Open Access Article
Open Access ArticleA material-basis study of Aloe vera on the wnt/β-catenin signaling pathway using a knockin/knockout method with high-speed countercurrent chromatography†
Cong Daia,
Meng-ping Liua,
Wei-jia Zhanga,
Christopher Wai Kei Lamb,
Jian-ru Guob,
Wa Lia,
Juan Wua,
Jie-feng Chena,
Zuan-guang Chena,
Wei Zhang‡
*b and
Mei-cun Yao‡ *a
*a
aSchool of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, P. R. China. E-mail: yaomeicun@gmail.com
bState Key Laboratory of Quality Research in Chinese Medicines, Macau Institute for Applied Research in Medicine and Health, Macau University of Science and Technology, Taipa, Macau, China. E-mail: Wzhang@must.edu.mo
First published on 8th August 2017
Abstract
Aloe vera has been widely used in cosmetics and as a health product. Although increasing research has indicated that chronic use of Aloe vera is closely associated with the development of colorectal cancer, the material basis and molecular mechanism of the pathogenesis have not been elucidated. The wnt/β-catenin signaling pathway plays an important role in the development of colorectal cancer. It should be useful to investigate whether Aloe vera has any impact on the wnt/β-catenin signaling pathway. In this study, we found that a water extract of Aloe vera at low concentration (0.98–12.50 μg ml−1) could activate the wnt/β-catenin signaling pathway in the presence of wnt3a ligand and up-regulated the level of active β-catenin protein in hek293 cells, as well as promoting the expression of wnt target genes (Axin2, DKK1, FGF20). Additionally, aloin and aloesin were the effective components interacting with other non-active ingredients in the water extract of Aloe vera that were identified by a knockout/knockin method with high-speed counter current chromatography (HSCCC). Meanwhile, no effects of the water extract of Aloe vera and aloin on the wnt/β-catenin signaling pathway were found, either in the presence or absence of other activators such as activator CHIR99021. In conclusion, the water extract of Aloe vera could activate the wnt/β-catenin signaling pathway in the presence of wnt3a and the effective components were aloin and aloesin. These findings might facilitate the understanding of the material basis and molecular mechanisms of Aloe vera in causing colorectal cancer.
1. Introduction
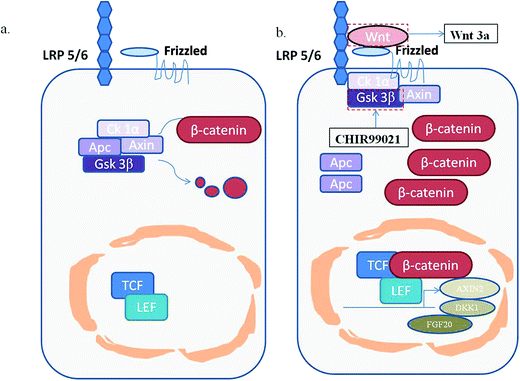
Aloe vera, a common traditional Chinese medicine, is widely used in cosmetics and as a health food due to its various biological functions including wound healing,1 and antiviral,2,3 antioxidant,4 and blood glucose lowering activities.5 Although it has diverse pharmacological activities, studies in recent years have focused on the association between long-term use of Aloe vera whole leaf extract and the development of colorectal cancer.6–8 Studies conducted by the National Toxicology Program confirmed that a non-decolorized whole leaf extract of Aloe vera exerted carcinogenic activity on the large intestine of F344/N rats.7 The colonic carcinogenicity of Aloe vera was also confirmed independently by several other studies.8–10 However, the material basis and molecular mechanism of Aloe vera in the pathogenesis of colorectal cancer have remained unclear.The wnt/β-catenin signaling pathway plays an indispensable role in biological evolution and human diseases. In the normal state of the wnt signaling pathway without wnt ligands, the key protein β-catenin is persistently phosphorylated and degraded by a destructive complex mainly composed of casein kinase 1α (Ck1α), the tumor suppressor adenomatous polyposis coli (APC), the scaffold protein Axin, and the glycogen synthase kinase 3β(Gsk 3β). However, if the wnt ligands were bound to their specific co-receptors which contain frizzled protein and low-density receptor related protein 5/6(LRP 5/6), β-catenin would be stabilized and accumulated in the cytoplasm. Part of the free β-catenin would enter the nucleus and combine with T cell factors (TCF) or lymphoid enhancer-binding factor (TEF) to promote the expression of the wnt target genes, such as Axin2, DKK1 and FGF20,11 which is an abnormal state of the wnt signaling pathway related to the occurrence and development of many cancers.12
Colon cancer is closely related to the wnt/β-catenin signaling pathway. A recent research showed that 93% of colorectal cancer had the aberrant activation of wnt/β-catenin signaling pathway.13 Mutations of wnt pathway genes such as Axin2, SOX17 or APC were found in sporadic colorectal cancer.14 The APC gene defect in familial adenomatous polyposis accelerated tumor initiation, and loss of APC function activated constitutive transcription of TCF target genes.15,16 The expression of β-catenin protein was increased in colorectal carcinomas.17 We hypothesized that the potential carcinogenicity Aloe vera to colorectal cancer may be contributed by its influence on the wnt/β-catenin signaling pathway.
Traditional Chinese medicines contain complex components responsible for their various pharmacological actions and it is helpful to identify what components are effective in a holistic view in achieving better quality control of these herbs. Knockin and knockout technology is a useful tool to screen for the effective components in a holistic view, which has been applied successfully in several studies recently.18,19 High-speed counter current chromatography (HSCCC) has been widely used to separate components from traditional Chinese medicines with advantages of nearly zero adsorption and strong preparation capacity.19 Current research findings testified that this chromatography technology can knock out the target components from the extracts successfully without complex operation and utilization of the experimental equipment.20,21
The modern approach of developing new drugs via scaffold repurposing of old drugs22,23 has inspired us to firstly explore any new bioactivities of known active components of traditional Chinese medicines when we screen for the material basis of their therapeutic applications.
In this study, we focused on the relationship between Aloe vera and wnt/β-catenin signaling pathway. Firstly we investigated the activation effect of the water extract of Aloe vera on the wnt signaling pathway, and then explored the key active components by an approach combining the knockin and knockout technology (HSCCC) with activity screening assay based on hek293 cells. Hek293 cells are transformed cell line with adenovirus.42 More recent reports demonstrated that use of these cells to evaluate the effect of traditional Chinese medicine on wnt/β-catenin signaling pathway was feasible.39–41 Finally we verified that the water extract of Aloe vera activated wnt/β-catenin signaling pathway in the presence of wnt3a ligand, which simultaneously improved the level of active β-catenin and upregulated the expression of Axin2, FGF20 and DKK1 genes. We also confirmed that aloin and aloesin were the effective components in the water extract of Aloe vera contributing to activating the wnt/β-catenin signaling pathway.
2. Materials and methods
2.1 Plant materials
The Aloe vera particulates (product number: 1503232) studied in this study were purchased from Yifang Pharmaceutical Co., Ltd. (Guangzhou, China), which were dried exudate of Aloe vera. Corresponding voucher specimens (voucher number: LH-YN-20160305) were preserved in the Laboratory of Pharmaceutical Analysis and Quality Assessment, School of Pharmaceutical Science, SunYat-sen University, Guangzhou, China.2.2 Chemicals and reagents
The reference materials of aloin and aloesin (both of 98% purity) were purchased from Baoji Herbest Bio-Tech Co., Ltd. (Baoji, China). Isoaloeresin D (94%) was prepared by us and checked with HPLC-DAD. And these three substances were used in this whole study. The powder of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was purchased from MP Biomedicals (Santa Ana, USA). wnt3a protein and CHIR99021 were obtained from Stem RD (Burlingame, USA). Steady-glo® luciferase assay system kit was obtained from Promega Corporation (Madison, USA). The primary antibody against active β-catenin (#19807) and β-actin (#4970) were purchased from Cell Signaling Technology (Beverly, MA, USA), while the second antibody (#AP132P) was from EMD Millipore Corporation (Temecula, CA). PrimeScript RT reagent kit with gDNA Eraser and SYBR Premix Ex Tap™ kit were from Takara Biotechnology Co., Ltd. (Minamikusatsu, Japan).2.3 Cell lines and culture
The human HT29 cells were a kind gift from Prof. Jun Du (Sun Yat-sen University) and the human HEK293 cells transfected with top flash plasmid were purchased from Curegenix Inc (Guangzhou, China). Both cell lines were cultured with DMEM medium added with 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco, China) in a 5% CO2 and 37 °C incubator.2.4 Sample preparation and HPLC analysis
The Aloe vera particulates were extracted ultrasonically in water at 45 °C for 1.5 h, and the suspension was filtered. The supernatant solution was evaporated at 50 °C under 0.08 MPa using a rotary evaporator, and the concentrate lyophilized to power form and stored at −80 °C. For subsequent analysis, the extract powder and standard materials were dissolved in ultrapure water or methanol to constitute the test samples, which were filtered through a 0.22 μm membrane before HPLC. A Luna (C18) 100A column (250 mm × 4.6 mm, 5 μm) was used. The mobile phase comprised ultrapure water and methanol. During elution, methanol content was adjusted according to a published method as follows: 0–30 min, 23–35%; 30–60 min, 35–45%; 60–80 min, 45–70%; 80–100 min, 70–80%; and 100–110 min, 80–23%.242.5 Luciferase reporter assay for wnt/β-catenin signaling pathway
HEK293 cells transfected with topflash plasmid (containing specific lcf/lef regions binding to β-catenin) were plated into 96-well plates at a density of 2 × 104 cells per well. After overnight culture, the cultured medium was removed, then medium with different drug concentration and wnt3a protein solution (100 ng ml−1) were added into the wells. The cells were lysed and processed according to the instructions of the Steady-glo® luciferase assay system kit. Their luciferase activities were determined by the luminescence function of an automatic microplate reader.2.6 Two-step high-speed counter current chromatography (HSCCC) for knockout assay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate
ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (0.2
water (0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0
5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0, v/v/v/v), which was adopted from the method of Wu.25 The elution time 200 min was used to ensure total separation of aloin from isoaloeresin D. The second step system containing ethyl acetone
5.0, v/v/v/v), which was adopted from the method of Wu.25 The elution time 200 min was used to ensure total separation of aloin from isoaloeresin D. The second step system containing ethyl acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butanol
n-butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v) was used for separating aloesin.
5, v/v/v) was used for separating aloesin.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate
ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (0.2
water (0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0
5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0, v/v/v/v). Four solvents were added to a separation funnel, the mixture was shaken followed by standing overnight. The upper and lower phases were separated before use. The stationary phase was the upper phase and the lower phase was used as the mobile phase when the revolution speed of the HSCCC instrument was regulated at 860 rpm and the flow rate was 1.5 ml min−1, and the separation was conducted at room temperature. After the first step separation, we obtained the single components and the extruded sample (without the single components). All these samples were evaporated at 45 °C under vaccum followed by lyophilization to powder form. The extruded sample was dissolved in 20 ml solvent comprised of 10 ml each of the upper and lower phases of ethyl acetone
5.0, v/v/v/v). Four solvents were added to a separation funnel, the mixture was shaken followed by standing overnight. The upper and lower phases were separated before use. The stationary phase was the upper phase and the lower phase was used as the mobile phase when the revolution speed of the HSCCC instrument was regulated at 860 rpm and the flow rate was 1.5 ml min−1, and the separation was conducted at room temperature. After the first step separation, we obtained the single components and the extruded sample (without the single components). All these samples were evaporated at 45 °C under vaccum followed by lyophilization to powder form. The extruded sample was dissolved in 20 ml solvent comprised of 10 ml each of the upper and lower phases of ethyl acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butanol
n-butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v) for the second step separation which was conducted at the same resolution speed, flow rate and temperature as the first separation.
5, v/v/v) for the second step separation which was conducted at the same resolution speed, flow rate and temperature as the first separation.2.7 Knockin assay
The target components (A and B) were knocked out by two-step HSCCC technology, the remaining components in the water extract of Aloe vera was designated as the residue part (C). To develop the knockin assay, the concentration of the target components (A and B) of the water extract of Aloe vera was first determined by HPLC using the elution method that was described above in Section 3.4. Secondly, 0.25 fold A, 0.5 fold A, 1 fold A, 0.25 fold B, 0.5 fold B, 1 fold B, 0.25 fold A + B, 0.5 fold A + B, 1 fold A + B were added respectively to C, constituting the different knockin samples. Thirdly, these knockin samples were measured by the luciferase reporter gene assay for their activities on the wnt/β-catenin signaling pathway.2.8 Western blotting assay
Cells were collected after incubation with the drugs for 24 h, and lysed with RIPA buffer. The protein concentration of the lysate was determined by bicinchoninic acid (BCA) assay. 20 μg protein of each cell lysate was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked at room temperature for 1 h and then blotted with the specific primary antibody for 2 h at room temperature. To detect the bound antibody, membranes were incubated with a horse-radish peroxidase (HRP)-conjugated anti-rabbit secondary antibody for 1 h at room temperature, followed by visualization of protein bands by SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, MA, USA) and exposure with a ChemiDoc XRS+ system (Bio-Rad, Hercules, CA, USA).2.9 Quantitative real time RT PCR assay
Quantitative real time (RT) PCR assay was applied to measure the wnt target gene mRNA levels. Total RNA was isolated by Trizol from HEK293 cells untreated or treated with different drug for 16 h. Reverse transcription of purified RNA was conducted using the PrimeScript RT reagent Kit (Takara) according to manufacture's instructions. Quantitative RT PCR was performed with SYBR Premix Ex Tap™ kit (Takara) for Axin2, DKK1, FGF20, GAPDH, and analyzed on Quantitative RT PCR system (Bio-Rad). GAPDH was the reference gene and the specific primers to amplify mRNA fragments were Axin2 (forwards: AGGCTAGCTGAGGTGT, towards: AGGCTTGGATTGGAGAA), DKK1 (forwards: CTGCAAAAATGGAATATGTGT, towards: CTTCTTGTCCTTTGGTGTGA), FGF20 (forwards: ATTCATCAGTGTGGCAGTGG, towards: GCTCCCTAAAGATGCATTCG), GAPDH (forwards: AGGTCGGAGTCAACGGATTTG, towards: TGTAAACCATGTAGTTGAGGTCA). The expression regulation of those target genes was evaluated with respect to cells of control group after normalized by GAPDH.2.10 MTT assay for cell viability
Cells at a density of 5 × 103 cells per well were seeded in 96-well plates. After 18 h incubation, they were treated with the medium containing different drug solutions. After 24 h incubation, cell viability was evaluated by MTT assay, as described previously.262.11 Statistical analysis
Graphprism 5 statistical analysis software was used. All experiments were repeated in triplicates, and then all data were performed as mean (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ) ± SD (n = 3). Two-tailed Student's t-test was applied to evaluate the significant difference and P values less than 0.05 were regarded as statistically significant.
) ± SD (n = 3). Two-tailed Student's t-test was applied to evaluate the significant difference and P values less than 0.05 were regarded as statistically significant.
3. Results and discussions
3.1 Standardization of Aloe vera extract by HPLC
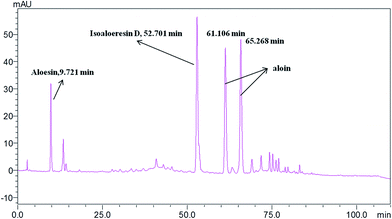
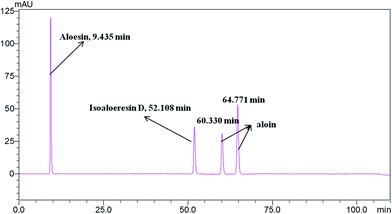
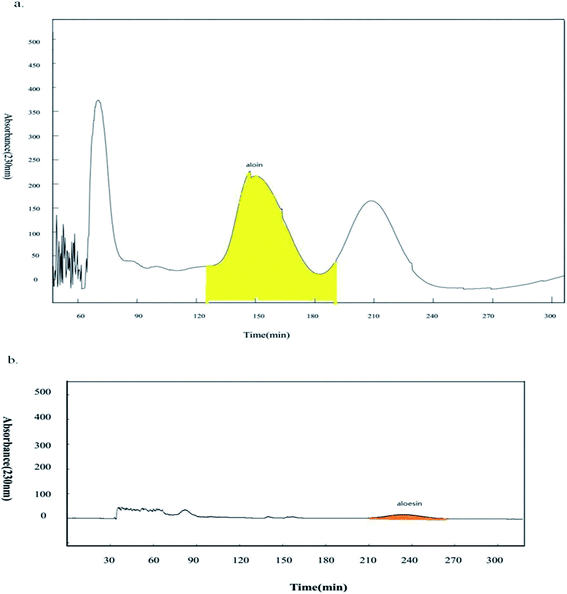
Standardization of traditional Chinese medicines should be conducted before further studies. HPLC analysis of the water extract of Aloe vera showed four main chromatographic peaks which were aloin A, aloin B, aloesin and isoaloeresin D (Fig. 1 and 2). Aloin A and aloin B are isomers that can transmute into each other, therefore we regarded them as a whole in further studies. The contents of aloin, aloesin and isoaloeresin D in the water extract were 680, 110 and 200 mg g−1 respectively.3.2 Effects of the water extract of Aloe vera and its components on the wnt/β-catenin signaling pathway
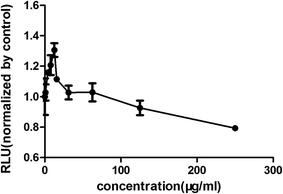
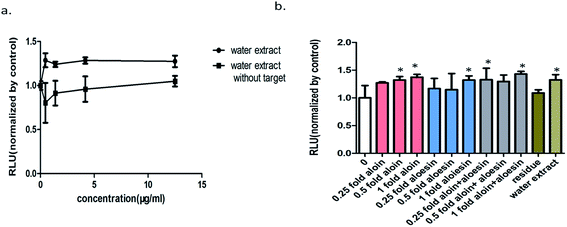
Increasing research studies have reported that long-term administration of Aloe vera can induce colorectal cancer in rats, and most colorectal cancers manifest aberrant activation of the wnt/β-catenin signaling pathway.7,8,13 We investigated the effects of Aloe vera on the wnt/β-catenin signaling pathway using the luciferase reporter gene assay. Results showed that the extract and its active components exhibited different effects on this signaling pathway. The extract activated the wnt/β-catenin signaling pathway at low concentration (0.98–12.50 μg ml−1). Interestingly, this activation became weakened with increasing extract concentration that was eventually even converted to an inhibitory effect upon reaching a certain concentration (Fig. 3). Considering of this complex situation, we have focused on studying low extract concentration in this article. To find out the components of Aloe vera activating the wnt/β-catenin signaling pathway, our experiments firstly focused on aloin, aloesin and isoaloeresin D, which are the major components of Aloe vera. The content of isoaloeresin D was the second most abundant component in the water extract without reported biological activities. Aloin and aloesin were the two components that have been studied substantially. Some studies have reported that aloin induced MC3T3-E1 cells into osteoblasts through activating MAPK mediated wnt signaling pathway,27 and it inhibited the NF-kappa B pathway to prevent osteoclastgenesis in RAW264.7 cells.28 Our results found that aloin and aloesin activated the wnt/β-catenin signaling pathway in the presence of wnt3a ligands, while isoaloeresin D had no obvious effect (Fig. 4). In addition, several studies have verified that aloesin and a chromone-enriched aloe composition had glucose-lowering activity in diet-induced obese-diabetic mice,29,30 and the activation effect of aloesin on the wnt/β-catenin signaling pathway may provide a molecular mechanism for its effects on diabetes, which should be confirmed by further studies. In addition, the highest activation rate of the water extract of Aloe vera on the wnt/β-catenin signaling pathway was nearly 32%. However, the highest activation rate of aloin and aloesin was only about 25% and 15% respectively (Fig. 4), these imply the existence of other components related to the wnt signaling pathway.3.3 Knockout of aloin and aloesin by two-step high-speed countercurrent chromatography
Based on the previous results, aloin and aloesin were proven to be the active components in the water extract of Aloe vera. Further studies were conducted to investigate possible impact of other components in the water extract on wnt signaling pathway. HSCCC technology has been used increasingly for knocking out components from herbal medicines.18,19,31 It was impossible to separate the components with a broad range of polarities by a solvent system of high-speed counter-current chromatography (HSCCC). Based on the position of chromatographic peak, there was the obvious polarity difference between aloin and aloesin. Then we applied two-step HSCCC to fish out aloin and aloesin, and the first step HSCCC separated aloin with the solvent system composed of n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate
ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (0.2
water (0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v/v), then the second step separated aloesin with the solvent system composed of ethyl acetone
5, v/v/v/v), then the second step separated aloesin with the solvent system composed of ethyl acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butanol
n-butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v, Fig. 5a and b). Finally, the activation effects of the two knockout samples and their residues were investigated by a luciferase reporter assay.
5, v/v/v, Fig. 5a and b). Finally, the activation effects of the two knockout samples and their residues were investigated by a luciferase reporter assay.
3.4 Activity validation of the lead active components and interaction between active components and nonactive components in Aloe vera by knockin technology
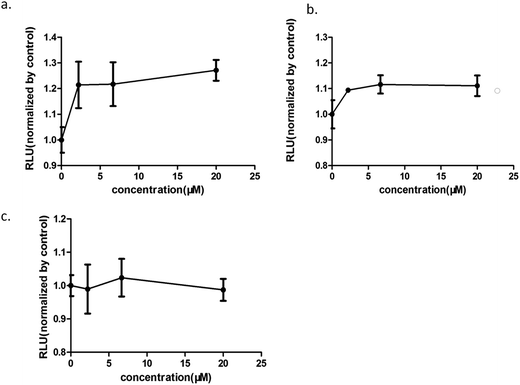
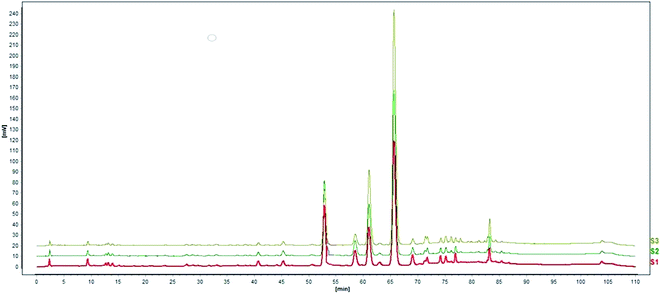
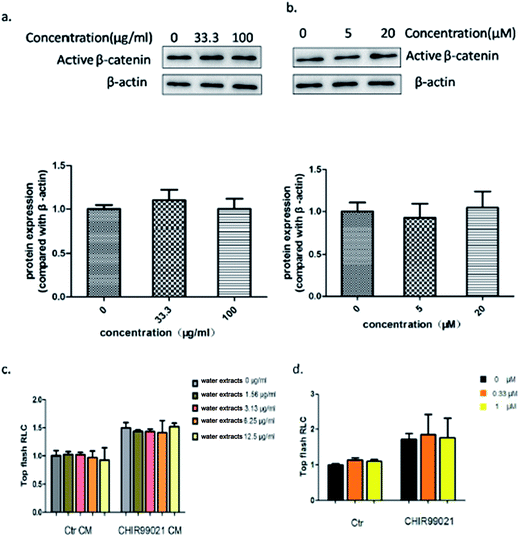
The expected results arising from the above knockout assay combined with activity screening assay indicated that the residue without the main two components nearly had no effects on the wnt/β-catenin signaling pathway (Fig. 6a and 7). To further determine the effects of these components on this signaling pathway, 0.25, 0.5 and 1 fold of the active components and their various combinations were fished into the residue (Fig. 8). Results of these knockin assays showed that the activity of the residue without the main active components was improved in a dose of target component-activity manner with the active components being knocked into separately or together. In addition, compared with their respective effects, the activation effects of aloin and aloesin on wnt/β-catenin signaling pathway were more significant when they were added into the residue part in different concentrations. These results verified that there were activity-assisting components in the residue part exerting an enhancing effect to aloin and aloesin. Moreover, our results showed that no obvious increase of activation activity on wnt/β-catenin signaling pathway appeared when aloin and aloesin were fished into the residue part together, suggesting that aloin may interact with aloesin resulting in restriction of their activation effects (Fig. 6b).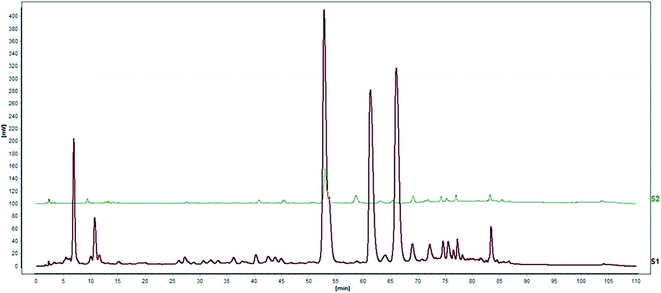 | ||
| Fig. 7 Comparison of HPLC chromatogram. S1: water extract of Aloe vera, S2: the residue without aloin and aloesin. | ||
3.5 Aloe vera and aloin increased the level of active β-catenin protein and promoted the expression of wnt target genes
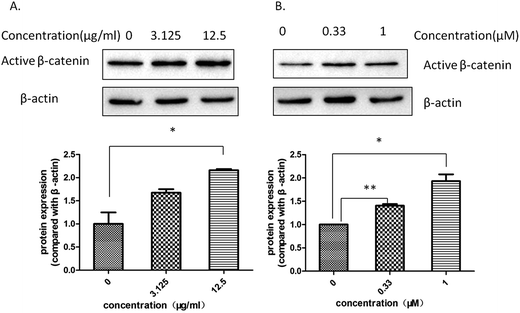
The level of active β-catenin plays a critical role in the wnt/β-catenin signaling pathway. When β-catenin is not phosphyrated and degraded by the complex, the active β-catenin enters the nucleus and binds to the T cell factor or lymphoid enhancer-binding factor, thereby activating the wnt signaling pathway. Our western blotting assay showed that both Aloe vera and aloin up-regulated the expression of active β-catenin in hek293 cells (Fig. 9a and b). Meanwhile, we found that the water extract of Aloe vera and aloin promoted the transcription of wnt target genes (Axin2, DKK1, FGF20) through a quantitative real time RT-PCR assay (Fig. 10a and b). Axin2,32–34 DKK1,37,38 FGF20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35,36 genes are the target genes of the wnt/β-catenin signaling pathway, which are related to the colon cancer and have various of biological function.34,36,38
35,36 genes are the target genes of the wnt/β-catenin signaling pathway, which are related to the colon cancer and have various of biological function.34,36,38
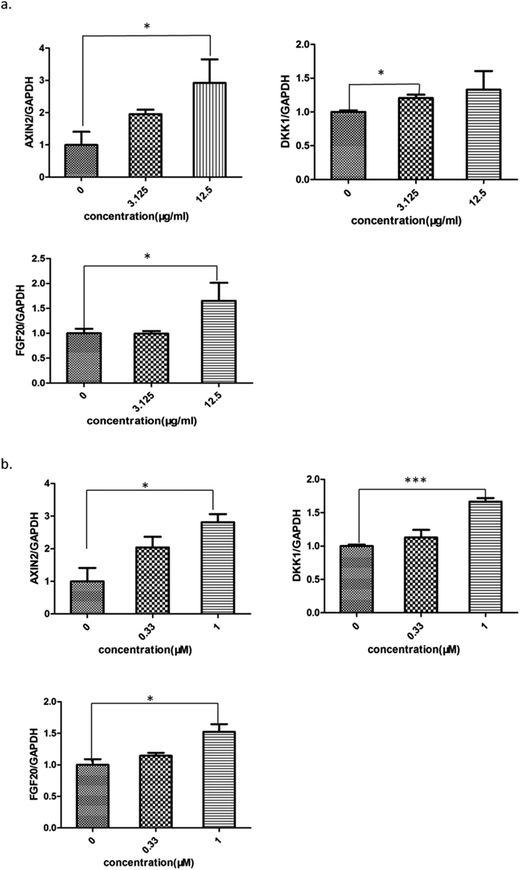 | ||
| Fig. 10 The Aloe vera extract and aloin promoted the expression of wnt target genes. (a) The Aloe vera; (b) aloin. All values were expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. | ||
3.6 Aloe vera and aloin activated the wnt/β-catenin signaling pathway synergistically with wnt3a ligands
To better understand the effect of Aloe vera extract and aloin on the wnt/β-catenin signaling pathway, a luciferase reporter assay was conducted with or without the wnt3a ligand. Results showed that Aloe vera and aloin only activated the wnt/β-catenin signaling pathway in the presence of the wnt3a ligand. Without the wnt3a ligand, the water extract of Aloe vera and aloin had no effect on the wnt signaling pathway (Fig. 11c and d). To demonstrate that the activity of Aloe vera and aloin on the wnt signaling pathway is dependent on wnt3a ligand, further studies were conducted. CHIR99021, an inhibitor of glycogen synthase kinase 3β, was applied in luciferase reporter assay to activate the wnt signaling pathway. Both wnt3a and CHIR99021 were the activators of the wnt/β-catenin signaling pathway. Wnt3a activated the wnt/β-catenin signaling pathway targeting at the upstream of the pathway, while CHIR99021 activated this pathway targeting at the midstream (Fig. 12). Aloe vera and aloin exerted no effects on the signaling pathway with CHIR99021 (Fig. 11c and d). Several studies have demonstrated that the level of active β-catenin indirectly reflects the state of the wnt/β-catenin signaling pathway.37,38 Further studies on the HT29 cells (a kind of colorectal cancer cell with APC mutation) showed that the water extract of Aloe vera and aloin had no influence on the level of active β-catenin, which illustrated that Aloe vera extract and aloin had no influence on the wnt/β-catenin signaling pathway in HT29 cells (Fig. 11a and b). These results demonstrated the water extract of Aloe vera and aloin synergistically with wnt3a ligands activated the wnt/β-catenin signaling pathway (Fig. 9 and 11a–d). Though our present results indicated that the Aloe vera extract did not activate the wnt signaling pathway in HT29 cells, more careful analyses of the relationship between the Aloe vera extract and the wnt/β-catenin signaling pathway in other colorectal cancer cells should be carried out.3.7 Cell viability assays of Aloe vera and its active components
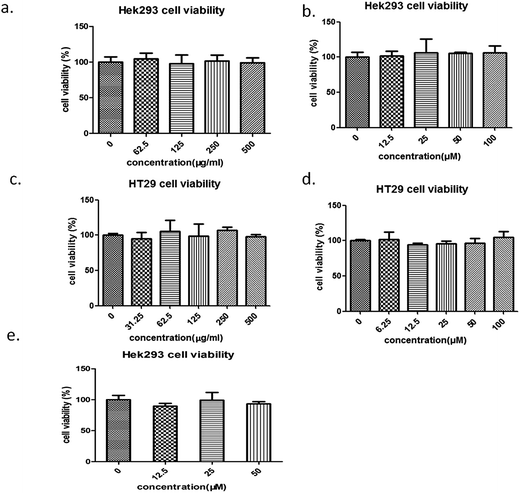
We investigated the effects of the water extract of Aloe vera and its active components on the proliferative activities of hek293 cells and HT29 cells using the MTT assay. The water extract and aloin had no significant effects on the proliferations of hek293 cells and HT29 cells in the studied range of concentration (0–500 μg ml−1 for the water extract and 0–100 μM for aloin) (Fig. 13a–d). In addition, our result also indicated that aloesin (0–50 μM) had no significant impact on the proliferation of hek293 cells (Fig. 13e).4. Conclusions
In conclusion, our study has verified that aloin and aloesin are the effective components in Aloe vera extract mainly via their activation of the wnt/β-catenin signaling pathway. The interactions between these active components and inactive ingredients have been explored combining knockin and knockout technologies using HSCCC and luciferase reporter assay based on a holistic review, and we found that other non-active ingredients exerting an enhancing effect to aloin and aloesin in water extract of Aloe vera. Increasing studies have demonstrated that some extracts of traditional Chinese medicines can activate the wnt/β-catenin signaling pathway,39–41 however, their material basis and action mechanisms have remained unclear. Our study provided a feasible way to investigate the material basis of herbal regulators on wnt/β-catenin signaling pathway in a holistic view. In addition, we verified that the water extract of Aloe vera and aloin activated wnt/β-catenin signaling pathway only in the presence of wnt3a ligands. The molecular mechanism of aloesin on wnt/β-catenin signaling pathway is under exploration.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Prof. Jun Du (Sun Yat-sen University) for kindly providing us the HT29 cells. This work has been supported by the National Natural Science Foundation of China (grants 81573689) and the Macao Science and Technology Development Fund (Project No. 006/2015/A1).References
- L. T. Fox, A. Mazumder, A. Dwivedi, M. Gerber, J. du Plessis and J. H. Hamman, J. Ethnopharmacol., 2017, 200, 1–7 CrossRef CAS PubMed.
- J. H. Kim, J. Y. Yoon, S. Y. Yang, S. K. Choi, S. J. Kwon, I. S. Cho, M. H. Jeong, Y. H. Kim and G. S. Choi, J. Enzyme Inhib. Med. Chem., 2017, 32, 78–83 CrossRef CAS PubMed.
- S. Ghayempour, M. Montazer and M. M. Rad, RSC Adv., 2016, 113, 111895–111902 RSC.
- G. Jairath, M. K. Chatli and A. K. Biswas, J. Food Process. Preserv., 2016, 40, 607–614 CrossRef CAS.
- M. Yimam, J. F. Zhao, B. Corneliusen, M. Pantier, L. Brownell and Q. Jia, Diabetol. Metab. Syndr., 2014, 6, 61 CrossRef PubMed.
- C. P. Siegers, E. V. Hertzberglottin, M. Otte and B. Schneider, Gut, 1993, 34, 1099–1101 CrossRef CAS PubMed.
- M. D. Boudreau, F. A. Beland, J. A. Nichols and M. Pogribna, Natl. Toxicol. Program Tech. Rep. Ser., 2013, 577, 1–266 Search PubMed.
- A. R. Pandiri, R. C. Sills, M. J. Hoenerhoff, S. D. Peddada, T. T. Ton, H. L. Hong, G. P. Flake, D. E. Malarkey, G. R. Olson, I. P. Pogribny, N. J. Walker and M. D. Boudreau, Toxicol. Pathol., 2011, 39, 1065–1074 CrossRef PubMed.
- M. D. Boudreau, P. W. Mellick, G. R. Olson, R. P. Felton, B. T. Thorn and F. A. Beland, Toxicol. Sci., 2013, 131, 26–39 CrossRef CAS PubMed.
- M. Yokohira, Y. Matsuda, S. Suzuki, K. Hosokawa, K. Yamakawa, N. Hashimoto, K. Saoo, K. Nabae, Y. Doi, T. Kuno and K. Imaida, J. Food Sci., 2009, 74, T24–T30 CrossRef CAS PubMed.
- M. Katoh and M. Katoh, Clin. Cancer Res., 2007, 14, 4042–4045 CrossRef PubMed.
- H. Clevers and R. Nusse, Cell, 2012, 149, 1192–1205 CrossRef CAS PubMed.
- D. M. Muzny, M. N. Bainbridge, K. Chang, H. Dinh, J. Drummond, G. Fowler, C. L. Kovar, L. a. Lewis, M. Morgan, I. Newsham, J. G. Reid, J. Santibanez, E. Shinbrot, L. R. Trevino, Y. Wu, M. Wang, P. H. Gunaratne, L. A. Donehower and C. J. Creighton, et al., Nature, 2012, 487, 330–337 CrossRef CAS PubMed.
- N. Suraweera, J. Robinson, E. Volikos, T. Guenther, I. Talbot, I. M. Tomlinson and A. Silver, Int. J. Cancer, 2006, 119, 1837–1842 CrossRef CAS PubMed.
- K. W. Kinzler and B. Vogelstein, Cell, 1996, 87, 159–170 CrossRef CAS PubMed.
- V. Korinek, N. Barker, P. J. Morin, D. V. Wichen, R. D. Weger, K. W. Kinzler, B. Vogelstein and H. Clevers, Science, 1997, 275, 1784–1787 CrossRef CAS PubMed.
- Q. F. Han, W. Zhao, J. Bentel, A. J. Shearwood, N. Zeps, D. J. Joseph, B. Iacopetta and A. Dharmarajan, Cancer Lett., 2006, 231, 129–137 CrossRef CAS PubMed.
- H. Zhao, X. Hu, X. Chen, S. Shi, X. Jiang, X. Liang, W. Chen and S. Zhang, J. Chromatogr. A, 2015, 1398, 57–65 CrossRef CAS PubMed.
- J. Jin, Y. Li, E. K. Tanui, L. Han, Y. Jia, L. Zhang, Y. Wang, X. Zhang and Y. Zhang, J. Ethnopharmacol., 2013, 147, 357–365 CrossRef CAS PubMed.
- J. Chen, Y. Xu, Z. Ge, W. Zhu, Z. Xu and C. Li, J. Agric. Food Chem., 2014, 62, 9744–9750 CrossRef CAS PubMed.
- Z. Jiang, Y. Wang, Y. Zhu, L. Zhang, X. Chai, M. Jiang and L. Shan, J. Pharm. Biomed. Anal., 2015, 115, 130–137 CrossRef CAS PubMed.
- H. Chen, J. Wu, Y. Gao, H. Chen and J. Zhou, Curr. Top. Med. Chem., 2016, 16, 2017–2114 CrossRef.
- G. M. Cragg, P. G. Grothaus and D. J. Newman, J. Nat. Prod., 2014, 3, 703–723 CrossRef PubMed.
- J. Zhong, Y. Huang, T. Zhang, Y. Liu, W. Ding, X. Wu, Z. Xie, H. Luo and J. Wan, Fitoterapia, 2015, 100, 68–74 CrossRef CAS PubMed.
- X. Wu, J. Zhong, Z. Xie, W. Ding and J. Wan, J. Liq. Chromatogr. Relat. Technol., 2013, 36, 2589–2600 CAS.
- L. Xin, J. Wang, G. Fan, B. Che, Y. Wu, S. Guo and J. Tong, Environ. Toxicol., 2016, 31, 1691–1699 CrossRef CAS PubMed.
- Y. Pengjam, H. Madhyastha, R. Madhyastha, Y. Yamaguchi, Y. Nakajima and M. Maruyama, Biomol. Ther., 2016, 24, 123–131 CrossRef CAS PubMed.
- Y. Pengjam, H. Madhyastha, R. Madhyastha, Y. Yamaguchi, Y. Nakajima and M. Maruyama, Phytomedicine, 2016, 23, 417–428 CrossRef CAS PubMed.
- M. Yimam, L. Brownel and Q. Jia, World J. Diabetes, 2015, 6, 1097–1107 CrossRef PubMed.
- M. Yimam, J. Zhao, B. Corneliusen, M. Pantier, L. Brownell and Q. Jia, Diabetol. Metab. Syndr., 2014, 6, 61 CrossRef PubMed.
- Q. Liu, S. Shi, L. Liu, H. Yang, W. Su and X. Chen, J. Chromatogr. A, 2013, 1304, 183–193 CrossRef CAS PubMed.
- R. C. Ransom, D. J. Hunter, S. Hyman, G. Singh, S. Ransom, E. Z. Shen, K. C. Perez, M. Gillette, J. Li, B. Liu, J. B. Brunski and J. A. Helms, Sci. Rep., 2016, 6, 36524 CrossRef CAS PubMed.
- S. M. Mazzoni, E. M. Petty, E. M. Stoffel and E. R. Fearon, Neoplasia, 2015, 17, 463–472 CrossRef CAS PubMed.
- U. Schaal, S. Grenz, S. Merkel, T. T. Rau, M. V. Hadjihannas, E. Kremmer, P. Chudasama, R. S. Croner, J. Behrens, M. Sturzl and E. Naschberger, Int. J. Colorectal Dis., 2013, 28, 1469–1478 CrossRef PubMed.
- M. Katoh and M. Katoh, Int. J. Oncol., 2006, 29, 163–168 CAS.
- H. Tian, Y. Zhao, N. i. Chen, M. Wu, W. Gong, J. Zheng, D. G. Fernig, A. Jungbauer, D. Wang, X. Li and C. Jiang, Appl. Microbiol. Biotechnol., 2016, 100, 3023–3034 CrossRef CAS PubMed.
- O. Lyros, P. Rafiee, L. Nie, R. Medda, N. Jovanovic, J. Schmidt, A. C. Mackinnon, N. Venu and R. Shaker, Am. J. Physiol.: Gastrointest. Liver Physiol., 2014, 306, G557–G574 CrossRef CAS PubMed.
- L. Qi, B. Sun, Z. Liu, R. Cheng, Y. Li and X. Zhao, J. Exp. Clin. Cancer Res., 2014, 33, 107 CrossRef PubMed.
- C. M. Gurroladiaz, P. Garcialopez, K. Gulewicz, R. Pilarsk and S. Dihlmann, Phytomedicine, 2011, 18, 683–690 CrossRef CAS PubMed.
- J. Hwang, P. Cha, G. Han, D. S. Min and K. Choi, Exp. Mol. Med., 2015, 47, e152 CrossRef CAS PubMed.
- P. Park, B. Moon, S. Lee, S. Kim, A. Kim, H. Kim, W. Park, K. Choi, E. Cho and T. R. Lee, Life Sci., 2012, 91, 935–943 CrossRef CAS PubMed.
- F. L. Graham, J. R. Smiley, W. C. Russell and R. Nairn, J. Gen. Virol., 1977, 36, 59–74 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06761e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |