Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
γ-Cyclodextrin metal–organic framework for efficient separation of chiral aromatic alcohols†
Cheng-Xiong Yang *a,
Yu-Zhen Zhenga and
Xiu-Ping Yan
*a,
Yu-Zhen Zhenga and
Xiu-Ping Yan ab
ab
aCollege of Chemistry, Research Center for Analytical Science, State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Molecular Recognition and Biosensing, Nankai University, Tianjin 300071, China. E-mail: cxyang@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering, Tianjin 300071, China
First published on 20th July 2017
Abstract
A γ-cyclodextrin metal–organic framework was applied as an efficient chiral stationary phase for high-performance liquid chromatographic separation of chiral aromatic alcohols.
Chirality is one of the essential properties of nature.1 Chiral separation is an interesting and challenging topic in diverse areas as pure enantiomers may profoundly differ in biological activity, pharmacology and toxicity.2 Among all the chiral separation techniques, chiral high-performance liquid chromatography (HPLC) has been proved to be the most robust and widely applicable one.3 Chiral stationary phase (CSP) is the core in chiral HPLC.4 Development of novel CSPs for chiral HPLC is an eternal research subject either in chemical industry and pharmaceutical production or in life science.1–4
Metal–organic frameworks (MOFs) are an emerging class of multifunctional porous materials in the past two decades.5–8 Chiral MOFs have received great attention due to their wide potential applications in catalysis and separation.9–11 Recently, quite a few chiral MOFs have been investigated as novel CSPs in gas chromatography, capillary electrochromatography, and HPLC with good selectivity and resolution for diverse chiral compounds.12–29 Nuzhdin et al. reported the first example of chiral MOF [Zn2(bdc)(L-lac)(dmf)]·DMF in liquid chromatographic separation of chiral sulfoxides.19 Padmanaban et al. utilized the Bn-ChirUMCM-1 for separation of 1-phenylethanol.20 Tanaka et al. demonstrated a series of (R)-MOF–silica composites for HPLC separation of chiral sulfoxides, sec-alcohols, lactams, benzoins, flavanones and epoxides.21–23 Kuang et al. showed a three-dimensional homochiral MOF {[ZnLBr]·H2O}n for separation of racemic drugs.24 Hailili R. et al. studied the planar Mn4O cluster homochiral MOF for HPLC separation of ibuprofen racemate.25 Yuan's group reported several chiral MOFs including [(CH3)2NH2][Cd(bpdc)1.5]·2DMA, [Zn2(D-Cam)2(4,4′-bpy)]n, [Cd2(D-Cam)3]·2HDMA·4DMA, and [In3O(obb)3(HCO2)(H2O)], [Cu2(D-Cam)2(4,4′-bpy)]n for HPLC of diverse chiral compounds.26–29 All the above mentioned chiral HPLC were performed on the chiral MOFs constructed from chiral ligands. However, some chiral ligands are expensive or are synthesized via complex steps.24,25 In addition, high temperature or long time is usually needed to synthesize chiral MOFs.19–29 Exploring novel chiral MOFs alternatives is quite significant.
In 2010, Smaldone et al. reported a new class of MOFs constructed from naturally available building blocks of cyclodextrin (CD) and salt substitute (e.g. KCl) under mild conditions.30–33 To date, the CD MOFs have been investigated as promising porous materials in drug delivery, separation and sensing.34–37 Recently, Hartlieb et al. reported the versatile HPLC separation performance of the γ-CD MOF for alkylaromatic compounds, haloaromatics, terpinenes and pinenes.33 In addition, they also tried the preliminarily attempt of γ-CD MOF for HPLC separation of chiral limonene and 1-phenylethanol.33 However, CD MOFs is still in its infancy in chiral HPLC. CD are the natural chiral selector.38–40 Therefore, further exploring the potential application of CD MOFs for chiral separation is quite significant not only for the expansion of novel CSPs in HPLC but also for the practical use of CD MOFs.
Herein, we report the application of γ-CD MOF for efficient HPLC separation of chiral aromatic alcohols. Chiral aromatic alcohols are important chiral mediates or chiral drugs in chemistry, pharmaceuticals, and industry. Separation of chiral aromatic alcohols is quite difficult and significant in the above areas.13 The γ-CD MOF was constructed by combining 1.0 equiv. of γ-CD with 8.0 equiv. of alkali metal cations at room temperature. Six γ-CD molecules occupy the face of a primary (γ-CD)6 cube with the assistance of alkali metal cations leading to the formation of γ-CD MOF as a chiral molecular building block with spherical pore and large pore window.30 The hydrophobic cavity and the hydrophilic rim of the γ-CD are the ideal chiral selectivity sites for chiral aromatic alcohols.38–40
In this work, twelve chiral aromatic alcohols were well separated on γ-CD MOF packed column with good precision and selectivity. These results demonstrate the practical application of γ-CD MOF as a specific CSP for important chiral aromatic alcohols and the development of CD MOFs in chiral HPLC. The easy, mild, and economic synthesis conditions of CD MOFs also make them highly potential in chiral separation.
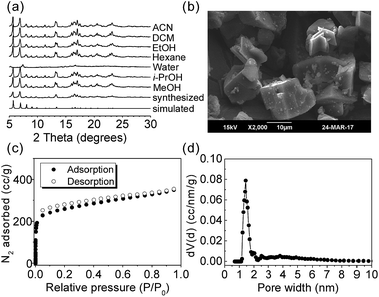
The γ-CD MOF was synthesized according to Hartlieb et al.33 X-ray diffraction (XRD), scanning electron microscopy (SEM), and N2 adsorption experiments were used to characterize the synthesized γ-CD MOF (Fig. 1). The good agreement of the XRD patterns between the synthesized γ-CD MOF and the simulated one reveals the successful synthesis of γ-CD MOF (Fig. 1a). The SEM image shows the particle size of the synthesized γ-CD MOF is about 20 μm (Fig. 1b). The synthesized γ-CD MOF gave a BET surface area of 866 m2 g−1 (Fig. 1c) and a pore size of 1.4 nm (Fig. 1d).
Solvent stability is the primary demand of CSP in HPLC. The stability of γ-CD MOF in diverse solvents was first studied. The γ-CD MOF was separately immersed in the common used solvents as chiral HPLC mobile phase including hexane, dichloromethane (DCM), methanol (MeOH), ethanol (EtOH), isopropanol (i-PrOH), acetonitrile (ACN) and water for 1 week. The collected γ-CD MOF were monitored with XRD experiments (Fig. 1a). The XRD patterns of γ-CD MOF immersed in hexane, DCM, MeOH, EtOH, i-PrOH, and ACN are the same as the synthesized one, suggesting the frameworks of γ-CD MOF are intact and stable in these solvents. However, the frameworks of γ-CD MOF were collapsed in water as indicated by the changing of the XRD pattern compared to the synthesized one (Fig. 1a). Therefore, the HPLC separation of chiral aromatic alcohols on γ-CD MOF was then carried out in normal phase mode (without water as the mobile phase).
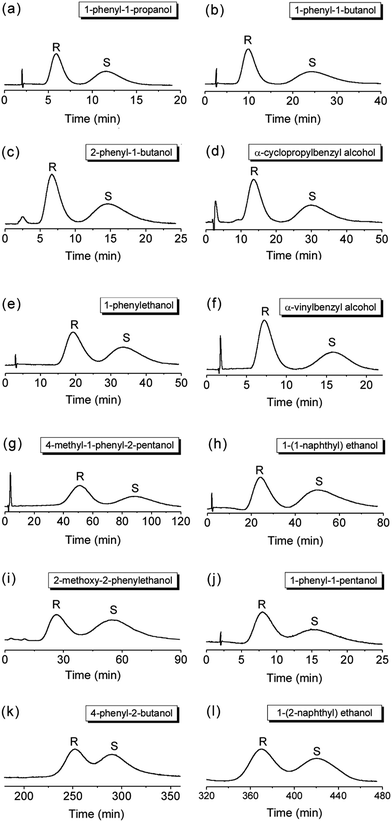
The γ-CD MOF packed column gave good resolution for the separation of chiral aromatic alcohols (Fig. 2). Twelve chiral aromatic alcohols including (R,S)-1-phenyl-1-propanol, (R,S)-1-phenyl-1-butanol, (R,S)-2-phenyl-1-butanol, (R,S)-α-cyclopropylbenzyl alcohol, (R,S)-1-phenylethanol, (R,S)-α-vinylbenzyl alcohol, (R,S)-4-methyl-1-phenyl-2-pentanol, (R,S)-1-(1-naphthyl) ethanol, (R,S)-2-methoxy-2-phenylethanol, (R,S)-1-phenyl-1-pentanol, (R,S)-4-phenyl-2-butanol, (R,S)-1-(2-naphthyl) ethanol were well separated on the γ-CD MOF packed column (Fig. 2). The elution order of all the studied aromatic alcohols follows the order of R-enantiomers < S-enantiomers, suggesting the γ-CD MOF have the larger affinity to S-enantiomers than their R-enantiomers. The chromatography parameters such as retention factor, selectivity and resolution for the separation of these chiral aromatic alcohols are summarized in Table 1. Mobile phase composition is a key factor in chiral HPLC.24,26 Therefore, separations of these chiral aromatic alcohols were performed under different conditions (Fig. 2).
| Chiral aromatic alcohols | K′1 | α | Rs |
|---|---|---|---|
| 1-Phenyl-1-propanol | 2.10 | 2.43 | 1.55 |
| 1-Phenyl-1-butanol | 4.20 | 2.76 | 1.74 |
| 2-Phenyl-1-butanol | 2.50 | 2.66 | 1.53 |
| α-Cyclopropylbenzyl alcohol | 6.17 | 2.38 | 1.66 |
| 1-Phenylethanol | 9.21 | 1.79 | 1.34 |
| α-Vinylbenzyl alcohol | 2.80 | 2.59 | 1.71 |
| 4-Methyl-1-phenyl-2-pentanol | 25.3 | 1.81 | 1.46 |
| 1-(1-Naphthyl) ethanol | 11.7 | 2.14 | 1.41 |
| 2-Methoxy-2-phenylethanol | 12.9 | 2.18 | 1.28 |
| 1-Phenyl-1-pentanol | 3.19 | 2.66 | 1.21 |
| 4-Phenyl-2-butanol | 131 | 1.15 | 0.70 |
| 1-(2-Naphthyl) ethanol | 194 | 1.14 | 0.83 |
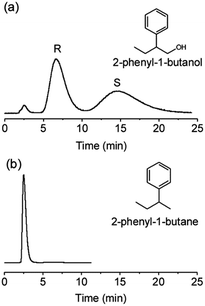
Although it is hard to precisely elucidate the chiral recognition mechanism on γ-CD MOF, the microenvironment of γ-CD MOF, the hydrophobic and hydrogen-bonding interaction between chiral aromatic alcohols and γ-CD MOF may play significant roles in high selectivity and good resolution of γ-CD MOF for chiral aromatic alcohols. The symmetric arrangement (C8) within the γ-CD torus of eight asymmetric (C1) α-1,4-linked D-glucopyranosyl residues led to the formation of γ-CD MOF as a chiral molecular building block.30 As the molecular dimensions of the studied chiral aromatic alcohols are smaller than the pore size of γ-CD MOF (14 Å) (Fig. S1, ESI†), the chiral aromatic alcohols can get into the pores of γ-CD MOF. The interaction between the aromatic alcohols with the most appropriate size and steric fit and the inner pore space of γ-CD MOF led to the good stereoselectivity. The hydrophobic nature of γ-CD cavity may lead to the hydrophobic interaction with the phenyl groups on chiral aromatic alcohols. In addition, the hydrogen-bonding interaction between chiral aromatic alcohols and γ-CD MOF may also occur during the chiral separation. To elucidate this hypothesis, the HPLC separation of (R,S)-1-phenyl-1-butane was compared (Fig. 3). The γ-CD MOF packed column gave no retention and resolution of (R,S)-1-phenyl-1-butane which has no hydroxy group. The co-elution of the racemic 1-phenyl-1-butane also reveals the important roles of the hydrophilic rim of γ-CD MOF for the chiral separation.30 To show the poor resolution of (R,S)-1-phenyl-1-butane does not result from the HPLC separation conditions, effect of mobile phase composition on the HPLC separation of (R,S)-1-phenyl-1-butane was studied (Fig. S2, ESI†). As a result, the resolution does not change with mobile phase composition, further suggesting the important role of hydrogen-bonding interaction between chiral aromatic alcohols and γ-CD MOF for high resolution separation of chiral aromatic alcohols. Chiral enantiomers have identical physical and chemical properties. Separations of them into pure enantiomers normally rely on the weak molecule interaction such as hydrogen-bonding, hydrophobic, and steric interaction between analytes and stationary phases. Therefore, long time are needed for some analytes (i.e., 460 min for (R,S)-1-(2-naphthyl) ethanol, Fig. 2l) to achieve baseline separation. Such time consuming chiral separation was also observed on the chiral MOF-based column.26
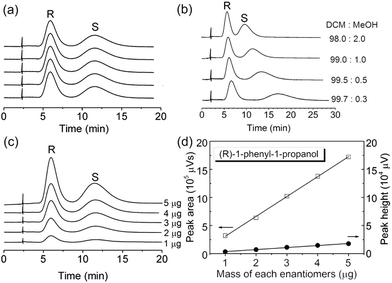
The γ-CD MOF packed column gave good reproducibility for the HPLC separation of chiral aromatic alcohols (Fig. 4a; Table S1, ESI†). The relative standard deviations (RSDs) for five replicate separations of (R,S)-1-phenyl-1-propanol are 0.3–0.4%, 1.5–2.1%, and 1.1–1.9% for the retention time, peak area, and peak height, respectively (Table S1, ESI†). Effect of mobile phase composition was also studied (Fig. 4b). Decrease of the MeOH content in the DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH binary mobile phase results in improved selectivity and resolution (Table S2, ESI†), showing the hydrogen-bonding interaction between the hydroxy groups on γ-CD MOF framework and 1-phenyl-1-propanol. The MeOH in the mobile phase should compete for the hydrogen-bonding interaction sites, leading to the decrease of the retention of (R,S)-1-phenyl-1-propanol. The chromatographic peak area and peak height of the 1-phenyl-1-propanol linearly increased as the analyte mass increased (Fig. 4c and d). The retention time for 1-phenyl-1-propanol enantisomers did not depend on the analyte mass though the increase in analyte mass.
MeOH binary mobile phase results in improved selectivity and resolution (Table S2, ESI†), showing the hydrogen-bonding interaction between the hydroxy groups on γ-CD MOF framework and 1-phenyl-1-propanol. The MeOH in the mobile phase should compete for the hydrogen-bonding interaction sites, leading to the decrease of the retention of (R,S)-1-phenyl-1-propanol. The chromatographic peak area and peak height of the 1-phenyl-1-propanol linearly increased as the analyte mass increased (Fig. 4c and d). The retention time for 1-phenyl-1-propanol enantisomers did not depend on the analyte mass though the increase in analyte mass.
To verify the good performance of the γ-CD MOF packed column for chiral aromatic alcohols, separation of these chiral aromatic alcohols were also studied on two typical commercial chiral HPLC columns41 (CHIRALPAK IA and IB, 25 cm long × 4.6 mm i.d.) (Fig. S3 and S4, ESI†). The CHIRALPAK IA column shows much higher resolution to (R,S)-4-phenyl-2-butanol and (R,S)-1-(2-naphthyl) ethanol than the γ-CD MOF packed column (Fig. S3k and l, ESI†). However, the γ-CD MOF packed column gave comparable resolution of (R,S)-1-phenyl-1-propanol, (R,S)-2-phenyl-1-butanol, (R,S)-α-cyclopropylbenzyl alcohol, (R,S)-1-phenylethanol, (R,S)-4-methyl-1-phenyl-2-pentanol and (R,S)-2-methoxy-2-phenylethanol, and even higher resolution to (R,S)-1-phenyl-1-butanol, (R,S)-α-vinylbenzyl alcohol, (R,S)-1-(1-naphthyl) ethanol and (R,S)-1-phenyl-1-pentanol than the CHIRALPAK IA column. In addition, the γ-CD MOF packed column gave better resolution to (R,S)-1-phenyl-1-butanol, (R,S)-α-vinylbenzyl alcohol, and (R,S)-1-phenyl-1-pentanol than the CHIRALPAK IB column (Fig. S4, ESI†). These results further confirm the promising application of γ-CD MOF for HPLC of chiral aromatic alcohols. The γ-CD MOF packed column was also tested on HPLC separation of antiarrhythmic drugs such as albuterol, epinephrine, noradrenaline, etc. However, these antiarrhythmic drugs cannot be separated on γ-CD MOF column.
In summary, we have reported the application of γ-CD MOF as an efficient CSP for HPLC separation of chiral aromatic alcohols with good precision, selectivity and resolution. The good performance of γ-CD MOF for chiral aromatic alcohols highly depends on the microenvironment of γ-CD MOF, the hydrophobic and hydrogen-bonding interaction between aromatic alcohols and γ-CD MOF. The results not only show the feasibility of γ-CD MOF as a specific CSP for chiral aromatic alcohols, but also reveal the potential of CD MOFs in chiral HPLC. Further works should pay more attention to the post-modification of CD MOFs or synthesis of spherical CD MOFs and CD MOFs composites for chiral separation or catalysis.
Acknowledgements
This work was supported by the National Basic Research Program of China (grant 2015CB932001), the National Natural Science Foundation of China (grant 21305071), and NFFTBS (No. J1103306).Notes and references
- N. M. Maier, P. Franco and W. Lindner, J. Chromatogr. A, 2001, 906, 3 CrossRef CAS PubMed.
- Y. Okamoto and T. Ikai, Chem. Soc. Rev., 2008, 37, 2593 RSC.
- E. R. Francotte, J. Chromatogr. A, 2001, 906, 379 CrossRef CAS PubMed.
- D. W. Armstrong and B. Zhang, Anal. Chem., 2001, 73, 557A CAS.
- M. Eddaoudi, D. B. Moler, H. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- Y. Liu, W. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112 CrossRef CAS PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS PubMed.
- P. Pelusoa, V. Mamane and S. Cossu, J. Chromatogr. A, 2014, 1363, 11 CrossRef PubMed.
- S.-M. Xie, Z.-J. Zhang, Z.-Y. Wang and L.-M. Yuan, J. Am. Chem. Soc., 2011, 133, 11892 CrossRef CAS PubMed.
- X.-H. Zhang, S.-M. Xie, A.-H. Duan, B.-J. Wang and L.-M. Yuan, Chromatographia, 2013, 76, 831 CAS.
- J.-R. Yang, S.-M. Xie, H. Liu, J.-H. Zhang and L.-M. Yuan, Chromatographia, 2015, 78, 557 CAS.
- Z.-X. Fei, M. Zhang, S.-M. Xie and L.-M. Yuan, Electrophoresis, 2014, 35, 3541 CrossRef CAS PubMed.
- Z.-X. Fei, M. Zhang, J.-H. Zhang and L.-M. Yuan, Anal. Chim. Acta, 2014, 830, 49 CrossRef CAS PubMed.
- S.-M. Xie, M. Zhang, Z.-X. Fei and L.-M. Yuan, J. Chromatogr. A, 2014, 1363, 137 CrossRef CAS PubMed.
- A. L. Nuzhdin, D. N. Dybtsev, K. P. Bryliakov, E. P. Talsi and V. P. Fedin, J. Am. Chem. Soc., 2007, 129, 12958 CrossRef CAS PubMed.
- M. Padmanaban, P. Muller, C. Lieder, K. Gedrich, R. Grunker, V. Bon, I. Senkovska, S. Baumgartner, S. Opelt, S. Paasch, E. Brunner, F. Glorius, E. Klemm and S. Kaskel, Chem. Commun., 2011, 47, 12089 RSC.
- K. Tanaka, T. Muraoka, D. Hirayama and A. Ohnish, Chem. Commun., 2012, 48, 8577 RSC.
- K. Tanaka, N. Hotta, S. Nagase and K. Yoza, New J. Chem., 2016, 40, 4891 RSC.
- K. Tanaka, T. Muraoka, Y. Otubo, H. Takahashi and A. Ohnishi, RSC Adv., 2016, 6, 21293 RSC.
- X. Kuang, Y. Ma, H. Su, J. Zhang, Y.-B. Dong and B. Tang, Anal. Chem., 2014, 86, 1277 CrossRef CAS PubMed.
- R. Hailili, L. Wang, J.-Z. Qv, R.-X. Yao, X.-M. Zhang and H.-W. Liu, Inorg. Chem., 2015, 54, 3713 CrossRef CAS PubMed.
- M. Zhang, Z.-J. Pu, X.-L. Chen, X.-L. Gong, A.-X. Zhu and L.-M. Yuan, Chem. Commun., 2013, 49, 5201 RSC.
- M. Zhang, X.-D. Xue, J.-H. Zhang, S.-M. Xie, Y. Zhang and L.-M. Yuan, Anal. Methods, 2014, 6, 341 RSC.
- M. Zhang, X.-L. Chen, J.-H. Zhang, J. Kong and L.-M. Yuan, Chirality, 2016, 28, 340 CrossRef CAS PubMed.
- M. Zhang, J.-H. Zhang, Y. Zhang, B.-J. Wang, S.-M. Xie and L.-M. Yuan, J. Chromatogr. A, 2014, 1325, 163 CrossRef CAS PubMed.
- R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 8630 CrossRef CAS PubMed.
- Y. Furukawa, T. Ishiwata, K. Sugikawa, K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2012, 51, 10566 CrossRef CAS PubMed.
- J. M. Holcroft, K. J. Hartlieb, P. Z. Moghadam, J. G. Bell, G. Barin, D. P. Ferris, E. D. Bloch, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, K. M. Thomas, J. R. Long, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 5706 CrossRef CAS PubMed.
- K. J. Hartlieb, J. M. Holcroft, P. Z. Moghadam, N. A. Vermeulen, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2016, 138, 2292 CrossRef CAS PubMed.
- H. Li, M. R. Hill, R. Huang, C. Doblin, S. Lim, A. J. Hill, R. Babaraoa and P. Falcaro, Chem. Commun., 2016, 52, 5973 RSC.
- X.-N. Xu, C.-F. Wang, H.-Y. Li, X. Li, B.-T. Liu, V. Singh, S.-X. Wang, L.-X. Sun, R. Gref and J.-W. Zhang, J. Chromatogr. A, 2017, 1488, 37 CrossRef CAS PubMed.
- Y.-H. Wei, D. Sun, D.-Q. Yuan, Y.-J. Liu, Y. Zhao, X.-Y. Li, S.-N. Wang, J.-M. Dou, X.-P. Wang, A.-Y. Hao and D.-F. Sun, Chem. Sci., 2012, 3, 2282 RSC.
- H.-M. Ma, X.-J. Li, T. Yan, Y. Li, H.-Y. Liu, Y. Zhang, D. Wu, B. Du and Q. Wei, ACS Appl. Mater. Interfaces, 2016, 8, 10121 CAS.
- E. Schneiderman and A. M. Stalcup, J. Chromatogr. B: Biomed. Sci. Appl., 2000, 745, 83 CrossRef CAS.
- T. Araki, Y. Kashiwamoto, S. Tsunoi and M. Tanaka, J. Chromatogr. A, 1999, 845, 455 CrossRef CAS.
- W. L. Hinze, T. E. Riehl, D. W. Armstrong, W. DeMond, A. Alak and T. Ward, Anal. Chem., 1985, 57, 237 CrossRef CAS.
- S. Caccamese, S. Bianca and G. T. Carter, Chirality, 2007, 19, 647 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06558b |
| This journal is © The Royal Society of Chemistry 2017 |