Open Access Article
Open Access ArticleFullerene C60 conjugated with phenols as new hybrid antioxidants to improve the oxidative stability of polymers at elevated temperatures†
R. Czochara ,
J. Kusio and
G. Litwinienko
,
J. Kusio and
G. Litwinienko *
*
University of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093 Warsaw, Poland. E-mail: litwin@chem.uw.edu.pl
First published on 12th September 2017
Abstract
Four derivatives of fullerene C60 with covalently bonded simple phenols were tested as inhibitors of oxidation of polyethylene at elevated temperatures 150–250 °C under non-isothermal conditions. Temperatures of the start of oxidation as well as the Arrhenius kinetic parameters of overall oxidation indicate a significant improvement of the oxidative stability of composites with fullerene derivatives.
Introduction
Several types of substances can be applied to enhance the oxidative stability of polymers, plastics, resins and lubricants, but the most commonly used include phenolic, amine and organosulfur derivatives1 as chain-breaking antioxidants, i.e. molecules able to trap peroxyl radicals mediating the autoxidation.2 Their effectiveness depends on the mechanism of inhibition (for example, H atom abstraction as a one- or multiple step process),3–6 the reactivity of secondary products formed from antioxidants,7 the type of material to be stabilized, the conditions of usage and the amount of the antioxidant in the polymer or the lubricant.8,9 It is important to obtain a good match between the reactivity of an antioxidant and the nature of the oxidation process with respect to applicability and exploitation conditions of the material to be protected. For example, some kinds of high-performance plastics are applied at high temperature (thermal or electrical insulators, bearings and seals), however most of the antioxidants are characterized by a relatively high volatility, making them difficult to be used at temperatures above 100 °C.10,11 Under such conditions typical “food antioxidants” are not effective and at higher temperatures they may be a source of re-initiation of the autoxidation process. On the other side, amines and organosulfur antioxidants produce sulphur or nitrogen oxides that can cause corrosion of metal parts. Another negative effect is a migration of the antioxidant or other additives from the plastic material into the liquid, food or medicines. This process depends on concentration of a substance in polymer matrix, temperature, and the time of interfacial contact12 and it has been found13 that common antioxidants like 2,6-di-tert-butyl-4-methylphenol (BHT), 2,6-di-tert-butyl-4-methoxyphenol (BHA) or Irganox 1330 can migrate from polymer into aqueous/oil at 40 °C during several days, and the process is faster as the fat content of food increases.14 Therefore, an ideal antioxidant for polymers, lubricants and resins should be active, non-volatile, and not corrosive. Another important feature of a potent antioxidant able to break the oxidative destruction of polymers is ability to react not only with peroxyl radicals (usual chain mediators of low temperature peroxidation) but also to scavenge other, non-peroxyl radical intermediates responsible for propagation of kinetic chain. Recent works suggest that high temperature oxidative degradation of polymers proceeds with participation of wide variety of radicals with alkoxyl radicals from decomposition of tetraoxides (non Russel termination products).15,16There are a few reports concerning the pristine fullerene C60 or its derivatives as a scavenger of several kinds of radicals. The carbon sphere of C60 is thermally stable (up to 600 °C),17 traps several radicals,18–20 and, therefore, is a good starting structure for development of new radical-scavengers to be used as stabilizing agents during thermal oxidation. We described the antioxidant behaviour of C60 during the autoxidation of stearic acid (STA) over a wide range of temperatures, as a non-corrosive agent effectively increasing the oxidative stability of lubricants.21 For high temperature oxidation of STA, the phenolic conjugates of C60 are more active antioxidants than the pristine C60 and phenols used alone.22 This lead us to hypothesis that conjugates of C60 with phenols are good candidates for hybrid antioxidants to be used in polymers at temperatures above 100 °C. Herein we present experimental study in which kinetic parameters of thermo-oxidation of pure high density polyethylene (HDPE) were determined and compared with kinetic parameters of HDPE containing pristine fullerene C60 and C60 adducts with four phenols I–IV (see Chart 1) at concentration 0.065–1%.
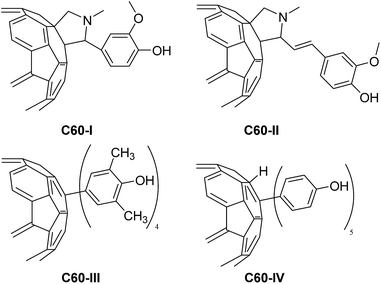 | ||
| Chart 1 Structures of fullerene C60 derivatives: N-methyl-2-[4-hydroxy-3-methoxyphenyl]-3,4-[60]fulleropyrrolidine (C60-I), N-methyl-2-[2-(4-hydroxy-3-methoxyphenyl)vinyl]-3,4-[60]fulleropyrrolidine (C60-II), tetra-2,6-methyl-4-hydroxyphenyl[60]fullerene (C60-III), and penta-(4-hydroxyphenyl)-hydro[60]fullerene (C60-IV). Synthesis and characterization of C60 derivatives is described in ref. 22, and quoted in ESI.† | ||
Experimental
Synthesis of C60-(I–IV) is described in ref. 22 see also ESI.† Thermal stability of obtained C60 derivatives was measured using a thermogravimeter (TA Q50) with platinum vessels under nitrogen flow. Samples of fullerene C60 derivatives C60-(I–IV) were dried in vacuum in 40 °C for 12 h. In a typical TG measurement a sample (6–7 mg) was heated at 5 K min−1 from 50 to 600 °C. Universal V4.54 TA Instruments software was used for data collection and analysis.Du Pont 910 apparatus with Du Pont 9900 thermal analyzer and normal pressure cell was used. Experiments were performed under oxygen flow 6 dm3 h−1 in open aluminum pans with an empty aluminum pan used as a reference. Samples (3.1–3.8 mg) of HDPE or HDPE with additives were heated from 50 to 250 °C with linear heating rate β. Preparation of samples: HDPE (0.10 ± 0.01 g) was crushed in a mortar then calculated mass of pristine fullerene C60 or its derivative (Chart 1) and a few mL of CS2 was added with continuous grinding of the mixture until the homogeneous powder was obtained. The composite was moved into a glass vial and left for one hour to evaporate CS2. More information can be found in ESI.†
Results and discussion
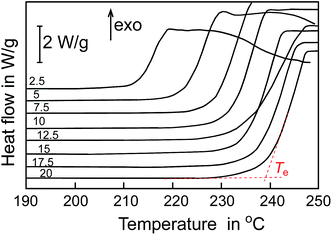
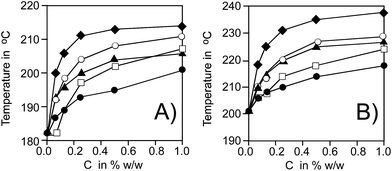
Thermal effect of oxidation can be recorded as the heat flow versus temperature and we employed Differential Scanning Calorimetry (DSC) for monitoring the oxidation process in non-isothermal mode. Typical DSC curves for oxidation of HDPE containing 1% w/w C60-I for different β (2.5–20.0 K min−1) are shown in Fig. 1. In general, the oxidative stability can be expressed as the length of the lag phase from start of experiment to the moment when thermal effect of spontaneous oxidation is detected. In non-isothermal DSC method the temperature is a linear function of time, thus, a temperature of start of oxidation (Te, in °C) can be easily determined instead of time. Fig. 2 presents Te values for HDPE containing C60 and its derivatives C60-(I–IV) at concentration range 0.065–1% w/w. Indeed, when unsubstituted C60 or compounds C60-(I–IV) are present in the polymer, Te is shifted to higher temperatures and a magnitude of such shift is non-linearly proportional to concentration of the additive.Addition of small amount of pristine fullerene C60 (0.065%) did not improve the oxidation stability while at concentration higher than 0.125% the parameter Te increases, see Fig. 2. A similar increase can be observed when conjugates C60-(I–IV) are added to HDPE: the values of Te for HDPE with additives are about 30–40 degrees higher than Te for pure HDPE (for β = 2.5 K min−1 a difference is 32 °C, for β = 10 K min−1 the difference is 37 °C).
In our previous publications21,23,24 we interpreted the shape of DSC curves of thermal effect of lipids and hydrocarbons oxidation and we proved that Te parameter corresponds to constant degree of conversion (start of oxidation). Therefore, Te collected for series of different β's (see, for example, Fig. 1) after recalculation into absolute temperature can be used for the calculation of the kinetic parameters of the process by iso-conversional methods. For a series of oxidations carried out with different β a linear correlation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β with reciprocal of Te (in kelvins) is found:21,23,24
β with reciprocal of Te (in kelvins) is found:21,23,24
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = a × Te−1 + b β = a × Te−1 + b
| (1) |
The slope a and intersection b can be used for calculation of the activation energy (Ea) and pre-exponential factor (Z) from equations proposed by Ozawa and independently, by Flynn and Wall (OFW method):25–28
Ea = −2.19R(d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/dT) β/dT)
| (2) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = log(Ea/R) − b − 2.315 Z = log(Ea/R) − b − 2.315
| (3) |
Rate constants (k, in min−1) for oxidation were calculated from the Arrhenius equation k = Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT) for temperatures 50–250 °C.
exp(−Ea/RT) for temperatures 50–250 °C.
Table 1 presents the overall Arrhenius kinetic parameters of oxidation of HDPE with and without additives. The activation parameters Ea = 129 ± 7 kJ mol−1 and Z = 1.11 × 1014 min−1 obtained for non-inhibited process (no additives) are in good agreement with 127 ± 6 kJ mol−1 and 7.15 × 1013 min−1 measured earlier24 as well as with other literature values.10,29 The values of activation energy of HDPE oxidation vary with the concentration of the added derivatives. Addition of pristine fullerene C60 to HDPE causes the growth of Ea with maximum at 0.5% w/w. Compounds C60-II and C60-III exhibit maximal value of activation energy (134 ± 6 and 140 ± 3 kJ mol−1, respectively) at concentration 0.065% w/w, whereas for C60-I and C60-IV the maximal Ea is observed at concentrations 0.25% and 1%.
| Cb [% w/w] | Eac [kJ mol−1] | log (Z/min−1) | log (k/min−1) 150 °C |
|---|---|---|---|
| a Full data including statistic and kinetic parameters are given in ESI.b Concentration in % w/w.c Errors of the activation energy Ea were calculated from the standard deviations of slopes calculated for confidence level 90%. | |||
| Pure HDPE | |||
| — | 129 ± 7 | 14.04 | −1.83 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Fullerene C60 | |||
| 0.065 | 127 ± 5 | 13.85 | −1.83 |
| 0.125 | 136 ± 8 | 14.66 | −2.13 |
| 0.250 | 142 ± 8 | 15.04 | −2.45 |
| 0.500 | 161 ± 8 | 17.06 | −2.88 |
| 1.000 | 156 ± 7 | 16.33 | −2.97 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Fullerene derivative C60-I | |||
| 0.065 | 141 ± 5 | 15.08 | −2.28 |
| 0.125 | 142 ± 8 | 15.04 | −2.46 |
| 0.250 | 154 ± 4 | 16.13 | −2.84 |
| 0.500 | 143 ± 4 | 14.79 | −2.85 |
| 1.000 | 142 ± 7 | 14.65 | −2.94 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Fullerene derivative C60-II | |||
| 0.065 | 134 ± 6 | 14.35 | −2.23 |
| 0.125 | 131 ± 4 | 13.83 | −2.32 |
| 0.250 | 124 ± 5 | 12.99 | −2.37 |
| 0.500 | 124 ± 3 | 12.81 | −2.49 |
| 1.000 | 126 ± 3 | 12.95 | −2.59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Fullerene derivative C60-III | |||
| 0.065 | 140 ± 3 | 14.77 | −2.56 |
| 0.125 | 132 ± 3 | 13.66 | −2.64 |
| 0.250 | 128 ± 7 | 13.09 | −2.74 |
| 0.500 | 130 ± 7 | 14.12 | −2.87 |
| 1.000 | 120 ± 5 | 12.07 | −2.75 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Fullerene derivative C60-IV | |||
| 0.065 | 129 ± 5 | 13.89 | −2.00 |
| 0.125 | 133 ± 5 | 14.27 | −2.12 |
| 0.250 | 140 ± 3 | 15.03 | −2.28 |
| 0.500 | 142 ± 8 | 15.09 | −2.40 |
| 1.000 | 148 ± 4 | 15.57 | −2.64 |
Ea and Z can be easily converted into rate constants calculated for given temperature. The values of the overall oxidation rate constant for pure HDPE and for the oxidation of polymer containing C60 and its derivatives C60-(I–IV) are listed in Table 1 and they confirm the inhibitory properties of studied compounds. As can be seen in Table 1 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k for 150 °C) and in Fig. 3 (log
k for 150 °C) and in Fig. 3 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k for 100 and 200 °C), pristine fullerene C60 causes a decrease of k's within the whole range of concentrations (ten-fold decrease in comparison with pure HDPE). When polyethylene sample contains any of the four C60-(I–IV) derivatives, the oxidation is slower than oxidation of pure HDPE. The observed stabilizing effect is stronger than for polyethylene containing 0.5% of BHT as a typical antioxidant widely used in polymers: values of log(k/min) for the oxidation of polyethylene with 0.5% BHT at temp. 100, 150 and 200 °C are: −1.841, −0.342, and +0.868, respectively (see ref. 24 and Table S27†).
k for 100 and 200 °C), pristine fullerene C60 causes a decrease of k's within the whole range of concentrations (ten-fold decrease in comparison with pure HDPE). When polyethylene sample contains any of the four C60-(I–IV) derivatives, the oxidation is slower than oxidation of pure HDPE. The observed stabilizing effect is stronger than for polyethylene containing 0.5% of BHT as a typical antioxidant widely used in polymers: values of log(k/min) for the oxidation of polyethylene with 0.5% BHT at temp. 100, 150 and 200 °C are: −1.841, −0.342, and +0.868, respectively (see ref. 24 and Table S27†).
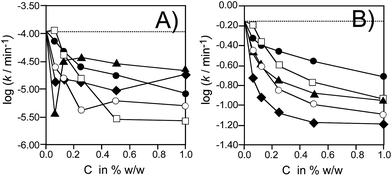 | ||
Fig. 3 Comparison of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k for the oxidation of HDPE (dashed line) and HDPE containing: pristine C60 (□) and derivatives: C60-I (○), C60-II (▲), C60-III (◆), C60-IV (●) at temperature 100 °C (panel A) and 200 °C (panel B). Rate constants were calculated from Ea and Z parameters listed in Table 1. k for the oxidation of HDPE (dashed line) and HDPE containing: pristine C60 (□) and derivatives: C60-I (○), C60-II (▲), C60-III (◆), C60-IV (●) at temperature 100 °C (panel A) and 200 °C (panel B). Rate constants were calculated from Ea and Z parameters listed in Table 1. | ||
Comparison of the rate constants is more reasonable than comparison of Ea values, in order to eliminate the possible misinterpretation connected with the isokinetic inversion of reaction rates (simple calculations for two processes described by two series of the Arrhenius parameters Ea and Z indicates that at temperatures below the isokinetic temperature, Tiso, the process with higher Ea is slower whereas at temp. above Tiso the process with higher Ea proceeds faster).22,24 For example, oxidation of polyethylene inhibited by 1% C60-III is described by Ea = 120 kJ mol−1 and Z = 1.2 × 1012 min−1, giving the rate constants 1.85 × 10−5 min−1 at 100 °C and 0.066 min−1 at 200 °C. If 1% C60-IV is used, the kinetic parameters Ea = 148 kJ mol−1 and Z = 3.7 × 1015 min−1 allow to calculate k100°C = 8.38 × 10−6 min−1 and k200°C = 0.193 min−1. Thus, at 100 °C C60-IV is more active than C60-III whereas at 200 °C the derivative C60-III is more active than C60-IV. For this particular pair of kinetic parameters Tiso = ΔEa/(8.314 × Δln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z) = 145 °C and indeed, rate constants presented in Table 1 for 150 °C are almost the same for C60-III and for C60-IV. Such inversions of the rates of the oxidation can be clearly observed if the results shown in Fig. 3A are compared with results presented in Fig. 3B: the rates in Fig. 3A are calculated below Tiso while Fig. 3B presents the results calculated above Tiso. This apparent discrepancy creates awareness and understanding of the kinetic behaviour of the systems monitored at various temperatures.
Z) = 145 °C and indeed, rate constants presented in Table 1 for 150 °C are almost the same for C60-III and for C60-IV. Such inversions of the rates of the oxidation can be clearly observed if the results shown in Fig. 3A are compared with results presented in Fig. 3B: the rates in Fig. 3A are calculated below Tiso while Fig. 3B presents the results calculated above Tiso. This apparent discrepancy creates awareness and understanding of the kinetic behaviour of the systems monitored at various temperatures.
As can be seen in Fig. 3B, first three derivatives (C60 with I, II, III) exhibit significantly better activity than C60-IV. Perhaps, the differences in phenolic O–H bond strength are the main reason responsible for this effect. C60-I and C60-II contain ortho-methoxyphenol moiety, C60-III contain o-,o-dimethylphenol moiety, whereas the phenol residues in C60-IV contain non-hindered hydroxyl groups. The Bond Dissociation Enthalpies (BDE) for O–H in the series:30 phenol, 2,6-dimethoxyphenol,‡ 2,6-dimethylphenol are: 88.3, 83.2, and 84.5 kcal mol−1, respectively, with the highest BDE for unsubstituted phenol, that will be the worse H-atom donating agent within this series. Therefore, among all four derivatives, the derivative C60-IV exhibits the weakest synergy of phenolic component (H-atom donor to a radical) and C60 moiety (being a scavenger able to form the adducts with non-peroxyl radicals).
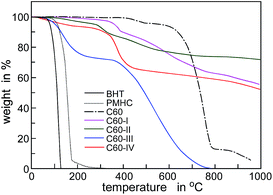
We also determined thermal stability (volatility) of the obtained conjugates and compared with volatility of low molecular phenolic antioxidants BHT and 2,2,5,7,8-pentamethyl-6-hydroksychromanol (PMHC). On the basis of thermogravimetric curves presented in Fig. 4 it can be stated that the conjugates of C60 (with exception of C60-III) are not volatile and they are thermally stable below 300 °C, in contrast to simple phenols that completely evaporate at temperatures below 180 °C.
Combined TG and DSC result demonstrate that at temperatures corresponding to Te (180–240 °C) the additives C60-I, C60-II and C60-IV are still present within the polymer and they are responsible for increase of the oxidative stability of the system. The exception is C60-III, that is less stable. However, the antioxidant effect of C60-III is also significant and we suppose this compound decomposes to the products able to enhance the oxidative stability of the whole system.
Earlier experimental results indicated that C60 does not exhibit antioxidant properties in such model systems as unsaturated hydrocarbons in air-saturated solution at moderate temperatures (30 °C in chlorobenzene, oxidation initiated with azobisisobutyronitrile)31 suggesting rather weak reactivity of fullerene toward peroxyl radicals: rate constant of reaction of C60 with cumylperoxyl radicals, (3.1 ± 1.1) × 102 M−1 s−1,31 is extremely slow comparing to rate constant (2.0 ± 0.8) × 108 M−1 s−1 for reaction with cumyl radicals.20,32 Another explanation of different behavior of fullerene in saturated and unsaturated hydrocarbons was discussed in our previous work as an effect of different ratio of rate constant of two competitive processes: chain-breaking (kinh) and chain propagation (kp). Depending on the kp for propagation of lipid or hydrocarbon, the same molecule can behave as a good antioxidant in hydrocarbon (kinh/kp > 1000) but with no antioxidant activity (no induction period will be observed) in unsaturated hydrocarbon (kinh/kp ≪ 1000).22
Additional explanation of good antioxidant behaviour of the studied conjugates of C60 with phenols is that we monitored a real process of oxidation carried out at temperature above 150 °C where some other radical species can effectively mediate the kinetic chain process of propagation step during hydrocarbon autoxidation. We assign this inhibitory effect as a result of low volatility of C60 conjugates, ability of phenolic moieties to react with peroxyl radicals and ability of fullerene spheres to react with other radicals like alkoxyl, alkyl, hydroxyl radicals as well as singlet oxygen formed during high temperature decomposition of peroxides (including non-Russel type reaction of tetraoxides).15,16
Conclusions
In order to find a new kind of hybrid antioxidants able to protect the polymers against oxidation in broad range of temperatures we tested four derivatives of fullerene C60 with covalently bonded simple phenols during non-isothermal oxidation of polyethylene at temperatures 150–250 °C. The fullerene derivatives exhibit better antioxidant activity than C60 or phenols used alone, thus, increased efficacy of C60-phenol conjugates is a result of a synergy between phenolic residues (reacting with peroxyl radicals by H-atom transfer) and C60 moiety (a “radical sponge” responsible for scavenging non-peroxyl radicals at elevated temperatures). Our findings implicate that hybrid materials made of fullerene and phenols can be potentially applied as agents increasing oxidative stability of polymers and other hydrocarbon materials (e.g., lubricants) exploited under severe thermal and oxidative conditions.Conflicts of interest
The authors declare that there are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the National Science Center of Poland (NCN grant No. 2014/15/B/ST4/04835).Notes and references
- E. T. Denisov and I. V. Khudyakov, Chem. Rev., 1987, 87, 1313–1357 CrossRef CAS.
- K. U. Ingold and D. A. Pratt, Chem. Rev., 2014, 114, 9022–9046 CrossRef CAS PubMed.
- M. Lucarini and G. F. Pedulli, Chem. Soc. Rev., 2010, 39, 2106–2119 RSC.
- G. Litwinienko and K. U. Ingold, J. Org. Chem., 2003, 68, 3433–3438 CrossRef CAS PubMed.
- G. Litwinienko and K. U. Ingold, Acc. Chem. Res., 2007, 40, 222–230 CrossRef CAS PubMed.
- M. Musialik and G. Litwinienko, Org. Lett., 2005, 7, 4951–4954 CrossRef CAS PubMed.
- V. W. Bowry, K. U. Ingold and R. Stocker, Biochem. J., 1992, 288, 341–344 CrossRef CAS PubMed.
- S. Q. A. Rizvi, A Comprehensive Review of Lubricant Chemistry, Technology, Selection, and Design, ASTM International, 2009 Search PubMed.
- L. R. Rudnick, Lubricant Additives: Chemistry and Applications, CRC Press, 2nd edn, 2009 Search PubMed.
- Y. Bolbukh, P. Kuzema, V. Tertykh and I. Laguta, J. Therm. Anal. Calorim., 2008, 94, 727–736 CrossRef CAS.
- R. Gensler, C. J. G. Plummer, H. H. Kausch, E. Kramer, J. R. Pauquet and H. Zweifel, Polym. Degrad. Stab., 2000, 67, 195–208 CrossRef CAS.
- K. Bhunia, S. S. Sablani, J. Tang and B. Rasco, Compr. Rev. Food Sci. Food Saf., 2013, 12, 523–545 CrossRef CAS.
- Y. Gao, Y. Gu and Y. Wei, J. Agric. Food Chem., 2011, 59, 12982–12989 CrossRef CAS PubMed.
- G. Beldì, S. Pastorelli, F. Franchini and C. Simoneau, Food Addit. Contam., Part A, 2012, 29, 836–845 CrossRef PubMed.
- R. Lee and M. L. Coote, Phys. Chem. Chem. Phys., 2016, 18, 24663–24671 RSC.
- R. Lee, G. Gryn'ova, K. U. Ingold and M. L. Coote, Phys. Chem. Chem. Phys., 2016, 18, 23673–23679 RSC.
- W. Vogel, Appl. Phys. A, 1996, 62, 295–301 Search PubMed.
- P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Science, 1991, 254, 1183–1185 CAS.
- J. R. Morton, F. Negri and K. F. Preston, Acc. Chem. Res., 1998, 31, 63–69 CrossRef CAS.
- E. B. Zeinalov and G. Koßmehl, Polym. Degrad. Stab., 2001, 71, 197–202 CrossRef CAS.
- R. Czochara, P. Ziaja, P. Piotrowski, R. Pokrop and G. Litwinienko, Carbon, 2012, 50, 3943–3946 CrossRef CAS.
- R. Czochara, J. Kusio, M. Symonowicz and G. Litwinienko, Ind. Eng. Chem. Res., 2016, 55, 9887–9894 CrossRef CAS.
- G. Litwinienko and T. Kasprzycka-Guttman, J. Therm. Anal. Calorim., 1998, 54, 203–210 CrossRef CAS.
- P. Ziaja, K. Jodko-Piorecka, R. Kuzmicz and G. Litwinienko, Polym. Chem., 2012, 3, 93–95 RSC.
- T. Ozawa, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- T. Ozawa, J. Therm. Anal., 1970, 2, 301–324 CrossRef CAS.
- L. A. Wall, S. Straus, J. H. Flynn, D. McIntyre and R. Simha, J. Phys. Chem., 1966, 70, 53–62 CrossRef CAS.
- G. Litwinienko, A. Daniluk and T. Kasprzycka-Guttman, Ind. Eng. Chem. Res., 2000, 39, 7–12 CrossRef CAS.
- M. Iring, F. Tüdős, Z. Fodor and T. Kelen, Polym. Degrad. Stab., 1980, 2, 143–153 CrossRef CAS.
- M. Lucarini, P. Pedrielli, G. F. Pedulli, S. Cabiddu and C. Fattuoni, J. Org. Chem., 1996, 61, 9259–9263 CrossRef CAS.
- R. F. Enes, A. C. Tomé, J. A. S. Cavaleiro, R. Amorati, M. G. Fumo, G. F. Pedulli and L. Valgimigli, Chem.–Eur. J., 2006, 12, 4646–4653 CrossRef CAS PubMed.
- E. B. Zeynalov, N. S. Allen and N. I. Salmanova, Polym. Degrad. Stab., 2009, 94, 1183–1189 CrossRef CAS.
Footnotes |
† Electronic supplementary information (ESI) available: Recorded DSC curves, plots of eqn (1) (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β versus 1000/Te), tables with statistical and kinetic parameters, and experimental procedures. See DOI: 10.1039/c7ra08764k β versus 1000/Te), tables with statistical and kinetic parameters, and experimental procedures. See DOI: 10.1039/c7ra08764k |
| ‡ Those experimental BDE values were taken from one series of EPR equilibration studies from ref. 30 For mono-ortho-MeO-phenol BDE(O–H) is 86 or 84 kcal mol−1, available in Luo: Comprehensive handbook of bond dissociation energies, CRC Press, 2007. |
| This journal is © The Royal Society of Chemistry 2017 |