Open Access Article
Open Access ArticleElectronic and excitonic properties of two-dimensional and bulk InN crystals
Dan Lianga,
Ruge Quhe *b,
Yingjie Chenc,
Liyuan Wua,
Qian Wanga,
Pengfei Guan*d,
Shumin Wangef and
Pengfei Lu
*b,
Yingjie Chenc,
Liyuan Wua,
Qian Wanga,
Pengfei Guan*d,
Shumin Wangef and
Pengfei Lu *ae
*ae
aState Key Laboratory of Information Photonics and Optical Communications, Beijing University of Posts and Telecommunications, Beijing 100876, China. E-mail: photon.bupt@gmail.com
bSchool of Sciences, Beijing University of Posts and Telecommunications, Beijing 100876, China
cSchool of Information and Communication Engineering, Beijing University of Posts and Telecommunications, Beijing 100876, China
dBeijing Computational Science Research Center, Beijing 100193, China
eState Key Laboratory of Functional Materials for Informatics, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai 200050, China
fPhotonics Laboratory, Department of Microtechnology and Nanoscience, Chalmers University of Technology, 41296 Gothenburg, Sweden
First published on 1st September 2017
Abstract
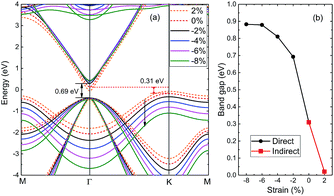
Motivated by potential extensive applications in nanoelectronics devices of III–V materials, we calculate the structural and optoelectronic properties of two-dimensional (2D) InN as well as its three-dimensional (3D) counterparts by using density functional theory (DFT). Compared with the 3D form, the In–N bonding in the 2D InN layer is stronger in terms of the shorter bond length, and the formation of the 2D one is higher in terms of the lower cohesive energy. The bandgap of monolayer InN is 0.31 eV at PBE level and 2.02 eV at GW0 level. By many-body GW0 and BSE within RPA calculations, monolayer InN presents an exciton binding energy of 0.12 eV. The fundamental bandgap increases along with layer reduction and is converted from direct (0.7–0.9 eV) in bulk InN to indirect (2.02 eV) in monolayer InN. Under biaxial compressive strain, the bandgap of 2D-InN can be further tuned from indirect to direct.
1. Introduction
Since the discovery of graphene,1,2 2D materials have attracted great interest due to their large surface area and unusual mechanical, electronic and optical properties.3–13 These novel properties make the 2D materials promising for applications in photocatalysis, nanoelectronic devices, energy storage, thermal conductors and even topological insulators. The III–V materials, in their bulk form developed over a rather long time, have also joined the 2D materials family. Boron-nitride (BN) sheets have the same planar honeycomb structure as graphene, and their ionic character causes an energy gap of 4.64 eV at the K point.2 Layered hexagonal AlN, which was experimentally realized by Tsipas et al.14 recently, displays transparent features in the visible light range. Monolayer honeycomb structured GaN, even if at high temperature, can remain stable, confirmed by high-temperature ab initio molecular-dynamics simulations.15 The experimental realization of 2D materials by chemical vapor deposition and molecular beam epitaxy, etc, requires suitable substrates. A. K. Singh et al.16 theoretically predicts that several transition-metal and rare-earth-metal substrates can be used to synthesize and stabilize 2D hexagonal III–V materials.Among III–V materials, bulk InN has a less than 1.0 eV direct bandgap which could be suitable for low-bandgap device applications such as future-generation solar cells because the nitride alloys can cover the whole solar spectrum range.17 Doping other III or V element into the host could achieve an extent potential application prospect in optoelectronic and high power/temperature electronic devices including light emitting diodes (LEDs), laser diodes (LDs), solar blind photodetectors and heterostructure field effect transistors.18–21 2D materials display different properties from their 3D counterparts, and the knowledge of the 3D form cannot be directly delivered to the 2D one. To the best of our knowledge, the studies on the 2D-InN are rare and most of them are focused on the geometry, growth dynamics, and intrinsic electronic properties of monolayers. The optical properties, and the modification of optoelectronic properties of 2D-InN, which are of great importance for potential nanoelectronic and optical applications, remain open.
In this study, the mechanical, stability, electronic and optical properties of bulk and 2D hexagonal planar InN based on density functional theory (DFT) are investigated to elucidate the difference between bulk and 2D structure, to determine the effect of strain on bandgap of 2D-InN as well as the bandgap variation of bilayer and multilayer structures (include van der Waals). Besides, by many-body calculations, exciton binding energy of 2D-InN is also obtained. The paper is organized as follows. In Section 2, we describe details of computational methods. The results and discussions are provided in Section 3. Finally, a brief summary is summarized in Section 4.
2. Methods and computational details
All the theoretical calculations are carried out by using DFT22 of the projector augmented wave method (PAW)23,24 as implemented in the Vienna ab initio simulation package (VASP).25 The exchange-correlation potential is in the form of generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE).26 The plane-wave basis set is defined by an energy cutoff of 520 eV for all calculations. The structural optimization is allowed to relax until the maximum force on each atom becomes less than 0.001 eV Å−1 and maximum energy change between two steps is smaller than 10−6 eV. The Γ-centered 7 × 7 × 7 and 12 × 12 × 1 K-point meshes are used in the first Brillouin zone for 3D and 2D InN respectively. The height of each unit cell of 2D InN is maintained at 20 Å to eliminate the interaction between periodic images of slabs in z-direction. The van der Waals interactions that have a significant role on layered materials are taken into account for the multilayered structures.27,28In order to overcome the bandgap problem of GGA potential, we employ HSE06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and quasiparticle (QP) GW0 corrections,30–32 because of their accuracy in describing the electronic structures of semiconductors and insulators. Regarding the optical response calculations, the random phase approximation (RPA)33,34 is undertaken on top of HSE06 and GW0 approaches. The attraction between quasi-electron and hole (on top of GW0 approximation) by solving BSE35,36 is taken into account. A unified K-point mesh 36 × 36 × 1 is adopted for the GW0 and BSE methods, which is enough for BSE calculation. The total number of empty bands is 336 used for the GW0 calculations. The six highest valence bands and the eight lowest conduction bands are included as basis for the excitonic state. BSE is solved using the Tamm–Dancoff approximation.
29 and quasiparticle (QP) GW0 corrections,30–32 because of their accuracy in describing the electronic structures of semiconductors and insulators. Regarding the optical response calculations, the random phase approximation (RPA)33,34 is undertaken on top of HSE06 and GW0 approaches. The attraction between quasi-electron and hole (on top of GW0 approximation) by solving BSE35,36 is taken into account. A unified K-point mesh 36 × 36 × 1 is adopted for the GW0 and BSE methods, which is enough for BSE calculation. The total number of empty bands is 336 used for the GW0 calculations. The six highest valence bands and the eight lowest conduction bands are included as basis for the excitonic state. BSE is solved using the Tamm–Dancoff approximation.
3. Results and discussions
3.1 3D InN crystals
We carry out structure optimization and electronic structures calculations of wurtzite (wz) and zinc-blende (zb) InN crystals with GGA-PBE and HSE06 potentials, respectively. The equilibrium lattice constants, In–N bond length d, cohesive energy per In–N pair Ec, bulk modulus B and direct bandgap EΓ–Γ of wz and zb-InN are presented in Table 1 and Table 2, together with other theoretical and experimental data. The present calculations predict a = 3.62 Å, c = 5.83 Å for wz-InN, the c/a ratio is 1.61, which are in good agreement with previous GGA calculations of a = 3.61, 3.55 Å and c = 5.88, 5.73 Å, while slightly larger than those of LDA calculations, a = 3.54, 3.53 Å and c = 5.76, 5.54 Å.37,38 With respect to experimental values of a = 3.53 Å and c = 5.69 Å,41–45 the calculated results are large by 2.55% and 2.46% respectively. And the measured bond length of In–N is 2.21 Å. Here, the cohesive energy per In–N pair (Ec) can be given by,| Ec = E(In) + E(N) − E(InN) | (1) |
| a (Å) | c (Å) | c/a | d (Å) | Ec (eV/InN) | B (GPa) | Eg (eV) | |
|---|---|---|---|---|---|---|---|
| PBE/HSE06 | 3.62 | 5.83 | 1.61 | 2.21 | 10.12 | 82.04 | 0/0.50 |
| LDA37,38 | 3.54, 3.53 | 5.76, 5, 54 | 1.62, 1.57 | 8.69 | 140, 146.2 | 0.27, 0.017 | |
| GGA37,38 | 3.61, 3.55 | 5.88, 5.73 | 1.63, 1.61 | 6.87 | 116.1, 121.58 | 0.37, 0.029 | |
| mBJ38 | 0.399 | ||||||
| HSE39,40 | 0.57, 0.68 | ||||||
| Expt.41–45 | 3.53 | 5.69 | 1.61 | 7.97 | 125 | 0.78–0.90 |
As for zb-InN, the lattice constant obtained from previous theoretical result is between 4.94 and 5.11 Å![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37,38 and is predicted here as 5.10 Å, which is 2.41% larger than experimental value of 4.98 Å.43–47 The equivalent In–N bond length is 2.21 Å. The cohesive energy per In–N pair calculated as Ec = 9.57 eV is slightly smaller than that of wz-InN because thermodynamically stable phase of 3D InN crystal has a wurtzite structure, which corresponds to a global minimum. Furthermore, the bulk modulus of 97.74 GPa is smaller than experimental value of 137 GPa.
37,38 and is predicted here as 5.10 Å, which is 2.41% larger than experimental value of 4.98 Å.43–47 The equivalent In–N bond length is 2.21 Å. The cohesive energy per In–N pair calculated as Ec = 9.57 eV is slightly smaller than that of wz-InN because thermodynamically stable phase of 3D InN crystal has a wurtzite structure, which corresponds to a global minimum. Furthermore, the bulk modulus of 97.74 GPa is smaller than experimental value of 137 GPa.
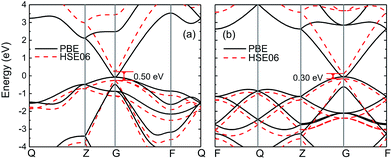
Fig. 1 shows the band structures of wz and zb-InN crystals obtained by using PBE and HSE06 methods. The energy bands of wz-InN calculated by PBE predict a near zero bandgap, which is largely underestimated with respect to the reported experimental values of 0.78–0.9 eV.41–45 With corrections by using HSE06, the direct bandgap increases to 0.50 eV, while it maintains 0.28 eV below the experimental value. Similar to wz structure, as shown in Fig. 1(b), zb-InN exhibits a near zero bandgap with PBE. After HSE06 corrections, it has a direct bandgap 0.30 eV, which is still 0.40 eV smaller than experimentally measured value. Our prediction agrees with the previous calculations within PBE and HSE corrections.37–40
3.2 2D InN
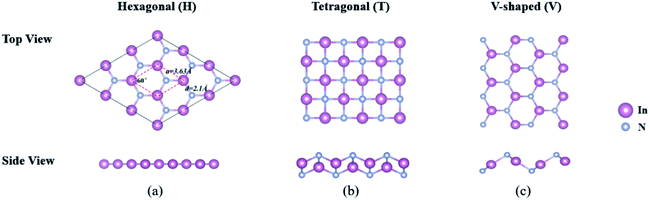
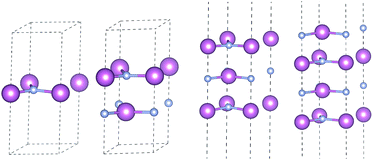
![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) M2 and of which the orbital hybridization is sp2. The T structure (Fig. 2(b)) has the space group P4/NMM, unlike the threefold coordinated planar hexagonal structure, each cation and anion in the T structure is bonded to four neighboring anions and cations, respectively.48 The V structure (Fig. 2(c)) with the space group PMN21, which has inequilateral hexagonal rings from the top view. The orbital hybridization in the V structure is neither sp2 nor sp3. In order to determine the dynamical stability of each structure, the phonon spectra are calculated in Fig. 3. For the T and V structures, there exist imaginary frequency in the calculated frequencies of phonon modes, which reveal instability. The phonon dispersions of H structure are positive and indicate stability, which are similar to previous calculated results.48–50
M2 and of which the orbital hybridization is sp2. The T structure (Fig. 2(b)) has the space group P4/NMM, unlike the threefold coordinated planar hexagonal structure, each cation and anion in the T structure is bonded to four neighboring anions and cations, respectively.48 The V structure (Fig. 2(c)) with the space group PMN21, which has inequilateral hexagonal rings from the top view. The orbital hybridization in the V structure is neither sp2 nor sp3. In order to determine the dynamical stability of each structure, the phonon spectra are calculated in Fig. 3. For the T and V structures, there exist imaginary frequency in the calculated frequencies of phonon modes, which reveal instability. The phonon dispersions of H structure are positive and indicate stability, which are similar to previous calculated results.48–50
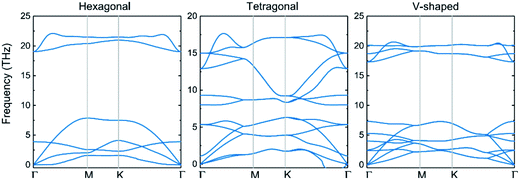 | ||
| Fig. 3 Calculated phonon-dispersion curves of hexagonal, tetragonal and V-shaped structures, Γ versus K, along major symmetry directions of the Brillouin zone. | ||
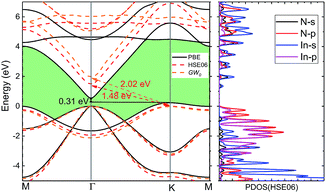
For optimized H structure, hybrid orbitals of In-sp2 and N-sp2 form σ-bonds along In–N bonds arranged as a hexagon. Due to the planar sp2 bonding, the bond angle between In–N bonds is 120°. In addition to three sp2 hybrid orbitals of each constituent, In and N, their pz orbitals are perpendicular to the plane of InN. While the σ-bonds attain the strength of InN, the π-bonds between nearest In-pz and N-pz orbitals maintain the planar geometry of InN.15 The optimized atomic structure and its lattice constant a, bond length d, cohesive energy Ec and minimum value of the energy gap, Eg calculated by using PBE, HSE06 and GW0 together with previous theoretical results are shown in Fig. 2(a) and Table 3. Our calculations predict a = 3.63 Å, which agrees well with previous values.48,49 The In–N bond of 2D-InN constructs from planar sp2 hybrid orbitals and pz orbitals, the value here we obtain is 2.10 Å, which is 0.11 Å shorter than the In–N bond of 3D wz(zb)-InN constructed from tetrahedrally coordinated sp3 hybrid orbitals. This indicates that In–N bonds in 2D-InN are stronger than those in wz(zb)-InN. Despite the stronger bonding in planar InN, the cohesive energy of 3D wz-InN crystal is 0.72 eV higher than that of 2D-InN (Ec = 9.40 eV).
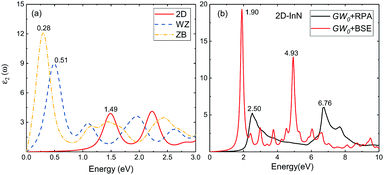
The absorption spectrum of 2D-InN given by GW0-BSE calculation is displayed in Fig. 5(b), together with GW0-RPA spectrum. We can obtain that the oscillator strengths of these peaks are enhanced considerably with electron–hole interactions taken into account. Under the quasiparticle direct bandgap (2.02 eV), there is a very significant light absorption peak (1.90 eV). This result is substantially dominated by the excitonic effect, due to the e–h interactions stemming from weak screening in low-dimensional InN. Comparing the lower GW0-BSE peak with quasiparticle bandgap excitation indicates the exciton binding energy is 0.12 eV, which is apparently higher than that of its wurtzite bulk structure (25 meV).52 The strongly bound excitons underline that for 2D-InN, e–h interaction can be significant to enhance the optical features in the absorption spectrum. The exciton effect is slightly weaker than that of other two-dimensional III–V materials, such as 1.7 eV in AlN monolayer honeycomb.51
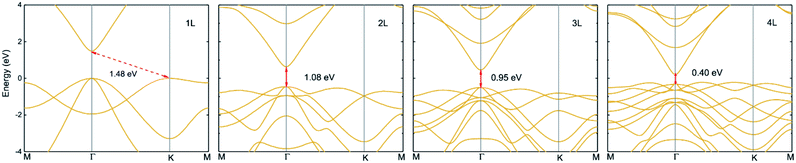 | ||
| Fig. 8 Electronic band structures of monolayer, bilayer, trilayer and four-layered InN within HSE06 potential. The fundamental bandgaps are shown by arrows. | ||
The lattice constant and bond length have no significant change with increasing layer up to three layers, an increase is found in four-layered InN, as listed in Table 4. However, interlayer distance and minimum bandgap display the different trend that they decrease with increasing layers, since interlayer interaction increases. The fundamental bandgap which is shown in Fig. 8 decreases from 1.48 eV to 1.08 eV in bilayer, to 0.95 eV in trilayer and to 0.40 eV in four-layered, these bandgaps are direct. There exists a crossover from indirect to direct in multilayered InN. Our calculations give a trend of band structure evolution with layers and the results shown here indicate rapid bandgap reduction. It indicates that the optoelectronic properties of layered 2D-InN can be controlled and tuned by modifying their structures.
| Layer | a (Å) | d (Å) | h (Å) | Eg (eV) |
|---|---|---|---|---|
| 1 | 3.63 | 2.10 | — | 1.48 |
| 2 | 3.64 | 2.10 | 2.67 | 1.08 |
| 3 | 3.64 | 2.10 | 2.66/2.78 | 0.95 |
| 4 | 3.84 | 2.21 | 2.56/2.58/2.48 | 0.40 |
4. Conclusions
We perform mechanical, electronic and optical properties of monolayer and few layers 2D-InN as well as 3D counterparts based on density functional theory. The In–N bond of 2D-InN is 0.11 Å shorter than that of 3D wz(zb)-InN indicating stronger bonding, despite the stronger bonding in planar InN, 3D wz-InN crystal has a higher cohesive energy. Going from 3D to 2D InN, the bandgap increases and is converted from direct to indirect. In optical properties, the absorption spectrum yields a blueshift compared to (wz)zb-InN. By many-body calculations, 2D-InN presents an exciton binding energy of 0.12 eV. When a biaxial compressive strain is exerted, monolayer InN changes from an indirect to direct semiconductor, which is promising for optoelectronic applications due to their tunable bandgaps. Finally, we investigate the bandgap variation of bilayer and multilayer structures (include van der Waals) that decreases from 1.48 eV to 0.40 eV in four-layered InN, these bandgaps are direct. This indicates that the optoelectronic properties of layered 2D-InN can be controlled and tuned by modifying their structures.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO.61675032, 61671085), the National Key Research and Development Program of China (No. 2017YFB0405100), National Basic Research Program of China (973 Program) under Grant No. 2014CB643900, the Open Program of State Key Laboratory of Functional Materials for Informatics. We acknowledge the computational support from the Beijing Computational Science Research Center (CSRC).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- D. Liang, H. He, P. F. Lu, L. Y. Wu, C. F. Zhang, P. F. Guan and S. M. Wang, J. Mater. Sci., 2017, 52, 5799–5806 CrossRef CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- H. He, P. F. Lu, L. Y. Wu, C. F. Zhang, Y. X. Song, P. F. Guan and S. M. Wang, Nanoscale Res. Lett., 2016, 11, 330 CrossRef PubMed.
- P. F. Lu, L. Y. Wu, C. H. Yang, D. Liang, R. G. Quhe, P. F. Guan and S. M. Wang, Sci. Rep., 2017, 7, 3912 CrossRef PubMed.
- Y. W. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- J. Dai, Z. Y. Li, J. L. Yang and J. G. Hou, Nanoscale, 2012, 4, 3032–3035 RSC.
- L. Y. Wu, P. F. Lu, J. Y. Bi, C. H. Yang, Y. X. Song, P. F. Guan and S. M. Wang, Nanoscale Res. Lett., 2016, 11, 525 CrossRef PubMed.
- J. Feng, X. Sun, C. Z. Wu, L. L. Peng, C. W. Lin, S. L. Hu, J. L. Yang and Y. Xie, J. Am. Chem. Soc., 2011, 133, 17832–17838 CrossRef CAS PubMed.
- W. Q. Xu, P. F. Lu, L. Y. Wu, C. H. Yang, Y. X. Song, P. F. Guan, L. H. Han and S. M. Wang, IEEE J. Sel. Top. Quantum Electron., 2016, 23, 9000305 Search PubMed.
- C. Y. Zhi, Y. Bando, C. C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS.
- G. Wang, X. G. Zhu, Y.-Y. Sun, Y. Y. Li, T. Zhang, J. Wen, X. Chen, K. He, L. L. Wang, X. C. Ma, J. F. Jia, S. B. Zhang and Q. K. Xue, Adv. Mater., 2011, 23, 2929–2932 CrossRef CAS PubMed.
- P. Tsipas, S. Kassavetis, D. Tsoutsou, E. Xenogiannopoulou, E. Golias, S. A. Giamini, C. Grazianetti, D. Chiappe, A. Molle, M. Fanciulli and A. Dimoulas, Appl. Phys. Lett., 2013, 103, 251605 CrossRef.
- A. Onen, D. Kecik, E. Durgun and S. Ciraci, Phys. Rev. B, 2016, 93, 085431 CrossRef.
- A. K. Singh, H. L. L. Zhuang and R. G. Hennig, Phys. Rev. B, 2014, 89, 245431 CrossRef.
- P. Carrier and S. H. Wei, J. Appl. Phys., 2005, 97, 033707 CrossRef.
- S. Nagahama, T. Yanamoto, M. Sano and T. Mukai, Jpn. J. Appl. Phys., 2001, 40, 3075–3081 CrossRef CAS.
- H. Morkoc, Nitride Semiconductors and Devices, Springer, New York, 1999 Search PubMed.
- P. M. Asbeck, E. T. Yu, S. S. Lau, G. J. Sullivan, J. Van Hove and J. Redwing, Electron. Lett., 1997, 33, 1230 CrossRef CAS.
- E. T. Yu, X. Z. Dang, L. S. Yu, D. Qiao, P. M. Asbeck and S. S. Lau, Appl. Phys. Lett., 1998, 73, 1880–1882 CrossRef CAS.
- W. Kohn and L. J. Sham, Phys. Rev. A, 1965, 140, 1133–1138 CrossRef.
- P. E. Blöchl, Phys. Rev. B, 1994, 50, 17953 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B, 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B, 1996, 54, 11169 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 183865 CrossRef PubMed.
- S. Grimme, Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- T. Bucko, J. Hafner, S. Lebegue and J. G. Angyan, J. Phys. Chem. A, 2010, 114, 11814–11824 CrossRef CAS PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- L. Hedin, Phys. Rev., 1965, 139, 663–696 CrossRef.
- M. S. Hybertsen and S. G. Louie, Phys. Rev. B, 1986, 34, 5390–5413 CrossRef CAS.
- M. Shishkin and G. Kresse, Phys. Rev. B, 2006, 74, 035101 CrossRef.
- D. Bohm and D. Pines, Phys. Rev., 1953, 92, 609–625 CrossRef CAS.
- H. Ehrenreich and M. H. Cohen, Phys. Rev., 1959, 115, 786–790 CrossRef.
- M. Rohlfing and S. G. Louie, Phys. Rev. B, 2000, 62, 4927–4944 CrossRef CAS.
- G. Onida, L. Reining and A. Rubio, Rev. Mod. Phys., 2002, 74, 601–659 CrossRef CAS.
- C. Stampfl and C. G. Van de Walle, Phys. Rev. B, 1999, 59, 5521–5535 CrossRef CAS.
- R. Graine, R. Chemam, F. Z. Gasmi, R. Nouri, H. Meradji and R. Khenata, Int. J. Mod. Phys. B, 2015, 29, 1550028 CrossRef CAS.
- Y. F. Duan, L. X. Qin, L. W. Shi, G. Tang and H. L. Shi, J. Phys.: Condens. Matter, 2014, 26, 025501 CrossRef PubMed.
- Q. M. Yan, P. Rinke, A. Janotti, M. Scheffler and C. G. Van de Walle, Phys. Rev. B, 2014, 90, 125118 CrossRef.
- K. Kim, W. R. Lambrecht and B. Segall, Phys. Rev. B, 1996, 53, 16310 CrossRef CAS.
- B. Paulus, F. H. Shi and H. Stoll, J. Phys.: Condens. Matter, 1997, 9, 2745–2758 CrossRef CAS.
- J. H. Edgar, Properties of Group-III Nitrides. EMIS Datareviews Series, IEE, London ( 1994) Search PubMed.
- P. Carrier and S. H. Wei, J. Appl. Phys., 2005, 97, 033707 CrossRef.
- J. Wu, et al., Appl. Phys. Lett., 2002, 80, 3967–3969 CrossRef CAS.
- V. Y. Davydov, et al., Phys. Status Solidi B, 2002, 229, R1–R3 CrossRef CAS.
- T. Matsuoka, H. Okamoto, M. Nakao, H. Harima and E. Kurimoto, Appl. Phys. Lett., 2002, 81, 1246–1248 CrossRef CAS.
- H. L. Zhuang, A. K. Singh and R. G. Hennig, Phys. Rev. B, 2013, 87, 165415 CrossRef.
- H. Şahin, S. Cahangirov, M. Topsakal, E. Bekaroglu, E. Akturk, R. T. Senger and S. Ciraci, Phys. Rev. B, 2009, 80, 155453 CrossRef.
- C. J. Tong, H. Zhang, Y. N. Zhang, H. Liu and L. M. Liu, J. Mater. Chem. A, 2014, 2, 17971–17978 CAS.
- D. Kecik, C. Bacaksiz, R. T. Senger and E. Durgun, Phys. Rev. B, 2015, 92, 165408 CrossRef.
- M. Dvorak, S. H. Wei and Z. G. Wu, Phys. Rev. Lett., 2013, 110, 016402 CrossRef PubMed.
- H. L. Zhuang and R. G. Hennig, Appl. Phys. Lett., 2012, 101, 153109 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |