Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multifunctional organic dyes: anion-sensing and light-harvesting properties of curcumin boron complexes†
Masahiro Tsuchikawaa,
Aya Takaoa,
Takashi Funakib,
Hideki Sugiharab and
Katsuhiko Ono *a
*a
aGraduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan. E-mail: ono.katsuhiko@nitech.ac.jp
bNational Institute of Advanced Industrial Science and Technology, Higashi, Tsukuba 305-8565, Japan
First published on 24th July 2017
Abstract
Organoboron compounds containing curcumin (i.e. natural phenols) were synthesised as a new type of multifunctional organic dye. These compounds behaved as sensing indicators for pH and anions in mineral waters while exhibiting sensitiser ability in dye-sensitised solar cells.
Introduction
The fabrication of high-performance, low-cost dye-sensitised solar cells (DSCs) has attracted considerable attention in the past decade.1–3 DSCs have been recently proposed as highly attractive subjects for the research and development of wearable technologies owing to their flexibility and simple fabrication process.4 For example, DSCs have been studied for textile-formed devices.5 Their utilisation in wearable devices have posed new requirements on DSCs. Thus, wearable devices require components having human and environmental safety and sustainability characteristics that can be met by using natural materials. However, natural dyes satisfying such demands have not been found yet in this field.6,7Organoboron compounds are of interest as sensing8 and light-harvesting9,10 materials, and these properties can be useful for the development of wearable technologies. Curcumin, a yellow dye found in turmeric and curry powders,6b is one of the natural phenols containing a 1,3-diketone skeleton that chelates boron reagents to afford red dyes. Rosocyanin is a well-known curcumin boron complex exhibiting chromism characteristics depending on the pH conditions.11 Curcumin difluoroboron complexes have been reported to show colour change to aqueous cyanine solution and serve as its indicator.12 CB is also of interest because it has a polar structure similar to those of rosocyanin and the related compounds (Scheme 1).13 In resonance hybrids, a positive charge is delocalised on the β-diketonate moiety, while a negative charge is localised on the boron atom. Thus, we synthesised compounds 1–4 as a new type of multifunctional organic dye for sensing device and DSC applications. Some of these compounds showed chromism depending upon solvents, pH conditions and adding mineral waters, and they exhibited higher sensitiser performances in DSCs than in simple natural dyes. We report herein the sensing and light-harvesting properties of 1–4 and CB.
 | ||
| Scheme 1 Structure of curcumin boron complexes with resonance hybrids, in which a positive charge is delocalised on the β-diketonate moieties and a negative charge is localised on the boron atoms. | ||
Results and discussion
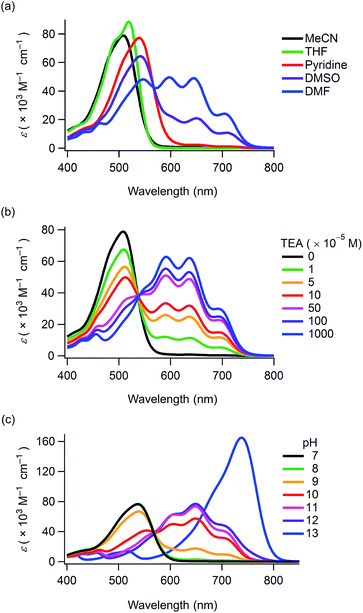
Compounds 1–4 and CB were synthesised in 60–88% yields by boron-chelation of curcumin, catechol derivatives and tributyl borate under refluxing toluene conditions (ESI†). These compounds were obtained as deep red crystals which were stable in air in their solid state. Their structures were determined by spectral data and elemental analyses (ESI†). The 11B NMR spectra revealed that the electronic properties at the boron-chelating moieties were almost similar to each other, with minor down- and up-field shifts produced by the carboxyl and octyl groups being observed, respectively (δ/ppm = 9.27 (1), 9.00 (2), 9.13 (3), 9.11 (4) and 9.08 (CB) as a standard of BF3·OEt2). The compounds were stable in aprotic solvents for periods ranging from a week to over a month (Fig. S1 and S2, ESI†). However, they underwent hydrolysis within a few days in ethanol and methanol yielding the corresponding curcumin derivatives.The UV-Vis absorption maxima of 1–4 and CB in acetonitrile were observed in the wavelength region of 503–514 nm (ε > 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) (Table 1). Thus, boron-chelating curcumin possessed a good visible light-harvesting ability. 1–3 and CB displayed solvatochromism in various solvents (Fig. S3, ESI†), and the absorption spectra of 1 are shown in Fig. 1a. This phenomenon was attributed to the deprotonation of the phenol groups because 4 did not show solvatochromism. When dissolved in DMF, 1 showed new absorption bands having maxima at 704, 645 and 597 nm apart from the absorption band at 546 nm (Table 1). When dissolved in DMSO, new absorption bands were also observed for 1. The intensity of these absorption bands increased while decreasing the solution concentration (Fig. S5, ESI†). The long-wavelength absorption bands rapidly disappeared upon ultrasonication, and the colour shifted from blue to red (Fig. S6a and b and Movie S1, ESI†). Since chromism was not observed in a buffer solution at pH = 10 (Fig. S6c, ESI†), this phenomenon was attributed to a decrease in the pH values of the solvents upon ultrasonication in air.
000 M−1 cm−1) (Table 1). Thus, boron-chelating curcumin possessed a good visible light-harvesting ability. 1–3 and CB displayed solvatochromism in various solvents (Fig. S3, ESI†), and the absorption spectra of 1 are shown in Fig. 1a. This phenomenon was attributed to the deprotonation of the phenol groups because 4 did not show solvatochromism. When dissolved in DMF, 1 showed new absorption bands having maxima at 704, 645 and 597 nm apart from the absorption band at 546 nm (Table 1). When dissolved in DMSO, new absorption bands were also observed for 1. The intensity of these absorption bands increased while decreasing the solution concentration (Fig. S5, ESI†). The long-wavelength absorption bands rapidly disappeared upon ultrasonication, and the colour shifted from blue to red (Fig. S6a and b and Movie S1, ESI†). Since chromism was not observed in a buffer solution at pH = 10 (Fig. S6c, ESI†), this phenomenon was attributed to a decrease in the pH values of the solvents upon ultrasonication in air.
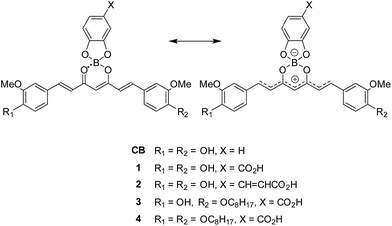
With the aim to promote deprotonation of the phenol groups, triethylamine (TEA) was added to the solution of 1 in acetonitrile (Fig. 1b). Upon increasing TEA concentration, the intensity of the absorption band at ca. 510 nm decreased and new absorption bands appeared in the wavelength region of 537–750 nm. The spectral change revealed an isosbestic point at 537 nm, thereby suggesting that the new absorption bands were produced upon generation of a phenoxide monoanion on the curcumin skeleton. The 1H NMR spectra of CB were determined while increasing the concentration of TEA (Fig. 2). All the peaks assigned to the π-conjugated system upfield shifted.13 In particular, large shifts were observed at the positions of H1, H2, H3 and H4, thereby indicating that the π-conjugated system had a significant contribution in the resonance of the hybrids (Scheme 1). The TEA-induced chromism was also found in a titanium oxide (TiO2) film, as shown in Fig. S8 (ESI†). The original colour was immediately recovered upon evaporation of TEA, whereas coating the TEA-treated dye substrate with manicure allowed to maintain the colour (Fig. S9, ESI†). Fig. 1c shows the absorption spectra of 1 under several pH conditions in DMSO containing 10 vol% of water. Neutral species of 1 was found under neutral and acidic solutions at pH = 7 or below, and its absorption maximum was observed at 537 nm. The absorption bands of 1 and its phenoxide monoanion were both observed at basic pH values ranging from 8 to 10. At higher pH values (i.e. 11 and 12), the spectra of the solutions indicated that 1 mainly existed as a phenoxide monoanion. The spectral change at pH = 7–11 revealed an isosbestic point at 568 nm. When dissolved in a strongly basic solution (pH = 13), a new absorption band having a maximum at 738 nm was observed for 1. Since this new band was not observed for a solution containing 3 (Fig. S10, ESI†), we inferred that the onset of this new absorption band was produced upon generation of a phenoxide dianion. Addition of acid to the solution of the phenoxide dianion recovered the neutral species of 1. In the above conditions, the first acid dissociation constants (pKa1) were determined to be 9.5, 9.7 and 9.7 for 1, 3 and CB, respectively. The second acid dissociation constants (pKa2) were determined to be 12.5 and 11.5 for 1 and CB, respectively (Fig. S11, ESI†). The UV-Vis absorption spectra of CB and its monoanion and dianion species were simulated by using time-dependent density functional theory (TDDFT) calculations at the B3LYP/6-31G(d) level (Fig. S12, ESI†). The theoretical spectra supported the absorption redshifts in the experimental spectra of the monoanion and dianion species.
 | ||
| Fig. 2 1H NMR spectra of CB (1.7 × 10−2 M) in DMSO-d6 solutions containing TEA (0, 1, 3, 5 and 10 mol equiv.). | ||
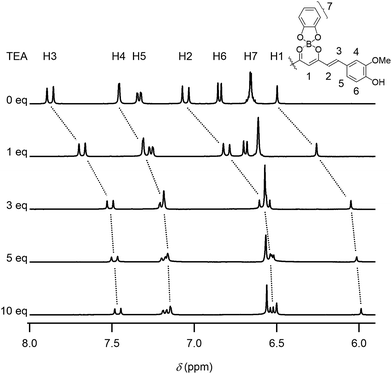
The chromism was attributed to the polar structure generated on the curcumin skeleton (Scheme 1). This π-electron system can be used for detecting ions in water. When water was dropwise added to a dye DMSO solution, the red solution turned blue, as shown in Fig. 3a (see also Movie S2, ESI†). In contrast, this colour change was not observed when pure water was added. The UV-Vis absorption spectra of DMSO solutions of 1 containing mineral waters are shown in Fig. 3b and S13 (ESI†). The absorption bands in the wavelength region of 567–780 nm increased with the amount of minerals added. With the aim to determine the ion species causing chromism, the ion selectivity of CB was investigated (Table S1 and S2, ESI†). These studies revealed that cationic species did not generate chromism. Indeed, anions such as OH−, PO43−, CO32−, HPO42−, HCO3−, CH3CO2−, F− and SO42− were responsible for this phenomenon. On the other hand, no colour change was observed when very weak bases such as BF4−, NO3−, ClO4− and Cl− were used, thereby suggesting a basicity boundary between active and inactive anions at ca. pKb = 12 in water. According to the Job's plots for CB and sodium salts, a CB to Na2CO3 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed (Fig. 3c). Thus, both CO32− and HCO3− species were able to deprotonate the phenol groups of the curcumin moiety to generate the monoanion of CB. On the other hand, a molar ratio of 1
1 was observed (Fig. 3c). Thus, both CO32− and HCO3− species were able to deprotonate the phenol groups of the curcumin moiety to generate the monoanion of CB. On the other hand, a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was measured when NaOH and Na2SO4 were used. In Na2SO4 solution, SO42− (and not HSO4−) was the active species to CB. Furthermore, when using Na3PO4, a CB to Na3PO4 molar ratio of 2
1 was measured when NaOH and Na2SO4 were used. In Na2SO4 solution, SO42− (and not HSO4−) was the active species to CB. Furthermore, when using Na3PO4, a CB to Na3PO4 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (instead of 3
1 (instead of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was observed, thereby indicating that both PO43− and HPO42− species were active (and H2PO4− was inactive) for generating chromism. Thus, these dyes were useful indicators for sensing anions in mineral waters.
1) was observed, thereby indicating that both PO43− and HPO42− species were active (and H2PO4− was inactive) for generating chromism. Thus, these dyes were useful indicators for sensing anions in mineral waters.
 | ||
Fig. 3 Anion-sensing properties: (a) chromism of 1 (conc. 5.0 × 10−5 M in DMSO) upon addition of mineral water (see also Movie S2, ESI†), (b) UV-Vis absorption spectra of 1 (conc. 1.0 × 10−5 M) in DMSO solutions containing several amounts of mineral water (hardness 40 mg L−1, pH = 6.8) and (c) Job's plots for CB (conc. 3.0 × 10−5 M) and sodium salts in DMSO containing 10 vol% of water (monitored at 648 nm), indicating CB to sodium salts molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NaOH and Na2SO4) and 2 1 (NaOH and Na2SO4) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Na2CO3 and Na3PO4). 1 (Na2CO3 and Na3PO4). | ||
With the aim to investigate the light-harvesting properties of the curcumin boron complexes, dyes 1–4 were used in DSCs (ESI†). Fig. 4a shows the current density–voltage characteristics of the DSCs containing dyes 1–4 (denoted as DSCs 1–4). The short-circuit photocurrent density (Jsc), open-circuit photovoltage (Voc), fill factor (FF) and solar-to-electric power conversion efficiency (PCE) of these DSCs are listed in Table 2. The PCEs varied with Jsc, and DSCs 2 and 3 showed higher PCEs as compared to DSCs employing curcumin (0.36%)6b or other natural dyes (<2%)6a with the exception of chlorophyll7a and a few more.7b These PCEs were also much higher than that of a similar boron complex (0.34%).14 DSC 2 showed higher PCEs as compared to DSC 1 as a result of dye modification by substitution of 3,4-dihydroxybenzoic acid with caffeic acid. However, the Jsc of DSC 2 gradually decreased with time. The highest PCE was obtained for DSC 3 containing a mono-alkylated curcumin dye. Furthermore, DSC 4 (containing a di-alkylated curcumin dye) did not achieve effective photoelectric conversion. The incident photo-to-current conversion efficiency (IPCE) spectra are shown in Fig. 4b. DSCs 1, 2 and 3 showed maximum efficiencies of 41, 41 and 48%, respectively. These values were relatively low against their light-harvesting abilities. The electrochemical characteristics of these dyes are shown in Fig. S14 (ESI†). The dyes showed reduction waves at −0.6 V versus the normal hydrogen electrode (NHE) potential. These reduction potentials were close to −0.5 V of the TiO2 photoanode, suggesting that a driving force for effective electron-injection was insufficient in the DSC performance.15 Therefore, the PCEs would be improved by optimization of LUMO energies.
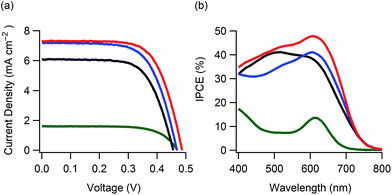 | ||
| Fig. 4 Photovoltaic performance for DSCs 1–4: (a) current density–voltage characteristics and (b) IPCE spectra; black line: DSC 1, blue line: DSC 2, red line: DSC 3 and green line: DSC 4. | ||
| DSC | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| a DSC conditions: irradiation of 100 mW cm−2 (AM 1.5 solar light); photoelectrode: TiO2 films (17 μm thickness and 0.25 cm2 area) coated with dye acetonic solutions (0.2 mM); electrolyte: 0.60 M 1,2-dimethyl-3-propylimidazolium iodide (DMPII), 0.05 M I2 and 0.10 M LiI in acetonitrile. | ||||
| 1 | 6.1 | 0.46 | 0.66 | 1.8 |
| 2 | 7.2 | 0.47 | 0.65 | 2.2 |
| 3 | 7.3 | 0.49 | 0.67 | 2.4 |
| 4 | 1.6 | 0.47 | 0.69 | 0.5 |
We investigated the effect of adding TEA, trihexylamine (THA) or 4-tert-butylpyridine (TBP) to the electrolyte solutions of DSCs 1–3. DSCs 1–3 containing 0.01 M of TEA exhibited PCEs of 1.8, 2.2 and 2.4%, respectively. These values were similar to those in Table 2 despite the TEA concentration (0.01 M) was enough to deprotonate the phenol groups (Fig. S8, ESI†). This fact suggested that phenoxide monoanions were generated in DSCs 1–3 even without TEA. DSCs 1–3 containing 0.01 M of THA showed PCEs of 2.0, 2.2 and 2.4%, respectively. The PCE of DSC 1 slightly increased upon addition of THA, thereby suggesting a charge recombination inhibition effect on the TiO2 photoanode. Furthermore, DSCs 1–3 containing 0.01 M of TBP exhibited PCEs of 1.5, 1.7 and 2.4%, respectively. The PCEs of DSCs 1 and 2 decreased upon addition of TBP, thereby indicating that the interaction of TBP with the TiO2 photoanodes weakened the electron-injection from the excited dyes to the TiO2 photoanodes.16 The three additives had no influence on the PCEs of DSC 3.
Conclusions
We synthesised curcumin boron complexes possessing sensing and light-harvesting abilities. The solutions of these compounds exhibited chromism depending on the solvent, ultrasonication and pH conditions. Addition of amines or mineral waters were also effective in generating chromism because the curcumin moiety having two phenol groups was highly polarised via boron chelation. We investigated the structural changes among neutral, phenoxide monoanion and dianion species by UV-Vis absorption and 1H NMR spectral analyses. The first and second acid dissociation constants in DMSO containing 10 vol% of water were measured to be ca. pKa1 = 9.5 and pKa2 = 12, respectively. These dyes exhibited chromism via deprotonation with anions having pKb values in water below ca. 12. This anion recognition ability is useful for the analysis of mineral waters. When loaded on TiO2 substrates, these dyes also demonstrated chromism depending on the environment. Furthermore, they showed light-harvesting abilities in the visible wavelength region. DSCs containing these organoboron compounds exhibited PCEs of 1.8–2.4%, and a PCE of 2.2% was achieved by a DSC containing a boron complex of curcumin and caffeic acid. These PCEs were higher than those obtained with simple natural dyes. Modification of curcuminoids via boron chelation is useful for the research and development of new types of DSCs to be employed in the wearable technology field.Acknowledgements
This work was supported by the Asahi Glass Foundation, Takahashi Industrial and Economic Research Foundation and JSPS KAKENHI Grant Number JP15K05422. Theoretical calculations were performed using Research Center for Computational Science, Okazaki, Japan.Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- (a) S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed; (b) K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, Chem. Commun., 2014, 50, 6379 RSC; (c) Z. Yao, M. Zhang, H. Wu, L. Yang, R. Li and P. Wang, J. Am. Chem. Soc., 2015, 137, 3799 CrossRef CAS PubMed.
- M. Graetzel, R. A. J. Janssen, D. B. Mitzi and E. H. Sargent, Nature, 2012, 488, 304 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- (a) S. Pan, Z. Yang, P. Chen, J. Deng, H. Li and H. Peng, Angew. Chem., Int. Ed., 2014, 53, 6110 CrossRef CAS PubMed; (b) M. J. Yun, S. I. Cha, S. H. Seo, H. S. Kim and D. Y. Lee, Sci. Rep., 2015, 5, 11022 CrossRef CAS PubMed; (c) W. Weng, P. Chen, S. He, X. Sun and H. Peng, Angew. Chem., Int. Ed., 2016, 55, 6140 CrossRef CAS PubMed.
- (a) M. R. Narayan, Renewable Sustainable Energy Rev., 2012, 16, 208 CAS; (b) S. A. M. Al-Bat'hi, I. Alaei and I. Sopyan, Int. J. Renew. Energy Res., 2013, 3, 138 Search PubMed.
- (a) X.-F. Wang, Y. Koyama, O. Kitao, Y. Wada, S. Sasaki, H. Tamiaki and H. Zhou, Biosens. Bioelectron., 2010, 25, 1970 CrossRef CAS PubMed; (b) W. Maiaugree, S. Lowpa, M. Towannang, P. Rutphonsan, A. Tangtrakarn, S. Pimanpang, P. Maiaugree, N. Ratchapolthavisin, W. Sang-aroon, W. Jarernboon and V. Amornkitbamrung, Sci. Rep., 2015, 5, 15230 CrossRef CAS PubMed.
- (a) J. Hu, Z. He, Z. Wang, X. Li, J. You and G. Gao, Tetrahedron Lett., 2013, 54, 4167 CrossRef CAS; (b) K. Tanaka and Y. Chujo, NPG Asia Mater., 2015, 7, e223 CrossRef CAS.
- (a) Y.-D. Lin, B.-Y. Ke, Y. J. Chang, P.-T. Chou, K.-L. Liau, C.-Y. Liu and T. J. Chow, J. Mater. Chem. A, 2015, 3, 16831 RSC; (b) Y. Kubo, D. Eguchi, A. Matsumoto, R. Nishiyabu, H. Yakushiji, K. Shigaki and M. Kaneko, J. Mater. Chem. A, 2014, 2, 5204 RSC; (c) S. B. Mane, J.-Y. Hu, Y.-C. Chang, L. Luo, E. W.-G. Diau and C.-H. Hung, Chem. Commun., 2013, 49, 6882 RSC; (d) M. Mao, X.-L. Zhang, X.-Q. Fang, G.-H. Wu, Y. Ding, X.-L. Liu, S.-Y. Dai and Q.-H. Song, Org. Electron., 2014, 15, 2079 CrossRef CAS; (e) J. A. Mikroyannidis, M. S. Roy and G. D. Sharma, J. Power Sources, 2010, 195, 5391 CrossRef CAS; (f) M. Mao, X.-L. Zhang, X.-Q. Fang, G.-H. Wu, S.-Y. Dai, Q.-H. Song and X.-X. Zhang, J. Power Sources, 2014, 268, 965 CrossRef CAS; (g) Y. Mizuno, Y. Yisilamu, T. Yamaguchi, M. Tomura, T. Funaki, H. Sugihara and K. Ono, Chem.–Eur. J., 2014, 20, 13286 CrossRef CAS PubMed.
- (a) K. Cnops, B. P. Rand, D. Cheyns, B. Verreet, M. A. Empl and P. Heremans, Nat. Commun., 2014, 5, 3406 Search PubMed; (b) S. P. Singh, C. H. P. Kumar, G. D. Sharma, J. A. Mikroyannidis, M. Singh and R. Kurchania, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 1612 CrossRef CAS; (c) T. Bura, N. Leclerc, S. Fall, P. Lévêque, T. Heiser, P. Retailleau, S. Rihn, A. Mirloup and R. Ziessel, J. Am. Chem. Soc., 2012, 134, 17404 CrossRef CAS PubMed.
- G. S. Spicer and J. D. H. Strickland, J. Chem. Soc., 1952, 4644 RSC.
- (a) A. Chaicham, S. Kulchat, G. Tumcharern, T. Tuntulani and B. Tomapatanaget, Tetrahedron, 2010, 66, 6217 CrossRef CAS; (b) D. R. Sherin, S. G. Thomas and K. N. Rajasekharan, Heterocycl. Commun., 2015, 21, 381 CrossRef CAS.
- A. T. Balaban, C. Párkányi, I. Ghiviriga, J.-J. Aaron, Z. Zajíčková and O. R. Martínez, ARKIVOC, 2008, 1 CAS.
- T. Ganesh, J. H. Kim, S. J. Yoon, B.-H. Kil, N. N. Maldar, J. W. Han and S.-H. Han, Mater. Chem. Phys., 2010, 123, 62 CrossRef CAS.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597 CrossRef CAS.
- K. Hara, Y. Dan-oh, C. Kasada, Y. Ohga, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, Langmuir, 2004, 20, 4205 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation of dyes, analytical studies, theoretical calculations, photovoltaic fabrication, spectra and movies. See DOI: 10.1039/c7ra06778j |
| This journal is © The Royal Society of Chemistry 2017 |