Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Growth phase diagram and upconversion luminescence properties of NaLuF4:Yb3+/Tm3+/Gd3+ nanocrystals†
Yangyang Lia,
Yanhui Donga,
Tuerxun·Aidilibikeab,
Xiaohui Liua,
Junjie Guoa and
Weiping Qin *a
*a
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, Jilin 130012, China. E-mail: wpqin@jlu.edu.cn
bYili Normal University, Electronic and Information Engineering, Yining, Xinjiang 835000, China
First published on 15th September 2017
Abstract
Sub-25 nm β-NaLuF4 nanocrystals (NCs) with high homogeneity, monodispersity, and good crystallinity were synthesized through thermal decomposition at different temperatures (285, 295, 305, and 315 °C), using an automatic nanomaterial synthesizer. The phase transition of NaLuF4 NCs from α-phase to β-phase suggests that the entire growth process consists of four stages: a cubic phase, the coexistence of cubic and hexagonal phases, a homogeneous hexagonal phase, and an inhomogeneous hexagonal phase, caused by Ostwald ripening. Based on this observation, we obtained the growth phase diagram of NaLuF4:Yb3+/Tm3+/Gd3+ NCs, relating to the reaction time, temperature, crystal size, and crystal phase. From spectral analysis, the products prepared in the third stage were shown to exhibit the characteristics of having small size (sub-25 nm), uniformity, and high upconversion luminescence (UCL) intensity. On the other hand, the luminescence properties of NaLuF4:Yb3+,Tm3+ were studied via incorporating different concentrations of Gd3+ ions. These results indicated that 10% Gd3+ was the best doping concentration for obtaining NaLuF4 NCs with relatively small size and high brightness. Further increasing the Gd3+ concentration induced a drastic decrease in UCL intensity.
Introduction
Rare earth (RE) doped nanomaterials have received considerable interest due to their potential applications in optics, microelectronics, optoelectronics, and biomedical sciences, because of their unique optical properties.1–3 In recent years, scientists have paid much attention to typical RE doped NaREF4 materials due to their potential applications in solid-state lasers, bioimaging, photodynamic therapy, etc.4–11 As a commonly studied object, β-NaYF4 was acknowledged as the most efficient UC host material before 2011.12–14 However, Qin and co-workers reported that β-NaLuF4:18% Yb3+,0.5% Tm3+ exhibited more intense ultraviolet and blue UC luminescence than β-NaYF4:18% Yb3+,0.5% Tm3+ of the same size.15 As a typical RE doped NaREF4 material, β-NaLuF4 may be a more promising host for UC behavior than β-NaYF4, especially for high-order multi-photon UCL. Furthermore, it is generally accepted that β-phase NaLuF4 provides much stronger UCL compared with its α-phase counterpart.12,16 Meanwhile, the hexagonal form of NaLuF4 usually appears with larger nanoparticles than the cubic form.7,17,18 Appropriate size and good luminescence properties are the fundamental requirements for the application of UC nanomaterials to bioimaging, biotherapy, bioassaying, etc. Hence it is of great significance to prepare small size and high brightness β-NaLuF4 for biological applications. However, as we know, there is still no established approach for discussing the growth process of NaLuF4 NCs. Therefore, a clear growth phase diagram of NaLuF4 NCs will be helpful for obtaining the desired NaLuF4 nanomaterials.In general, nanocrystal size can be controlled by adjusting the raw material ratio, reaction time, and reaction temperature. During the past decade, impurity doping technologies have become a research hotspot because of their significant role in modifying morphology and decreasing size in many nanomaterials, which has been demonstrated by some groups.17,19–25 For instance, Li et al. reported that the doping of 24 mol% Gd3+ ions could control the size of lanthanide-doped NaLuF4 nanocrystals to sub-10 nm.20 Doping Gd3+ ions in an NaLuF4 host lattice can reduce the size of the nanocrystals, but meanwhile it will also reduce their luminescence performance. However, this weakness has been rarely mentioned in relevant reports. Therefore, research consideration must be given to the balance between crystal size and luminescence performance in order to obtain high-quality NaLuF4 nanomaterials. At present, the thermal decomposition method has been widely used in the preparation of NaREF4 nanomaterials.26–30 The advantages of this method are the shorter experimental time and that it is easier to get small size products compared with the traditional hydrothermal method. Thermolysis usually requires a higher temperature, resulting in crystal growth rate that is faster and more sensitive to external factors than when using hydrothermal methods. For this reason, it is hard to ensure the uniqueness of experimental variables and the repeatability of products during parallel synthesis. However, these problems can be easily resolved for us through the use of an independently-developed automatic nanomaterial synthesizer (ANS02, Changchun Micro Era Technology Co. LTD.). With the help of this instrument, all experimental parameters, such as the heating rate, stirring speed, air flow rate, and dropping rate of the sodium source and fluoride source, can be exactly set in a computer program. Therefore, it provides technical support for precise research into the growth and phase transition processes for nanocrystals.
Herein, we studied the nucleation, phase transition, Ostwald ripening, and luminescence properties of NaLuF4 nanocrystals through precisely changing experimental parameters. According to the effects of different reaction times and temperatures on the growth processes of NaLuF4 crystals, we obtained a time–temperature–phase–size (T–T–P–S) phase diagram. Combined with spectral analysis, these results indicate that material with small size, uniform shape, monodispersity, and high UCL intensity can be obtained in a specific region of the diagram at any temperature from 285 to 315 °C. In addition, we investigated the influence of Gd3+ ions on the sizes of the crystals and the luminescence properties of NaLuF4:Yb3+,Tm3+,Gd3+ nanomaterials. It was found that a high concentration of Gd3+ could drastically reduce the luminescence performance of NaLuF4, and the doping of 10 mol% Gd3+ is suitable for the preparation of this nanomaterial with relatively small size and strong upconversion emission.
Results and discussion
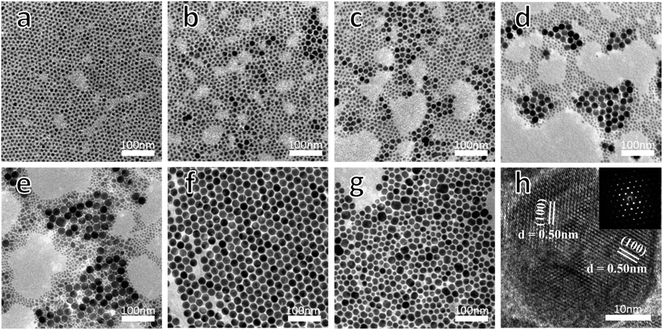
Using the automatic nanomaterial synthesizer mentioned above, NaLuF4:20% Yb3+,0.5% Tm3+,x% Gd3+ (x = 0, 3, 5, 10, 20, or 30) NCs were obtained at 305 °C via a thermal decomposition method. The morphologies and sizes of the nanocrystals were characterized via TEM, as shown in Fig. 1. In Fig. 1a–c, the nanocrystals with lower doped amounts of Gd3+ ions are relatively large and irregular, while the sample with a Gd3+ doping concentration of 10% shows a uniform and regular shape with an average diameter of around 23 nm and a narrow size distribution (Fig. 1d and g). Increasing the amount of Gd3+ ions to 30%, the crystal size becomes non-uniform again, with a larger distribution varying from 7–12 nm (Fig. 1f and g). Here, we found that an increase in the doping amount of Gd3+ ions has obvious modifying effects on the growth of the nanocrystals. Combined with the results from XRD studies (Fig. S1†), these effects mainly show in two aspects: the decrease in crystal size; and the crystal shape changing, following the order irregularity–regularity–irregularity.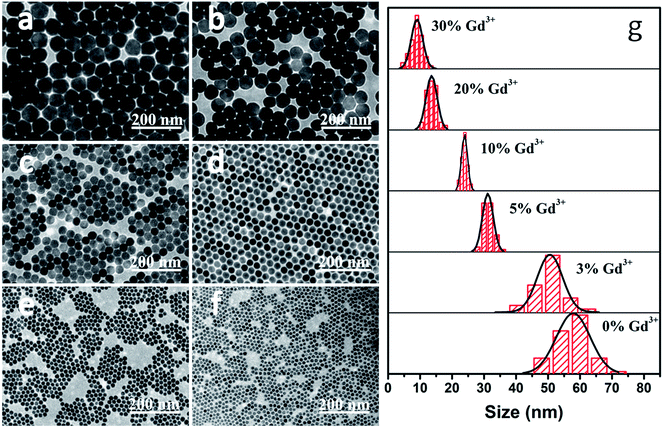 | ||
| Fig. 1 TEM images of NaLuF4:20% Yb3+,0.5% Tm3+,x% Gd3+ with (a) x = 0, (b) x = 3, (c) x = 5, (d) x = 10, (e) x = 20, and (f) x = 30. (g) Size distributions of the aforementioned NCs. | ||
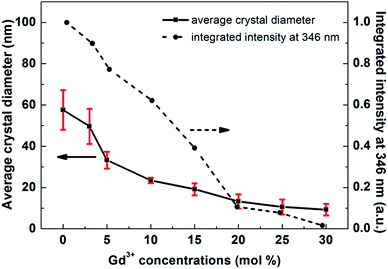
In order to study the influence of Gd3+ concentration on the crystal size and UCL properties of NaLuF4, relevant data are plotted in Fig. 2. The UCL intensity decreased obviously with an increase in Gd3+ concentration. This decrease can attributed to two factors: a decrease in crystal size and the reduced luminescence performance of the material from introducing Gd3+ into the NaLuF4 host lattice. Compared with the UCL intensity at 346 nm for a sample with no Gd3+, the luminescence intensities of samples with larger amounts of Gd3+ (3%, 5%, and 10%) decreased by 10.1%, 22.7% and 37.8%, respectively. Meanwhile, the average crystal size decreased by 61%, from 59 nm to 23 nm. There is a similar dependence of crystal size, as well as UCL, on Gd3+ concentration, indicating the dominant role of size on the UCL decrease. Upon increasing the Gd3+ concentration to 15, 20, 25 and 30%, the average crystal sizes are 19, 13, 10, and 9 nm, respectively. The corresponding UCL intensities of samples were calculated to be 39.2, 10.7, 7.9, and 1.6% of the value for NaLuF4:20% Yb3+,0.5% Tm3+ NCs. It can be seen that these UCL intensities decrease significantly upon a relatively small change in size and a large change in Gd3+ concentration. Contrary to the decrease in UCL intensity for samples with smaller amounts of Gd3+ ions, a degradation in material performance due to a relatively large amount of Gd3+ ions may be the main factor for this drastic decrease. To further verify that Gd3+ can reduce the luminescence performance of NaLuF4, we annealed the Gd3+ variable samples to eliminate the effects of size variation on luminescence performance. SEM images and UCL spectra from these annealed samples are shown in Fig. S2 and S3.† These samples exhibit similar irregular morphologies and sizes, but show obvious differences in spectral properties. The UCL intensities of the samples have a downward trend as the amount of Gd3+ increases, especially in the UV region, but this trend is not obvious for samples with low Gd3+ doping (≤10 mol%). In general, high-order multi-photon emission should be an important feature of a good upconversion material. Thus, too many Gd3+ ions entering Lu3+ sites will reduce the optical performance of NaLuF4 nanomaterials. To summarize, it is remarkable that 10 mol% Gd3+ is highly favorable for the preparation of high-quality hexagonal-phase NaLuF4 nanocrystals with relatively small size, uniform shape, and high UCL intensity. Therefore, we maintain this doping ion concentration for the following discussion.
Fig. 3 demonstrates the effects of reaction time on the crystal growth and nucleation processes, while other parameters were kept unchanged. Here, the reaction time was regarded as the only variable factor, because all the experimental steps can be precisely controlled by setting them into computer programs, including the stirring speed, air flow rate, and dropping rate of the sodium source and fluoride source. A typical TEM image (Fig. 3a) demonstrates that the NaLuF4 nanocrystals obtained at high temperature for 10 minutes have a pure cubic phase, which can also be seen from Fig. S2a.† The images in Fig. 3b–f and the XRD patterns in Fig. S2b–f† show the gradual phase transition of NaLuF4 from a cubic phase to a hexagonal phase. In these, α-phase and β-phase NCs are coexistent and the proportion of the latter increases gradually over the first four pictures (Fig. 3b–e), which is consistent as well with the XRD results. It is worth noting that the NaLuF4 NCs in Fig. 3f (23 min) show monodispersity and high uniformity. All these NCs display uniform hexagonal shape, with an average diameter of 23 nm, and a rather narrow size distribution, which indicates that a heat reaction time of about 23 minutes can result in the complete formation of pure β-phase NaLuF4 using the current technology. HRTEM (Fig. 3h) characterization also provides further evidence that interplanar spacing of 0.50 nm can be indexed as being from (100) lattice fringes of β-phase NaLuF4. TEM images of all samples prepared between 23 and 60 minutes (Fig. S5†) show samples similar in size and shape to that shown in Fig. 3f. This may be due to an ionic equilibrium state of free ions in the solvent, which leads to crystal growth at a relatively stable stage. Therefore, this time period is ideal for synthesizing β-NaLuF4 with small size and high uniformity. Upon further prolonging the reaction time to 65 minutes, some of the crystals grow larger at the expense of smaller ones, which is known as Ostwald ripening (Fig. 3g). Ripening typically leads to a broadening of the size distribution, however, nanocrystals with a large size distribution are not as we expected. Therefore, the reaction time should not exceed 65 minutes under the current experimental conditions. The effects of reaction time on the phase and size of NaLuF4 NCs are listed in Table 1.
| Reaction time/minutes | Phase | Size |
|---|---|---|
| 10 | Cubic | Very tiny |
| 15–21 | Cubic/hexagonal | Very tiny cubic phase and hexagonal phase, with diameters between 12 and 24 nm |
| 23–60 | Homogeneous hexagonal | ∼23 nm |
| 65 | Inhomogeneous hexagonal | 7–53 nm |
To reveal the phase change and growth processes of NaLuF4 NCs in detail, the same time-varying experiments were done at different temperatures of 285, 295 and 315 °C, and the relevant TEM images are shown in Fig. S6–S8,† respectively. The nucleation processes of NaLuF4 NCs at different reaction temperatures can be seen in these TEM images. In order to facilitate comparison and analysis, we plotted a T–T–P–S phase diagram (Fig. 4) that could indicate the relationship between size, phase, temperature and reaction time. The phase transition process for NaLuF4 NCs can be divided into four parts: a cubic phase, the coexistence of cubic and hexagonal phases, a homogeneous hexagonal phase, and an inhomogeneous hexagonal phase caused by Ostwald ripening. This phase diagram, on the whole, demonstrates that the higher the temperature, the faster the growth rate of the nanocrystals. In the first two stages, the crystal size increases monotonically, because of the phase transition from α-phase to β-phase. Notably, small and homogeneous β-NaLuF4 NCs (sub-25 nm) can be obtained after different time periods, corresponding to different temperatures from 285 to 315 °C. Upon increasing the reaction temperature, the length of the third stage gradually becomes shorter: from 110 min to 280 min at 285 °C, from 60 min to 160 min at 295 °C, from 23 min to 65 min at 305 °C, and from 12 min to 30 min at 315 °C. It is generally accepted that hexagonal-phase nanocrystals show more efficient UCL than their cubic-phase counterparts, thus, this result is meaningful for preparing high-quality β-NaLuF4 nanomaterials. Moreover, it is interesting to find that β-phase NCs prepared at different temperatures have similar dimensions of about 23 nm (Fig. S6e, S7e, 3f, and S8c†), which indicates that temperature mainly affects the growth rate, while the raw material ratio mainly affects the shape and size of NaLuF4 nanocrystals. Further increasing the reaction time would lead to the ripening of the nanocrystals, resulting in inhomogeneous nanocrystals with a rather large size distribution. Ripened nanoparticles are not suitable for biological applications. In contrast, for materials prepared in the third stage, nanocrystals with small and uniform size have been found to show greater potential for applications.31 Therefore, we have obtained synthetic details for small and homogeneous β-NaLuF4 NCs, which may be further generalized to synthesize other ideal RE fluoride nanomaterials.
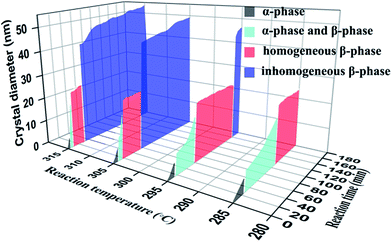 | ||
| Fig. 4 Growth phase diagram of NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+. Relationships between size, phase, temperature, and reaction time for the growth process of NaLuF4 nanocrystals are shown. | ||
In order to characterize the luminescence properties of samples at various stages, we collected upconversion emission spectra from the as-obtained NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+ NCs at 305 °C after different reaction times, as shown in Fig. 5. It can be seen that the overall luminescence intensities of the samples increase monotonically upon increasing the reaction time, under near infrared irradiation at 980 nm. A comparison of the intensities of emission at 346 nm shows that the UCL intensity of β-NaLuF4 (23 min) is increased about 17-fold when compared to that of pure α-NaLuF4 (10 min). This significant UCL enhancement in the β-NaLuF4 NCs comes from their more ordered hexagonal structure and larger crystal size. As can be seen in the spectra, there are six main emission peaks at 290, 346, 361, 451, 474, and 801 nm, which are assigned to the 1I6 → 3H6, 1I6 → 3F4, 1D2 → 3H6, 1D2 → 3F4, 1G4 → 3H6, and 3H4 → 3H6 transitions of Tm3+, respectively. In addition, the peak at 311 nm corresponds to the 6P7/2 → 8S7/2 transition of Gd3+, which is a five-photon UCL process sensitized by Tm3+.32 Under 980 nm laser excitation, the emission spectra of all the as-synthesized samples were measured and all showed intensive high-order multi-photon UCL in the ultraviolet and near ultraviolet regions. Taking NaLuF4 synthesized at 60 min as an example, the ratios of I290/I801, I311/I801, I346/I801, and I361/I801 (I represents the intensity of the emission peak) are 1, 1.2, 4.3, and 4.7, respectively. Apart from the traditional application to in vivo imaging, using the intense emission of Tm3+ at 801 nm,33,34 our nanomaterials exhibit other excellent characteristics and may have a wider range of applications. Among the upconverted UV luminescence signals, the intense emissions at 346 and 361 nm are useful for activating UV-sensitive materials. Therefore, our investigations focus on the relationship between the UCL intensity at 346 nm and crystal phase of NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+, as shown in Fig. 6.
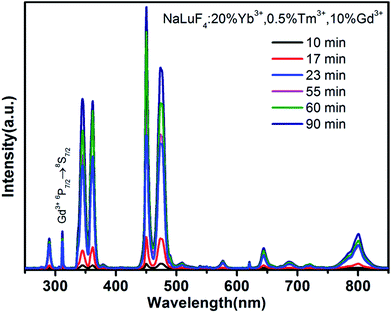 | ||
| Fig. 5 Upconversion emission spectra of NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+ synthesized at 305 °C after different reaction times under 980 nm near-infrared excitation. | ||
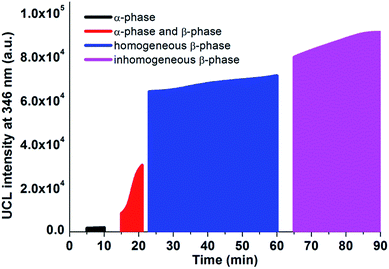 | ||
| Fig. 6 The UCL integrated intensity at 346 nm and the crystal phase distribution of NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+ synthesized at 305 °C, both with respect to reaction time. | ||
Hexagonal-phase nanocrystals usually exhibit higher luminescence efficiency than their cubic-phase counterparts. The samples prepared in the α-phase stage show poor luminescence properties. If we define the UCL intensity (346 nm) of a sample prepared after 90 min as 100%, the intensity of the α-NaLuF4 NCs is only about 2%. In the second stage, the UCL intensities of the samples increase rapidly due to the phase transition from the α-phase to β-phase. This phase transition leads to an increase in the UCL intensities of the time-varying samples from 9% to 33%. There is obvious spectral enhancement when the α-phase disappears completely and a pure and homogeneous β-phase is formed. The materials prepared in the period between 23 and 60 minutes have similar crystal sizes, and show a moderate increase in their luminescence intensity (from 70% to 78%) with an increase in reaction time. This is probably because the crystals obtained after a longer reaction time have higher crystallinity and a slightly larger size. Prolonging the reaction time over 65 minutes would lead to Ostwald ripening and cause a larger average size and broader size distribution. As indicated in Fig. 6, there is no significant enhancement in emission intensity after 65 minutes compared with the homogeneous β-NaLuF4 material. Consequently, high-quality NaLuF4 can be obtained in the third stage of the graph, and samples with a longer crystallization time have better luminescence properties.
Conclusions
In this work, a series of NaLuF4 nanomaterials were prepared using an automatic nanomaterial synthesizer to precisely control the experimental parameters. First we discussed the influence of varying the Gd3+ doping amount on the size and UCL properties of NaLuF4:Yb3+,Tm3+,Gd3+. It has been shown that 10 mol% doping of Gd3+ can be used to prepare NaLuF4 NCs with relatively small size (sub-25 nm) and strong UCL intensity. Although higher Gd3+ concentrations can produce smaller sized NaLuF4 NCs, their UCL intensities are greatly decreased. Based on this observation, and combined with a large number of experimental results, we obtained the T–T–P–S phase diagram for NaLuF4:20% Yb3+,0.5% Tm3+,10% Gd3+ over the range from 285 to 315 °C. This phase diagram can guide us in the synthesis of samples with definite properties, in order to obtain the desired NaLuF4 nanomaterials. For example, sub-25 nm β-NaLuF4 NCs with uniform shape, monodispersity, and high UCL intensity can be obtained in the homogeneous β-phase regions of the diagram. More importantly, the phase diagram may be further generalized to study the growth progress of other NaREF4 NCs.Experimental section
β-NaLuF4:20% Yb3+,0.5% Tm3+,x% Gd3+ nanocrystals were synthesized via a thermal decomposition method. The combined amount of RECl3·6H2O (RE = Lu, Yb, Tm, Gd) was set to 2 mmol in total, and the next step was to mix RECl3·6H2O with 12 ml of oleic acid and 30 ml of 1-octadecene and add this into a three-neck flask. The mixture was heated to 160 °C and kept at this temperature for 30 min under dry argon; it was then cooled down to room temperature. After that, 10 ml of methanol solution containing NaOH (5 mmol) and 20 ml of methanol solution containing NH4F (8 mmol) were slowly dropped into the flask with constant stirring for 30 min. Then the reaction temperature was raised to 108 °C and maintained at this temperature for 20 min under a vacuum environment to remove the methanol. Finally, the solution was heated rapidly to the heating temperature (285 °C, 295 °C, 305 °C, or 315 °C) and kept at this temperature for a different number of minutes for each kind of product. It is worth noting that all of the above steps were automatically completed using the automatic nanomaterial synthesizer.The samples were characterized via transmission electron microscopy (TEM), using an H-600 microscope at an acceleration voltage of 100 kV. High-resolution TEM (HRTEM) characterization was performed with a JEM-2200FS operated at 200 kV. The powder X-ray diffraction (XRD) patterns were recorded using a Model Rigaku Ru-200b diffractometer, using Cu Kα radiation (λ = 0.15406 nm) in the 2-theta range from 10° to 70°. Power adjustable and continuous wave (CW) laser diodes (980 nm, 3 W) and a Hitachi F-4500 fluorescence spectrophotometer (PMT voltage is 400 V) were employed for upconversion spectral analysis. The spectral scan range was from 250 to 850 nm, with a resolution of 1 nm.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (11274139).References
- J. Zhou, Z. Wu, Z. Zhang, W. Liu and H. Dang, Wear, 2001, 249, 333–337 CrossRef CAS.
- X. Wang and Y. Li, Angew. Chem., Int. Ed., 2003, 42, 3497–3500 CrossRef CAS PubMed.
- S. Sivakumar, F. C. M. van Veggel and M. Raudsepp, J. Am. Chem. Soc., 2005, 127, 12464–12465 CrossRef CAS PubMed.
- A. Cooke, D. Jones, J. Silva and M. Wells, J. Phys. C: Solid State Phys., 1975, 8, 4083 CrossRef CAS.
- B. Choi, B. Moon, H. Seo, J. Jeong, H. Lee and W. Seo, Mater. Des., 2000, 21, 567–570 CrossRef CAS.
- S. Ghosh, T. Rosenbaum, G. Aeppli and S. Coppersmith, Nature, 2003, 425, 48–51 CrossRef CAS PubMed.
- S. Heer, K. Kömpe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102–2105 CrossRef CAS.
- L. Liang, H. Xu, Q. Su, H. Konishi, Y. Jiang, M. Wu, Y. Wang and D. Xia, Inorg. Chem., 2004, 43, 1594–1596 CrossRef CAS PubMed.
- G. Yi, H. Lu, S. Zhao, Y. Ge, W. Yang, D. Chen and L.-H. Guo, Nano lett., 2004, 4, 2191–2196 CrossRef CAS.
- M. Karbowiak, A. Mech, A. Bednarkiewicz, W. Stręk and L. Kępiński, J. Phys. Chem. Solids, 2005, 66, 1008–1019 CrossRef CAS.
- J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119–2123 CrossRef CAS.
- K. W. Kramer, D. Biner, G. Frei, H. U. Gudel, M. P. Hehlen and S. R. Luthi, Chem. Mater., 2004, 16, 1244–1251 CrossRef.
- Y. Wei, F. Lu, X. Zhang and D. Chen, Chem. Mater., 2006, 18, 5733–5737 CrossRef CAS.
- Z. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606 CrossRef PubMed.
- F. Shi, J. Wang, X. Zhai, D. Zhao and W. Qin, CrystEngComm, 2011, 13, 3782–3787 RSC.
- G. Wang, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 14200–14201 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- C. Li, J. Yang, P. Yang, X. Zhang, H. Lian and J. Lin, Cryst. Growth Des., 2008, 8, 923–929 CAS.
- G. Xiang, J. Zhang, Z. Hao, X. Zhang, Y. Luo, S. Lü and H. Zhao, CrystEngComm, 2014, 16, 2499–2507 RSC.
- Q. Liu, Y. Sun, T. Yang, W. Feng, C. Li and F. Li, J. Am. Chem. Soc., 2011, 133, 17122–17125 CrossRef CAS PubMed.
- A. Xia, M. Chen, Y. Gao, D. Wu, W. Feng and F. Li, Biomaterials, 2012, 33, 5394–5405 CrossRef CAS PubMed.
- S. Zeng, J. Xiao, Q. Yang and J. Hao, J. Mater. Chem., 2012, 22, 9870–9874 RSC.
- D. Chen and Y. Wang, Nanoscale, 2013, 5, 4621–4637 RSC.
- Y. Chen, X. Yan, Q. Liu and X. Wang, J. Alloys Compd., 2013, 562, 99–105 CrossRef CAS.
- J. A. Damasco, G. Chen, W. Shao, H. Ågren, H. Huang, W. Song, J. F. Lovell and P. N. Prasad, ACS Appl. Mater. Interfaces, 2014, 6, 13884–13893 CAS.
- H.-X. Mai, Y.-W. Zhang, R. Si, Z.-G. Yan, L.-d. Sun, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2006, 128, 6426–6436 CrossRef CAS PubMed.
- G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324–2329 CrossRef CAS.
- C. Liu, H. Wang, X. Zhang and D. Chen, J. Mater. Chem., 2009, 19, 489–496 RSC.
- H. Lian, Y. Dai, D. Yang, Z. Cheng, C. Li, Z. Hou, M. Shang and J. Lin, Nanoscale, 2014, 6, 9703–9712 RSC.
- A. Naduviledathu Raj, T. Rinkel and M. Haase, Chem. Mater., 2014, 26, 5689–5694 CrossRef CAS.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- C. Cao, W. Qin, J. Zhang, Y. Wang, P. Zhu, G. Wei, G. Wang, R. Kim and L. Wang, Opt. Lett., 2008, 33, 857–859 CrossRef CAS PubMed.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano lett., 2008, 8, 3834–3838 CrossRef CAS PubMed.
- G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Agren and P. N. Prasad, ACS Nano, 2010, 4, 3163–3168 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional results of TEM, SEM, XRD and UC spectra. See DOI: 10.1039/c7ra07902h |
| This journal is © The Royal Society of Chemistry 2017 |