Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Macromolecular interactions and synergy in xanthan/HPAM aqueous solutions
Shuwei Caia,
Xianru He *a,
Kun Liub,
Alisson M. Rodrigues
*a,
Kun Liub,
Alisson M. Rodrigues c and
Rui Zhang
c and
Rui Zhang d
d
aCollege of Materials Science and Engineering, State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu, 610500, China. E-mail: xrhe@swpu.edu.cn; gyfzcsw@126.com
bGeological Scientific Research Institute of Shengli Oilfield, SINOPEC, Dongying, 257015, China. E-mail: eorlab@hotmail.com
cCERTEV – Center for Research, Technology and Education in Vitreous Materials, Department of Materials Engineering, Federal University of São Carlos, 13565-905 São Carlos, SP, Brazil. E-mail: alisson_mendes@ymail.com
dInstitut für Physik, Universität Rostock, Albert-Einstein-Str. 23-24, Rostock, Germany 18051. E-mail: rui.zhang@uni-rostock.de
First published on 25th August 2017
Abstract
It is known that the rheological properties of a polymer solution are strongly dependent on the polymer structure, composition, conformation and interactions between the polymer and the solvent. Indeed, the macromolecular interactions change the polymer conformation which can result in an improvement of rheological behavior. Herein, the synergy and interactions between hydrolyzed polyacrylamide (HPAM) and xanthan were explored by rheology experiments and dynamic light scattering. The results indicated that hydrogen bonding interactions occur between the HPAM and xanthan existing in saline solution. It was showed that negative or positive synergistic effects occurred when the proportions of partially hydrolyzed polyacrylamide and xanthan were changed and the maximum positive synergy occurred when the content of xanthan was ca. 80% for the present experimental conditions. In addition, the synergy of mixed solution was markedly influenced by temperature and the HPAM molecular weight and mineralization degree. The two types of synergistic effects tended to recede with increasing shear rate in the rheological experiments. It is noted the calculation of relaxation time (t) for the mixed solutions also demonstrated an interaction between the HPAM and xanthan in aqueous solutions.
1 Introduction
Partially hydrolyzed polyacrylamide (HPAM) is widely used in tertiary oil recovery. For polymer flooding, it is well known that the viscoelasticity of the HPAM solution has significant effects on the microscopic oil displacement efficiency and can improve the oil displacement efficiency.1,2 For common HPAM, the viscoelasticity often declines and shows shear thinning when the shear rate increases. This occurs because the molecular chains are easily oriented when it flows in porous media under a higher shear rate. The reason for this is that the backbone of HPAM is flexible and its chain segments have lower flow activation energy. In addition, the carboxyl groups on HPAM are extremely sensitive to salts; especially high valence salts. It is well known that inorganic salts can compress the double electrode layer of a polyelectrolyte, shield the effective charge and weaken the static repulsion between the HPAM lateral groups. Thus the conformation becomes more constricted and the viscosity of the polymer solution decreases sharply. Moreover, a higher temperature would also decrease the apparent viscosity of HPAM.3 In summary, it is highly desirable to improve the viscoelasticity at higher salt concentrations, higher temperature and higher shear rate, and to improve the oil displacement efficiency of HPAM. Therefore, modification of HPAM is extremely necessary.Chemical modification is a conventional method that through grafting rigid or large volume lateral groups to achieve the desired improvement, e.g. salt tolerant sulfonic acid groups, hydrolyzed resistant and hydrophobic. Jiang and his co-workers4 grafted rigid lateral groups onto HPAM and made the HPAM more heat stable. In the process of aging, the backbone of HPAM may fracture, but the steric effect of the rigid lateral groups, increases the resistance to molecular motion of the macromolecules and lowers the loss of the apparent viscosity.5 In general, common rigid monomers such as styrene sulfonic acid, N-alkyl maleic imide etc. are chosen. Grafting salt insensitive sulfonic acid groups, for instance, can obviously improve the salt tolerance. Wang6 et al. synthesized a 2-acrylamide-2-methyl propane sulfonic acid (AMPS) and acrylamide copolymer and obtained a temperature salt-tolerant copolymer. It is benefit from special molecular structure of AMPS. Chang7 et al. synthesized the AMPS/AM copolymer at low temperature with a redox initiation system and achieved relatively high molecular weight copolymer with good temperature salt-tolerant properties. Furthermore, introduction of the hydrolysis resistance group can enhance the temperature salt-tolerant properties to some degree. Hydrophobically associated polymer is a kind of supermolecule that enhances its properties by bringing in hydrophobic group onto lateral chain. It has been shown to be an effective method for HPAM improvement. Ye8 et al. synthesized a hydrophobically associated polymer and found a physical cross-linking network formed in aqueous solution and displaying a good effect. In addition, in saline solution, an electrolyte such as sodium chloride can enhance hydrophobicity and increase the hydrodynamic volume. This may increase solution viscosity. Though these ways have been realized in the lab over the years, its radical based synthetic mechanism leads to uncontrollable products, complicated processes and then high cost of industrial manufacture. In particular, the side products cause serious environment pollution, which is difficult to handle. Therefore, in order to keep a stable yield, increase production and solve the contradiction between oil yield and market demand, developing a simple, eco-friendly and low-cost technology for the oil displacement agent is essential.
Polysaccharides are a kind of condensation compound that consist of five or six membered ring repetitive units.9–11 The ring repetitive unit is bioside and contains a large amount of hydroxy. Because of the special structure of the ring, polysaccharide molecules are more rigid and shear tolerant compared to HPAM.12,13 Besides, polysaccharide also has good salt tolerance and thickening effects in solutions. Molecular construction, conformation, and interaction of polymers may have radical effects on the physical–chemical properties of polymer solution. Similar to HPAM, polysaccharides have a large number of carboxyl and acylamino groups. Taking advantage of hydrogen bonding interactions between polysaccharide and HPAM may improve the rheological properties of their blend solutions. Furthermore, as a high molecular weight exopolysaccharide produced by the bacterium Xanthomonas campestris, xanthan (XG) is widely used in the food industry and oil fields. It is most important properties are high viscosity at low shear rates and good resistance to shear degradation. Xanthan molecules may become rigid when amount of an electrolyte is added and the side chains (shown in Fig. 2 below) twine around the backbone of xanthan.
The viscoelastic behavior of the aqueous solution compound of poly(acrylic acid) and poly(acrylamide) has been widely investigated. On the contrary, the viscoelastic behavior of the aqueous solution compound of XG and poly(acrylamide) has been rarely investigated. Poly(acrylic acid) is a type of polyelectrolyte and its molecular conformation is very sensitive to both the pH of solution and the ionic strength. Generally, the pH of solution and the ionic strength could significantly influence both the intermolecular interaction of poly(acrylic acid) and the viscoelastic behavior of its compounds.14–18 Because of its unique molecular structure, XG is insensitive to both the pH of solution and the ionic strength. Partially hydrolyzed polyacrylamides, used as an oil displacement agent, only has a little amount of carboxyl groups. As a result, the sensitivity to the pH of solution of the aqueous solution compound of XG and HPAM is relatively low. Therefore, solution of the aqueous solution compound of XG and HPAM are prepared in neutral conditions.19–21 During the practical application in the oil filed, the oil displacement agents are generally mixed with saline solutions and the requirement of the concentration of the oil displacement agent is 1000–2000 mg L−1. In real oil fields, the main salt elements are sodium salts, magnesium salts and calcium salts. According to the requirement of the practical application, the effect of concentration and types of salt on the viscoelastic behavior of XG/HPAM aqueous solution has been studied in this article. In addition, the effect of the molecular weight of HPAM on the viscoelastic behavior of aqueous solution has also been studied. Three typical molecular weight of HPAM, which is commonly used in real oil field has been adopted.
HPAM has excellent viscosity but poor temperature tolerance and salt resistance, while XG has good temperature tolerance and salt resistance but lower viscosity than that of HPAM. In case of that there are hydrogen bonds between XG and HPAM under neutral conditions, HPAM and XG can form some assemble structures in aqueous solution. In this study, an aqueous solution compound consists of XG and HPAM has been prepared to improve the temperature tolerance and salt resistance of oil displacement agents. The positive synergistic effect in aqueous solution is expected, so synergistic effects were investigated under low shear rates state in this study, because it is convenient to illustrate the interaction between XG and HPAM and the hydrodynamic radii of their blends.
Furthermore, studying the synergistic effect and the rheology under shear can provide the basis for application of these two mixed oil displacement agents, which has better salt resistance and shearing resistance than traditional oil displacement agents.
Through blending these two types of oil displacement agents, we expect to be synergistic effects in aqueous solution and with their respective advantages complementary to each other. Furthermore, studying the synergistic effect and the rheology under shear can provide the basis for application of these two mixed oil displacement agents, which has better salt resistance and shearing resistance than traditional oil displacement agents.
2 Experiments
2.1 Materials
Three industry grade partially hydrolyzed polyacrylamides (HPAM) was provided by the Geological Research Institute of Shengli Oil Field, China, the hydrolysis degree (HD) was ca. 27% and weight-average molecular weight (measured by light scattering) were 2.3 × 107 g mol−1, 1.3 × 107 g mol−1, 5.0 × 106 g mol−1, respectively. These three HPAM samples are all well-applied industrial products. The HPAM can be used after further purification. Salts (NaCl, MgCl2 and CaCl2) were purchased from Chengdu Kelong Chemical Agents Co. (PR China), analytically pure. Xanthan (food grade, WM = 2.1 × 106 g mol−1) was obtained from Guangfu Fine Chemical Engineering Institution (Tianjing, PR China). The water used in the preparation of solutions was homemade ultrapure water.2.2 Preparation of sample solutions
HPAM and xanthan were dried at 30 °C in a drying closet before use. The solvents were saline solutions with concentrations of 0.05 mol L−1, 0.1 mol L−1, 0.15 mol L−1 and 0.2 mol L−1. The dissolving temperatures of HPAM and XG were consistently set at 35 °C with a slow rate mechanical stirring for 3 days. They were then removed and XG was stored at 4 °C in a refrigerator before use. Mother solutions with various volumes were then mixed to obtain desired blend solution. Sample solutions were left overnight to equilibrate before being used.2.3 Relative viscosimetry measurements
Viscosimetry measurements for a series of HPAM/XG solutions were carried out with an Ubbelohde viscometer (globule volume 4 mL, capillary diameter 0.65 mm) in a water bath with automatic temperature control. All the measurements were conducted at 25 °C. Calibration was done at 25 °C: acetone (ρ = 0.7851 g mL−1; η = 3.075 × 10−3 Pa s) and n-butyl alcohol (ρ = 0.8051 g mL−1; η = 2.6390 × 10−2 Pa s) and the calibration parameters (A = 0.000202420996; B = 0.013729785) were obtained. All the relative viscosities were calculated by eqn (1) as follows:| ηr = (At − Bt)/(Ats − Bts) | (1) |
2.4 Shear rheology tests
The rheology tests using a peak hold model, steady state flow model and oscillation model were conducted using an AR-2000ex Rheometer (TA Instruments Co., USA).40 mm diameter standard steel parallel plate was used, the gap was set at 200 micrometer and the equilibrium time was 1 min. All the data were taken with log–log coordinate. The experiment parameters for the steady state model: shear rate were 0.1–100 s−1; for the oscillation model: angular frequency: 0.1–50 Hz, oscillation stress 0.1 Pa. All the measurements were implemented at 25 °C.22–24 The discrete relaxation time was calculated by Rheology Advantage Data Analysis supermolecular (product version v5.7.0; file version v5.7.0, TA Instruments Co., USA). Continuous relaxation time spectra were calculated and processed by Matlab (version 7.8.0.347(R2009) win64, MathWorks company) and Originpro 8.0 software (OriginLab company).2.5 DLS measurements
The hydrodynamic radius for all polymer samples were measured by a BI-200SM wide-angle laser scattering at 25 °C, the wavelength of the monochromatic laser light was 532 nm, delay time was constant, 3 minutes, detection angle was 90°, pin hole size was 2 μm and the transmittance was 50%. All of the NaCl solvents were filtered with 0.45 μm filter and all the samples were filtered with a 0.22 μm Millipore filter (Navigator, USA) to eliminate dust to the best of our ability.3 Results and discussion
3.1 Synergy study of XG/HPAM solutions in a Ubbelohde viscometer
For a single component polymer solution, its apparent viscosity increases with increasing polymer concentration. The contribution of a single macromolecule to the solution could be measured as the specific viscosity calculated at 0 concentration by extrapolation. For a solution of two polymers without specific molecular interactions between the polymers, the relative viscosity, ηr,m, can be calculated as a weight average of the viscosities of the individual components:| ηr,m = (CAηA + CBηB)/C0 | (2) |
| ηr,i = ηr,exp − ηr,m | (3) |
| R = ηr,exp/ηr,m − 1 | (4) |
 | ||
| Fig. 1 Viscosity enhancement factor versus weight percentage of XG (t = 25 °C; CNaCl = 0.2 mol L−1). | ||
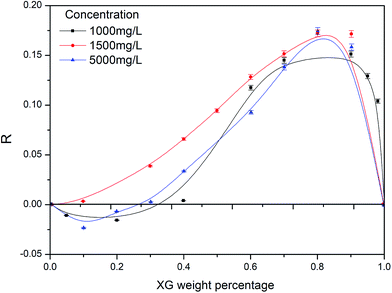
As shown in Fig. 1, when the content of XG was low, the value of ηr,exp was smaller than their ηr,i's, especially for total polymer concentration(Cp) of 1000 mg L−1 and 2000 mg L−1. According to the result of Rahbari,17 this indicates that HPAM and XG had negative synergy, viscosity of blend solution decreased slightly. However, in the high XG content area, the viscosity increased and showed positive synergy. When the XG weight percentage was equal to 0.8, all three solutions showed the maximum positive synergy. A possible explanation is: interaction of two types polymers may be influenced by solvation and intermolecular hydrogen bonds. On one hand, the hydrophility of XG was better than that of HPAM. This was determined by the large numbers of hydroxy groups on the XG backbone and side chain. The molecular structures of HPAM and XG are shown in Fig. 2. The addition of XG may change the solvent properties and decrease the free water molecules around HPAM, that equals to increasing the hydrophobicity of HPAM.26
On the other hand, large numbers of XG molecules are absorbed onto HPAM coil by hydrogen bonding, and a supermolecular structure forms.29 Fig. 1 reveals that it is the result of competition of these two factors with each other. The probability of formation of supermolecular structure is low and the former interaction had the dominant position when the content of XG in solution was low. Therefore, the conformation of HPAM became more constricted, the hydrodynamic volume decreased and a negative synergy occurred. With increasing content of XG, the XG could be absorbed onto HPAM coil due to hydrogen bonding. Thus, the hydrodynamic volume increased, as well as the viscosity of the solution. However, when the content of XG was more than 80%, the positive synergistic effect weakened due to reduction of the number of the supermolecular structures.
In order to further investigate effects of coil size on the synergistic effects, experiments were carried out with different molecule weight HPAM samples to study the interaction and their rheology behavior. The results are shown in Fig. 3.
 | ||
| Fig. 3 Viscosity enhancement factor versus weight percentage of xanthan gum (t = 25 °C, Cp = 2000 mg L−1, CNaCl = 0.2 mol L−1). | ||
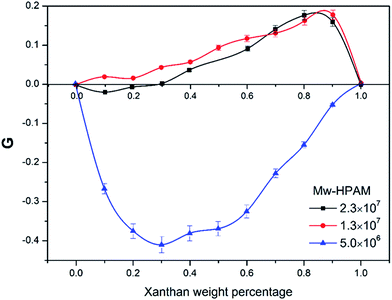
For mixed solutions with the HPAM molecular weight of 2.3 × 107 g mol−1, the former interaction had a significant effect on hydrodynamic volume of HPAM coil due to its large molecule size. This may explain why there was a negative synergic effect in low XG content area. Nevertheless, when the molecular weight of HPAM was 1.3 × 107 g mol−1, the HPAM coil size was smaller than that of the 2.3 × 107 g mol−1 sample, and the variation of the solvent property had lesser effect on the HPAM and the formation of supermolecular structures held the dominant position. As a consequence, the viscosity of solutions for all concentrations of the XG increased and showed a positive synergic effect. However, when the HPAM molecular weight relative to 5 × 106 g mol−1, the number of carbonyl groups on the side of the HPAM lateral chain decreased, as well as the coil size, so that formation of supermolecular structures through hydrogen bonds becomes difficult, thus the former interaction becomes more obvious.29,30 In addition, the solution behavior of the polymers in different types of the salt solutions is shown in Fig. 4.
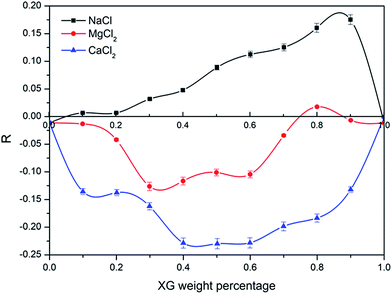 | ||
| Fig. 4 Viscosity enhancement factor versus weight percentage of xanthan gum (25 °C, Cp = 2000 mg L−1). | ||
It indicated that divalent cations caused the solution viscosity to decrease compared with monovalent cations when the salt concentration was 0.2 mol L−1 for all XG concentrations. Both XG and HPAM are anionic polyelectrolyte, but the XG molecular structure is more rigid compared with HPAM. Thus, the HPAM coil size is significantly impacted by the cation, especially divalent cations. Because the shielding effect of divalent cations is stronger than that of monovalent cations, the HPAM coil become more contracted, even though a supermolecular structure forms; the size decrease is more than the increment of complex formation. That results in negative synergy appearing. What is noteworthy is that the absolute value of R for CaCl2 was bigger than that for MgCl2, which might be related with intramolecular bridging of Ca2+ via electrostatic attraction.31
3.2 Synergy study of XG/HPAM solution in a torque rheometer
A torque rheometer was used to observe the effects of shear of the HPAM; based on the results with the Ubbelohde viscometer, the shear rheology behavior of the HPAM molecular weight 2.3 × 107 g mol−1 HPAM blend solutions was investigated. A series of viscosity under different shear rates were measured, with the value of R were displayed in Fig. 5. The Fig. 5 shows the R value of the 2000 mg L−1 blend solution under different shear rates (0.25–73 S−1) as a function of XG weight percentage. It was found that with increasing XG weight percentage, the synergy effect changed from negative synergy to positive synergy with the variation of the shear rate. The biggest positive synergy appeared at ca. 80%, this accorded with results of the Ubbelohde viscometer measurements. Furthermore, with increasing shear rate, the absolute value of R decreased for all compositions of the XG. This may be ascribed to the shear stress damaging supermolecular structure in the high XG content area. The higher the shear rate, the stronger was the damage degree. However, the reason why a high shear rate caused the absolute value of R to decrease in low XG content area needs our further study. Possibly, it can be explained by a relationship between mechanical stirring and hydration.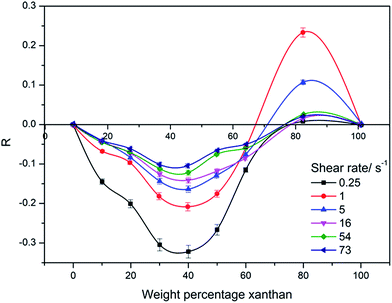 | ||
| Fig. 5 Viscosity enhancement factor versus weight percentage of xanthan gum under different shear rate (25 °C, Cp = 2000 mg L−1, CNaCl = 0.2 mol L−1). | ||
On the basis of the results above, the blend solution whose XG content in the HPAM/XG was 80% was selected to investigate the effects of the concentrations of the saline solutions on cooperative behavior. Referring to Zhang et al.'s work,28 Rh of HPAM decreased by 25% when the concentration of NaCl increased from 0.02 to 0.2 mol L−1, however, it decreased only by 30% when the concentration increased from 0.02 to 1 mol L−1. This demonstrated that the influence of the salt concentration on the viscosity mainly occurred when the concentration of the NaCl was less than 0.2 mol L−1. The viscosity enhancement factor for 80% XG content blend solution at different shear rates as a function of NaCl concentration is displayed in Fig. 6. It can be seen that an obvious negative synergy appeared for the NaCl concentration of 0.05 mol L−1. Then it changed to a positive synergy with the absolute value of R decreasing gradually. According to Meada et al.' work,32 hydrogen bonding between polymers would be broken down if a small quantity of inorganic salt was added, and then supermolecular structures were breaking down. For HPAM, the Na+ in aqueous solution can shield static repulsion effects on polyelectrolyte lateral groups. Thus, the coil conformation of HPAM would contract. However, for rigid XG molecules, the electrostatic shielding effect of Na+ causes its lateral groups on the main chains to twine around the backbone, and due to this behavior, the conformation of XG converts into a rigid rod, the hydrophilic groups on the backbone are shielded and the hydrophobicity effect of XG increases.9,10,33 Therefore, the intermolecular hydrogen bonding might be partially damaged when the sodium chloride concentration was 0.05 mol L−1 and there was little supermolecular structure in the blend solution; the first interaction above would hold the dominant position, resulting in the negative synergy. When more salt was added, the hydrophobicity of XG increased further and the XG molecule aggregated on the two HPAM coils.33 Many rigid XG molecules had a tendency to parallelly and densely arrange around HPAM coil.34,35 As a consequence, positive synergy appeared. However when the concentration of NaCl was more than 0.1 mol L−1, the electrostatic shielding and the HPAM conformation contraction increased sufficiently that the positive synergy tended to decrease.
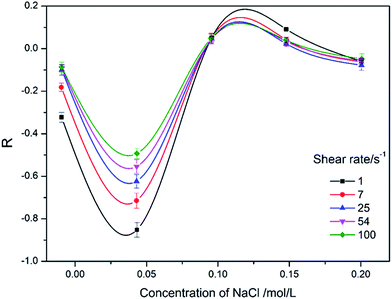 | ||
| Fig. 6 Viscosity enhancement factor versus sodium chloride concentration under different shear rate (25 °C, Cp = 2000 mg L−1). | ||
The Fig. 7 exhibits the effects of temperature on viscosity enhancement factor. With rising salt concentration, the variation tendency of R was similar to that in the curves in Fig. 6. The reasons have been described above. For a lower concentration (at 0.05 mol L−1), the absolute value of R increased first and then decreased with the increase of temperature. Hydrogen interactions can form more easily between amino and carbonyl groups than between hydroxyl and carbonyl groups at 40–45 °C.36–38 It makes the intramolecular hydrogen bonding partly replace the hydrogen bonding between water molecules and HPAM. Thus it makes HPAM coil contractive and decreases solution viscosity. When the temperature was higher than 45 °C, the intramolecular hydrogen bonding between amino and carbonyl in HPAM has been gradually destroyed with the increasing of temperature. The hydrodynamic radius of HPAM increases. The viscosity of the solution increases. So the negative synergistic effect weakened gradually, and the positive synergistic effect increased gradually.
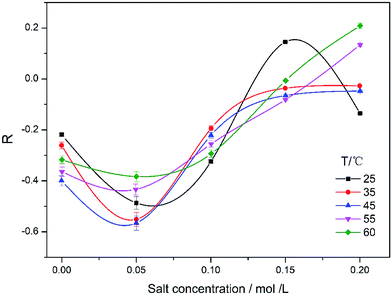 | ||
| Fig. 7 Viscosity enhancement factor versus sodium chloride concentration under different temperature (Cp = 2000 mg L−1). | ||
3.3 Effect of different mixture ratio of HPAM-XG on its viscoelastic properties
The viscosity of a polymer is the result of the large scale molecular motions. It is correlated with the hydrodynamic volume of the polymer and its intermolecular interactions. The modulus of the blend solutions with different XG contents versus angular frequency is shown in Fig. 8. Fig. 8(a) illustrates the dependence of the G′ on the angular frequency in term of the log–log coordinate. The G′ of the pure XG solution was the largest while the G′ of the pure HPAM solution was the lowest over the entire frequency range; the G′ increased continuously with the increase of the XG content. It indicates that the value of the G′ depended on the content of the XG in this system.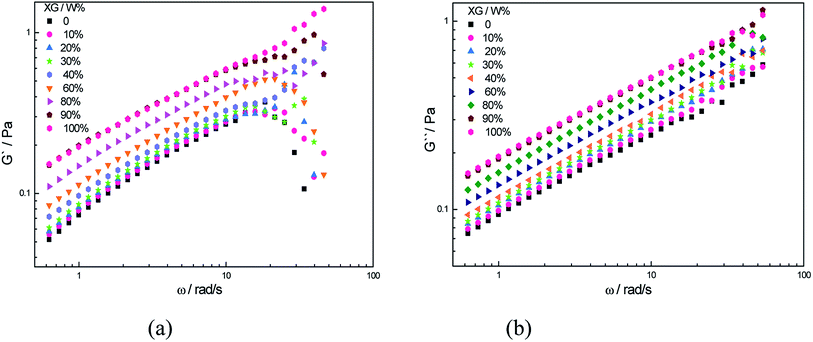 | ||
| Fig. 8 Dependence of the G′ and G′′ on angular frequency (T = 25 °C, C = 2000 mg L−1, CNaCl = 0.2 mol L−1, Mw = 2.3 × 107 g mol−1). | ||
When the XG content was low, especially lower than 30%, the G′ of the blend solutions were nearly the same as the value of the pure HPAM, and in this case, there was only a small amount of the absorbed XG in the above discussion, and the polymer coils could deform through the variations of the HPAM conformation when subjected to the action of shear force. Therefore, G′ of the solution was similar to that of the pure HPAM solution. When the XG content was higher than 30%, the flexible part of HPAM with no absorbed XG, relative. So the polymer deformation became difficult compared with the preceding situation. Thus the G′ of blend solutions increased in a large extent. When the content of XG was more than 80%, the G′ of pure XG and blend solution overlapped. The possible reason was that HPAM coil was encased by the XG molecules, a supermolecular structure was formed and its ability to resist deformation was mainly controlled by the XG. In addition, Fig. 8(b), a plot of the G′′ vs. the angular frequency for the same set of the solutions showed the same variation pattern. This is in accord with synergy study and indicates that an interaction by hydrogen bond between HPAM and XG was confirmed. The viscoelasticity of solution was changed by the molecular interactions.
The Fig. 9 shows the apparent viscosity of the different blend ratio HPAM/XG solutions versus shear rate via log–log coordinates. It was obvious that with increasing shear rate, the apparent viscosity of all the solution decreased, exhibiting shear thinning behavior. In the area of the negative synergy (the XG content < 30%), the apparent viscosity of HPAM/XG solution approached the pure HPAM. When the XG content was more than 40%, the viscosity increased. This, thus, corroborates the Ubbelohde viscometer measurements.
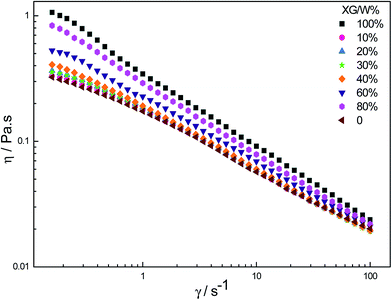 | ||
| Fig. 9 The dependence of apparent viscosity, η, on shear rate (T = 25 °C, C = 2000 mg L−1, CNaCl = 0.2 mol L−1, Mw = 2.3 × 107 g mol−1). | ||
The apparent viscosities of HPAM, HPAM/XG and XG in different salinity aqueous solutions are displayed in Fig. 10, respectively. The Fig. 10(a) illustrates the apparent viscosity of HPAM as a function of shear rate for different NaCl concentrations. With rising Na+ concentration in the aqueous solutions, the electrostatic shielding effect for the HPAM side chains was strengthened, and the conformation became more contracted, and the viscosity decreased, showing a worse salt resistance. In Fig. 10(b), the viscosity of the XG in saline solution was higher than that in an aqueous solution for the reason that the electrostatic shielding effect by Na+ resulted in its side chains winding around the backbone and forming intermolecular two-dimensional ordinal rod structures by hydrogen bonds.9,10 However, for the blend solutions in Fig. 10(c), its viscosity decreased and then increased with NaCl concentration. As discussed above relative to the Fig. 6, the negative synergy appeared for low NaCl concentration. There existed supermolecular structure in solution, and the contracted coils of the HPAM caused the viscosity to decrease. When the NaCl concentration was further increased, the positive synergy and the viscosity were increased.
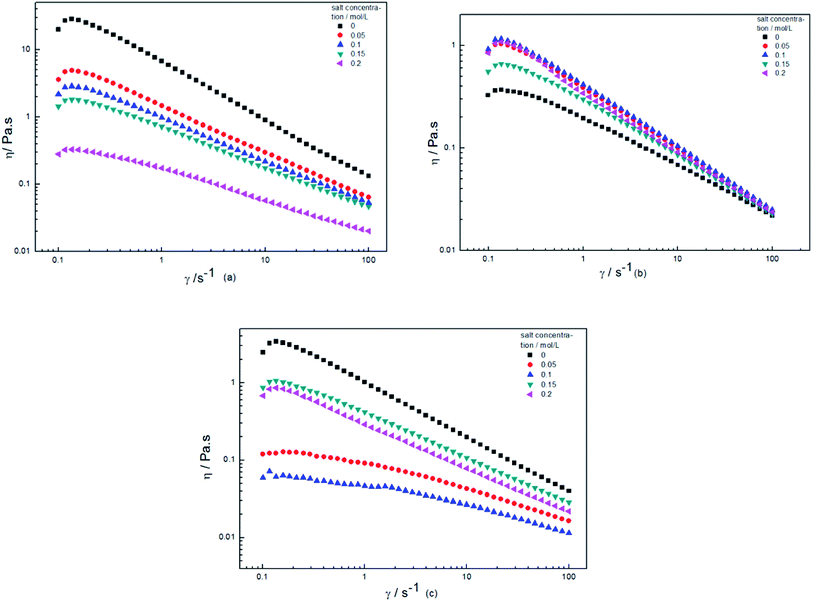 | ||
| Fig. 10 The dependence of apparent viscosity η on shear rate, (a) HPAM, (b) XG, (c) blend solution (80% XG) (C = 2000 mg L−1, Mw = 2.3 × 107 g mol−1, 25 °C). | ||
3.4 Hydrodynamic diameter distribution of the HPAM/XG solution
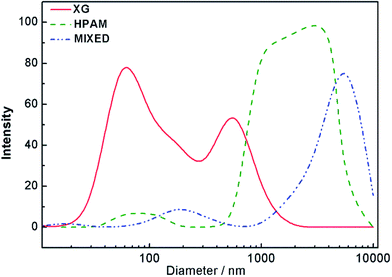
In order to obtain further information concerning the interaction of two polymers, dynamic light scattering experiments were carried out to measure their hydrodynamic size. The hydrodynamic diameter of the HPAM, the XG and their mixture in the solution were tested and is displayed in Fig. 11. The hydrodynamic diameter of XG was lower corresponding to its relatively small molecular weight; its mean diameter was ca. 1610 nm at the concentration of ca. 2000 mg L−1. HPAM hydrodynamic diameter was ca. 3050 nm. However, the hydrodynamic diameter was 4470 nm for the blend solution at the same total concentration, being higher than those of the pure components. These results further confirmed that there was a strong interaction between the HPAM and the XG and that aggregates were formed via intermolecular hydrogen bonding; it was also in accordance with the above discussion of synergy.There are many studies of other interpolymer complexes using anisotropic techniques that study the decreased relaxation due to hydrogen bonding between polymers. Relaxation time can reflect the interpolymer complexes. Based on the corresponding oscillation experimental data in Fig. 8, discrete relaxation spectra were calculated. The results are listed in Table 1. The relaxation time τ includes two parts: relaxation times of the coils and relaxation times of the chain segments. The coil relaxation time had a bigger value of τ due to its large size whereas the segmental relaxation time had a smaller τ. The size of the motion units determines the value of τ. From Table 1, it could be seen that the relaxation time of the coil in the blend solution was longer than that for the pure HPAM, this also indicated a larger coil size in blend solution compared with pure HPAM solution.
| Pure HPAM | 40% XG | 80% XG | |
|---|---|---|---|
| τ of segment(s) | 1.97 × 10−3 | 1.97 × 10−3 | 1.83 × 10−3 |
| τ of coil(s) | 0.06416 | 0.1122 | 1.384 |
In order to obtain further evidence of the relaxation times and their distribution, the relaxation spectra were calculated according to our previous study.39–42 The relaxation time spectra were calculated using eqn (5) and (6) (ref. 43) and the corresponding oscillation experimental data in the Fig. 8.
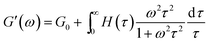 | (5) |
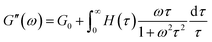 | (6) |
The Fig. 12 showed a continuous relaxation time spectrum for the pure HPAM solution, the blend solution and the pure XG solution. In the Fig. 12, there are two peaks corresponding to smaller motion units which have a short relaxation time. This most probable distribution represents the segmental relaxation time distributions of the molecules or supermolecules. Because the motion units were of various compositions, it had a large distribution. The peaks in the middle of the curve probably represented the relaxation times of the distorted complete coils under shear stress. This part contained several peaks and illustrated that the distortion of coil was staged. The coil relaxation time or the chain relaxation time was longer and had a small distribution due to their large size and small amount. Moreover, the molecular distribution would broaden the peaks. It was worth noting that the relaxation time distribution of the blend solution was the widest. Thus it manifested a bigger motion unit in blend solutions.
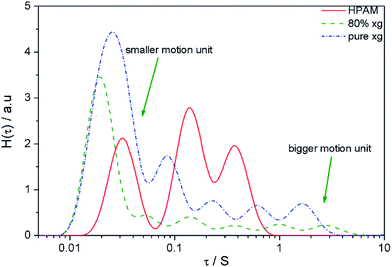 | ||
| Fig. 12 Continuous relaxation time spectra for pure HPAM, mixed solution and pure xanthan solution obtained from corresponding oscillation data. | ||
4 Conclusions
Ubbelohde viscometry, torque rheometry and laser light scattering were used to study the interactions between HPAM and XG and their rheology behavior in saline solutions. The main conclusions are as follows:(1) Hydrogen bond interaction between HPAM side groups and XG was found in saline solution. A negative synergy occurred when XG content was less than 30%; however, an obviously maximum positive synergy effect occurred when the XG content was 80% and the total polymer concentration was 2000 mg L−1.
(2) The synergy effects were affected by temperature, molecular and degree of mineralization. The higher the molecular weight, the more obvious the synergy was; however, only the negative synergy was observed when the molecular weight is 5.0 × 106 g mol−1. Greater positive synergy was observed at higher NaCl concentration. At the same NaCl concentration, the negative synergy had a tendency to increase firstly and then decrease with increasing temperature. The tendency of positive synergy was opposite to the former.
(3) It showed negative synergy at lower mineralization and a positive one at higher NaCl concentration when XG content is 80%. In addition, good salt resistance of the blend solutions was confirmed.
(4) The two types of synergy effects tended to recede with increasing shear rate. We suggest this may have been caused by hydrogen bond damage.
Conflicts of interest
There are no conflicts to declare.References
- H. F. Xia, Viscoelastic polymer solution study for flowing in reservoir and improving micro mechanism of oil displacement, PhD Dissertation, Daqin Petroleum Institution, Daqin, China, 2001.
- H. F. Xia, Study On The Mechanism Of Polymer Solution With Visco Elastic Behavior Increasing Microscopic Oil Displacement Efficiency, Acta Pet. Sin., 2001, 22, 60–65 CAS.
- M. J. He, H. D. Zhang, W. X. Chen and X. X. Dong, Introduction of Polymer Physics, Fudan University Press, Shanghai, 2006 Search PubMed.
- T. Jiang and G. C. Xin, Research Progress of Polyacrylamide for Thrice Oil Production, Adv. Fine Petrochem., 2000, 1, 30–32 Search PubMed.
- L. V. And and R. G. Gilbert, Electrosteric Stabilization with Poly(Acrylic) Acid in Emulsion Polymerization: Effect on Kinetics and Secondary Particle Formation, Macromolecules, 2000, 33, 6693–6703 CrossRef.
- Z. H. Wang, Water-soluble AMPS copolymers for use in fields of China, Oilfield Chem., 1999, 16, 81–85 Search PubMed.
- Z. Chang, F. Peng, J. Nie, H. Chen and M. Li, Synthesis of AMPS/AM copolymer at low temperature and its properties, Polym. Mater.: Sci. Eng., 1997, 13, 16–20 CAS.
- Y. Lin and R. H. Huang, Synthesis and studies on the solution properties of AM-AMPS-DMDA hydrophibic ampholytic copolymer, Polym. Mater.: Sci. Eng., 1998, 14, 67–70 Search PubMed.
- L. Wu, T. Xu, X. Han and X. Pan, Research on high temperature stability effects of xanthan gum, Drill. Fluid Completion Fluid, 2011, 28, 77–80 CAS.
- R. Guo and E. Y. Ding, Structure performance and applications of xanthan gum, China Surfactant Deterg. Cosmet., 2006, 36, 42–45 CAS.
- K. W. Song, Y. S. Kim and G. S. Chang, Rheology of concentrated xanthan gum solutions: Steady shear flow behavior, Fibers Polym., 2006, 7, 129–138 CrossRef.
- C. Haem and Y. Byoungseung, Steady and dynamic shear rheology of sweet potato starch-xanthan gum mixtures, Food Chem., 2009, 116, 638–643 CrossRef.
- S. Maurer, A. Junghans and T. A. Vilgis, Impact of xanthan gum, sucrose and fructose on the viscoelastic properties of agarose hydrogels, Food Hydrocolloids, 2012, 29, 298–307 CrossRef CAS.
- G. A. Mun, Z. S. Nurkeeva, V. V. Khutoryanskiy, G. S. Sarybayeva and A. V. Dubolazov, pH-effects in the complex formation of polymers I. Interaction of poly(acrylic acid) with poly(acrylamide), Eur. Polym. J., 2003, 39, 1687–1691 CrossRef CAS.
- Y. Shen, X. Zhang, J. Lu, A. Zhang, K. Chen and X. Li, Effect of chemical composition on properties of pH-responsive poly(acrylamide-co-acrylic acid) microgels prepared by inverse microemulsion polymerization, Colloids Surf., A, 2009, 350, 87–90 CrossRef CAS.
- M. Kowblansky and P. Zema, Interactions of sodium ions with the sodium salts of poly(acrylic acid/acrylamide) copolymers of varying charge density, Macromolecules, 1981, 14, 166–170 CrossRef CAS.
- R. Rahbari and J. Francois, Interactions between aluminium ions and acrylic acid–acrylamide copolymers in aqueous solution: 3. Influence of ionic strength on gelation and phase separation, Polymer, 1992, 33, 1449–1458 CrossRef CAS.
- R. Rahbari and J. François, Interactions between aluminium ions and acrylic acid–acrylamide copolymers in aqueous solution: 1. Study by 27 Al nuclear magnetic resonance, Polymer, 1988, 29, 845–850 CrossRef CAS.
- H. Kheradmand, J. François and V. Plazanet, Hydrolysis of polyacrylamide and acrylic acid–acrylamide copolymers at neutral pH and high temperature, Polymer, 1988, 29, 860–870 CrossRef CAS.
- K. D. Branham, H. S. S. And and C. L. Mccormick, Water-Soluble Copolymers. 64. Effects of pH and Composition on Associative Properties of Amphiphilic Acrylamide/Acrylic Acid Terpolymers, Macromolecules, 1996, 29, 254–262 CrossRef CAS.
- W. R. Cabaness, Y. C. Lin and C. Párkányi, Effect of pH on the reactivity ratios in the copolymerization of acrylic acid and acrylamide, J. Polym. Sci., Part A-1: Polym. Chem., 1971, 9, 2155–2170 CrossRef CAS.
- X. U. Guan-Li, G. Sun, Z. B. Shao, C. F. Shi and J. I. Bing-Yu, Study on Aqueous Solution Viscosity and Elasticity of Blends of Commercial Partially Hydrolyzed Polyacrylamides with Different Relative Molecular Mass, Oilfield Chem., 2006, 23, 341–344 Search PubMed.
- Z. B. Shao, J. S. Zhou, S. Gang, J. G. Niu, F. Q. Liu and Z. R. Han, Studies on Mechanical Degradation of Partially Hydrolized Polyacrylamide in Course of Polymer Flooding: Changes in Relative Molecular Mass, Viscosity and Related Parameters, Oilfield Chem., 2005, 22, 72–77 Search PubMed.
- D. L. Zhang, Y. Q. Li and Z. D. Bao, A laboratory experimental study of feasibility of polymer flood for middle-low permeability reservoirs in Daqing, Oilfield Chem., 2005, 22, 78–87 Search PubMed.
- X. Lu and R. A. Weiss, Solution behavior of lightly sulfonated polystyrene and poly(styrene-co-4-vinylpyridine) complexes in dimethylformamide, Macromolecules, 1991, 24, 5763–5768 CrossRef CAS.
- L. Ting and G. Shan, Viscometric Studies On The Interaction Between Polyacrylamide And Poly(Ethylene Glycol) In Water, Acta Polym. Sin., 2010, 010, 156–159 CrossRef.
- X. P. Kong, J. Wang and H. T. Lv, Study of the interaction of between polyacrylamide and poly(ethlene glycol) in aqueous solution by using apparent viscosity method, Res. Explor. Lab., 2012, 31, 29–31 CAS.
- Q. Zhang, J. S. Zhou, Y. A. Zhai, F. Q. Liu and G. Gao, Effect of salt solutions on chain structure of partially hydrolyzed polyacrylamide, J. Cent. South Univ., 2008, 15, 80–83 CrossRef.
- H. T. Oyama, W. T. Tang and C. W. Frank, Complex formation between poly(acrylic acid) and pyrene-labeled polyethylene glycol in aqueous solution, Macromolecules, 1987, 20, 422–433 CrossRef.
- H. T. Oyama, W. T. Tang and C. W. Frank, Effect of the Hydrophobic Interaction in the Poly(methacrylic acid)/Pyrene End-Labeled Poly(ethylene glycol) Complex, Macromolecules, 1986, 20, 1839–1847 CrossRef.
- X. Xin, G. Xu, D. Wu, Y. Li and X. Cao, The effect of CaCl 2 on the interaction between hydrolyzed polyacrylamide and sodium stearate: Rheological property study, Colloids Surf., A, 2007, 305, 138–144 CrossRef CAS.
- Y. Maeda, A. Tomomi Higuchi and I. Ikeda, Change in Hydration State during the Coil–Globule Transition of Aqueous Solutions of Poly(N-isopropylacrylamide) as Evidenced by FTIR Spectroscopy, Langmuir, 2000, 16, 7503–7509 CrossRef CAS.
- Y. Zeng, Relationship between salt resistance and structure of polysaccharide, PhD Dissertation, Guangdong University of Technology, Guangzhou, China, 2002.
- M. Jiang, A. Eisenberg, G. Liu and X. Zhang, Macromolecular self-assembly, Science Press, Beijing, 2006 Search PubMed.
- S. A. Jenekhe and X. L. Chen, Self-assembled aggregates of rod-coil block copolymers and their solubilization and encapsulation of fullerenes, Science, 1998, 279, 1903 CrossRef CAS PubMed.
- S. A. Jenekhe and X. L. Chen, Self-Assembly of Ordered Microporous Materials from Rod–Coil Block Copolymers, Science, 1999, 283, 372 CrossRef CAS PubMed.
- Y. Katsumoto, T. Tanaka and Y. Ozaki, Relationship between the coil-globule transition of an aqueous poly(N-isopropylacrylamide) solution and structural changes in local conformations of the polymer, Macromol. Symp., 2004, 205, 209–224 CrossRef CAS.
- Y. Katsumoto, T. Tanaka, H. Sato and Y. Ozaki, Conformational Change of Poly(N-isopropylacrylamide) during the Coil−Globule Transition Investigated by Attenuated Total Reflection/Infrared Spectroscopy and Density Functional Theory Calculation, J. Phys. Chem. A, 2002, 106, 3429–3435 CrossRef CAS.
- X. He, R. Zhang, K. Liu, S. Cai and G. Huang, Rheological behaviors and molecular motions of semi-diluted Xanthan solutions under shear: Experimental studies, Polym. Sci., 2014, 56, 687–696 CAS.
- S. Cai, X. He, K. Liu, R. Zhang and L. Chen, Interaction between HPAM and urea in aqueous solution and rheological properties, Iran. Polym. J., 2015, 1–8 Search PubMed; S. W. Cai, H. G. Zhao, T. X. Li, X. R. He, X. Wang, A. M. Rodrigues and R. Zhang, Influence of molecular interplay on the HPAM/UR rheological properties in an aqueous solution, RSC Adv., 2017, 7, 37055 RSC.
- R. Zhang, X. He, S. Cai and K. Liu, Rheology of Diluted and Semi-diluted Partially Hydrolyzed Polyacrylamide Solutions under Shear: Experimental Studies, Petroleum, 2016 DOI:10.1016/j.petlm.2016.08.001.
- R. Zhang, X. He and H. Yu, Why tanδ of poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric?, Polymer, 2014, 55, 4720–4727 CrossRef CAS.
- J. D. Ferry, Viscoelastic properties of polymers, Wiley, 1980, New York Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |