Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A MOF-derived ZIF-8@Zn1−xNixO photocatalyst with enhanced photocatalytic activity
Yanqiu Jing†
a,
Jianan Wang†a,
Baohua Yu†b,
Jin Luna,
Yuyuan Chengc,
Bin Xiongd,
Qiang Leie,
Yongfeng Yangf,
Liangyuan Cheng and
Mingqin Zhao *a
*a
aCollege of Tobacco Science, Henan Agricultural University, Zhengzhou, Henan province, China. E-mail: MingqinZhao17@163.com
bEconomics and Management College, National Tobacco Cultivation and Physiology and Biochemistry Research Centre, Henan Agricultural University, Zhengzhou, Henan province, China
cNanyang Branch of Henan Tobacco Corporation, Nanyang, Henan province, China
dTechnology Center of China Tobacco Hubei Industrial Co. Ltd., Wuhan, Hubei province, China
eScience and Technology Department of Sichuan of China National Tobacco Corporation, Chengdu, Sichuan province, China
fTechnology Center of China Tobacco Henan Industrial Co. Ltd., Zhengzhou, Henan province, China
gKey Laboratory of Tobacco Processing Morphology Research in Tobacco Industry, Zhengzhou, Henan province, China
First published on 30th August 2017
Abstract
Today, metal doped ZnO exhibits good performances and attracts worldwide attention. In this paper, a ZIF-8@Zn1−xNixO photocatalyst was successfully prepared using MOF as precursor and AgNO3 as catalyst. We explored the influence of Ni content on the photocatalytic activity of ZIF-8@Zn1−xNixO. The structure and properties of ZIF-8@Zn1−xNixO were characterized by XRD, SEM, FT-IR, BET, UV-vis diffuse reflectance spectroscopy and so on. The results show that Ni is well loaded in ZIF-8 and part of ZIF-8 is oxidized to form Zn1−xNixO composites. The photocatalytic performance of ZIF-8@Zn1−xNixO was evaluated by degradation of rhodamine B (RhB) solution under UV light. It was found that ZIF-8@Zn0.95Ni0.05O exhibited the highest photocatalytic degradation efficiency and can degrade 99.19% rhodamine B solution after 20 min under UV light. Furthermore, ZIF-8@Zn0.95Ni0.05O exhibits high stability. After five repeated cycles, its photocatalytic activity remains at 97.43%.
1. Instruction
Today, organic dyes, such as methylene blue, methyl orange, methyl red, rhodamine B, etc., are widely used in the textile, cosmetic and pharmaceutical industries.1–3 They play a vital role in our daily life, but they constitute some of the important types of pollutants and can cause permanent injury to humans and animals upon inhalation and ingestion.4,5 Until now, there have been many ways to solve this problem, such as micro-biological degradation methods, adsorption methods, photocatalytic degradation and so on.6–8 Among these, the photocatalytic degradation method effective in the remediation of toxic organic pollutants owing to the high mineralization efficiency of semiconductor photocatalysts.9–13 Until now, lots of semiconductor photocatalysts have been found, such a TiO2, WO3, SnO2, ZnO.14–17ZnO has excellent physicochemical properties, such as for its high chemical stability, non-toxic properties, and excellent photocatalytic activity. It has been widely studied as heterogeneous photocatalyst to degrade organic dyes. With the development of science and technology, it is important to improve its photocatalytic activity. As we know, the photocatalytic activity of ZnO is widely influenced by its band gap and electron–hole pair recombination. So many effects have been made to narrow its band gap and slow its electron–hole pair recombination, such as doping with metals ions,18 non-metal ions,19 anchoring with porphyrins and coupling with other semiconductors.20,21 Furthermore, many researchers have made effects to prepare ZnO composite materials, which possess high specific surface area and have excellent photocatalytic activity.22
Zeolitic imidazolate framework-8 (ZIF-8), as a kind of MOFs, has high specific surface area, excellent thermal and chemical stability.23 It is constructed by Zn(II) and 2-methylimidazole ligands and widely used in adsorption, catalytic process and gas separation, etc.24 Bux et al. used ZIF-8 membrane to separate ethane from ethane. ZIF-8 membrane exhibits excellent separating property.25 Zhu et al. used ZIF-8 as catalyst to synthesize styrene carbonate from carbon dioxide and styrene oxide. ZIF-8 crystals displayed catalytic activity even at temperatures as low as 50 °C, with styrene carbonate yields as high as ∼54% at 100 °C.26 Nevertheless, the photocatalytic performance of ZIF-8 is not well applicated.
Ni2+, as a kind of transition metal ions, has been widely used as doping element to prepare Ni doped ZnO, which effectively eliminate the electron hole recombination during photocatalysis.27,28 In this paper, we firstly used ZIF-8 as precursor and AgNO3 as catalyst to prepare ZIF-8@Zn1−xNixO photocatalyst and explored the influence of Ni content for its photocatalytic efficiency when it used as photocatalyst for degradation of rhodamine B solution. Finally, we systematically analyzed the synergistic effect between ZIF-8 and Zn1−xNixO.
2. Experimental
2.1 Chemicals
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), nickel nitrate hexahydrate (Ni(NO3)2·6H2O), 2-methylimidazole (2MI), rhodamine B (RhB), silver nitrate (AgNO3), methanol and ethanol were all analytical reagents and obtained from Sinopharm Chemical Reagent Co. Ltd. All chemicals were directly used without any further purification. Distilled water was used throughout the experiments.2.2 Preparation of ZIF-8@Zn1−xNixO photocatalyst
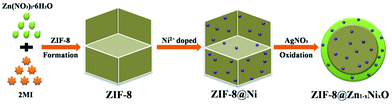
The preparation of ZIF-8@Zn1−xNixO includes two procedures. First, we adopted the modified method to synthesize ZIF-8.29 40 mmol 2-methylimidazole (2MI) was absolutely dissolved in 100 ml methanol and then slowly poured into another 100 ml methanol containing 10 mmol Zn(NO3)2·6H2O. The solution was stirred for 5 min and then kept standing at room temperature. After 24 h crystallizing, it was centrifuged and dried at 70 °C to obtain ZIF-8. Second, the synthesized ZIF-8 was used to prepare a series of ZIF-8@Zn1−xNixO photocatalysts containing different of Ni content (x = 0, 0.03, 0.05, 0.1). Taking ZIF-8@Zn0.95Ni0.05O for example, 0.95 g ZIF-8 was immersed in the 20 ml ethanol containing 0.05 g Ni(NO3)2·6H2O and kept stirring for 60 min. Then, it was directly dried at 70 °C to get the Ni doped ZIF-8 (labelled as ZIF-8@Ni0.05). Finally, 1 g ZIF-8@Ni0.05 was immersed in 40 ml ethanol solution (38 ml ethanol and 2 ml H2O) containing 38 mM silver nitrate. After 60 min stirring, it was centrifuged and washed by ethanol for three times to remove the redundant Ag+ absorbing on the surface and pores of ZIF-8. Subsequently, the sample was dried at 50 °C to obtain the ZIF-8@Zn0.95Ni0.05O photocatalyst. Meanwhile, we used the same procedures to obtain the ZIF-8@ZnO, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.9Ni0.1O photocatalysts. The synthetic procedure of ZIF-8@Zn1−xNixO was shown in Fig. 1.2.3 Characterization of the products
X-ray powder diffraction (XRD) was carried out to detect the phase of the samples using Bruker-AxsD8 diffractometer with Cu-Kα radiation (operated at 40 kV and 40 mA) in the angular range (2θ) from 5 to 80°. Scanning electron microscopy (SEM) was operated on a Hitachi SU70 microscope to observe the morphology of the samples. The N2 adsorption–desorption data were obtained from Micromeritics ASAP2020 at −196 °C after degassing the samples at 100 °C for 3 h. The specific surface area of all samples was calculated form the N2 adsorption–desorption data using Brunauer–Emmett–Teller (BET) equation. UV-vis absorption spectra were determined using a UV-1901 UV-visible spectrophotometer.2.4 Catalytic activity testing
The photocatalytic activity of all photocatalysts was evaluated by degradation of rhodamine B (RhB) solution under ultraviolet irradiation. The reaction was carried out in a closed box, which contained a 200 ml beaker and a Philips lamp irradiating UV light with the power of 300 W. The distance between the beaker and Philips lamp is 10 cm. In each experiment, 100 ml rhodamine B solution (20 mg L−1), containing 0.1 g ZIF-8@Zn1−xNixO, was put into the breaker and stirred continuously in the dark to ensure the adsorption–desorption equilibrium between rhodamine B and ZIF-8@Zn1−xNixO. Then, the solution was irradiated under UV light for 20 min. Meanwhile, seven milliliters of the solution was withdrawn from the beaker every five minutes to monitor the residual dye concentration in solution by recording the corresponding changes in the intensity of the absorbance peak at 550 nm as a function of reaction time.3. Result and discussion
The XRD patterns of all samples are shown in Fig. 2. From Fig. 2, it can be seen that all of the samples exhibit characteristic peaks at 7.38°, 10.42°, 12.77°, 14.75°, 16.50° and 18.08°, which are ascribable to (101), (002), (112), (200), (013) and (222) reflections of ZIF-8.30,31 When ZIF-8 is treated by AgNO3, it shows new peaks at about 31.77°, 34.42° and 36.25°, which are ascribable to (100), (002) and (101) reflections of ZnO.32 The doping of Ni do not change the diffraction peaks of ZIF-8@ZnO. It may be the reason that the content of Ni, doped in ZnO, is low. From the previous research, we can know that Ag+ can break coordinative bonds and combine with IM− to form HAg(IM)2 (HAg(IM)2 represents any silver species coordinated in a linear fashion by IM which also includes polymeric complexes). And Ag+ can also create a hydroxyl rich environment to transform Zn2+ into ZnO. The chemical reactions are proposed to occur as follow:33| Zn(IM)2 + 2Ag+ + 2NO3− + H2O ↔ Zn2+ + HAg(IM)2 + 2OH− | (1) |
| Zn2+ + 2OH− ↔ Zn(OH)2 ↔ ZnO + H2O | (2) |
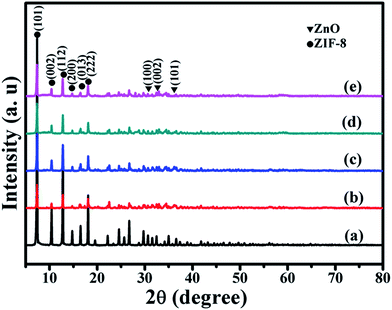 | ||
| Fig. 2 XRD patterns of (a) ZIF-8, (b) ZIF-8@ZnO, (c) ZIF-8@Zn0.97Ni0.03O, (d) ZIF-8@Zn0.95Ni0.05O, (e) ZIF-8@Zn0.9Ni0.1O. | ||
Fig. 3 shows the SME images of ZIF-8, ZIF-8@ZnO, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O and ZIF-8@Zn0.9Ni0.1O. Fig. 3a is the SEM image of ZIF-8. It can be seen that the synthesized ZIF-8 shows the uniform particle size. Fig. 3b is the high magnification image of ZIF-8. ZIF-8 has cubic morphology. And the particle size of ZIF-8 is about 300 nm. When ZIF-8 is treated by AgNO3, its edges are rounded (Fig. 3c). The morphology of ZIF-8@Zn1−xNixO, doped with different amount of Ni, shows almost the same.
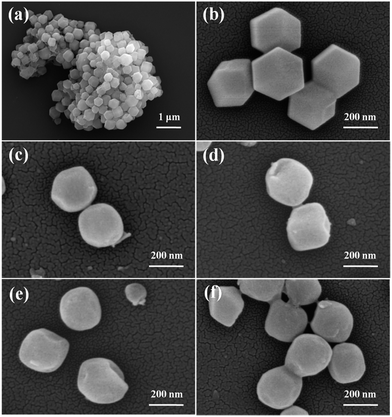 | ||
| Fig. 3 SEM images of (a and b) ZIF-8, (c) ZIF-8@ZnO, (d) ZIF-8@Zn0.97Ni0.03O, (e) ZIF-8@Zn0.95Ni0.05O, (f) ZIF-8@Zn0.9Ni0.1O. | ||
Fig. 4 is the EDS maps of ZIF-8@Zn0.95Ni0.05O. It can be seen that ZIF-8@Zn0.95Ni0.05O exist Zn, O, C, N, Ni elements. The Ni element is well dispersed in ZIF-8@Zn0.95Ni0.05O nanoparticles. Fig. 4f is the EDS map of Ag in ZIF-8@Zn0.95Ni0.05O. Ag element is not very clear. It indicates that the content of Ag in ZIF-8@Zn0.95Ni0.05O is low, which is in agreement with the XRD patterns (Fig. 2) and Wee et al.'s research.33
The nitrogen sorption isotherms of ZIF-8@Zn1−xNixO are shown in Fig. 5. From Fig. 5, it can be seen that ZIF-8@ZnO, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O and ZIF-8@Zn0.9Ni0.1O exhibit a type I isotherm pattern and show a significant high uptake in P/P0 < 0.1 region, which is characteristic of microporous materials.34 When ZIF-8 is oxidized into ZIF-8@ZnO by AgNO3, its microporous volume and BET are 0.32 cm3 g−1 and 803 cm2 g−1, respectively. When ZIF-8@ZnO is doped with Ni from 3% to 10%, its microporous volume decreases from 0.29 cm3 g−1 to 0.17 cm3 g−1. And its BET date decreases from 682 cm2 g−1 to 398 cm2 g−1.
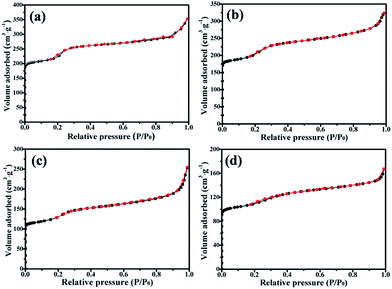 | ||
| Fig. 5 Nitrogen sorption isotherms of (a) ZIF-8@ZnO, (b) ZIF-8@Zn0.97Ni0.03O, (c) ZIF-8@Zn0.95Ni0.05O, (d) ZIF-8@Zn0.9Ni0.1O. | ||
UV-visible absorption spectra of ZIF-8, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O andZIF-8@Zn0.9Ni0.1O are shown in Fig. 6. ZIF-8 shows a strong absorption at about 260 nm. When ZIF-8 is treated by AgNO3, it shows two strong absorptions at 260 nm and 390 nm, which are ascribable to the absorption spectra of ZIF-8 and ZnO, respectively. It indicates that part of ZIF-8 is transformed into ZnO. When ZIF-8@ZnO is doped with 3% Ni, it shows two strong absorptions at 260 nm and 397 nm. The strong absorption of ZnO is shifted to 397 nm. With the increase of Ni content (from 3% to 10%), the absorption spectra of ZIF-8@ZnO increases too (from 390 nm to 425 nm). It indicates that the doping of Ni can decrease the band gap of ZnO.35
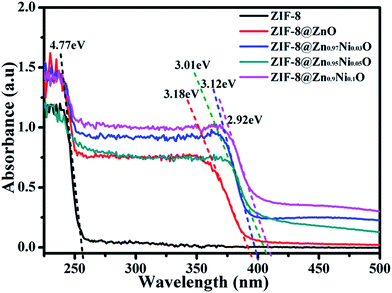 | ||
| Fig. 6 UV-vis spectra of ZIF-8, ZIF-8@ZnO, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O, ZIF-8@Zn0.9Ni0.1O. | ||
Rhodamine B was chosen as a model organic dye to evaluate the photocatalytic activity of all photocatalysts. Firstly, every experiment was operated in the dark for 30 min to eliminate the influence of the adsorption of the photocatalyst. The results of RhB adsorption by photocatalysts are shown in Fig. 7a. From Fig. 7a, it can be seen that the after stirring 30 min in the dark, 37.82% RhB is absorbed by ZIF-8. And ZIF-8@ZnO can absorb 32.95% RhB. When ZIF-8 is doped with Ni and oxidized to form ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O, ZIF-8@Zn0.9Ni0.1O, its adsorptive property is 30.24%, 28.33%, 24.65%, respectively. The decrease of the adsorptive property of the photocatalysts is attributed to the decrease of its specific surface area. Then, they were exposed under the UV light. The results of RhB degradation by photocatalysts are shown in Fig. 7a. From Fig. 7a, it can be seen that RhB exists self-degradation and can be self-degraded 21.32% after 20 min of UV light irradiation. Meanwhile, the solution, mixture with ZIF-8, just degraded 15.44% of RhB, which is attributed the self-degradation of RhB under UV light. ZIF-8 cannot degrade RhB under UV light. When ZIF-8 is oxidized to form ZIF-8@ZnO, it can degrade 91.25% RhB. When ZIF-8@ZnO is doped with 3%, 5%, 10% Ni, its photocatalytic efficiency is 95.36%, 99.19%, 89.08%, respectively. ZIF-8@Zn0.95Ni0.05O shows the highest photocatalytic activity. Fig. 7b is the kinetics of photocatalysts for absorption of RhB. The photocatalysis degradation kinetic reaction can be described by ln(C0/C) = kt (k is a pseudo-first-rate kinetic constant and t is the irradiation time). The calculated k values of ZIF-8, ZIF-8@ZnO, ZIF-8@Zn0.97Ni0.03O, ZIF-8@Zn0.95Ni0.05O and ZIF-8@Zn0.9Ni0.1O are 0.0145, 0.1085, 0.1375, 0.175 and 0.095 min−1, respectively. ZIF-8@Zn0.95Ni0.05O shows the highest kinetic constant.
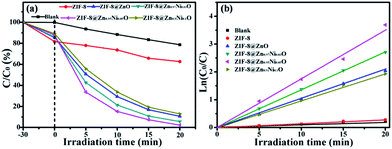 | ||
| Fig. 7 Photocatalytic degradation (a) and kinetics (b) of photocatalysts for absorption of RhB in the dark and degradation under UV light irradiation. | ||
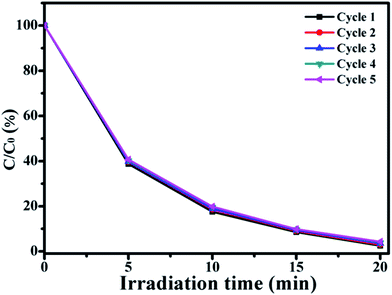
In order to evaluate the separation and reusability of ZIF-8@Zn1−xNixO, ZIF-8@Zn0.95Ni0.05O was chosen to degrade RhB solution in five repeated photocatalytic degradation cycles. The results are shown in Fig. 8. From Fig. 8, it can be seen that after five repeated cycles, the photocatalytic activity of ZIF-8@Zn0.95Ni0.05O is 97.43%. ZIF-8@Zn0.95Ni0.05O has high separation and reusability. A small decrease in the photocatalytic activity of ZIF-8@Zn0.95Ni0.05O in the five cycles is possibly due to the inevitable loss of the catalyst during the washing process.36
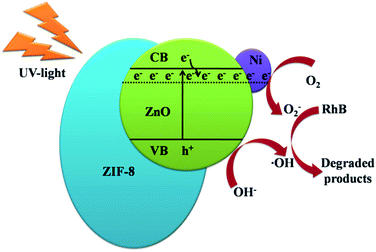
A possible mechanism has been proposed and shown in Fig. 9 to explain the synergistic effects of Ni and ZIF-8 on the photocatalytic activity of ZnO. ZIF-8 has high specific surface area, which can absorb RhB molecules on its surface and pores to form a layer of RhB with high concentration. And the doping of Ni can generate more defects in ZnO. These defects can make the effective separation of electrons and holes, which is irradiated by UV light.37 The doping of Ni and the incorporation of ZIF-8 make ZnO having high photocatalytic activity.
4. Conclusions
This paper reports a sample way to synthesize ZIF-8@ZnO and explores the influence of Ni on the structure and properties of ZIF-8@ZnO. The results show that the out surface of ZIF-8 can be transformed into ZnO by using AgNO3 as catalyst. And Ni element has great influence on the photocatalyst activity of ZIF-8@ZnO. When ZIF-8@ZnO is doped with 5% Ni, it shows the highest photocatalyst activity and can degrade 99.19% RhB in 20 min. And ZIF-8@Zn0.95Ni0.05O exhibits high photostability. After five repeated cycles, the photocatalytic activity of ZIF-8@Zn0.95Ni0.05O just reduces by 1.76%. ZIF-8@Zn0.95Ni0.05O has high separation and reusability. The excellent properties of ZIF-8@Zn0.95Ni0.05O make it potential to be used in the treatment of dye pollutants.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are very grateful for the technical guidance from Li Wen professor.Notes and references
- N. O. San, A. Celebioglu, Y. Tümtaş, T. Uyar and T. Tekinay, RSC Adv., 2014, 4, 32249–32255 RSC.
- T. Kou, C. H. Jin, C. Zhang, J. Z. Sun and Z. H. Zhang, RSC Adv., 2012, 2, 12636–12643 RSC; H. P. Zhao, Y. F. Zhang, G. F. Li, F. Tian, H. Tang and R. Chen, RSC Adv., 2016, 6, 7772–7779 RSC.
- A. Pal and B. Bag, RSC Adv., 2014, 4, 10118–11022 RSC.
- S. H. Chen, J. Zhang, C. L. Zhang, Q. Y. Yue, Y. Li and C. Li, Desalination, 2010, 252, 149–156 CrossRef CAS.
- X. F. Xu, M. Wang, Y. Y. Pei, C. C. Ai and L. J. Yuan, RSC Adv., 2014, 4, 64747–64755 RSC.
- P. Q. Long, Y. H. Zhang, X. X. Chen and Z. G. Yi, J. Mater. Chem. A, 2015, 3, 4163–4169 CAS.
- M. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114(20), 10575–10612 CrossRef PubMed.
- E. Kowalska, M. Janczarek, L. Rosa, S. Juodkazis and B. Ohtani, Catal. Today, 2014, 230, 131–137 CrossRef CAS.
- C. L. Yu, Z. Wu, R. Y. Liu, D. D. Dionysiou, K. Yang, C. Y. Wang and H. Liu, Appl. Catal., B, 2017, 209, 1–11 CrossRef CAS.
- C. L. Yu, W. Q. Zhou, L. H. Zhu, G. Li, K. Yang and R. C. Jin, Appl. Catal., B, 2016, 184, 1–11 CrossRef CAS.
- X. C. Meng, Z. Z. Li, H. M. Zeng, J. Chen and Z. S. Zhang, Appl. Catal., B, 2017, 210, 160–172 CrossRef CAS.
- Ş. Ş. Türkyilmaz, N. Güy and M. Özacar, J. Photochem. Photobiol., A, 2017, 341, 39–50 CrossRef.
- C. L. Yu, L. F. Wei, W. Q. Zhou, D. D. Dionysiou, L. H. Zhu, Q. Shu and H. Liu, Chemosphere, 2016, 157, 250–261 CrossRef CAS PubMed.
- Y. C. Huang, H. B. Li, M. S. Balogun, H. Yang, Y. Tong, X. H. Lu and H. B. Ji, RSC Adv., 2015, 5, 7729–7733 RSC.
- H. Katsumata, Y. Tachi, T. Suzuki and S. Kaneco, RSC Adv., 2014, 4, 21405–21409 RSC.
- X. S. Zhu, H. Shi, J. W. Yin, H. M. Zhu, Y. M. Zhou, Y. Tang, P. Wu and T. H. Lu, RSC Adv., 2014, 4, 34417–34420 RSC.
- R. Saravanan, M. M. Khan, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, RSC Adv., 2015, 5, 34645–34651 RSC.
- H. J. Wang, F. Raziq, Y. Qu, C. L. Qin, J. S. Wang and L. Q. Jing, RSC Adv., 2015, 5, 85061–85064 RSC.
- P. Liang, C. Zhang, H. Q. Sun, S. M. Liu, M. Tadé and S. B. Wang, RSC Adv., 2016, 6, 95903–95909 RSC.
- Y. Z. Chen, M. B. Yue, Z. H. Huang, L. N. Wang and F. Y. Kang, RSC Adv., 2015, 5, 23174–23180 RSC.
- M. Y. Nassar, A. A. Ali and A. S. Amin, RSC Adv., 2017, 7, 30411–30421 RSC.
- Q. Q. Yin, W. J. Wu, R. Qiao, X. X. Ke, Y. Hu and Z. Q. Li, RSC Adv., 2016, 6, 38653–38661 RSC.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., 2009, 121, 7221–7223 CrossRef.
- D. F. Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons and T. Dürent, J. Am. Chem. Soc., 2011, 133, 8900–8902 CrossRef PubMed.
- H. Bux, C. Chmelik, R. Krishna and J. Caro, J. Membr. Sci., 2011, 369, 284–289 CrossRef CAS.
- M. Q. Zhu, D. Srinivas, S. Bhogeswararao, P. Ratnasamy and M. A. Carreon, Catal. Commun., 2013, 32, 36–40 CrossRef CAS.
- M. Xiao, Y. F. Lu, Y. G. Li, H. Song, L. P. Zhu and Z. Z. Ye, RSC Adv., 2014, 4, 34649–34653 RSC.
- J. N. Li, F. Zhao, L. Zhang, M. Y. Zhang, H. F. Jiang, S. Li and J. F. Li, RSC Adv., 2015, 5, 67610–67616 RSC.
- C. S. Wu, Z. H. Xiong, C. Li and J. M. Zhang, RSC Adv., 2015, 5, 82127–82137 RSC.
- L. Y. Wang, M. Q. Fang, J. Liu, J. He, L. H. Deng, J. D. Li and J. D. Lei, RSC Adv., 2015, 5, 50942–50954 RSC.
- J. T. Yoo, S. H. Lee, C. K. Lee, C. R. Kim, T. Fujigaya, H. J. Park, N. Nakashima and J. K. Shim, RSC Adv., 2014, 4, 49614–49619 RSC.
- X. C. Dong, Y. F. Cao, J. Wang, M. B. C. Park, L. H. Wang, W. Huang and P. Chen, RSC Adv., 2012, 2, 4364–4369 RSC.
- L. H. Wee, N. Janssens, S. P. Sree, C. Wiktor, E. Gobechiya, R. A. Fischer and C. E. A. Kirschhock, Nanoscale, 2014, 6, 2056–2060 RSC.
- Y. Q. Jing, Y. C. Wang, Y. Z. Gao, H. Q. Li, Y. Y. Cheng, P. Lu, Y. H. Zhang and C. Ma, RSC Adv., 2016, 6, 42495–42501 RSC.
- Z. Y. Zhang, C. L. Shao, X. H. Li, C. H. Wang, M. Y. Zhang and Y. C. Liu, ACS Appl. Mater. Interfaces, 2010, 2, 2915–2923 CAS.
- S. Senapati, S. K. Srivastava and S. B. Singh, Nanoscale, 2012, 4, 6604–6612 RSC.
- S. M. Mousavi, A. R. Mahjoubm and R. Abazari, J. Mol. Liq., 2017, 242, 512–519 CrossRef CAS.
Footnote |
| † Yanqiu Jing, Jianan Wang and Baohua Yu contributed equally to this work and they are all first author. |
| This journal is © The Royal Society of Chemistry 2017 |