Open Access Article
Open Access ArticlePrepared MnO2 with different crystal forms as electrode materials for supercapacitors: experimental research from hydrothermal crystallization process to electrochemical performances
Xiaoyu Zhao *a,
Yongdan Houb,
Yanfei Wang*a,
Libin Yanga,
Liang Zhua,
Ruge Caoc and
Zuoliang Shaa
*a,
Yongdan Houb,
Yanfei Wang*a,
Libin Yanga,
Liang Zhua,
Ruge Caoc and
Zuoliang Shaa
aTianjin Key Laboratory of Marine Resources and Chemistry, College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology, Tianjin 300457, China. E-mail: xyz@tust.edu.cn; wangyanfei@tust.edu.cn
bCollage of Engineering, Kwame Nkrumah University of Science and Technology, Ghana
cCollege of Food Engineering and Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China
First published on 17th August 2017
Abstract
The aim of this study was to prepare manganese dioxide with different crystal forms through hydrothermal treatment of MnSO4·H2O–KMnO4 precursors at various precursor ratios, temperatures, time periods, and pH values. The effect of the preparation parameters on the crystal structure and electrochemical performance of MnO2 was also studied. The conditions for the preparation of each crystal of MnO2 were experimentally optimized. The results indicate that the varied parameters affect the crystal form of MnO2; the ratio of the precursor and reaction temperature are the significant factors affecting the crystal form of MnO2, and the reaction time and pH value mainly affect the integrity of crystallization. Moreover, the presence of the cations K+ and NH4+ assists the crystallization of α-MnO2. δ-MnO2 and amorphous MnO2 have higher specific capacitance. The specific capacitance is determined not only by the crystal form, but also by the parameters of hydrothermal treatment.
1 Introduction
Supercapacitors can be categorized into electric double-layer capacitors and faradaic pseudocapacitors based on the mechanism of charge storage.1 The former store energy by forming electric double layers between the electrode and the electrolyte, whereas the latter make use of highly reversible chemical intercalation and deintercalation of electrolytic ions at the surface, or in-phase of electroactive species or redox reactions of electrolytic ions at the electrode to store electric charges.2 The charging and discharging behaviours of faradaic pseudocapacitors are quite similar to those of electric double-layer capacitors; however, in the case of electric double-layer capacitors, the electrode potential is proportional to the loaded electric charges, the voltage is linear to the applied time, and the detected current is almost constant.3 It has been proven4 that faradaic process can not only prolong the working time, but also increase the specific capacitance of the capacitor. Due to both the interactions of the electrolytic ions with the surface and in-phase of electroactive species, the energy density stored in this process will be 10–100 times higher than that stored via only a superficial process.5As electrode materials of supercapacitors, metallic oxides are believed to have higher faradaic pseudocapacitance rather than the electric double-layer capacitance formed at the surface of the carbon electrode. The faradaic pseudocapacitance can be induced not only at the interface of the electrode|electrolyte, but also inside metallic oxides via fast and reversible redox reactions; therefore, the energy is stored in a three-dimensional space, which improves the utilization rate of materials to provide a relatively high specific capacitance and energy density.6
Manganese dioxide (MnO2) as an electrode material for supercapacitors is of great potential. In faradaic pseudocapacitors, the merits of the MnO2 electrode material are as follows: high theoretical specific capacitance, wide potential window, rich reserves, environmental friendliness, etc. However, the low conductivity (10−5 to 10−6 S cm−1) and unstable structure of MnO2, leading to poor electrochemical cyclability, limit its application. To overcome these problems, many researchers have tried to design a nano-MnO2 structure to improve the electrochemical behaviour. It has been reported that the electrochemical performance of MnO2 depends on the surface area, morphology, and crystal structure and its features.7,8
The mechanism of energy storage by MnO2 materials is generally of two types. First is via surface adsorption–desorption: H+ or basic ions C+ (Li+, Na+, and K+) adsorb at the surface of electrode to store charges. Second is via electron–proton transfer: charges is stored via fast and reversible redox reactions, via which H+ or basic ions C+ (Li+, Na+, and K+) intercalate in or deintercalate out of both the surface materials and materials inside the crystal structure. Reaction equations are given as follows (eqn (1-1) and (1-2)):
| (MnO2)surface + M+ + e− ↔ (MnO2·M)surface | (1-1) |
| MnO2 + M+ + e− ↔ MnOOM | (1-2) |
Zhang et al.15 prepared MnO2 with different kinds of crystal structures and morphologies via redox reactions of potassium permanganate and sodium nitrite using a hydrothermal process. They also found that nano-α-MnO2 ball with a low crystalline degree demonstrated a very high specific capacitance of 200 F g−1. Rusi et al.16 also prepared α-MnO2 with a low crystalline degree, but through electrochemical deposition in a manganese acetate tetrahydrate electrolyte. The prepared α-MnO2 characterized by cyclic voltammetry test in the Na2SO4 electrolyte at a scan rate of 1 mV s−1 showed a high specific capacitance of 238 F g−1 and good stability; after cycling for 1900 times, the capacitance retention was 84%. Myeongjin Kim et al.17 also prepared graphene oxide (GO)/MnO2 via one-step hydrothermal crystallization as compared to reduced graphene oxide (rGO) prepared by reducing GO with hydrazine hydrate and phosphate acid; needle-shaped MnO2 grew on reduced graphene oxide to form H-rGO/MnO2 and S-rGO/MnO2. In cyclic voltammetry test, the needle-shaped MnO2 loaded onto reduced graphene oxide forming nano-composites of H-rGO/MnO2 shows a high specific capacitance of 327.5 F g−1 in a 1 M NaSO4 electrolyte at a scan rate of 10 mV s−1. Previous researchers have achieved ideal results with beautiful nano-architectures and excellent electrochemical performance with the aim of enhancing the capacity via few specific technologies, for example, heterogeneous doping,18–24 morphological design and control,25–30 crystal structure design and selection,31–33 and so on, which are indeed effective in enhancing the capacity and conductance of MnO2. However, no systematic research on the hydrothermal crystallization process of powder MnO2 considering all operating parameters and electrochemical performances for different crystal forms of MnO2 has been reported to date. Herein, MnSO4·H2O–KMnO4 was applied as a precursor. By varying the ratio of the precursor, reaction temperature, time, and pH value, α-MnO2, β-MnO2, and δ-MnO2 were prepared. The effect of cationic dopant on the crystal form of MnO2 was also examined. Finally, the electrochemical properties of prepared MnO2 with different crystal forms were studied for its applications in supercapacitors.
2 Experiments
2.1 Preparation of MnO2
All the chemicals used were of analytical grade. Non-purified polycrystalline MnO2 was simply prepared via one-step hydrothermal synthesis. The classical synthesis procedures are as follows: different designed amounts of potassium permanganate (KMnO4) were weighed and dissolved in 60 mL distilled water. When it was fully dissolved, the pH value of the solution was adjusted by adding 1 M H2SO4 or NH3·H2O. After the addition of MnSO4·H2O to the above mentioned solution, a black precipitate was formed. The solution was then stirred at room temperature for 30 min and then transferred into a 100 mL Teflon reactor, which was later placed in an incubator for hydrothermal reaction. The reactor was removed after different reaction times and then naturally cooled down to room temperature. The filtered residue was washed with distilled water till the filtrate became neutral. Finally, the solid product was frozen and dried. By changing the ratio of MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4, time duration of the hydrothermal process, pH value, and amount of additive cations, powder MnO2 with different crystal forms was obtained.
KMnO4, time duration of the hydrothermal process, pH value, and amount of additive cations, powder MnO2 with different crystal forms was obtained.
2.2 X-ray diffraction characterization of MnO2
X-ray diffraction was used to characterize the phase and weight percentage of manganese dioxides prepared under the conditions of different MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 ratios, hydrothermal treatment time durations, pH values, and cations doped.
KMnO4 ratios, hydrothermal treatment time durations, pH values, and cations doped.
2.3 Assembly of electrodes
(I) Porous nickel foam was used as a current collector and cut into 2.5 × 2 cm2 as a matrix of electrode sheet, which was later washed with acetone for 10 min, distilled water for 5 min, 1 M HCl for 20 min, distilled water for 5 min, and anhydrous ethanol for 5 min. After washing, the cut foam was dried in an incubator at 50 °C for eight hours and then pressed and weighed. The weight was obtained as m.(II) Powered active species, acetylene black, and polyvinylidene fluoride (PVDF) were mixed in the ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An appropriate amount of N-methyl pyrrolidone (NMP), as a binder, was added to sufficiently wet the powder. Through magnetic stirring and ultrasonication, the powder mixture and the binder were uniformly mixed.
1. An appropriate amount of N-methyl pyrrolidone (NMP), as a binder, was added to sufficiently wet the powder. Through magnetic stirring and ultrasonication, the powder mixture and the binder were uniformly mixed.
(III) The abovementioned mixture was blade coated on nickel foam and then placed in a drying oven at 50 °C to remove the solvent. The active species-loaded nickel foam was then pressed into an electrode sheet, the weight of which was obtained as m1; the mass of the active species was calculated from the difference in mass before and after they were coated on nickel foam.
(IV) The electrode sheet was placed in the prepared 1 M Na2SO4 electrolyte till it was completely wet; after this, the electrochemical test was carried out.
2.4 Electrochemical test
Ivium potentiostat was used for cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), electrochemical impedance spectroscopy (EIS) analyses of electrode materials. A three-electrode system was applied, in which the active species, platinum wire (Pt), and saturated calomel electrode (SCE) were used as the working electrode, counter electrode, and reference electrode, respectively. The electrolyte applied was 1 M NaSO4. The potential window for the CV test was 0–0.8 V, and the frequency range for the EIS test was from 100 kHz to 0.01 Hz.3 Results and discussion
3.1 Factors of hydrothermal process influencing the MnO2 crystal forms
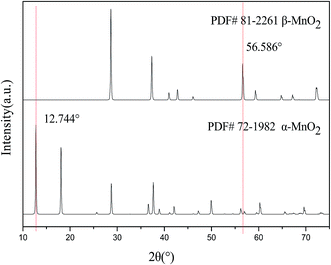
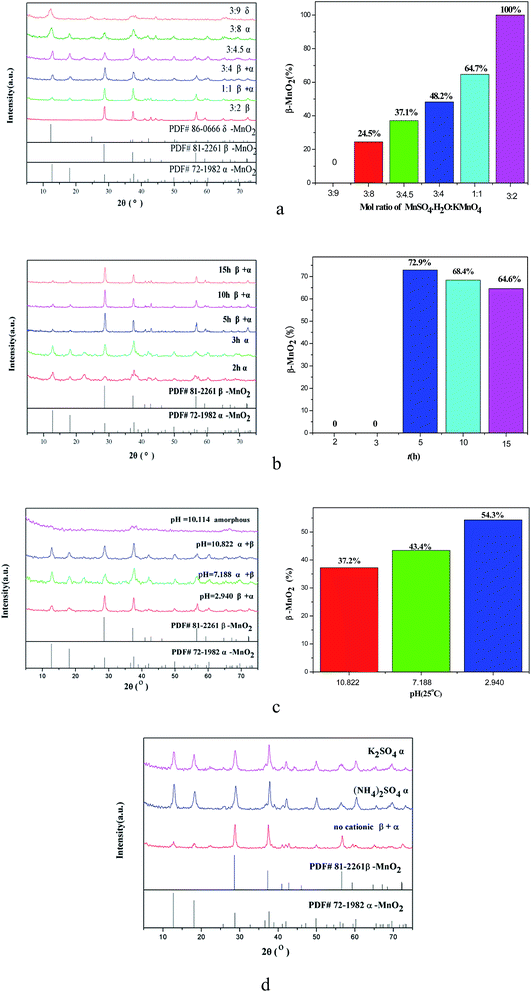
Fig. 1 shows the XRD patterns of both α-MnO2 and β-MnO2.15 On comparing the graphs of two different crystals, it was observed that the diffraction angles of characteristic peaks of both α-MnO2 and β-MnO2 were 12.744° and 56.586°, respectively, via ratio of which, for instance, I12.744°/(I12.744° + I56.586°), the relative amount of α-MnO2 was obtained.Fig. 2(a) displays the XRD patterns of MnO2 obtained via hydrothermal synthesis at different ratios of the MnSO4·H2O–KMnO4 precursor. It can be observed from Fig. 2(a) that when the ratio was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the product was β-MnO2; when the ratio was 1
2, the product was β-MnO2; when the ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, α-MnO2 appeared; when the ratio was 3
1, α-MnO2 appeared; when the ratio was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5, the amount of α-phase increased; with a decrease in the amount of MnSO4·H2O, the ratio became 3
4.5, the amount of α-phase increased; with a decrease in the amount of MnSO4·H2O, the ratio became 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, and the peaks of the products δ-MnO2 and trace amount of Mn3O4 were observed at the diffraction angles of 36.091°, 36.487°, 38.033°, and 44.425°. The presence of Mn3O4 with poor crystallinity might be caused by the little amount formed immediately when MnSO4·H2O and KMnO4 reacted with each other, or by crystal defects.
9, and the peaks of the products δ-MnO2 and trace amount of Mn3O4 were observed at the diffraction angles of 36.091°, 36.487°, 38.033°, and 44.425°. The presence of Mn3O4 with poor crystallinity might be caused by the little amount formed immediately when MnSO4·H2O and KMnO4 reacted with each other, or by crystal defects.
 | ||
| Fig. 2 XRD patterns of MnO2 obtained by hydrothermal synthesis (a) at different ratios, (b) different reaction times, (c) different pH values, and (d) different cations doped. | ||
The analysis indicates that when the ratio of MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 is reduced, MnO2 with a β-phase and mixtures of β- and α-phase and α-phase and δ-phase is obtained. There are three reasons for the formation of these products: first, K+ intercalates into the crystal lattice of MnO2 to support the lattice and form a larger [2 × 2] tunnel structure. Second, K+ preferentially adsorbs at the surface of [MnO6]; this decreases the surface energy of [MnO6], leading the co-edge of [MnO6] unit to form a [2 × 2] tunnel structure. Third, from the viewpoint of crystallization, with a constant quantity of KMnO4 but reduced quantity of MnSO4·H2O, the degree of supersaturation of the crystallized product decreased, which influenced the nucleation of the crystal to form MnO2 with different phases. The slower the reaction, the lower the supersaturation level; under this condition, α-MnO2 is more likely to be formed. With the increasing amount of KMnO4, when MnSO4·H2O
KMnO4 is reduced, MnO2 with a β-phase and mixtures of β- and α-phase and α-phase and δ-phase is obtained. There are three reasons for the formation of these products: first, K+ intercalates into the crystal lattice of MnO2 to support the lattice and form a larger [2 × 2] tunnel structure. Second, K+ preferentially adsorbs at the surface of [MnO6]; this decreases the surface energy of [MnO6], leading the co-edge of [MnO6] unit to form a [2 × 2] tunnel structure. Third, from the viewpoint of crystallization, with a constant quantity of KMnO4 but reduced quantity of MnSO4·H2O, the degree of supersaturation of the crystallized product decreased, which influenced the nucleation of the crystal to form MnO2 with different phases. The slower the reaction, the lower the supersaturation level; under this condition, α-MnO2 is more likely to be formed. With the increasing amount of KMnO4, when MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 reaches 3
KMnO4 reaches 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, δ-MnO2 is obtained via the hydrothermal treatment at 180 °C for 5 h. The formed δ-MnO2 with a larger surface area and stratified structure was shaped via the rearrangement of [MnO6] units induced by an increase in the concentration of potassium ions.
9, δ-MnO2 is obtained via the hydrothermal treatment at 180 °C for 5 h. The formed δ-MnO2 with a larger surface area and stratified structure was shaped via the rearrangement of [MnO6] units induced by an increase in the concentration of potassium ions.
Based on the abovementioned discussion, the summary can be drawn as follows: in the MnSO4·H2O–KMnO4 system, if MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 ≥3
KMnO4 ≥3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the product of hydrothermal crystallization is β-MnO2. If the ratio of MnSO4·H2O to KMnO4 falls between 3
2, the product of hydrothermal crystallization is β-MnO2. If the ratio of MnSO4·H2O to KMnO4 falls between 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 and 1
4.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the product is a mixture of β- and α-MnO2. If MnSO4·H2O
1, the product is a mixture of β- and α-MnO2. If MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 falls between 3
KMnO4 falls between 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and 3
12 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the product is δ-MnO2. Furthermore, [MnO6] unit in β-MnO2 forms single-stranded octahedron via the common edge and extends along the crystal axis to form a [1 × 1] tunnel structure. However, the channel is very small (0.189 nm in diameter) with few crystal defects and water to support the movement of cations. However, the [MnO6] unit in α-MnO2 forms single- or double-stranded octahedron via the common edge and [1 × 1] and [2 × 2] tunnel structures, between which the [1 × 1] channel is hollow, but small (0.189 nm in diameter), and the [2 × 2] channel with a diameter of 0.46 nm is helpful for basic ions (such as K+) to intercalate and deintercalate. Interestingly, δ-MnO2, with a special stratified structure with an interspacing of 0.7 nm, is beneficial for the free transportation of charged ions, protons, electrons, etc., which further enhances the transfer coefficient of electrons for ion exchange or redox reactions, leading to no damage of the crystal stratified structure.
9, the product is δ-MnO2. Furthermore, [MnO6] unit in β-MnO2 forms single-stranded octahedron via the common edge and extends along the crystal axis to form a [1 × 1] tunnel structure. However, the channel is very small (0.189 nm in diameter) with few crystal defects and water to support the movement of cations. However, the [MnO6] unit in α-MnO2 forms single- or double-stranded octahedron via the common edge and [1 × 1] and [2 × 2] tunnel structures, between which the [1 × 1] channel is hollow, but small (0.189 nm in diameter), and the [2 × 2] channel with a diameter of 0.46 nm is helpful for basic ions (such as K+) to intercalate and deintercalate. Interestingly, δ-MnO2, with a special stratified structure with an interspacing of 0.7 nm, is beneficial for the free transportation of charged ions, protons, electrons, etc., which further enhances the transfer coefficient of electrons for ion exchange or redox reactions, leading to no damage of the crystal stratified structure.
Fig. 2(b) displays the XRD results of the effect of time duration of hydrothermal treatment on the crystal forms of MnO2. It can be observed from Fig. 2(b) that the relative amounts of β-MnO2 are 72.9%, 68.4%, and 64.6% for 5 h, 10 h, and 15 h treatment, respectively. The amount of β-MnO2 decreases with the increasing treatment time; this indicates that the mixture formed in the first three hours has poor thermal stability and, therefore, continuously transforms into β-MnO2 under treatment. Therefore, hydrothermal treatment of 3 h will be preferred for forming α-MnO2 with good crystallinity. During the hydrothermal treatment, the reaction time was proven to have a significant influence on the growth of nano-materials; moreover, Yang et al.14 have reported that the growth of crystal and its morphology were controlled by a curing process. As the curing time increases, large grains continue to grow completely, but small grains gradually disappear to feed the growth of large grains due to different solubilities of large and small grains, which was driven by reduction of surface energy. Within 3–5 h, the transformation from α- to β-phase can occur due to a relatively higher K+ concentration induced by re-crystallization.
Fig. 2(c) shows the XRD results of crystal forms of MnO2 prepared at different pH values. It can be observed from Fig. 2(c) that the product is a mixture of 66.7% β-MnO2 and α-MnO2 with good crystallinity at a pH value of 2.940 adjusted by 1 mol L−1 H2SO4; however, the product at neutral is 43.4% β-MnO2 with poor crystallinity, whereas the β-MnO2 content in the product becomes 37.2% with good crystallinity at a pH value of 10.822 adjusted by 1 mol L−1 NH3·H2O. The percentage of product under different pH conditions indicates that when the concentration of cations is higher than the concentration of H+ ions, the product is mainly α-MnO2 with a [2 × 2] tunnel structure. It has been reported that by adjusting the pH value, the optimum matched degree of hydrolysis can be controlled;34 this further clarifies that the pH value mainly affects the solubility of the precursor, causing the presence of [MnO6] unit in different aggregates with various solubilities and degrees of hydrolysis. Therefore, this is consistent with the experimental result that acidic environment is helpful for the growth of β-MnO2, but basic environment is beneficial for the formation of α-MnO2. With the MnSO4·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KMnO4 ratio of 1
KMnO4 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a temperature of 150 °C, reaction time of 5 h, and a pH value ranging from 2 to 10, the mixtures of β- and α-MnO2 were obtained as the products of the hydrothermal process.
1, a temperature of 150 °C, reaction time of 5 h, and a pH value ranging from 2 to 10, the mixtures of β- and α-MnO2 were obtained as the products of the hydrothermal process.
Fig. 2(d) illustrates the XRD results of the crystal forms of MnO2 prepared with different amounts of cations doped. The products obtained under the conditions of a precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 180 °C, and 5 h were mixtures of β- and α-MnO2. However, the crystal form started to transform when the cations were doped into the system. In detail, [MnO6] octahedron with a common edge forms β-MnO2 with a [1 × 1] single-stranded channel along the C axis, which later transforms to a double-stranded channel. The double-stranded octahedron shares a common apical angle with the single-stranded octahedron to form α-MnO2 with a [1 × 1] + [2 × 2] tunnel structure. Besides this, K+, H+, NH4+, and H2O can support the large crystal lattice without damage, enlarge the path of cations and electrons, and enhance the transport coefficient. The main contribution to the improvement is likely to be the different surface energy of [MnO6] octahedrons, which caused the variation of the linking forms among the octahedrons units leading to the appearance of crystal defects, finally, causing the formation of channels and vugular channels with different unit cell parameters.
1, 180 °C, and 5 h were mixtures of β- and α-MnO2. However, the crystal form started to transform when the cations were doped into the system. In detail, [MnO6] octahedron with a common edge forms β-MnO2 with a [1 × 1] single-stranded channel along the C axis, which later transforms to a double-stranded channel. The double-stranded octahedron shares a common apical angle with the single-stranded octahedron to form α-MnO2 with a [1 × 1] + [2 × 2] tunnel structure. Besides this, K+, H+, NH4+, and H2O can support the large crystal lattice without damage, enlarge the path of cations and electrons, and enhance the transport coefficient. The main contribution to the improvement is likely to be the different surface energy of [MnO6] octahedrons, which caused the variation of the linking forms among the octahedrons units leading to the appearance of crystal defects, finally, causing the formation of channels and vugular channels with different unit cell parameters.
3.2 Electrochemical characterizations
Using cyclic voltammetry curves to calculate the specific capacitance, the value of charges is obtained by integrating the CV curve. Based on the weight of the active species on the electrode, the specific capacitance can be calculated using the eqn (2-1):
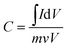 | (2-1) |
The calculation of the specific capacitance via GCD curves is shown in eqn (2-2):
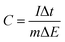 | (2-2) |
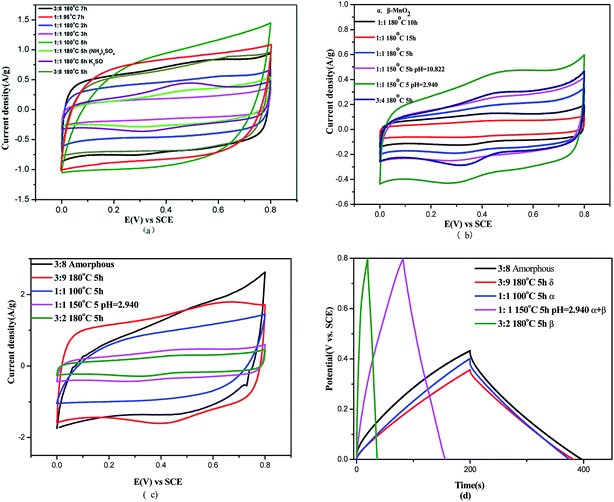
Fig. 3(a) shows the cyclic voltammetry curves of α-MnO2 prepared under various conditions at a scan rate of 10 mV s−1. It can be observed that all eight α-MnO2 samples display quasi-rectangular CV curves without obvious redox peaks between 0 and 0.5 V and 0 and 0.8 V against the SCE reference electrode; this indicates relatively good capacitive characteristics.35 In particular, the α-MnO2 sample prepared at 100 °C held for 5 h exhibits the highest absolute responding current within the commonly wide potential window from 0 to 0.8 V against the SCE reference electrode; this suggests the better capacitance characteristics under the control of potential limit of hydrogen and oxygen evolution in an aqueous electrolyte.
Fig. 3(b) and (c) show the cyclic voltammetry curves of α- and β-MnO2 and δ-MnO2, respectively, prepared under various conditions at a scan rate of 10 mV s−1. The existence of both electric double-layer capacitance and faradaic pseudocapacitance can be realized through quasi-rectangular CV curves and minor redox reaction peaks in all the samples in both Fig. 3(b) and (c).
The quasi-rectangular CV curves indicate the presence of adsorption and desorption processes at the interface of electrode|electrolyte (electric double layers). In Fig. 3(b), in particular, the CV curve of the α + β-MnO2 sample, prepared at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of precursor and a pH value of 2.940 and hydrothermally treated at 150 °C for 5 h, shows the largest closed area among all the α + β-MnO2 samples, suggesting better electrochemical property in the potential window of 0–0.8 V.
1 ratio of precursor and a pH value of 2.940 and hydrothermally treated at 150 °C for 5 h, shows the largest closed area among all the α + β-MnO2 samples, suggesting better electrochemical property in the potential window of 0–0.8 V.
Symmetrical redox peaks in both Fig. 3(b) and (c) indicate good reversible faradaic processes.36 In Fig. 3(c), the minor redox peaks for δ-MnO2 are induced by storage of charges through free tunnel deintercalation of electrolytic ions (hydrated Na+, 0.358 nm in radius) within a two-dimensional stratified structure with a 0.7 nm spacing of layers. Generally, the more sufficient the redox reactions occurring through electrons and electrolytic ions with electrode crystals, the higher the reaction peaks; the faster the reactions, the sharper the peaks in the CV curves. Therefore, the CV curves of δ-MnO2 show a quick response (sharp peak) to current steering, as shown in Fig. 3(c), indicating a fast reaction resulting from small inner resistance; however, the CV curves of β-MnO2 show irregular shapes with a small closed area, but better rectangular shape, which indicates good capacitive properties.
To obtain more information on how suitable the products are for electrode materials of supercapacitors, galvanostatic charge–discharge tests were performed on different crystal forms of MnO2 in 1 M Na2SO4 at 0.5 A g−1, as illustrated in Fig. 3(d). The plots obtained are nearly linear, indicating that the prepared MnO2 have good pseudocapacitive characterization. The specific capacitances were 286, 111.6, 26.8, and 3.1 F g−1 for δ-, α-, α + β-, and β-MnO2, respectively. Obviously, δ-MnO2 exhibits best specific capacitance. α-MnO2 has a 1D structure with (2 × 2) and (1 × 1) tunnels,37 whereas δ-MnO2 has a 2D layered structure.38 Protons can be intercalated into the wide (2 × 2) tunnels (∼4.6 Å) of α-MnO2 and in between the layers (∼7 Å) of 2D δ-MnO2. The presence of narrow (1 × 1) tunnels in β-MnO2 makes it unsuitable for capacitor application because it cannot accommodate cations during charge–discharge cycling.37
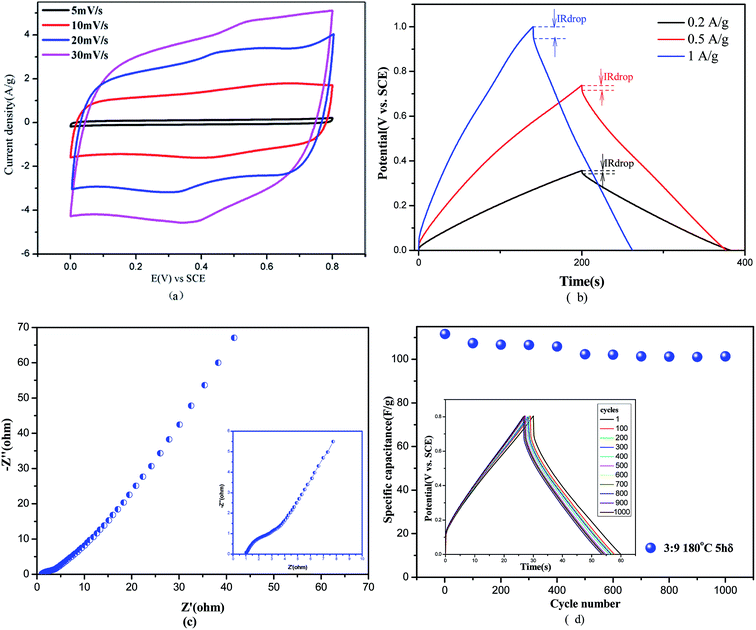
The cyclic voltammetry curves of δ-MnO2 prepared at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 precursors ratio at 100 °C held for 2 h, were studied at the scan rates of 5, 10, 20, and 30 mV s−1, as shown in Fig. 4(a), which displays the specific capacitances of 280, 270, 268, and 242 F g−1, respectively, according to the eqn (2-1). The well-presented quasi-rectangular CV curves can also be observed. With the increase in scan rate, the shape of the CV curve deforms; this suggests a conjunct influence of both electric double-layer and pseudofaradaic capacitances.36,39 At 30 mV s−1, the capacitance retention was still 86.4%; therefore, the product had very good capacitive performance and cycling stability.
1 precursors ratio at 100 °C held for 2 h, were studied at the scan rates of 5, 10, 20, and 30 mV s−1, as shown in Fig. 4(a), which displays the specific capacitances of 280, 270, 268, and 242 F g−1, respectively, according to the eqn (2-1). The well-presented quasi-rectangular CV curves can also be observed. With the increase in scan rate, the shape of the CV curve deforms; this suggests a conjunct influence of both electric double-layer and pseudofaradaic capacitances.36,39 At 30 mV s−1, the capacitance retention was still 86.4%; therefore, the product had very good capacitive performance and cycling stability.
The galvanostatic charge–discharge (GCD) tests of δ-MnO2 were further considered to examine the electrochemical performance, as shown in Fig. 4(b). It can be seen from Fig. 4(b) that the GCD curves present symmetrical triangles, which suggest good charging–discharging reversibility, and little IR drops (caused by interfacial potential of different contacting phases, E = IR, determined by current passing through the inner resistance). The specific capacitances can be obtained from Fig. 4(b) via eqn (2-2), which are 571 F g−1, 286 F g−1, and 140 F g−1 at the current densities of 0.2 A g−1, 0.5 A g−1, and 1 A g−1, respectively. It has been reported that with an increase in current density, the storage of electrolytic ions and electrons in the crystal of MnO2 drops. Most electrolyte ions and electrons can only approach the surface via adsorption–desorption to store energy, which have small specific capacitance.40
Fig. 4(c) illustrates the Nyquist plot of δ-MnO2, commonly composed of a half circle at high frequency and a straight line at low frequency. This combination indicates that the electrode process was controlled by electron transport (kinetics of electrochemical reaction) and diffusion of reactant(s) or product(s) of electrode reactions.41 At high-frequency range, the plot met the Z′ axis at Rs, which is the resistance of solution, including inner resistance of the electrode material, resistance of diffusion of electrolytic ions, and contact resistance between the active species and current collector.42,43 The resistance of solution shown in Fig. 4(c) is 1 Ω. The radius of the half circle at high frequency indicates a small resistance of electron transfer (Rct) in the Faraday's process and easy passage of electrons through electrodes for energy storage.44,45 It can also be observed from Fig. 4(c) that at low frequency, a straight line presents diffusion impedance, Zw, with the angle between 45° and 90°, illustrating pseudocapacitive reaction under diffusion control; a line with an angle of 45° indicates diffusion control and that with an angle of 90° indicates an ideal pseudocapacitive reaction.
Apart from specific capacitance, the cycling life of the material for supercapacitors is also an important aspect.46 Fig. 4(d) displays the specific capacitance at a current density of 3 A g−1 for δ-MnO2 prepared at the precursor ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, hydrothermally treated at 180 °C for 5 h. Generally, the fading of capacitance retention resulted from the irreversible product Mn(OH)2, which destroyed the structure of MnO2 and resulted in a decrease in reactive sites at the surface of electrodes, leading to an increase in inner resistance. The reduced reactive sites at the surface, causing a low utilization rate of electrode materials, lead to a drop of specific capacitance. However, in Fig. 4(d), capacitance retention of 91.8% can be observed after 1000 cycling times, and the specific capacitance only dropped by 8.6% after 1000 cycling times, presenting a relatively high cycling life.
9, hydrothermally treated at 180 °C for 5 h. Generally, the fading of capacitance retention resulted from the irreversible product Mn(OH)2, which destroyed the structure of MnO2 and resulted in a decrease in reactive sites at the surface of electrodes, leading to an increase in inner resistance. The reduced reactive sites at the surface, causing a low utilization rate of electrode materials, lead to a drop of specific capacitance. However, in Fig. 4(d), capacitance retention of 91.8% can be observed after 1000 cycling times, and the specific capacitance only dropped by 8.6% after 1000 cycling times, presenting a relatively high cycling life.
4 Conclusions
Manganese dioxide with different crystal forms was prepared by varying the ratio of the precursor MnSO4·H2O–KMnO4, reaction temperature, time, and pH values. The electrochemical performances of the prepared MnO2 were studied as well. The conclusions are as follows:(1) In the MnSO4·H2O–KMnO4 system, MnO2 with a controlled crystal form can be obtained via hydrothermal treatment from the following parameters:
α-MnO2; MnSO4·H2O–KMnO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 100 °C, t = 5 h, pH at neutral;
1, T = 100 °C, t = 5 h, pH at neutral;
β-MnO2; MnSO4·H2O–KMnO4 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, T = 180 °C, t = 5 h, pH at neutral;
2, T = 180 °C, t = 5 h, pH at neutral;
δ-MnO2; MnSO4·H2O–KMnO4 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, T = 100 °C, t = 3 h, pH at neutral.
8, T = 100 °C, t = 3 h, pH at neutral.
(2) Cationic dopants K+ and NH4+ are beneficial for the formation of α-MnO2.
(3) Specific capacitances of the prepared MnO2 are in the following order: δ-MnO2 > amorphous MnO2 > α-MnO2 > α + β-MnO2 > β-MnO2. Therefore, the optimal parameters for hydrothermal crystallization of powder MnO2 as a starting material for capacitor electrode plate are MnSO4·H2O–KMnO4 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, T = 100 °C, t = 3 h, and neutral pH.
8, T = 100 °C, t = 3 h, and neutral pH.
(4) Specific capacitance of δ-MnO2 displays a specific capacitance of 571 F g−1 at a current density of 0.2 A g−1 and a capacitance retention of 91.4% after cycling for 1000 times.
(5) The prepared MnO2 crystal powders from the MnSO4·H2O–KMnO4 system have relatively small equivalent series resistance, which is helpful for the transport of electrolytic ions and electrons. These observations display their potential capability as electrode materials for supercapacitors; however, it is necessary to significantly enhance their conductivity using heterogeneous doping during the fabrication of electrode plates from crystal powder for their practical applications in supercapacitors.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (NSFC Grant no. 21503146), the Yangtze Scholars and Innovative Research Team in Chinese University (IRT-17R81), and the Innovative Research Team of Tianjin Municipal Education Commission (TD12-5004) to support the work.References
- S. L. Zhang and N. Pan, Supercapacitors performance evaluation, Adv. Energy Mater., 2015, 5(6), 1–19 CrossRef.
- H. Song, H. Zhang and C. He, et al., Graphene sheets decorated with SnO2 nanoparticles:in situ synthesis and highly efficient materials for cataluminescence gas sensors, J. Mater. Chem., 2011, 21(16), 5972–5977 RSC.
- Y. Zhang, H. Feng and X. Wu, et al., Progress of electrochemical capacitor electrode materials:A review, Int. J. Hydrogen Energy, 2009, 34(11), 4889–4899 CrossRef CAS.
- Y. Zhang, H. Feng and X. Wu, et al., Progress of electrochemical capacitor electrode materials:A review, Int. J. Hydrogen Energy, 2009, 34, 4889–4899 CrossRef CAS.
- C. M. Chuang, C. W. Huang and H. Teng, et al., Effects of carbon nanotube grafting on the performance of electric double layer capacitors, Energy Fuels, 2010, 24, 6476–6482 CrossRef CAS.
- H. Song, H. Zhang and C. He, et al., Graphene sheets decorated with SnO2 nanoparticles:in situ synthesis and highly efficient materials for cataluminescence gas sensors, J. Mater. Chem., 2011, 21(16), 5972–5977 RSC.
- Q. Feng and H. Kanoh, Manganese oxide porous crystals, J. Mater. Chem., 1999, 9(2), 319–333 RSC.
- Y. J. Zhang, C. T. Sun and P. Lu, et al., Crystallization design of MnO2 towards better supercapacitance, CrystEngComm, 2012, 14(14), 5892–5897 RSC.
- M. Toupin, T. Brousse and D. Belanger, Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor, Chem. Mater., 2004, 16(16), 3184–3190 CrossRef CAS.
- S. L. Kuo and N. L. Wu, Investigation of pseudocapacitive charge-storage reaction of MnO2·nH2O supercapacitors in aqueous electrolytes, J. Electrochem. Soc., 2006, 153(7), A1317–A1324 CrossRef CAS.
- M. K. Song, S. Cheng and H. Chen, et al. Anomalous pseudocapacitivebehavior of a nanostructured, mixed-valent manganese oxide film for electrical energy storage, Nano Lett., 2012, 12(7), 3483–3490 CrossRef CAS PubMed.
- Y. Zhang, C. Sun and P. Lu, et al. Crystallization design of MnO2 towards better supercapacitance, CrystEngComm, 2012, 14(14), 5892–5897 RSC.
- S. K. Meher and G. R. Rao, Enhanced activity of microwave synthesized hierarchical MnO2 for high performance supercapacitor applications, J. Power Sources, 2012, 215(4), 317–332 CrossRef CAS.
- M. Y. Yang, P. Ni and Y. Li, et al. Synthesis and electrochemical performance of β-MnO2 with semi-tubular morphology, Mater. Chem. Phys., 2010, 124(1), 155–158 CrossRef CAS.
- Y. J. Zhang, C. Sun and P. Lu, et al., Crystallization design of MnO2 towards better supercapacitance, CrystEngComm, 2012, 14(14), 5892–5897 RSC.
- S. R. Majid, Controllable synthesis of flowerlike α-MnO2 as electrode for pseudocapacitor application, Solid State Ionics, 2014, 262, 220–225 CrossRef.
- M. Kim, Y. Hwang and J. Kim, Graphene/MnO2-based composites reduced via different chemical agents for supercapacitors, J. Power Sources, 2013, 239, 225–233 CrossRef CAS.
- H. Sun, L. Mei and J. Liang, et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage, Science, 2017, 356(6338), 599–604 CrossRef CAS PubMed.
- N. Kurra, Q. Jiang and H. N. Alshareef, A general strategy for the fabrication of high performance microsupercapacitors, Nano Energy, 2015, 16, 1–9 CrossRef CAS.
- J. B. Sim, S. Mayavan and S. M. Choi, Scalable thermal synthesis of a highly crumpled, highly exfoliated and N-doped graphene/Mn-oxide nanoparticle hybrid for high-performance supercapacitors, RSC Adv., 2015, 5(53), 42516–42525 RSC.
- Q. Y. Shan, B. Guan and S. J. Zhu, et al. Facile synthesis of carbon-doped graphitic C3N4@MnO2 with enhanced electrochemical performance, RSC Adv., 2016, 6(86), 83209–83216 RSC.
- S. A. Sherrill, J. Duay and Z. Gui, et al. MnO2/TiN heterogeneous nanostructure design for electrochemical energy storage, Phys. Chem. Chem. Phys., 2011, 13(33), 15221–15226 RSC.
- R. Poonguzhali, N. Shanmugam and R. Gobi, et al. Influence of Zn doping on the electrochemical capacitor behavior of MnO2 nanocrystals, RSC Adv., 2015, 5(56), 45407–45415 RSC.
- C. Pan, Y. Lv and H. Gong, et al. Synthesis of Ag/PANI@MnO2 core–shell nanowires and their capacitance behavior, RSC Adv., 2016, 6(21), 17415–17422 RSC.
- N. Subramanian, B. Viswanathan and T. K. Varadarajan, A facile, morphology-controlled synthesis of potassium-containing manganese oxide nanostructures for electrochemical supercapacitor application, RSC Adv., 2014, 4(64), 33911–33922 RSC.
- N. Kumar, A. Sen and K. Rajendran, et al. Morphology and phase tuning of α-and β-MnO2 nanocacti evolved at varying modes of acid count for their well-coordinated energy storage and visible-light-driven photocatalytic behaviour, RSC Adv., 2017, 7(40), 25041–25053 RSC.
- D. Deng, B. S. Kim and M. Gopiraman, et al. Needle-like MnO2/activated carbon nanocomposites derived from human hair as versatile electrode materials for supercapacitors, RSC Adv., 2015, 5(99), 81492–81498 RSC.
- S. Devaraj and N. Munichandraiah, Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties, J. Phys. Chem. C, 2008, 112(11), 4406–4417 CAS.
- M. Zhou, X. Zhang and J. Wei, et al. Morphology-controlled synthesis and novel microwave absorption properties of hollow urchinlike α-MnO2 nanostructures, J. Phys. Chem. C, 2010, 115(5), 1398–1402 Search PubMed.
- D. Kong, J. Luo and Y. Wang, et al. Three-Dimensional Co3O4@MnO2 Hierarchical Nanoneedle Arrays: Morphology Control and Electrochemical Energy Storage, Adv. Funct. Mater., 2014, 24(24), 3815–3826 CrossRef CAS.
- S. Devaraj and N. Munichandraiah, Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties, J. Phys. Chem. C, 2008, 112(11), 4406–4417 CAS.
- H. Wang, Z. Lu and D. Qian, et al. Single-crystal α-MnO2 nanorods: synthesis and electrochemical properties, Nanotechnology, 2007, 18(11), 115616 CrossRef.
- M. Xu, L. Kong and W. Zhou, et al. Hydrothermal synthesis and pseudocapacitance properties of α-MnO2 hollow spheres and hollow urchins, J. Phys. Chem. C, 2007, 111(51), 19141–19147 CAS.
- S. H. Yu, B. Liu and M. S. Mo, et al., General synthesis of single-crystal tungstate nanorodes/nanowires: a facile, low-temperature solution approach, Adv. Funct. Mater., 2003, 13(8), 639–647 CrossRef CAS.
- Y. Chen, M. L. Zhang and Z. H. Shi, Electrochemical and capacitance properties of rod-shaped MnO2 for supercapacitor, J. Electrochem. Soc., 2005, 152(6), A1272–A1278 CrossRef.
- H. Chen, S. Zhou and M. Chen, et al., Reduced graphene oxide-MnO2 hollow sphere hybrid nanostructures as high-performance electrochemical capacitors, J. Mater. Chem., 2012, 22(48), 25207–25216 RSC.
- N. Sui, Y. Duan and X. Jiao, et al. Large-scale preparation and catalytic properties of one-dimensional α/β-MnO2 nanostructures, J. Phys. Chem. C, 2009, 113(20), 8560–8565 CAS.
- G. Zhu, H. Li and L. Deng, et al. Low-temperature synthesis of δ-MnO2 with large surface area and its capacitance, Mater. Lett., 2010, 64(16), 1763–1765 CrossRef CAS.
- B. Xu, S. Yue and Z. Sui, What is the choice for supercapacitors: graphene or graphene oxide?, Energy Environ. Sci., 2011, 4(8), 2826–2830 CAS.
- X. Zeng, X. Zhao and H. Wei, et al. Specific Capacitance and Supercapacitive Properties of Polyaniline-Reduced Graphene Oxide Composite, Acta Phys.-Chim. Sin., 2017, 33(10), 2035–2041 Search PubMed.
- X. Zhao, Y. Wang and Y. Zhu, et al. Frequency-dependence of electric double layer capacitance of TiO2-based composite nanotube arrays, J. Electroanal. Chem., 2016, 779, 199–206 CrossRef CAS.
- Y. Chen and M. L. Zhang, et al., Electrochemical and capacitance properties of rod-shaped MnO2 for supercapacitor, J. Electrochem. Soc., 2005, 152(6), A1272–A1278 CrossRef.
- J. Gamby, P. L. Taberna and P. Simon, et al., Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors, J. Power Sources, 2001, 101(1), 109–116 CrossRef CAS.
- D. L. Fang, Z. D. Chen and X. Liu, et al., Homogeneous growth of nano-sized-Ni(OH)2 on reduced graphene oxide for high-performance supercapacitors, Electrochim. Acta, 2012, 81, 321–329 CrossRef CAS.
- L. Li, K. H. Seng and H. K. Liu, et al., Synthesis of Mn3O4-anchored graphene sheet nanocomposites via a facile, fast microwave hydrothermal method and their supercapacitive behavior, Electrochim. Acta, 2013, 87(1), 801–808 CrossRef CAS.
- Y. J. Zhang, C. T. Sun and P. Lu, et al., Crystallization design of MnO2 towards better supercapacitance, CrystEngComm, 2012, 14(14), 5892–5897 RSC.
| This journal is © The Royal Society of Chemistry 2017 |