Chem. Commun., 2012, 48, 7350 RSC; (b) V. K. Sharma, T. J. McDonald, H. Kim and V. K. Garg, Adv. Colloid Interface Sci., 2015, 225, 229 CrossRef CAS PubMed; (c) P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413 CrossRef CAS PubMed; (d) P. Thanikaivelan, N. T. Narayanan, B. K. Pradhan and P. M. Ajayan, Sci. Rep., 2012, 2, 230 CrossRef PubMed; (e) Q. Zhu, F. Tao and Q. M. Pan, ACS Appl. Mater. Interfaces, 2010, 2, 3141 CrossRef CAS PubMed; (f) J. Hu, G. H. Chen and I. M. C. Lo, Water Res., 2005, 39, 4528 CrossRef CAS PubMed; (g) H. Y. Chen, K. L. Lv, Y. Du, H. P. Ye and D. Y. Du, J. Alloys Compd., 2016, 674, 399 CrossRef CAS; (h) Q. R. Zhang, J. Teng, G. D. Zou, Q. M. Peng, Q. Du, T. F. Jiao and J. Xiang, Nanoscale, 2016, 8, 7085 RSC; (i) R. Liu, J. F. Liu, L. Q. Zhang, J. F. Sun and G. B. Jiang, J. Mater. Chem. A, 2016, 4, 7606 RSC; (j) V. Chandra, J. Park, Y. Chun, J. W. Lee, I. C. Hwang and K. S. Kim, ACS Nano, 2010, 4, 3979 CrossRef CAS PubMed; (k) B. Peng, T. T. Song, T. Wang, L. Y. Chai, W. C. Yang, X. R. Li, C. F. Li and H. Y. Wang, Chem. Eng. J., 2016, 299, 15 CrossRef CAS; (l) J. T. Mayo, S. S. Lee, C. T. Yavuz, W. W. Yu, A. Prakash, J. C. Falkner and V. L. Colvin, Nanoscale, 2011, 3, 4560 RSC; (m) L. Yu. Novoselova, Powder Technol., 2016, 287, 364 CrossRef CAS; (n) L. Yu. Novoselova, E. E. Sirotkina and N. I. Pogadaeva, Pet. Chem., 2008, 48, 67 CrossRef; (o) L. Yu. Novoselova and E. E. Sirotkina, Russ. J. Phys. Chem. A, 2009, 83, 2127 CrossRef CAS.
- (a) R. V. Jagadeesh, A. E. Surkus, H. Junge, M. M. Pohl, J. Radnik, J. Rabeah, H. M. Huan, V. Schuenemann, A. Brueckner and M. Beller, Science, 2013, 342, 1073 CrossRef CAS PubMed; (b) D. Wang, C. Deraedt, J. Ruiz and D. Astruc, Acc. Chem. Res., 2015, 48, 1871 CrossRef CAS PubMed; (c) C. Bolm, Nat. Chem., 2009, 1, 420 CrossRef CAS PubMed; (d) J. M. Walker and J. M. Zaleski, Nanoscale, 2016, 8, 1535 RSC.
- (a) S. Yan and Q. S. Wu, J. Mater. Chem. A, 2015, 3, 5982 RSC; (b) Z. Dai, C. S. Lee, Y. Tian, I. D. Kim and J. H. Lee, J. Mater. Chem. A, 2015, 3, 3372 RSC; (c) X. L. Hu, J. C. Yu, J. M. Gong, Q. Li and G. S. Li, Adv. Mater., 2007, 19, 2324 CrossRef CAS; (d) N. D. Cuong, D. Q. Khieu, T. T. Hoa, D. T. Quang, P. H. Viet, T. D. Lam, N. D. Hoa and N. V. Hieu, Mater. Res. Bull., 2015, 68, 302 CrossRef CAS.
- (a) O. Zandi and T. W. Hamann, Nat. Chem., 2016, 8, 778 CrossRef CAS PubMed; (b) P. Dias, A. Vilanova, T. Lopes, L. Andrade and A. Mendes, Nano Energy, 2016, 23, 70 CrossRef CAS; (c) Z. Sun, E. Madej, A. Genc, M. Muhler, J. Arbiol, W. Schuhmann and E. Ventos, Chem. Commun., 2016, 52, 7348 RSC; (d) Y. Huang, Z. X. Lin, M. B. Zheng, T. H. Wang, J. Z. Yang, F. S. Yuan, X. Y. Lu, L. Liu and D. P. Sun, J. Power Sources, 2016, 307, 649 CrossRef CAS; (e) S. Tanaka, R. R. Salunkhe, Y. V. Kaneti, V. Malgras, S. M. Alshehri, T. Ahamad, M. B. Zakaria, S. X. Dou, Y. Yamauchi and M. S. A. Hossain, RSC Adv., 2017, 7, 33994 RSC.
- (a) K. L. Viola, J. Sbarboro, R. Sureka, M. De, M. A. Bicca, J. Wang, S. Vasavada, S. Satpathy, S. Wu, H. Joshi, P. T. Velasco, K. MacRenaris, E. A. Waters, C. Lu, J. Phan, P. Lacor, P. Prasad, V. P. Dravid and W. L. Klein, Nat. Nanotechnol., 2015, 10, 91 CrossRef CAS PubMed; (b) J. Xie, G. Liu, H. S. Eden, H. Ai and X. Y. Chen, Acc. Chem. Res., 2011, 44, 883 CrossRef CAS PubMed; (c) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS PubMed; (d) R. Hachani, M. Lowdell, M. Birchall, A. Hervault, D. Mertz, S. Begin-Coline and T. K. T. Nguyen, Nanoscale, 2016, 8, 3278 RSC; (e) H. Arami, A. Khandhar, D. Liggitt and K. M. Krishnan, Chem. Soc. Rev., 2015, 44, 8576 RSC; (f) S. A. Corr, S. J. Byrne, R. Tekoriute, C. J. Meledandri, D. F. Brougham, M. Lynch, C. Kerskens, L. O'Dwyer and Y. K. Gun'ko, J. Am. Chem. Soc., 2008, 130, 4214 CrossRef CAS PubMed.
- (a) H. Hashimoto, M. Nakanishi, H. Asaoka, T. Maeda, Y. Kusano, T. Fujii and J. Takada, ACS Appl. Mater. Interfaces, 2014, 6, 20282 CrossRef CAS PubMed; (b) H. Hashimoto, H. Inada, Y. Okazaki, T. Takaishi, T. Fujii and J. Takada, ACS Appl. Mater. Interfaces, 2016, 8, 10918 CrossRef CAS PubMed; (c) P. Thanikaivelan, R. Murali and K. Krishnaraj, RSC Adv., 2016, 6, 6496 RSC.
- (a) Y. Ding, L. Zhang, O. L. Liao, G. J. Zhang, S. Liu and Y. Zhang, Nano Res., 2016, 9, 2018 CrossRef CAS; (b) G. L. Wu, Y. H. Cheng, Y. Y. Ren, Y. Q. Wang, Z. D. Wang and H. J. Wu, J. Alloys Compd., 2015, 652, 346 CrossRef CAS; (c) H. Zhang, A. J. Xie, C. P. Wang, H. S. Wang, Y. H. Shen and X. Y. Tian, J. Mater. Chem. A, 2013, 1, 8547 RSC.
- (a) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed; (b) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC; (c) P. Anastas, B. X. Han, W. Leitner and M. Poliakoff, Green Chem., 2016, 18, 12 RSC.
- (a) T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. Bin Na, J. Am. Chem. Soc., 2001, 123, 12798 CrossRef CAS PubMed; (b) N. R. Jana, Y. F. Chen and X. G. Peng, Chem. Mater., 2004, 16, 3931 CrossRef CAS; (c) S. P. Gubin, Yu. A. Koksharov, G. B. Khomutov and G. Yu. Yurkov, Russ. Chem. Rev., 2005, 74, 489 CrossRef CAS; (d) S. T. Aruna and A. S. Mukasyan, Curr. Opin. Solid State Mater. Sci., 2008, 12, 44 CrossRef CAS; (e) A. Varma, A. S. Mukasyan, A. S. Rogachev and K. V. Manukyan, Chem. Rev., 2016, 116, 14493 CrossRef CAS PubMed; (f) A. I. Gusev and A. A. Rempel, Nanocrystalline Materials, Cambridge International Science Publishing, Cambridge, 2004 Search PubMed.
- (a) C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, Science, 2006, 314, 964 CrossRef PubMed; (b) W. W. Yu, J. C. Falkner, C. T. Yavuz and V. L. Colvin, Chem. Commun., 2004, 20, 2306 RSC; (c) A. Demortiere, P. Panissod, B. P. Pichon, G. Pourroy, D. Guillon, B. Donnio and S. Begin-Colin, Nanoscale, 2011, 3, 225 RSC.
- (a) F. Ooi, J. S. DuChene, J. J. Qiu, J. O. Graham, M. H. Engelhard, G. X. Cao, Z. Gai and W. D. Wei, Small, 2015, 11, 2649 CrossRef CAS PubMed; (b) D. Ho, X. L. Sun and S. H. Sun, Acc. Chem. Res., 2011, 44, 875 CrossRef CAS PubMed.
- (a) R. F. Chen, G. Q. Song and Y. Wei, J. Phys. Chem. C, 2010, 114, 13409 CrossRef CAS; (b) S. Zhao and S. Asuha, Powder Technol., 2010, 197, 295 CrossRef CAS; (c) J. P. Ge, Y. X. Hu, M. Biasini, W. P. Beyermann and Y. D. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342 CrossRef CAS PubMed.
- (a) J. B. Lian, X. C. Duan, J. M. Ma, P. Peng, T. I. Kim and W. J. Zheng, ACS Nano, 2009, 3, 3749 CrossRef CAS PubMed; (b) J. M. Ma, J. B. Lian, X. C. Duan, X. D. Liu and W. J. Zheng, J. Phys. Chem. C, 2010, 114, 10671 CrossRef CAS; (c) S. Sivakumar, D. Anusuya, C. P. Khatiwada, J. Sivasubramanian, A. Venkatesan and P. Soundhirarajan, Spectrochim. Acta, Part A, 2014, 128, 69 CrossRef CAS PubMed.
- (a) M. Arruebo, R. Fernandez-Pacheco, M. R. Ibarra and J. Santamaria, Nano Today, 2007, 2, 22 CrossRef; (b) A. Dreyer, A. Feld, A. Kornowski, E. D. Yilmaz, H. Noei, A. Meyer, T. Krekeler, C. G. Jiao, A. Stierle, V. Abetz, H. Weller and G. A. Schneider, Nat. Mater., 2016, 15, 522 CrossRef CAS PubMed.
- (a) K. V. Manukyan, Y. S. Chen, S. Rouvimov, P. Li, X. Li, S. N. Dong, X. Y. Liu, J. K. Furduna, A. Orlov, G. H. Bernstein, W. Porod, S. Roslyakov and A. S. Mukasyan, J. Phys. Chem. C, 2014, 118, 16264 CrossRef CAS; (b) H. Karami, J. Ordoukhanian and A. Nezhadali, Ceram. Int., 2015, 41, 14760 CrossRef CAS; (c) A. Manikandan, J. J. Vijaya and L. J. Kennedy, J. Nanosci. Nanotechnol., 2013, 13, 2986 CrossRef CAS PubMed.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28 CrossRef CAS PubMed.
- P. B. Hirsch, A. Howie, R. B. Nicholson, D. W. Pashley and M. J. Whelan, Electron Microscopy of Thin Crystals, Butterworths, London, 1965 Search PubMed.
- (a) L. Lu, L. P. Li, X. J. Wang and G. S. Li, J. Phys. Chem. B, 2005, 109, 17151 CrossRef CAS PubMed; (b) S. Hayashi and H. Kanamori, J. Phys. C: Solid State Phys., 1980, 13, 1529 CrossRef CAS; (c) C. J. Serna, M. Ocana and J. E. Iglesias, J. Phys. C: Solid State Phys., 1987, 20, 473 CrossRef CAS.
- (a) H. J. Wu, G. L. Wu and L. D. Wang, Powder Technol., 2015, 269, 443 CrossRef CAS; (b) M. Hu, J. S. Jiang and Y. Zeng, Chem. Commun., 2010, 46, 1133 RSC; (c) M. Hu, A. A. Belik, M. Imura, K. Mibu, Y. Tsujimoto and Y. Yamauchi, Chem. Mater., 2012, 24, 2698 CrossRef CAS.
- D. L. A. deFaria, S. V. Silva and M. T. deOliveira, J. Raman Spectrosc., 1997, 28, 873 CrossRef CAS.
- I. V. Chernyshova, M. F. Hochella Jr and A. S. Madden, Phys. Chem. Chem. Phys., 2007, 9, 1736 RSC.
- C. C. Lin, J. M. Ho and M. S. Wu, Powder Technol., 2015, 274, 441 CrossRef CAS.
- F. Q. Hu, K. W. MacRenaris, E. A. Waters, E. A. Schultz-Sikma, A. L. Eckermann and T. J. Meade, Chem. Commun., 2010, 46, 73 RSC.
- (a) Z. A. Zang, H. B. Yao, Y. X. Zhou, W. T. Yao and S. H. Yu, Chem. Mater., 2008, 20, 4749 CrossRef CAS; (b) P. C. Wu, W. S. Wang, Y. T. Huang, H. S. Sheu, Y. W. Lo, T. L. Tsai, D. B. Shieh and C. S. Yeh, Chem.–Eur. J., 2007, 13, 3878 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and characterization details; discussion of process details. See DOI: 10.1039/c7ra09062e |
| This journal is © The Royal Society of Chemistry 2017 |